Abstract
Occupational exposure to beryllium (Be) results in Be sensitization (BeS) that can progress to pulmonary granulomatous inflammation associated with chronic Be disease (CBD). Be-specific lymphocytes are present in the blood of patients with BeS and in the blood and lungs of patients with CBD. Sulfasalazine and its active metabolite, mesalamine, are clinically used to ameliorate chronic inflammation associated with inflammatory bowel disease. We tested whether sulfasalazine or mesalamine could decrease Be-stimulated peripheral blood mononuclear cell (PBMC) proliferation in subjects with CBD and BeS and Be-induced cytokine production in CBD bronchoalveolar lavage (BAL) cells. CBD (n = 25), BeS (n = 12) and healthy normal control (n = 6) subjects were enrolled and ex vivo proliferation and cytokine production were assessed in the presence of Be and sulfasalazine or mesalamine. Be-stimulated PBMC proliferation was inhibited by treatment with either sulfasalazine or mesalamine. Be-stimulated CBD BAL cell IFN-γ and TNF-α cytokine production was decreased by treatment with sulfasalazine or mesalamine. Our data suggest that both sulfasalazine and mesalamine interfere with Be-stimulated PBMC proliferation in CBD and BeS and dampens Be-stimulated CBD BAL cell proinflammatory cytokine production. These studies demonstrate that sulfasalazine and mesalamine can disrupt inflammatory pathways critical to the pathogenesis of chronic granulomatous inflammation in CBD, and may serve as novel therapy for human granulomatous lung diseases.
Keywords: granulomatous inflammation, IFN-γ, TNF-α, lymphocyte proliferation, berylliosis
CLINICAL RELEVANCE.
Mesalamine and sulfasalazine inhibit beryllium (Be) antigen–stimulated T cell proliferation in peripheral blood mononuclear cells and Be antigen–induced cytokine secretion in bronchoalveolar lavage cells from subjects with chronic Be disease ex vivo, suggesting a therapeutic potential of these agents in this and similar granulomatous lung diseases.
The U.S. National Institute for Occupational Safety and Health estimates that approximately 134,000 beryllium (Be) industry workers are potentially exposed to Be in the United States and are at risk for the development of Be sensitization (BeS) and chronic Be disease (CBD) (1). The majority of workers that are exposed to Be remain without sequelae from their exposure. A subset of individuals develops BeS that is defined as a Be-specific CD4+ T cell–mediated immune response directed against the Be antigen (2). These individuals are usually asymptomatic and have normal chest radiography, pulmonary physiology, and lung biopsies, but demonstrate Be antigen–specific proliferation in the Be lymphocyte proliferation test (BeLPT). In addition, they have a 6–8% per year risk of progression to CBD (3). Progression from BeS to CBD is characterized by the presence of interstitial mononuclear cell infiltrates and/or noncaseating granulomas on lung biopsy. It has been shown that peripheral blood mononuclear cells (PBMCs) from both patients with BeS and those with CBD contain a subset of CD4+ Be–specific T cells (4, 5). Upon Be stimulation ex vivo, these Be-specific T cells proliferate, as demonstrated by the DNA incorporation of tritiated thymidine in the BeLPT, and produce proinflammatory cytokines, including IFN-γ and TNF-α (4–6). The proliferative response of Be-specific T cells present in the PBMC and bronchoalveolar lavage (BAL) mixed cell populations provides the basis for the BeLPT (6, 7). The PBMC BeLPT is used in the clinical evaluation and diagnosis of Be-exposed patients to determine whether they are BeS. The BAL mixed cell population from subjects with BeS does not appear to contain a Be-specific T cell subset, whereas CBD BAL cells can contain relatively high numbers of these pathogenic T cells, and Be stimulation ex vivo induces their proliferation and the production of high levels of IFN-γ and TNF-α mRNA and protein (5–8).
Currently, there is no known cure for CBD. Patients with BeS require counseling and ongoing clinical evaluation to determine whether they are progressing to CBD. Treatment of CBD is aimed at controlling or reducing inflammation and immune reactions to Be that can persist in the CBD granuloma for decades after the last Be exposure (9). The mainstay treatment is prednisone, and is usually administered when there is evidence of pulmonary impairment based on symptoms, pulmonary function tests, or exercise testing. Life-long corticosteroids are needed, along with other supportive treatment, including oxygen, bronchodilators, and immunizations against respiratory pathogens. Patients who do not respond to steroids or who develop side effects may be administered methotrexate (10). Thus, patients with CBD might benefit from less toxic alternative approaches that limit inflammation and immune reactions to Be.
Sulfasalazine, or its metabolite, mesalamine (5-aminosalicylate), are commonly used drugs for the treatment of active inflammatory bowel disease (IBD) and rheumatoid arthritis (11). Mesalamine inhibits inflammatory mediators through mechanisms related to the down-regulation of the immune and inflammatory responses associated with IBD (12). The precise mechanism of action by which these agents inhibit inflammatory mediators is unclear. Mesalamine has been shown to decrease leukotriene synthesis, scavenge peroxynitrite and oxygen-derived reactive species, and inhibit IL-1β synthesis (13). We tested the hypothesis that Be-stimulated PBMC proliferation and Be-stimulated CBD BAL cell cytokine production could be inhibited by treatment with either sulfasalazine or mesalamine. In the present study, we determined if ex vivo sulfasalazine or mesalamine treatment of PBMC from subjects with CBD and BeS could inhibit Be-induced cell proliferation and Be-stimulated CBD BAL cell cytokine production. We observed that treatment with these agents decreased Be-stimulated CBD and BeS PBMC proliferation, and also decreased Be-stimulated CBD BAL cell production of IFN-γ and TNF-α. Our data suggest that sulfasalazine and mesalamine may disrupt critical inflammatory pathways in the pathogenesis of CBD, and offer a novel approach to treat chronic lung granulomatous inflammation in humans.
