Abstract
Changes in airway nerves associated with chronic inflammation may underlie the pathogenesis and symptoms of lower airway diseases, such as asthma. The molecules most likely causing such alterations are neurotrophins (NTs) and/or related neurokines. In several species, including humans, lower airway parasympathetic postganglionic neurons that project axons to airway smooth muscle are either cholinergic or nonadrenergic noncholinergic (NANC), the latter synthesizing vasoactive intestinal peptide and nitric oxide, but not acetylcholine. In guinea pig trachealis smooth muscle, cholinergic nerve terminals arise from ganglionic neurons located near the tracheal smooth muscle, whereas the source of NANC nerve fibers is from neurons in ganglia located in the adjacent myenteric plexus of the esophagus, making this an ideal species to study regulation of parasympathetic neurotransmitter phenotypes. In the present study, we determined that, 48 hours after repeated allergen challenge, the NANC phenotype of airway parasympathetic ganglionic neurons changed to a cholinergic phenotype, and NT-3 mimicked this change. Nerve growth factor, brain-derived neurotrophic factor, leukemia inhibitory factor, or IL-1β had no effect on either phenotype, and they did not induce these neurons to synthesize substance P or tyrosine hydroxylase. These results indicate a role for inflammation and NT-3 in regulating biochemical and anatomical characteristics of principal neurons in adult airway parasympathetic ganglia.
Keywords: airway remodeling, asthma, autonomic nerves, neurotransmitter phenotype, parasympathetic ganglia
CLINICAL RELEVANCE.
It is well know that chronic exposure to allergens or pollution leads to changes in airway structures and the release of neurotrophins (NTs). This study addresses how allergen and NTs can modulate airway parasympathetic nerves by altering the neurotransmitter(s) they release, which may affect airflow to the lungs. Results from these studies help us understand the complex pathophysiology of airway diseases, such as asthma and chronic obstructive pulmonary disease, and may ultimately determine new therapeutic treatments for these complex diseases.
Lower airway caliber is regulated predominantly by parasympathetic nerves in most mammals, including humans, and alterations of airway nerves are thought to underlie the pathogenesis and certain symptoms of lower airway diseases, such as asthma (1) and chronic obstructive pulmonary disease (2). The molecules most likely contributing to such alterations are neurotrophins (NTs), and related compounds referred to as neurokines (3).
There is overwhelming evidence that the level of certain NTs are increased in the inflamed airway (4, 5) and, as NTs have been identified in many cell and tissue types in contact with parasympathetic nerves (6), such changes in organ structure may affect parasympathetic neurotransmitter phenotype or activity.
NTs are polypeptides that support growth, differentiation, and survival of neurons in developing and adult nervous systems. The prototypical NT is nerve growth factor (NGF), and this family also includes brain-derived neurotrophic factor (BDNF), NT-3, NT-4, and NT-4/5. NTs act on a family of receptor tyrosine kinases first identified as tropomyosin-related kinases (Trks): NGF preferentially activates Trk-A receptors, BDNF and NT-3 are selective for Trk-B receptors, and NT-4 is selective for Trk-C receptors (6). NTs also act via an additional NT receptor (p75NTR), which has no tyrosine kinase activity and, also unlike Trk receptors, binds NTs with low affinity and no specificity. Cytokine-like molecules (neurokines), such as IL-1β, leukemia inhibitory factor (LIF), ciliary neurotrophic factor, glial-derived neurotrophic factors, and others, may also alter nerves directly or indirectly. Although there is sufficient evidence that neurokines and NTs are increased in the blood (7), skin (8), and lungs (4, 9, 10) in inflammatory diseases, there are relatively few studies showing how that these molecules directly affect airway parasympathetic nerves (11–13).
Inflammation and NTs may affect responses of airway parasympathetic nerves, but the mechanisms for these alterations remain to be determined. For example, chronic allergen exposure enhances cholinergic neurotransmission in sensitized guinea pigs (14), and studies with cultured mouse tracheal segments showed that the cholinergic response can be enhanced by NGF (15) and attenuated by NT-3 (16). NGF also directly affects synaptic responses and neurite growth on principal neurons in adult guinea pig lower airway parasympathetic ganglia (12). In the lower airways, parasympathetic nerve terminals in airway smooth muscle are either cholinergic or nonadrenergic, noncholinergic (NANC), and the balance between these two opposing systems is most likely regulated at the level of the parasympathetic cell body by NTs. In contrast to central and other peripheral (sensory, sympathetic, enteric) neurons, there are relatively few studies (e.g., Ref. 11) on the effects of airway inflammation or NTs on differentiation of the adult parasympathetic nervous system. In the present study, we determined that allergic inflammation reduced the NANC phenotype of airway parasympathetic ganglionic neurons and increased the cholinergic phenotype, and NT-3 mimicked this change.
