Abstract
Children have a lower incidence and mortality from acute lung injury (ALI) than adults, and infections are the most common event associated with ALI. To study the effects of age on susceptibility to ALI, we investigated the responses to microbial products combined with mechanical ventilation (MV) in juvenile (21-d-old) and adult (16-wk-old) mice. Juvenile and adult C57BL/6 mice were treated with inhaled Escherichia coli 0111:B4 lipopolysaccharide (LPS) and MV using tidal volume = 15 ml/kg. Comparison groups included mice treated with LPS or MV alone and untreated age-matched control mice. In adult animals treated for 3 hours, LPS plus MV caused synergistic increases in neutrophils (P < 0.01) and IgM in bronchoalveolar lavage fluid (P = 0.03) and IL-1β in whole lung homogenates (P < 0.01) as compared with either modality alone. Although juvenile and adult mice had similar responses to LPS or MV alone, the synergistic interactions between LPS and MV did not occur in juvenile mice. Computational analysis of gene expression array data suggest that the acquisition of synergy with increasing age results, in part, from the loss of antiapoptotic responses and the acquisition of proinflammatory responses to the combination of LPS and MV. These data suggest that the synergistic inflammatory and injury responses to inhaled LPS combined with MV are acquired with age as a result of coordinated changes in gene expression of inflammatory, apoptotic, and TGF-β pathways.
Keywords: adult respiratory distress syndrome, inflammation, oligonucleotide array sequence analysis
CLINICAL RELEVANCE.
Children are less susceptible to acute lung injury than adults. The age-dependent mechanisms by which inflammatory and injury responses in the lungs are acquired are unknown. This study shows that the synergistic inflammatory and injury responses to bacterial lipopolysaccharide and mechanical ventilation are acquired with age and identifies gene expression pathways that might be involved.
Acute lung injury (ALI) affects more than 150,000 people each year in the United States. The mortality of ALI ranges from over 60% in elderly patients to less than 20% in children, and ALI leads to more than $2 billion in health care costs (1). The only intervention to significantly reduce the mortality of ALI has been decreasing tidal volumes used in mechanical ventilation (MV) (2). The age-adjusted childhood incidence (12/100,000 person-years) and mortality (2.3/100,000 persons) of ALI are much lower than in adults (79/100,000 person-years and 25.4/100,000 respectively) (1, 3). Although comorbid conditions probably contribute to the higher mortality in elderly patients, middle-aged adults still have a significantly higher mortality than children (1).
Infections are the leading cause of ALI in adults and children (1, 3, 4). Furthermore, bacterial products are found in the bronchoalveolar lavage fluid (BALF) of patients with acute respiratory distress syndrome (ARDS) even in the absence of evidence of clinically defined infection (5). Although MV is a life-saving therapy for patients with ALI, the mechanical stretch from positive pressure MV has been shown to interact with infectious agents to increase lung injury in laboratory animals (6).
Postnatal development of the lung and the innate immune system are likely to be relevant to the age-dependent differences in the incidence and mortality of ALI. Although bulk alveolarization is probably complete by approximately 18 months of life, lung growth and development continues throughout childhood (7–9). The innate immune system also develops in the postnatal period (10–12). Recently, Alvira and colleagues showed lower inflammatory and apoptotic responses in the lungs of newborn mice treated with systemic bacterial products as compared with adults, and that these responses were associated with differences in NFκB dimerization (13). In addition to the postnatal development of the lung and innate immune system, there are common pathways in the regulation of lung development, innate immunity, repair mechanisms, apoptosis, and alveolar fluid clearance (14–17). Finally, animal studies have shown that newborns have lower inflammatory and injury responses to MV as compared with adults (18, 19).
We hypothesized that the increased incidence and severity of lung injury associated with increasing age is due in large part to acquired changes in the way in which inflammatory responses are activated in the lungs in response to microbial products and MV. To test this hypothesis, we developed a murine model of lung injury using adult (16-wk-old, approximating a 35- to 45-yr-old human) and juvenile (21-d-old, approximating a 2-yr-old human) mice (20). The mice were treated with inhaled lipopolysaccharide (LPS) alone or in combination with moderate tidal volume (15 ml/kg) MV. We found that the combination of LPS and MV resulted in significant increases in inflammation and injury in adult but not juvenile mice. The dose of LPS used caused significant increases in inflammation in the juvenile and adult mice, and the tidal stretch from MV alone did not result in significant pulmonary inflammation in adult or juvenile mice. Therefore, these data suggest that the age-dependent differences in this lung injury model occur at the intersection between innate immunity and mechanical stretch.
MATERIALS AND METHODS
Animal Protocol
The Animal Research Committee of the Veterans Affairs Puget Sound Health Care System approved all of the experiments. Male or female 14-day-old and 15-week-old C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in a specific pathogen–free animal facility until the day of the experiment. Twenty-one–day-old (7–10 g) and 16-week-old (19–23 g) mice were divided into four different treatment groups: (1) no treatment, (2) aerosolized LPS with spontaneous ventilation, (3) MV, or (4) aerosolized LPS combined with MV (LPS+MV). The duration of the experiments to collect whole-lung mRNA for expression array analysis was 2 hours, and the duration of the experiments to measure inflammation and injury was 3 hours. Spontaneously breathing mice were allowed free access to food and water in their cages and breathed room air for the duration of the experimental protocol. C57BL/6J-Ticam1Lps2/J (TRIF−/−) mice were bred in the vivarium of the VA Puget Sound Medical Center Animal Facility, and 21-d-old mice were treated with aerosolized LPS with spontaneous ventilation or aerosolized LPS+MV for 3 hours.