MATERIALS AND METHODS
Written, informed consent was obtained from each patient enrolled in this study with a protocol approved by the Human Subjects Institutional Review Board at National Jewish Health (Denver, CO). Subjects with CBD (n = 25) and BeS (n = 12) were clinically enrolled at National Jewish Health based on the availability of blood and BAL samples. Blood from healthy control subjects (n = 6) was drawn from non–Be-exposed volunteer subjects without lung disease. Subjects were excluded if they were active smokers, and all subjects were nonsmokers for at least 2 years before enrollment in the study. The diagnosis of CBD was established with previously defined criteria, including a history of Be exposure, the presence of noncaseating pulmonary granulomatous inflammation and/or mononuclear cell infiltrates on transbronchial or open-lung biopsy, and a positive proliferative response of PBMCs and/or BAL cells in the BeLPT (6, 7). For subjects that did not undergo biopsy, CBD was diagnosed based on a history of Be exposure, a positive proliferative response of BAL cells in the BeLPT, and alveolar lymphocytosis (3). The diagnosis of BeS was established based on a history of Be exposure, a positive proliferative response of PBMCs to Be stimulation in the BeLPT, absence of interstitial mononuclear cell infiltrates or noncaseating granulomatous inflammation on transbronchial or open-lung biopsy, and absence of lymphocytic alveolitis (6, 7). With access to a limited number of subjects with CBD and BeS enrolled in this study, individual experiments were performed with samples from subsets of study subjects, as specified in the Results. BeS and CBD blood was obtained by venipuncture, and BAL samples from subjects with CBD were obtained at bronchoscopy (4, 14). The BeLPT was performed to evaluate PBMC and BAL cell proliferation responses (Table 1), and the data are reported as the mean (± SEM) peak stimulation index (SI) for thymidine uptake in triplicate cultures as the ratio of the test sample counts per minute to the counts per minute in the unstimulated (medium alone) group (6).
TABLE 1.
CLINICAL CHARACTERISTICS OF THE STUDY POPULATION
| BeS | CBD | Control | |
|---|---|---|---|
| (n = 12) | (n = 25) | (n = 6) | |
| Age, yr | 61.3 ± 3.6 | 59.3 ± 1.6 | 31.8 ± 5.4* |
| Race, W/AA/H† | 11/0/1 | 23/1/1 | 4/2/0 |
| Sex, female/male | 2/10 | 6/19 | 4/2 |
| Smoking status, CS/FS/NS‡ | 0/7/5 | 0/10/15 | 0/2/4 |
| Oral corticosteriod treatment, yes/no | 0/12 | 6/19 | N/A |
| Granulomas, yes/no | 0/12 | 23† | N/A |
| BeLPT, Stimulation Index | |||
| Peak blood BeLPT SI | 4.9 ± 1.2 | 13.6 ± 4.8 | N/A |
| Peak BAL BeLPT SI | 1.3 ± 0.2 | 19.2 ± 6.7* | N/A |
| BAL Cells | |||
| Total white blood cell count (×106) | 21.0 ± 3.7 | 47.8 ± 5.4* | N/A |
| Macrophages, % | 86.8 ± 4.4 | 67.1 ± 4.3* | N/A |
| Lymphocytes, % | 12.3 ± 4.3 | 30.4 ± 4.4* | N/A |
Definition of abbreviations: AA = African American; BAL, bronchoalveolar lavage; BeLPT, beryllium lymphocyte proliferation test; BeS, beryllium sensitization; CBD, chronic beryllium disease; CS, current smoker; FS, former smoker; H, Hispanic; N/A, not applicable; NS, never smoked; SI, stimulation index; W, White.
Data are expressed as means (±SEM).
P < 0.05.
Two subjects did not undergo lung biopsy due to medical reasons.
All studies were performed with Ficoll-Hypaque–isolated PBMCs from subsets of study subjects. Unless indicated otherwise, isolated PBMCs and BAL cells were adjusted to 1 × 106 cells/ml in RPMI complete medium with 10% heat-inactivated calf serum (see the online supplement for more details). For all experiments, 200 μl aliquots were cultured in triplicate wells per treatment condition with 96-well round-bottom plates (Costar no. 3799; Corning Inc., Corning, NY), to yield a final concentration of 0.2 × 106 cells/well. Cells were cultivated at 37°C in a humidified air atmosphere containing 5% CO2. PBMC viability was assessed by measuring the cellular exclusion (viable cells) versus uptake (dead cells) of propidium iodide (PI; Invitrogen, Carlsbad, CA). PI-stained PBMCs were analyzed for fluorescence intensity with a FACSCalibur cytometer (BD Biosciences, San Diego, CA). Samples were gated on forward and side scatter to exclude debris, and viability was determined by the percentage of cells that had excluded the PI stain.
TNF-α and IFN-γ production in BAL supernatant was determined with ELISA kits (R&D Systems, Minneapolis, MN) with a sensitivity of 7.8 pg/ml, as previously described (4). Matched antibody pairs and standards (capture and detection) for TNF-α and IFN-γ were obtained from R&D Systems. These studies were performed 24 hours after Be exposure, an interval that yields maximum levels of Be-stimulated cytokine secretion in CBD BAL cells (4).