MATERIALS AND METHODS
The methods for animal treatment were approved by the Johns Hopkins University Animal Care and Use Committee (the Johns Hopkins University, Baltimore, MD). Pathogen-free guinea pigs (Hilltop Laboratory Animals, Scottsdale, PA) were housed in a filtered-air containment room before experimentation.
Immune Sensitization and Allergen Challenge
Adult male Hartley guinea pigs (150–200 g) were passively sensitized to antigen by intraperitoneal injection of serum (20 ml/kg) containing IgG1 that was collected from guinea pigs actively sensitized to ovalbumin (OVA) (17). Control guinea pigs (n = 6) were injected with serum from guinea pigs not sensitized to OVA (17). Control or passively sensitized guinea pigs were aerosol challenged by exposing animals to aerosolized antigen (0.01, 0.03, 0.1, and 0.3% OVA in 0.9% saline) during normal breathing in a Plexiglas chamber (8 L volume) for 5 minutes at each concentration (“Exposure(s)” in Table 1). The animals were removed to breath ambient air and allowed to recover after the highest concentration or if they showed signs of an overt allergic response (gasping, rapid breathing, cyanosis). This commonly employed animal model for allergen challenge resulted in an eosinophilic bronchitis within 24 hours of exposure (group A, Table 1; see Results) and 48 hours (group B, Table 1) after a single exposure. Another group of animals was first similarly exposed and then, 24 hours later, exposed again, but starting with a 0.003% OVA dose and following the dosing as described previously here. For all groups, the animals were killed by an overdose of pentobarbital (150 mg/kg, intraperitoneal), transcardially perfused with PBS (pH 7.4; 60 ml) containing 0.1% procaine and heparin (10 U/ml), followed by 4% formaldehyde in PBS (60 ml), and the trachea with the esophagus were removed and fixed for an additional 2 hours (4°C). The level of inflammation was determined in Giemsa-stained (30 min) cryostat sections (described subsequently here) by the density of eosinophils (cells/mm2) in the epithelium and mucosa.
TABLE 1.
ALLERGEN EXPOSURE AND RECOVERY GROUPS
| Group | No. of Exposure(s) | Time after Last Exposure when Tissue Was Recovered (h) |
|---|---|---|
| A | 1 | 24 |
| B | 1 | 48 |
| C | 2* | 24 |
| D | 2* | 48 |
In groups C and D, animals were exposed a second time 24 hours after the first exposure, starting with threefold-lower dose (0.003%) compared with the previous day(s) exposure, and increasing either for the entire dose range as the first exposure, or until the animal showed signs of anaphylaxis (see Materials and Methods, Immune Sensitization and Allergen Challenge). There were four animals in groups A–C, and six animals in group D.
NT Exposure
To demonstrate whether an exogenously administered NT could mimic the effect of allergen challenge, we developed a delivery system for exposing tracheal parasympathetic cholinergic and NANC postganglionic neurons to an NT in vivo in guinea pigs. To ensure that NTs injected into the trachealis muscle would reach the myenteric NANC neurons in the esophagus, we followed the protocol previously reported by Fischer and colleagues (18), except that we used rhodamine-dextran instead of 1,1′-didodecyl-. 3,3,3,3′-tetramethyl-indocarbocyanine perchlorate (DiI), because, with our protocol, DiI washes out of the tissue sections during immunostaining. Animals were anesthetized with 50 mg/kg ketamine and 2.5 mg/kg xylazine (intramuscular) and, after local nerve block, a 1-cm midline incision of the skin was made over the cervical trachea. The cervical trachea was exposed, and a single NT (NGF, n = 4; NT-3, n = 6; or BDNF, n = 4) or a neurokine (IL-1β or LIF, n = 4), each at 100 ng/ml PBS, along with 1% rhodamine-dextran (Invitrogen–Molecular Probes, Carlsbad, CA), a fluorescent tracer, were microinjected into five sights (2 μl per sight) into the cervical and thoracic trachealis muscle. Control animals (n = 4) were injected with NT vehicle in a similar fashion with tracer only. When the injections were completed, the incision was sutured and the animals recovered for 24 or 48 hours; the tissue was then harvested and fixed as described previously here.
Immunofluorescence Staining
Esophageal (in the ventral quadrant of the esophagus) and tracheal ganglia neurons that project axons to the tracheal smooth muscle were identified in cross-sections and transverse sections, respectively, either by the accumulation of rhodamine-dextran (for NT study) in their cell bodies, or by protein gene product (PGP) 9.5 immunostaining (for allergen challenge study) in 12-μm cryostat sections. Cryostat sections of tracheas and esophagi were blocked with a solution containing 10% donkey serum, 1% BSA, and 0.1% Tween 20 in PBS, applied for 1 hour at room temperature. For double immunofluorescent staining, the sections were then incubated (4°C) overnight in primary antibodies (source and dilutions in Table 2) diluted in PBS containing 1% BSA and 0.5% Trition X-100, rinsed, and then covered in species-specific (Table 2) secondary antibodies conjugated with Alexa dyes 350, 488, and 594 (Invitrogen–Molecular Probes) recognizing species-specific primary antibodies. The source of primary antibodies and dilutions are shown in Table 2.