Through the use of a subset of PBMCs, antigen-presenting cells (APCs) were separated by adherence to plastic, as previously described (14). Adherent cells served as APCs, whereas nonadherent cells served as responding T lymphocytes. Separated APCs and T cells were incubated overnight in 96-well round-bottom plates at 37°C in a humidified 5% CO2 atmosphere. Adherent APCs were washed three times with 200 μl of complete culture media per well, with the last 200 μl aliquot being discarded. The wells containing the matched lymphocyte targets were gently suspended and transferred to the wells containing the autologous, washed APCs in a 1:1 ratio to yield a final concentration of approximately 0.2 × 106 cells/well. Proliferation assays were performed in triplicate, as described previously here. The separated APCs or lymphocytes were exposed overnight to 100 μM sulfasalazine or mesalamine before the addition of either lymphocytes or APCs, respectively. Assays were then performed 120 hours after Be stimulation. For additional details, see the Materials and Methods section of the online supplement.
Statistical comparisons were made with repeated measures ANOVA. After the data were checked for significant treatment differences, individual contrasts were calculated to compare treatment means of interest. In cases where the data were non-Gaussian, normalizing transformations were used. All other statistical comparisons were made with Wilcoxon's rank sum test and the paired Student's t test. A P value of less than 0.05 was used to determine statistical significance.
RESULTS
Subject Demographics
Demographic and clinical characteristics are shown in Table 1. Due to the hiring practices of the Be industry, the majority of subjects with CBD and BeS are male (15). Furthermore, a large percentage of enrolled subjects with CBD and BeS are white. In comparison to the mean age of the normal control subjects enrolled in this study (31.8 ± 5.4 yr), the mean age of the BeS study subjects (61.3 ± 3.6 yr) and the CBD study subjects (59.3 ± 1.6 yr) was greater (15, 16). Although there is a significant difference in the ages of the healthy normal controls to the BeS and CBD groups, the BeS group serves as the main control to the CBD group, because individuals with BeS are healthy and do not present granulomatous lung disease (6, 7). No differences were noted in BeLPTs from steroid users versus those that were never on steroids (data not shown).
The peak BeLPT SI for PBMCs of subjects with CBD (13.6 ± 4.8) and that of subjects with BeS (4.9 ± 1.2) were elevated, indicating the presence of Be-specific lymphocytes in the blood. The peak SI for CBD BAL cells (19.2 ± 6.7) was significantly elevated in comparison to the peak for BeS BAL cells (1.3 ± 0.2), reflecting the presence of granulomatous lung disease in subjects with CBD, but not in subjects with BeS (7). Also consistent with the diagnosis of CBD was the presence of a significantly increased white blood cell count and lymphocytic alveolitis in CBD BAL (30.4 ± 4.4%) as compared with a normal BeS BAL cell count and lymphocyte differential (12.3 ± 4.3%). Bronchoscopy, BAL cell isolation, and the BAL BeLPT were not performed on normal control subjects, due to the risk of the procedure.
Sulfasalazine and Mesalamine Treatments Do Not Alter PBMC Viability
We used flow cytometry to determine whether sulfasalazine or mesalamine treatment would alter PBMC viability based on their ability to exclude PI. We determined the percentage of viable PBMCs from subjects with BeS (n = 3), subjects with CBD (n = 4), and healthy, normal control subjects (n = 3) that were untreated or treated with 100 μM sulfasalazine or 100 μM mesalamine for 120 hours in culture. Neither mesalamine nor sulfasalazine altered cell viability, with greater than 90% viable PBMCs from BeS (95.3 ± 0.3 with 100 μM mesalamine; 93.3 ± 0.4 with 100 μM sulfasalazine [mean ± SEM]), CBD (95.8 ± 0.5 with 100 μM mesalamine; 95.2 ± 0.5 with 100 μM sulfasalazine [mean ± SEM]), and healthy, normal control subjects (93.6 ± 0.3 with 100 μM mesalamine; 93.9 ± 0.5 with 100 μM sulfasalazine [mean ± SEM]).
Sulfasalazine and Mesalamine Treatments Inhibit Be-Stimulated CBD and BeS PBMC Proliferation in the BeLPT
The proliferation of Be-specific PBMCs from subjects with CBD (n = 7) and those with BeS (n = 6) was determined by the BeLPT. The objective of this study was to test the hypothesis that Be-stimulated PBMC proliferation could be inhibited by treatment with sulfasalazine or its active metabolite, mesalamine. Treatment concentrations of sulfasalazine or mesalamine were based on previously reported values (17, 18). Furthermore, a preliminary set of studies in which PBMCs were treated with different concentrations of sulfasalazine or mesalamine demonstrated that significant inhibition of Be-stimulated lymphocyte proliferation occurred with 100 μM sulfasalazine or 100 μM mesalamine (data not shown). Be stimulation (10 μM BeSO4) induced the proliferation of both CBD PBMC (with SI = 3.2 ± 0.2) and BeS PBMC (with SI = 2.7 ± 0.2) (Figures 1A and 1B). Neither CBD nor BeS PBMC proliferated in response to treatment with either sulfasalazine or mesalamine alone (Figures 1A and 1B), nor did they proliferate in response to stimulation with a control metal salt (10 μM aluminum sulfate; data not shown). In comparison, the SI was significantly decreased in Be-stimulated CBD PBMCs treated either with sulfasalazine (SI = 1.5 ± 0.3; P < 0.05 versus Be stimulation alone) or treated with mesalamine (SI = 1.5 ± 0.2; P < 0.05 versus Be stimulation alone). A similar decrease in the SI was observed with Be-stimulated BeS PBMCs treated with sulfasalazine (SI = 0.8 ± 0.1; P < 0.05 versus Be stimulation alone) or treated with mesalamine (SI = 1.1 ± 0.2; P < 0.05 versus Be stimulation alone) (Figures 1A and 1B).