TABLE 2.
PRIMARY ANTIBODIES USED IN THIS STUDY
| Antibody | Species | Source, Cat. No.* | Dilution |
|---|---|---|---|
| ChAT | goat | Chemicon, AB144 | 1:50 |
| VIP | Rabbit | Peninsula, IHC7161 | 1:200 |
| Nitric oxide synthase | Rabbit | Chemicon, AB5380 | 1:2,500 |
| Neuropeptide Y | Rabbit | Chemicon, AB1915 | 1:500 |
| Tyrosine hydoxylase | Rabbit | Chemicon, AB5986 | 1:1,000 |
| Substance P | Rabbit | Peninsula, AB7451 | 1:200 |
| Substance P | Rat | Peninsula, MAB356 | 1:200 |
| PGP9.5 | Rabbit | Ultraclone, PGP101 | 1:400 |
| PGP9.5 | Mouse | Ultraclone, 1C34 | 1:400 |
| Neurotrophin-3 | Rabbit | Santa Cruz, SC-547 | 1:500 |
Definition of abbreviations: ChAT, choline acetyltransferase; PGP, protein gene product; VIP, vasoactive intestinal peptide.
Chemicon International, Inc. (Temecula, CA); Peninsula Laboratories (Belmont, CA); Ultraclone (Isle of Wight, UK); Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
For negative controls, separate sections were processed similarly, but either the primary antibody was replaced with species-specific IgG to evaluate nonspecific staining or with primary antibodies preabsorbed with antigenic peptide (choline acetyltransferase [ChAT], NT3, substance P (SP), and vasoactive intestinal peptide [VIP]). In all initial experiments, immunostaining was done for sympathetic nerve markers, neuropeptide Y (NPY) or tyrosine hydroxylase (TH), or the C-fiber sensory nerve neuropeptide, SP, in both NANC esophageal ganglia and cholinergic tracheal ganglia. The number of rhodamine-dextran– or PGP9.5-positive neurons was counted, and the percent of neurotransmitter-positive cells was determined. In preliminary experiments, we determined that rhodamine-dextran had no effect on neurotransmitter phenotype in control animals (data not shown).
For NT-3 immunostaining, tyramide signal amplification (TSA) processing was used: cross-sections of tracheas were collected, endogenous peroxidase activity was blocked with hydrogen peroxide (5% in 50% methanol in PBS, 30 min), and blocked with 10% goat serum in PBS with 1% BSA and 0.1% Tween 20. Primary antibody was applied as above and, after rinsing, a TSA kit (Invitrogen) was used: Briefly, sections were incubated with peroxidase-conjugated goat anti-rabbit Ig (1 μg/ml) and then with Alexa Fluor 568–conjugated tyramide in TSA solution. For all immunostaining, rinsed slides were coverslipped with Tris-buffered glycerol (pH 8.6), viewed, and photographed with an epifluorescence microscope (Olympus BX50; Olympus America, Inc., Melville, NY).
Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO), except antibodies, as per the text and Table 2.
Statistical Analysis
All data are expressed as the arithmetic mean ± the standard deviation of the mean. Control (see above) values for eosinophil or neuron number was compared with peak changes evoked by allergen challenge or NT-injected tissue using unpaired Student's t tests. Statistical tests were performed using GraphPad Prism statistics program (San Diego, CA). Statistical significance was accepted at a P value of 0.05.
RESULTS
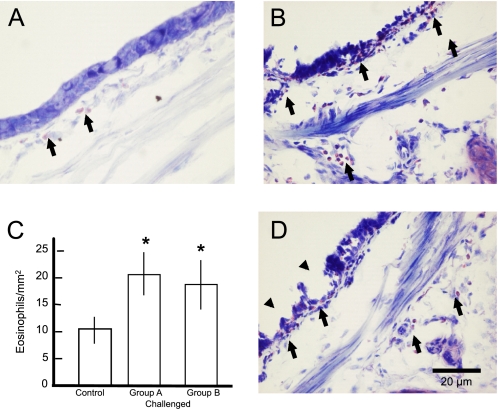
Compared with control animals (Figure 1A), single challenge (groups A or B) or double allergen challenge (groups C or D) caused the predicted allergic inflammation in the airways. Relative to the low number of eosinophils in sections of control tracheas, eosinophils increased by 90% in the trachea 24 hours (group A; Figures 1B and 1C), and by 72% 48 hours after a single allergen challenge (group B; Figure 1C) and a lesser amount 24 or 48 hours (groups C and D, respectively) after a second allergen challenge (data not shown). Also common with allergen challenge, there was disruption of the epithelial layer lining the lower airways 48 hours after a second allergen challenge (group D; Figure 1D), but not 24 hours (group A) after a single (Figure 1B) or double allergen challenge (data not shown).