Figure 1.
Sulfasalazine (SZ) and mesalamine (MSL) treatments inhibit Be-stimulated chronic beryllium (Be) disease (CBD) and Be sensitization (BeS) peripheral blood mononuclear cell (PBMC) proliferation. The stimulation index (SI) of Be-stimulated (A) CBD PBMCs (n = 7) and (B) BeS PBMCs (n = 6) that were treated with 10 μM BeSO4 plus 100 μM SZ or 10 μM BeSO4 plus 100 μM MSL. Controls were stimulated with 100 μM SZ, 100 μM MSL, or 10 μM BeSO4 alone. *P < 0.05, 10 μM BeSO4 versus 10 μM BeSO4 + 100 μM MSL; **P < 0.05, 10 μM BeSO4 versus 10 μM BeSO4 + 100 μM SZ. Values presented are mean SI.
Previous studies have shown that Be antigen is presented to Be-specific T cells in an major histompatibility complex class II (MHCII)–restricted manner (2). Our data suggest that Be-stimulated CBD and BeS PBMC proliferation could be inhibited by sulfasalazine and mesalamine treatment. By using PBMC from healthy normal control subjects (n = 3), we therefore tested the hypothesis that these agents could inhibit lymphocyte proliferation driven by tetanus toxoid (TT). The objective of this study was to determine whether these agents differed in their ability to inhibit MHCII-driven PBMC proliferation by the TT antigen (Figure 2). TT stimulation (2.5 flocculation unites (LfU)) induced the proliferation of healthy normal control (n = 3) PBMCs (SI = 4.4 ± 0.6 [mean ± SEM]). TT-stimulated healthy normal control PBMCs treated with 100 μM sulfasalazine had a significantly decreased SI (2.6 ± 0.3; P < 0.05 versus TT-stimulation alone). By comparison, TT-stimulated healthy normal control PBMCs treated with 100 μM mesalamine were not inhibited in their ability to proliferate, with an SI of 4.3 (±0.4) that was similar to the TT treatment alone.
Figure 2.
SZ treatment, but not MSL, inhibits tetanus toxoid (TT)–stimulated PBMC proliferation. TT-stimulated lymphocyte proliferation (SI) by healthy normal control (n = 3) PBMCs that were treated with 2.5 flocculation units (Lfu) TT, 2.5 Lfu TT plus 100 μM MSL, or 2.5 Lfu TT plus 100 μM SZ. *P < 0.033, TT + MSL versus TT + SZ. Values presented are mean SI (±SEM).
Be-Stimulated CBD and BeS PBMC Proliferation Is Inhibited by Treating either APCs or Lymphocytes with Sulfasalazine or Mesalamine
Our data suggest that sulfasalazine and mesalamine treatment could inhibit Be-stimulated PBMC proliferation in subjects with BeS and CBD. We tested the hypothesis that the inhibitory effect of these drugs could be directed toward the APC population, or toward the responding lymphocyte populations, or both. We used an APC–lymphocyte separation model developed previously (14). Isolated PBMC APCs were treated with either 100 μM sulfasalazine or 100 μM mesalamine. Autologous PBMC lymphocytes from these cultures were untreated and incubated in parallel to the treated APCs overnight. Alternatively, isolated PBMC lymphocytes were treated with either 100 μM sulfasalazine or 100 μM mesalamine, and the autologous isolated PBMC APCs from these cultures were untreated and incubated in parallel to the treated lymphocytes overnight. The autologous pretreated APCs or pretreated lymphocytes were then washed, added back together, and then stimulated overnight with 10 μM BeSO4, and the SI determined by the BeLPT.
Serving as the positive Be-stimulation control for these experiments, isolated APCs or isolated lymphocytes were incubated overnight in medium alone. The autologous, separated cells were then washed, added back together, Be stimulated (10 μM BeSO4) overnight, and the SI was determined by the BeLPT. We observed that added-back CBD PBMCs (n = 8; SI = 2.8 ± 0.3) and BeS PBMCs (n = 8; SI = 2.7 ± 0.2) proliferated in response to Be stimulation (Figure 3). Untreated isolated APCs or lymphocytes from subjects with CBD and BeS that were added back and stimulated with medium alone did not proliferate. Isolated APCs or lymphocytes were also incubated overnight in medium containing either 100 μM sulfasalazine or 100 μM mesalamine. Pretreatment of either isolated APCs or lymphocytes from CBD or BeS PBMCs still resulted in proliferation of the added-back PBMCs in response to Be stimulation, although the extent of proliferation was modified.
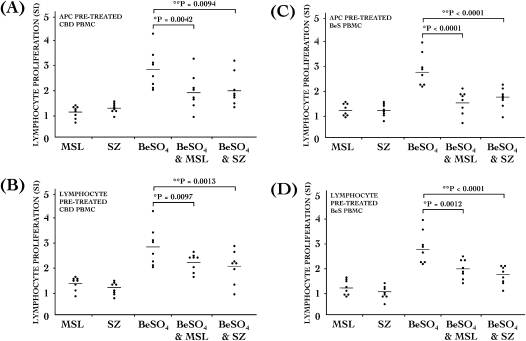
Figure 3.