Figure 1.
Allergen challenge of passively sensitized guinea pigs elicits airway inflammation and remodeling. Compared with control tracheas (A), eosinophils (arrows) were increased in the trachea 24 (B, arrows) and 48 hours (data not shown) after a single allergen challenge. (C) Summary of the effects of single allergen challenges (groups A and B) on eosinophil density. Eosinophils were also increased 24 hours (data not shown) and 48 hours (D, arrows) after a second allergen challenge. Also common with chronic allergen challenge, there was disruption of the epithelial layer lining the lower airways 48 hours after a second allergen challenge (arrowheads in D). Scale bar in D is for images in A, B, and D. Asterisks in C signify differences in the number of eosinophils in treated tissue as compared with control.
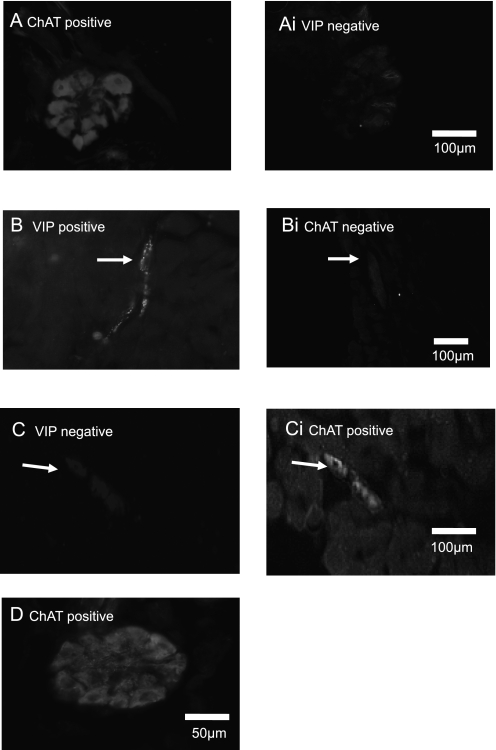
Control neurons located in tracheal parasympathetic ganglia are cholinergic (i.e., they contain ChAT immunoreactivity [ChAT-ir] [Figure 2A]), but do not express markers associated with NANC innervation (VIP, nitric oxide synthase [NOS]; VIP in Figure 2A, panel i). This is because the postganglionic neurons responsible for the noncholinergic parasympathetic innervation of guinea pig tracheal smooth muscle are situated in the myenteric plexus of the esophagus (18, 19). After rhodamine-dextran injection into the trachealis, the number of neurons labeled in the ventral quadrant of the esophagus was similar to that previously reported for DiI injection (18). In control esophageal ganglia, all neurons that project axons to the trachealis muscle (i.e., that were labeled with rhodamine-dextran) expressed VIP (Figure 2B) and NOS (data not shown), but the same neurons do not express ChAT (Figure 2B, panel i). Consistent with previous observations (18), there was no colocalization of cholinergic and NANC markers in single neurons innervating the trachea.
Figure 2.
Neurons in esophageal parasympathetic ganglia change to a cholinergic phenotype 48 hours after repeated allergen challenge. (A) All control neurons in tracheal ganglia are immunoreactive for choline acetyltransferase (ChAT), but, in the same section, as shown in Ai, not for markers associated with nonadrenergic, noncholinergic (NANC) innervation, such as vasoactive intestinal peptide (VIP) or for nitric oxide synthase (NOS; data not shown). (B) All control neurons in esophageal ganglia that project axons to the trachealis muscle express VIP (arrow) and NOS (data not shown), but not ChAT, as shown in the same section (Bi). (C) Esophageal neurons from animals exposed to allergen on 2 days and analyzed 48 hours later (group D) were no longer VIP immunoreactive and, in Ci, the same neurons were immunoreactive for ChAT. (D) A tracheal ganglion from an animal exposed to allergen on 2 days and analyzed 48 hours later (group D) remained cholinergic (three others showed similar ChAT immunoreactivity. Neither tracheal nor esophageal ganglia neurons became substance P (SP)–, tyrosine hydroxylase (TH)–, or neuropeptide Y (NPY)–immunoreactive for any group (data not shown). Scale bar on right image (A-C) is for corresponding image on the left.