SZ and MSL treatments inhibits Be-stimulated proliferation in both antigen-presenting cells (APCs) and lymphocytes. Proliferation (SI) by (A and B) CBD (n = 8) and (C and D) BeS (n = 8) PBMCs that were either isolated APCs (A and C) or isolated responding lymphocytes (B and D). PBMCs were exposed to 100 μM SZ, 100 μM MSL, 10 μM BeSO4, 10 μM BeSO4 plus 100 μM MSL, or 10 μM BeSO4 plus 100 μM SZ. *P < 0.05, 10 μM BeSO4 versus 10 μM BeSO4 + 100 μM MSL; **P < 0.05, BeSO4 versus BeSO4 + SZ. Values presented are mean SI.
Pretreated CBD and BeS PBMCs in which the isolated APCs had been pretreated with sulfasalazine were respectively inhibited by 31% and 41%, on average, in their ability to proliferate in response to Be stimulation (Figures 3A and 3C). CBD and BeS PBMCs in which the isolated lymphocytes had been pretreated with sulfasalazine were inhibited in their ability to proliferate in response to Be stimulation by 36% and 37%, on average, respectively (Figures 3B and 3D). Furthermore, CBD and BeS PBMCs in which the isolated APCs had been pretreated with mesalamine were inhibited in their ability to proliferate in response to Be stimulation by 32% and 44%, on average, respectively (Figures 3A and 3C). Finally, CBD and BeS PBMCs in which the isolated lymphocytes had been pretreated with mesalamine were also inhibited in their ability to proliferate in response to Be stimulation by approximately 29% and 30%, on average, respectively (Figures 3B and 3D).
Sulfasalazine and Mesalamine Inhibit Be-Stimulated IFN-γ and TNF-α Production in CBD BAL Cells
Our data suggest that sulfasalazine and mesalamine could inhibit the proliferation of CBD and BeS PBMCs in response to Be stimulation. However, the lungs are the target organ most affected in patients with CBD. CBD BAL T cells produce high levels of IFN-γ and TNF-α mRNA and protein after Be stimulation (8, 19). It is believed that these proinflammatory cytokines play a central role in granuloma pathogenesis of CBD (2, 10). We therefore tested the hypothesis that sulfasalazine and mesalamine could inhibit Be-induced CBD BAL cell IFN-γ and TNF-α protein production, as measured by ELISA. Previous detailed analysis showing that Be stimulation induces proinflammatory cytokine production by the Be-specific T cells from the lungs of subjects with CBD (5, 19), and not by CBD BAL alveolar macrophages (8). As such, it was reasonable to make the assumption that, for the purposes of this experiment, Be-stimulated CBD BAL mixed cell cytokine production would serve as a surrogate for cytokine production by Be-specific T cells. Because there is variable response in cytokine production upon Be stimulation among subjects with CBD (8), our data values were normalized according to each subject's Be-stimulated IFN-γ or TNF-α levels (Figures 4 and 5).
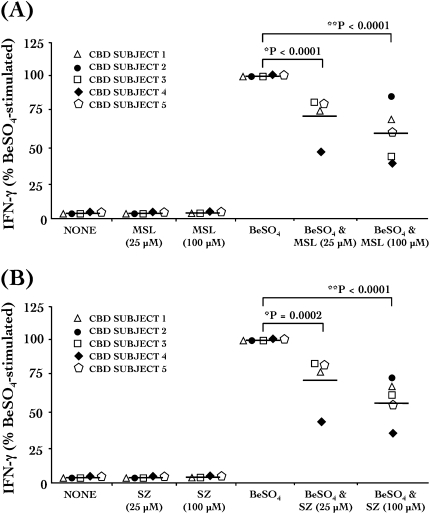
Figure 4.
SZ and MSL treatments inhibit Be-stimulated IFN-γ production in CBD bronchoalveolar lavage (BAL) cells. Be-stimulated production of IFN-γ by CBD BAL cells (n = 5) is inhibited with (A) MSL or (B) SZ treatment. BAL cells were unstimulated (NONE) or exposed to 25 μM or 100 μM SZ, 25 μM or 100 μM MSL, 100 μM BeSO4, 100 μM BeSO4 plus 25 μM or 100 μM MSL, or 100 μM BeSO4 plus 25 μM or 100 μM SZ. *P < 0.05 and **P < 0.05, BeSO4 versus BeSO4 + 25 μM MSL or SZ, or BeSO4 versus BeSO4 + 100 μM MSL or SZ, respectively. IFN-γ values are presented as percent of the BeSO4-stimulated IFN-γ response for each subject.
Figure 5.
SZ and MSL treatments inhibit Be-stimulated TNF-α production in CBD BAL cells. Be-stimulated production of TNF-α by CBD BAL cells (n = 5) is inhibited with (A) MSL or (B) SZ treatment. BAL cells were unstimulated (NONE) or exposed to 25 μM or 100 μM SZ, 25 μM or 100 μM MSL, 100 μM BeSO4, 100 μM BeSO4 plus 25 μM or 100 μM MSL, or 100 μM BeSO4 plus 25 μM or 100 μM SZ. *P < 0.05 and **P < 0.05, BeSO4 versus BeSO4 + 25 μM MSL or SZ, or BeSO4 versus BeSO4 + 100 μM MSL or SZ, respectively. TNF-α values are presented as percent of the BeSO4-stimulated TNF-α response for each subject.
Treatment of CBD BAL cells with either mesalamine or sulfasalazine caused a reduction in Be-stimulated IFN-γ secretion (n = 5; Figures 4A and 4B). Unstimulated control values for IFN-γ ranged from 0 to 54 pg/ml. Be-stimulated CBD BAL cells produced a median of 1,300 pg/ml (range, 433–3,966 pg/ml) of IFN-γ, observed by ELISA in culture supernatant after 24 hours of stimulation with 100 μM BeSO4 (Figure 4). We were unable to measure IFN-γ levels above the ELISA manufacturer's limits of IFN-γ detection in the culture supernatant of CBD BAL cells that were treated for 24 hours with either 100 μM sulfasalazine or mesalamine alone. The ability of CBD BAL cells to produce Be-stimulated IFN-γ was significantly reduced (40%) when cells were cotreated with 100 μM mesalamine (Figure 4A). A similar 45% decrease in Be-stimulated IFN-γ was also observed in cells that were cotreated with 100 μM sulfasalazine (Figure 4B).
Furthermore, mesalamine and sulfasalazine also reduced Be-stimulated TNF-α secretion in CBD BAL cells (n = 5; Figure 5). Unstimulated control values for TNF-α ranged from 0 to 1,141 pg/ml. Be-stimulated CBD BAL cells produced a median of 2,095 pg/ml (range, 547–4,914 pg/ml) of TNF-α, observed by ELISA in culture supernatant after 24 hours of stimulation with 100 μM BeSO4 (Figure 5). The ability of CBD BAL cells to produce Be-stimulated TNF-α with 100 μM mesalamine cotreatment was reduced by approximately 23.5% (Figure 5A). Similarly, cotreatment with 100 μM sulfasalazine caused a significant reduction in Be-stimulated TNF-α by 30% (Figure 5B).
DISCUSSION
Corticosteroids are the mainstay of treatment for patients with CBD. Individuals who fail to respond to steroids are often treated with the more toxic drug, methotrexate. Little attention has focused on evaluating potentially new therapeutic approaches to treat this chronic granulomatous lung disorder. In the current study, we tested the hypothesis that the commonly used anti-inflammatory compound, sulfasalazine, or its active metabolite, mesalamine, could inhibit Be-stimulated CBD and BeS PBMC proliferative responses in the BeLPT. We observed that both agents were able to inhibit CBD and BeS PBMC proliferation in the BeLPT, whereas only sulfasalazine, and not mesalamine, inhibited healthy normal control PBMC proliferation in response to TT stimulation. We also tested whether these agents could inhibit Be-stimulated cytokine production by CBD BAL cells acquired from the lung, the target organ most affected in CBD. Cytokine levels were not measured in PBMCs, because a previous study showed that the ability of Be to stimulate CBD PBMC and BeS PBMC proinflammatory cytokine production does not correlate with Be-stimulated PBMC proliferation in the BeLPT (5). Both sulfasalazine and mesalamine significantly reduced the ability of Be-stimulated CBD BAL cells to produce proinflammatory cytokines. Our data suggest that sulfasalazine and mesalamine may alter proinflammatory pathways critical to the pathogenesis of CBD. Based on these observations, we conclude that sulfasalazine and mesalamine could represent a new therapeutic approach to augment the treatment of patients with CBD or other similar granulomatous lung disorders, such as sarcoidosis.
We used the BeLPT as a pharmacological screening tool to determine if sulfasalazine or mesalamine could inhibit Be-stimulated proliferation in CBD and BeS PBMCs ex vivo. Be stimulation drives the activation of a subset of Be-specific CD4+ pathogenic T cells that proliferate upon Be stimulation and secrete proinflammatory cytokines, a response that, in turn, amplifies lung granuloma formation in CBD (2). The granulomatous inflammation in the lungs of patients with CBD is a complex process that likely involves the long-term persistence of Be metal at the anatomical site of disease and the continued exposure of Be to the innate and acquired immune systems (2, 21). Our study shows that the commonly used anti-inflammatory drugs, sulfasalazine and mesalamine were able to inhibit Be-stimulated CBD PBMC and BeS PBMC proliferation in the BeLPT. We also observed that these agents significantly inhibited Be-stimulated CBD BAL cell IFN-γ and TNF-α production.
Corticosteroid therapy is the most widely used treatment for CBD, but its therapeutic efficacy is variable and, in some individuals, steroids are not well tolerated (22). An important consideration in this study is whether corticosteroid therapy might confound determination of SI by using the BeLPT for cells also treated with sulfasalazine and mesalamine. Our study was not powered to determine whether corticosteroid therapy affected our results, but it should be noted that 6 of 25 subjects with CBD were currently receiving oral corticosteroids (Table 1). However, findings from this and previous studies demonstrate that corticosteroid therapy has not been shown to significantly reduce BeLPT or cytokine response to Be stimulation (4, 19, 20). Based on these observations, we believe it unlikely that corticosteroid therapy adversely affected proliferation of Be-treated PBMCs used in this study, although this remains a possibility. For patients with CBD undergoing corticosteroid therapy, possible adverse side effects of corticosteroids not only create important quality of life issues, but the side effects may be severe and preclude their use for treatment of these patients (23). We believe that our preliminary observations support the conclusion that sulfasalazine and mesalamine could represent an important new treatment option for patients with CBD. This conclusion must be tempered by the fact that this study was performed ex vivo with a small number of patient samples, and thus did not allow for more detailed pharmacologic and mechanistic studies. However, our preliminary observations support future investigations that will determine the mechanisms by which these agents inhibit Be-stimulated lymphocyte proliferation and cytokine production in CBD.