Allergen challenge was not associated with overt changes in neurotransmitter phenotype in cholinergic ganglion tracheal neurons 24 hours (group A, n = 4) or 48 hours (group B, n = 4) after a single allergen challenge, or 24 hours after the second allergen challenge (group C, n = 4; data not shown).
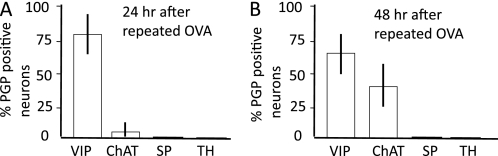
In the esophageal NANC ganglia, allergen challenge was associated with a decrease in VIP expression in a small percentage (6.7 ± 0.8% of 128 PGP9.5-positive neurons) of neurons (group C); these neurons remained ChAT negative (data not shown). In tissue from animals (n = 6) exposed to allergen on 2 days and analyzed 48 hours later (group D), of 97 labeled neurons (i.e., similar to those that normally synthesize the NANC neurotransmitter, VIP, in control tissue), 31 were no longer VIP immunoreactive (Figure 2C), and now the same neurons contained ChAT-ir (Figure 2C, panel i) (i.e., there was a switch from the NANC to the cholinergic phenotype). In group D animals, tracheal ganglionic neurons (n = 71) that normally synthesize acetylcholine (Figure 2A) remained cholinergic, and did not stain for the NANC neurotransmitters, VIP or NOS (data not shown). These data are summarized in Figure 3. Neither tracheal nor esophageal ganglia neurons became SP, TH, or NPY immunoreactive (n = 4) 24 hours after single (group A) or double (group B) allergen challenge or 48 hours after double allergen challenge (group D; data not shown). These data are summarized in Figure 3.
Figure 3.
Summary of the effects of different allergen challenges on neurotransmitter immunoreactivity in neurons located in esophageal ganglia. (A) VIP and ChAT-ir in group C esophageal neurons were unaffected 24 hours after a repeated allergen challenge. (B) VIP and ChAT-ir in group D esophageal neurons were decreased and increased, respectively, 48 hours after a repeated allergen challenge.
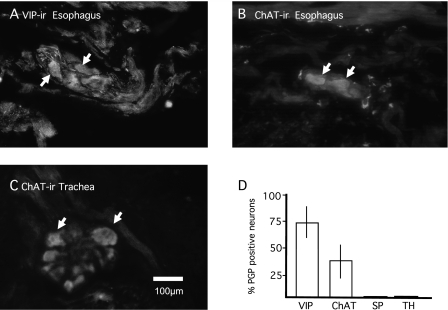
To determine if an NT could mimic the effect of allergen challenge, individual NTs, NGF, BDNF, or NT-3, or neurokines, LIF or IL-1β, were injected into the trachealis muscle and, after 48 hours, the neurotransmitter phenotype was determined immunohistologically in tracheal and esophageal parasympathetic ganglia. NT-3 (100 ng/ml), 48 hours after exposure, caused neurons that normally synthesize the NANC neurotransmitter, VIP (Figure 4A, vehicle control), to no longer synthesize VIP (18 of 67 neurons; data not shown) and several were ChAT immunoreactive (24 of 67 neurons; Figure 4B). Neurons near the trachealis muscle that normally synthesize acetylcholine remained cholinergic (Figure 4C), and did not stain for the NANC neurotransmitter, VIP (data not shown). These data are summarized in Figure 4D. NGF, BDNF, LIF, or IL-1β had no effect on the cholinergic or NANC neurotransmitter phenotype 48 hours after exposure, nor did they induce immunoreactivity for SP, TH, or NPY (data not shown).
Figure 4.
Neurotrophin (NT)-3 mimics the effect of allergen challenge. Individual NTs nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT-3, or neurokines, leukemia inhibitory factor (LIF) or IL-1β, were injected into the trachealis muscle and, after 48 hours, the neurotransmitter phenotype was determined immunohistologically in tracheal and esophageal parasympathetic ganglia. (A) Neurons that normally express VIP immunoreactivity (ir) in esophageal ganglionic neurons. (B) NT-3 (100 ng/ml), 48 hours after exposure, esophageal neurons were ChAT immunoreactive. (C) NT-3 (100 ng/ml), 48 hours after exposure, had no effect on ChAT-ir in tracheal ganglionic neurons. These data for esophageal neurons are summarized in D. NGF, BDNF, LIF, or IL-1β (all 100 ng/ml, 48 hours; n = 4 animals for each exposure) had no effect on the cholinergic or NANC neurotransmitter phenotype 48 hours after exposure, nor did they induce immunoreactivity for SP, TH, or NPY (data not shown). Scale bar in C is for images in A–C.
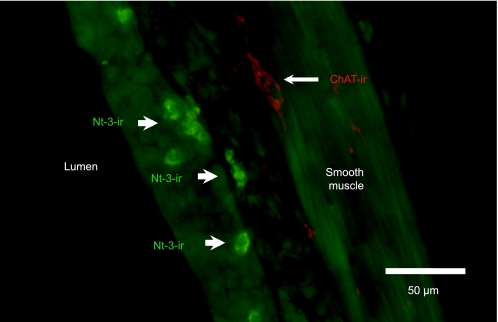
Positive immunofluorescent staining for NT-3 was observed in airways in guinea pigs from groups A and C. The staining appeared to be in small, inflammatory-like cells located in the mucosa (Figure 5). The size and location of these cells was very similar to eosinophil-like cells observed after single allergen challenge (Figure 1).
Figure 5.
Immunofluorescent staining for NT-3 in a frozen section of guinea pig trachea 2 days after a single allergen challenge. The staining appeared to be in small, inflammatory-like cells (arrows, green) located in and below the epithelium; the size and location of these cells was very similar to eosinophil-like cells observed after single allergen challenge (Figure 1). Fewer cells were immunoreactive in animals 1 day after a single allergen exposure (data not shown). ChAT-ir (red) near airway smooth muscle shows proximity of mucosal inflammatory cells near ChAT-ir nerves.
DISCUSSION
The purpose of the present study was to provide a better understanding of how inflammation affects the autonomic innervation, specifically parasympathetic nerves, of the lower airways. In this study, we address two specific hypotheses: (1) that airway inflammation leads to alterations in the neurochemistry of airway NANC parasympathetic nerves; and (2) that an NT mimics the change caused by inflammation.
Using passive sensitization and in vivo allergen challenge, we demonstrate that single or double allergen challenge caused allergic eosinophilic inflammation of the lower airways (Figure 1). Eosinophils were increased in the trachea 24 hours after a single allergen challenge and 48 hours after a second allergen challenge. Also common with chronic allergen challenge (20), there was disruption of the epithelial layer lining the lower airways, but only 48 hours after a second allergen challenge, and not 24 hours after a single or double allergen challenge. We thus established that this type of sensitization and challenge alters the structure of the lower airways of guinea pigs. Airway inflammatory cells degranulate and release NTs, including NGF (21), LIF (22), and BDNF and NT-3 (23), which may have either direct or indirect effects on parasympathetic nerves.
Several functional studies have demonstrated changes in parasympathetic tone after acute and chronic allergen challenge associated with either an increase in cholinergic nerve activity (14, 24, 25) or a decrease in NANC activity (26). In our study, animals exposed to repeated allergen challenge had a significant percentage of the NANC phenotype of airway parasympathetic ganglionic neurons change to a cholinergic phenotype after 48 hours. The major relaxant innervation to airway smooth muscle in guinea pigs (and the only relaxant innervation to human bronchial smooth muscle [26]) is derived from parasympathetic NANC neurons (18, 19). A decrease in neurons expressing the relaxant transmitters (VIP and nitric oxide) would be expected to decrease relaxant tone and, as a consequence, increase the contractile tone of the airways. Amplifying this effect is the observation that not only was the amount of NANC innervation decreased, it appeared to phenoptypically switch to cholinergic innervation. Thus, there was at the same time a decrease in relaxant innervation and an increase in contractile innervation in the allergen-challenged animals.
The inflammatory mediator responsible for this change in parasympathetic nerve phenotypes cannot be deduced from our data, although NT-3 immunoreactivity was observed in allergen-challenged airways (Figure 5). It is interesting, however, that among several neurotrophic factors, NT-3 alone was able to mimic this change. NGF, BDNF, LIF, or IL-1β had no effect on either NANC or cholinergic phenotype. This effect of NT-3 is similar to that reported for embryonic avian sympathetic ganglia where expression of the NT-3 receptor, Trk-C, overlaps substantially with ChAT expression, and culturing with NT-3 increases the level of ChAT in these neurons (28). The expression of neurotransmitters and neuropeptides by pelvic ganglionic neurons were altered under different culture conditions (29). Specifically in pelvic ganglia, under control conditions or after NGF exposure, VIP was up-regulated in noradrenergic and cholinergic neurons; testosterone reduced up-regulation in noradrenergic neurons. ChAT-ir could only be visualized when NGF was present in the cultures, and cholinergic neurons showed less neurite outgrowth than noradrenergic neurons under all culture conditions. NGF did not stimulate levels of this enzyme as strongly if testosterone was present (29). Whether such alterations in neurotransmitter phenotype alter functional responses to nerve stimulation remains to be determined.