A key feature of the BAL cells recovered from the lungs of patients with CBD is their ability to produce high levels of proinflammatory cytokines after Be stimulation. We found that Be-stimulated TNF-α and IFN-γ were significantly decreased in CBD BAL cells after treatment with sulfasalazine and mesalamine. This is an important observation, as these proinflammatory cytokines produced by Be-specific T cells from the anatomical site of disease are believed to play a key functional role in the severity of lung disease in CBD (2, 21). Thus, it is rational to suggest that anti-inflammatory compounds, such as sulfasalazine and mesalamine, compounds that are well tolerated by patients with other chronic inflammatory diseases (11), could serve to reduce or limit Be-stimulated TNF-α and IFN-γ production in the lungs of patients with CBD, and thereby reduce the severity of the chronic inflammatory process.
Our data also indicate an interesting difference in the inhibitory effect of sulfasalazine and mesalamine on T cell proliferation induced by two different MHCII-restricted antigens, Be-antigen and TT. Sulfasalazine and mesalamine both decreased Be-stimulated CBD and BeS PBMC proliferation, but we were surprised to observe that only sulfasalazine inhibited TT-induced proliferation in PBMCs from healthy, normal control subjects. Although the mechanisms by which this differential effect on PBMC proliferation are unknown, the difference in selectivity relative to these disparate MHCII-restricted antigens suggests that sulfasalazine and mesalamine are likely to operate through very complex and possibly different pathways.
Sulfasalazine has been used in the treatment of chronic inflammatory disorders, including rheumatoid arthritis and IBD, of which Crohn's disease and ulcerative colitis are the two main forms. Sulfasalazine is a conjugate of mesalamine (5-aminosalicyclic acid) and sulfapyridine, linked together by an azo bond. The active moiety for IBD is mesalamine (24), whereas sulfapyridine appears to be the active moiety for rheumatoid arthritis (25). It is also interesting that Crohn's disease can also present with granulomas of the gastrointestinal tract (26). Sulfasalazine and mesalamine have been shown to down-regulate and inhibit the immune and inflammatory responses associated with IBD (27). Although the underlying causes of IBD are unknown, inflammatory mediators, including oxidative stress and proinflammatory cytokines, are widely believed to contribute to the inflammatory cascade that, in turn, modulates the immune system (12, 14, 27–29). We have previously shown that Be increases reactive oxygen species production and decreases thiol levels in PBMCs (14).
Data in the present ex vivo study show that sulfasalazine and mesalamine were able to reduce key cellular responses triggered by Be. The data advance our current level of understanding of Be-induced inflammatory processes in CBD. This study demonstrates the feasibility of considering these agents as potential therapy aimed at reducing granulomatous inflammation in the lungs of patients with CBD. Clearly, additional studies are required to elucidate the exact mechanisms by which these agents exert their action on Be-specific T cells, but our data suggest a potential therapeutic utility for mesalamine and sulfasalazine in CBD.
Supplementary Material
This work was supported by National Institutes of Health grants RO1 ES-012504, RO1 ES-017582, PO1 ES11810, and 1 UL1 RR025780.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0150OC on November 9, 2009
Author Disclosure: D.R.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.J.D. received consultancy fees from Aeolus Pharmaceuticals for more than $100,001, a sponsored grant for $50,001–$100,000, and owns stock valued at $10,001–$50,000. He also received patents from National Jewish Health for methods for treatment of thiol containing compound deficient conditions and antioxidant therapies, and a sponsored grant from NIH for more than $100,001. L.A.M. received expert witness fees from Golomb & Honek of less than $1,000, a sponsored grant from Centocor for $5,001–$10,000, and a patent from National Jewish Health for Beryllium ELISPOT for use in diagnosis of beryllium-related health effects. She also received grants from NIH: one for $10,001–$50,000, two for $50,001–$100,000, and one for more than $100,001. She has been employed by National Jewish Health for more than $1,000. L.S.N. received a patent from National Jewish Health for methods for treatment of thiol-containing compound deficient conditions. He is employed by University of Colorado Denver for more than $100,001, and has received sponsored grants from Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health for more than $100,001, U.S. Department of Energy for more than $100,001, NIH for more than $100,001, and U.S.-Israel Binational Science Foundation for less than $1,001. R.T.S. received a patent from National Jewish Health for methods for therapy of cystic fibrosis. He owns mutual funds in Teachers Insurance and Annuity Association (TIAA), College Retirement Equities Fund (CREF) for more than $100,001, Federal Employee Retirement System for $50,001–$100,000, and his spouse owns mutual funds/stock in IBM for $50,001–$100,000. He is also employed by NIH for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Henneberger PK, Goe SK, Miller WE, Doney B, Groce DW. Industries in the United States with airborne beryllium exposure and estimates of the number of current workers potentially exposed. J Occup Environ Hyg 2004;1:648–659. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot AP, Kotzin BL. Chronic beryllium disease: Immune-mediated destruction with implications for organ-specific autoimmunity. Tissue Antigens 2003;62:449–458. [DOI] [PubMed] [Google Scholar]
- 3.Newman LS, Mroz MM, Balkissoon R, Maier LA. Beryllium sensitization progresses to chronic beryllium disease: a longitudinal study of disease risk. Am J Respir Crit Care Med 2005;171:54–60. [DOI] [PubMed] [Google Scholar]
- 4.Maier LA, Sawyer RT, Tinkle SS, Kittle LA, Barker EA, Balkissoon R, Rose C, Newman LS. IL-4 fails to regulate in vitro beryllium-induced cytokines in berylliosis. Eur Respir J 2001;17:403–415. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot AP, Palmer BE, Sullivan AK, Joslin FG, Wilson CC, Maier LA, Newman LS, Kotzin BL. Frequency of beryllium-specific, central memory CD4+ T cells in blood determines proliferative response. J Clin Invest 2005;115:2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol 1991;88:54–60. [DOI] [PubMed] [Google Scholar]
- 7.Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, Markham TN, Daniele RP. Proliferative response of bronchoalveolar lymphocytes to beryllium: a test for chronic beryllium disease. Ann Intern Med 1988;108:687–693. [DOI] [PubMed] [Google Scholar]
- 8.Sawyer RT, Parsons CE, Fontenot AP, Maier LA, Gillespie MM, Gottschall EB, Silveira L, Newman LS. Beryllium-induced TNF-alpha production by CD4+ T cells is mediated by HLA-DP. Am J Respir Cell Mol Biol 2004;31:122–130. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer RT, Abraham JL, Daniloff E, Newman LS. Secondary ion mass spectroscopy demonstrates retention of beryllium in chronic beryllium disease granulomas. J Occup Environ Med 2005;47:1218–1226. [DOI] [PubMed] [Google Scholar]
- 10.Rom WN, Markowitz S, editors. Environmental and occupational medicine. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007.