Airway inflammation has been reported to affect the level of SP and other sensory neuropeptides in airway-specific neurons in several species. For example, Fischer and colleagues (18) showed that, 24 hours after allergen challenge, the number of SP-immunoreactive nodose ganglionic neurons in guinea pigs increased by 25%, which was mimicked by exogenous NGF (30). In ferrets, airway ozone exposure increases SP levels in the cholinergic cell bodies and in parasympathetic nerves located in tracheal smooth muscle (31), which is mimicked by the cytokine/neurokine IL-1β (11). Our results show that, in guinea pigs, neither NGF nor IL-1β changed the parasympathetic neurons to sensory-like (SP) or sympathetic (TH- or NPY-positive) phenotypes.
Several studies on cholinergic neurons in the central nervous system have identified BDNF as the NT regulating this phenotype; for example, BDNF can abrogate the injury-induced loss of ChAT mRNA in mature motor neurons in vivo in the brainstem of adult rats (32). From that study, and the evidence that NT-3 changes sympathetic neurons to a cholinergic phenotype (28), it is possible that these two NTs regulate the cholinergic phenotype in peripheral parasympathetic neurons. Furthermore, BDNF immunoreactivity has been reported to be relatively higher than other NTs in and around human airway nerves (6). However, we found that BDNF had no effect on the cholinergic (or other) phenotype(s) of NANC neurons. Nor is it likely that the cholinergic phenotype is regulated by cholinergic preganglionic nerves from the central nervous system, as both esophageal NANC neurons and tracheal cholinergic neurons are innervated by cholinergic preganglionic nerves (19). It is also unlikely that the cholinergic phenotype is regulated by the VIP/NOS–containing nerve fibers, because, in organotypic cultures, the cholinergic phenotype of guinea pig tracheal neurons remains after 12–48 hours with or without the esophagus (33), which is the source of the VIP/NOS nerve fibers found in the cholinergic ganglia (ACM, Dr. Brendan Canning, unpublished observation).
It is known that inflammation affects airway structures, including growth of smooth muscle, inflammatory cell influx, and mucosal gland hypertrophy (reviewed by Pascual and Peters [20]). However, little is known about how alterations in these structures and cells affect innervating or nearby parasympathetic nerves. Furthermore, as stated previously here, the level of certain NTs is increased in the inflamed airways (4, 5). Our results provide direct evidence that part of the so-called airway remodeling processes associated with allergic inflammation may include a substantive remodeling of parasympathetic innervation, such that the net extent of contractile innervation is increased.
Some results included in this manuscript have been published previously in abstract form (34).
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants HL038095 (B.J.U.) and HL088608 (A.C.M.), and by the Johns Hopkins University Woodrow Wilson Fellowship (J.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0130OC on November 9, 2009
Author Disclosure: A.C.M. is employed by Johns Hopkins University and has received consultancy fees from Sanofi-Aventis for $1,001–$5,000, a sponsored grant for more than $100,001, and a sponsored grant from the National Institutes of Health for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J Allergy Clin Immunol 2006;117:67–71. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby DB, Fryer AD. Anticholinergic therapy for airway diseases. Life Sci 2001;68:2565–2572. [DOI] [PubMed] [Google Scholar]
- 3.Carr MJ, Hunter DD, Undem BJ. Neurotrophins and asthma. Curr Opin Pulm Med 2001;7:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Virchow JC, Julius P, Lommatzsch M, Luttman W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med 1998;58:2002–2005. [DOI] [PubMed] [Google Scholar]
- 5.Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med 2005;172:233–237. [DOI] [PubMed] [Google Scholar]
- 6.Ricci A, Felici L, Mariotta S, Mannino F, Schmid G, Terzano C, Cardillo G, Amenta F, Bronzetti E. Neurotrophin and neurotrophin receptor protein expression in the human lung. Am J Respir Cell Mol Biol 2004;30:12–19. [DOI] [PubMed] [Google Scholar]
- 7.Bonini S, Lambiase A, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA 1996;93:10955–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol 1998;78:84–86. [DOI] [PubMed] [Google Scholar]
- 9.Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol 2004;141:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassel O, de Blay F, Duvernelle C, Olgart C, Israel-Biet D, Krieger P, Moreau L, Muller C, Pauli G, Frossard N. Local increase in the number of mast cells and expression of nerve growth factor in the bronchus of asthmatic patients after repeated inhalation of allergen at low-dose. Clin Exp Allergy 2001;31:1432–1440. [DOI] [PubMed] [Google Scholar]
- 11.Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1beta–induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol 2002;283:L909–L917. [DOI] [PubMed] [Google Scholar]
- 12.Hazari MS, Pan J, Myers AC. Nerve growth factor acutely potentiates synaptic transmission in vitro and induces dendritic growth in vivo on adult neurons in airway parasympathetic ganglia. Am J Physiol 2007;292:L992–L1001. [DOI] [PubMed] [Google Scholar]
- 13.Verbout NG, Jacoby DB, Gleich GJ, Fryer AD. Atropine-enhanced, antigen challenge–induced airway hyperreactivity in guinea pigs is mediated by eosinophils and nerve growth factor. Am J Physiol Lung Cell Mol Physiol 2009;297:L228–L237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kageyama N, Igarashi A, Ichinose M, Miura M, Yamauchi H, Tomaki M, Ishikawa J, Sasaki Y, Shirato K. Chronic allergen exposure enhances cholinergic neurotransmission in sensitized guinea-pigs. Eur Respir J 1995;8:752–754. [PubMed] [Google Scholar]
- 15.Bachar O, Adner M, Uddman R, Cardell LO. Nerve growth factor enhances cholinergic innervation and contractile response to electric field stimulation in a murine in vitro model of chronic asthma. Clin Exp Allergy 2004;34:1137–1145. [DOI] [PubMed] [Google Scholar]
- 16.Bachar O, Adner M, Uddman R, Cardell LO. Prolonged exposure to NT-3 attenuates cholinergic nerve–mediated contractions in cultured murine airways. Respir Physiol Neurobiol 2005;147:81–89. [DOI] [PubMed] [Google Scholar]
- 17.Undem BJ, Buckner CK, Harley P, Graziano FM. Smooth muscle contraction and release of histamine and slow-reacting substance of anaphylaxis in pulmonary tissues isolated from guinea pigs passively sensitized with IgG1 or IgE antibodies. Am Rev Respir Dis 1985;131:260–266. [DOI] [PubMed] [Google Scholar]
- 18.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest 1996;98:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canning BJ, Undem BJ. Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea-pig trachealis. J Physiol 1993;471:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 2005;116:477–486. [DOI] [PubMed] [Google Scholar]
- 21.Solomon A, Aloe L, Pe'er J, Frucht-Pery J, Bonini S, Bonini S, Levi-Schaffer F. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol 1998;102:454–460. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Knight DA, Zhou D, Weir T, Peacock C, Schellenberg RR, Bai TR. Leukemia inhibitory factor is synthesized and released by human eosinophils and modulates activation state and chemotaxis. J Allergy Clin Immunol 1999;104:136–144. [DOI] [PubMed] [Google Scholar]
- 23.Noga O, Englmann C, Hanf G, Grutzkau A, Seybold J, Kunkel G. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin Exp Allergy 2003;33:649–654. [DOI] [PubMed] [Google Scholar]
- 24.Fryer AD, Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol 1991;71:2255–2261. [DOI] [PubMed] [Google Scholar]
- 25.Larsen GL, Fame TM, Renz H, Loader JE, Graves J, Hill M, Gelfand EW. Increased acetylcholine release in tracheas from allergen-exposed IgE-immune mice. Am J Physiol 1994;266:L263–L270. [DOI] [PubMed] [Google Scholar]
- 26.De Boer J, Pouw FM, Zaagsma J, Meurs H. Effects of endogenous superoxide anion and nitric oxide on cholinergic constriction of normal and hyperreactive guinea pig airways. Am J Respir Crit Care Med 1998;158:1784–1789. [DOI] [PubMed] [Google Scholar]
- 27.Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol 2001;125:113–127. [DOI] [PubMed] [Google Scholar]
- 28.Brodski C, Schnurch H, Dechant G. Neurotrophin-3 promotes the cholinergic differentiation of sympathetic neurons. Proc Natl Acad Sci USA 2000;97:9683–9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meusburger SM, Keast JR. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neuroscience 2001;108:331–340. [DOI] [PubMed] [Google Scholar]
- 30.Hunter DD, Myers AC, Undem BJ. Nerve growth factor–induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 2000;161:1985–1990. [DOI] [PubMed] [Google Scholar]
- 31.Wu ZX, Maize DF Jr, Satterfield BE, Frazer DG, Fedan JS, Dey RD. Role of intrinsic airway neurons in ozone-induced airway hyperresponsiveness in ferret trachea. J Appl Physiol 2001;91:371–378. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Salvaterra PM, Loera S, Chiu AY. Brain-derived neurotrophic factor spares choline acetyltransferase mRNA following axotomy of motor neurons in vivo. J Neurosci Res 1997;47:134–143. [DOI] [PubMed] [Google Scholar]
- 33.Canning BJ, Undem BJ, Karakousis PC, Dey RD. Effects of organotypic culture on parasympathetic innervation of guinea pig trachealis. Am J Physiol 1996;271:L698–L706. [DOI] [PubMed] [Google Scholar]
- 34.Pan J, Undem BJ, Myers A. Repeated allergen exposure induces neural plasticity of adult neurons in lower airway parasympathetic ganglia [abstract]. FASEB J 2007;21:912. [Google Scholar]