- 11.Sands BE. Therapy of inflammatory bowel disease. Gastroenterology 2000;118(2 Suppl 1)S68–S82. [DOI] [PubMed] [Google Scholar]
- 12.Joshi R, Kumar S, Unnikrishnan M, Mukherjee T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radic Res 2005;39:1163–1172. [DOI] [PubMed] [Google Scholar]
- 13.Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the X(c)− cystine transporter: a new action for an old drug. Leukemia 2001;15:1633–1640. [DOI] [PubMed] [Google Scholar]
- 14.Dobis DR, Sawyer RT, Gillespie MM, Huang J, Newman LS, Maier LA, Day BJ. Modulation of lymphocyte proliferation by antioxidants in chronic beryllium disease. Am J Respir Crit Care Med 2008;177:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreiss K, Mroz MM, Zhen B, Martyny JW, Newman LS. Epidemiology of beryllium sensitization and disease in nuclear workers. Am Rev Respir Dis 1993;148:985–991. [DOI] [PubMed] [Google Scholar]
- 16.Newman LS, Kreiss K, King TE Jr, Seay S, Campbell PA. Pathologic and immunologic alterations in early stages of beryllium disease: re-examination of disease definition and natural history. Am Rev Respir Dis 1989;139:1479–1486. [DOI] [PubMed] [Google Scholar]
- 17.Stevens C, Lipman M, Fabry S, Moscovitch-Lopatin M, Almawi W, Keresztes S, Peppercorn MA, Strom TB. 5-aminosalicylic acid abrogates T-cell proliferation by blocking interleukin-2 production in peripheral blood mononuclear cells. J Pharmacol Exp Ther 1995;272:399–406. [PubMed] [Google Scholar]
- 18.Doering J, Begue B, Lentze MJ, Rieux-Laucat F, Goulet O, Schmitz J, Cerf-Bensussan N, Ruemmele FM. Induction of T lymphocyte apoptosis by sulphasalazine in patients with Crohn's disease. Gut 2004;53:1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinkle SS, Kittle LA, Schumacher BA, Newman LS. Beryllium induces IL-2 and IFN-gamma in berylliosis. J Immunol 1997;158:518–526. [PubMed] [Google Scholar]
- 20.Pott GB, Palmer BE, Sullivan AK, Silviera L, Maier LA, Newman LS, Kotzin BL, Fontenot AP. Frequency of beryllium-specific, Th1-type cytokine-expressing CD4+ T cells in patients with beryllium-induced disease. J Allergy Clin Immunol 2005;115:1036–1042. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer RT, Maier LA, Kittle LA, Newman LS. Chronic beryllium disease: a model interaction between innate and acquired immunity. Int Immunopharmacol 2002;2:249–261. [DOI] [PubMed] [Google Scholar]
- 22.Sood A, Beckett WS, Cullen MR. Variable response to long-term corticosteroid therapy in chronic beryllium disease. Chest 2004;126:2000–2007. [DOI] [PubMed] [Google Scholar]
- 23.Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest 2005;127:1064–1071. [DOI] [PubMed] [Google Scholar]
- 24.van Hees PA, Bakker JH, van Tongeren JH. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulphasalazine. Gut 1980;21:632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann VC, Taggart AJ, Le Gallez P, Astbury C, Hill J, Bird HA. A study to determine the active moiety of sulphasalazine in rheumatoid arthritis. J Rheumatol 1986;13:285–287. [PubMed] [Google Scholar]
- 26.Freeman HJ. Granuloma-positive Crohn's disease. Can J Gastroenterol 2007;21:583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieto N, Torres MI, Fernandez MI, Giron MD, Rios A, Suarez MD, Gil A. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci 2000;45:1820–1827. [DOI] [PubMed] [Google Scholar]
- 28.Murata Y, Ishiguro Y, Itoh J, Munakata A, Yoshida Y. The role of proinflammatory and immunoregulatory cytokines in the pathogenesis of ulcerative colitis. J Gastroenterol 1995;30:56–60. [PubMed] [Google Scholar]
- 29.Wendland BE, Aghdassi E, Tam C, Carrrier J, Steinhart AH, Wolman SL, Baron D, Allard JP. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr 2001;74:259–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.