Reagents
A stock solution of LPS derived from Escherichia coli serotype 0111:B4 (List Biological Laboratories, Campbell, CA) was diluted in phosphate-buffered saline to 1 mg/ml and stored at −20°C. On each experiment day, LPS was thawed, and 2 mg were diluted in 20 ml phosphate-buffered saline (0.1 mg/ml). Multiplex bead-based immunoassays for TNF-α, MIP-2, chemokine (C-X-C) motif ligand 1 (CXC1/KC), MCP-1, IL-12P70, IL-6, IL-1β, IFNγ, IL-10, IL-2, IL-13, IL-17, VEGF, and GM-CSF were performed with murine specific antibodies (R&D Systems, Minneapolis, MN) and an automated analysis system (Luminex Corp, Austin, TX). Total protein was measured using the BCA Protein Assay Kit (Pierce, Rockford, IL), and the ELISA for IgM was performed using the Mouse IgM Quantitation Kit (Bethyl Laboratories, Montgomery, TX). Quantitative PCR was performed with the High Capacity cDNA reverse transcription kit (#4374966) and primers to Bcl2, S100a9, and Tlr4 from ABI (Foster City, CA).
Murine Model of Ventilator-Induced Lung Injury
LPS aerosol treatment.
Mice were exposed to an aerosol of LPS (20 ml of 0.1 mg/ml) for 30 minutes in a sealed aerosol chamber as described in the online supplement (21). The experimental protocol began immediately after completion of the aerosol treatment. Mice that were treated with LPS alone were recovered and returned to their cages and were allowed free access to food and water for the duration of the experimental protocol. Mice that were treated with LPS+MV were anesthetized, intubated, and mechanically ventilated immediately after exposure to aerosolized LPS.
Mechanical ventilation.
Mechanically ventilated mice were anesthetized with an intraperitoneal injection of ketamine (0.075 mg/g) and xylazine (0.015 mg/g) and orotracheally intubated. In the 2-hour studies for mRNA collection, the mice were anesthetized with 4% isoflurane. Mice were mechanically ventilated for 2 or 3 hours with FiO2 = 0.3, 2 cm H2O positive end-expiratory pressure; VT = 15 ml/kg, and rate = 80 breaths/min, providing a normal minute ventilation (22). A lung recruitment maneuver with 5 cm H2O positive end-expiratory pressure for 30 seconds was completed every 30 minutes for mice that were mechanically ventilated for 3 hours (23).
Physiological Monitoring
Temperature (Mon-a-therm; Nellcor, Pleasanton, CA), airway pressure, and heart rate (Animal BioAmp ML136, PowerLab 16/30; ADInstruments, Colorado Springs, CO) were measured continuously in all mechanically ventilated mice. Noninvasive blood pressure was measured every 30 minutes for the first hour and then hourly (NIBP Controller ML125, PowerLab 16/30; ADInstruments).
Sample Processing
After 3 hours, the animals were killed and exsanguinated. The tracheae were cannulated, and the lungs were lavaged as described (details provided in the online supplement). The lavage fluid was processed for cell counts and differentials (n ≥ 12 per group) and then stored in individual aliquots at −70°C as described (details provided in the online supplement). After the lavage procedure, the lungs were homogenized for cytokine measurements (n = 10 per group) as described (details provided in the online supplement).
Histology
The excised lungs were fixed by inflation with 4% paraformaldehyde at a transpulmonary pressure of 15 cm H2O and then processed for light microscopy (n = 5 per group) as described (details provided in the online supplement).
RNA Analysis
To measure early changes in gene expression, the lungs were removed immediately after 2 hours of treatment. Total lung mRNA was purified, and the samples were reverse transcribed, labeled, and hybridized on the Affymetrix mouse 430 2.0 expression array chip.
Measurements
Cell viability was assessed, and total and differential cell counts were performed (n ≥ 12 per group) (details provided in the online supplement). The total protein and IgM were measured in the BALF (n = 10 per group) as described in the online supplement. Cytokines and growth factors were measured in lung homogenates as described (n = 10 per group) in the online supplement.
Statistical Analysis
To determine the effect of each treatment in the juvenile and adult mice, whether there were synergistic interactions between LPS combined with MV in each age group, and whether age modified the responses to each treatment, we used regression analysis with a three-factorial model that included age, MV, and LPS using polymorphonuclear neutrophils (PMNs), total protein, IgM, and cytokines as the outcome variables (SPSS 16.0.1; SPSS Inc, Chicago, IL). Synergistic interactions between LPS and MV were identified when the difference between untreated mice and mice treated with LPS+MV were significantly different from the sum of the effects of each treatment alone. Age was determined to modify the effects of treatment (MV, LPS, or LPS+MV) when the measured outcome in the juvenile mice was significantly different from the adults. Model residuals were examined graphically to check for normality of the data. Dependent variables with nonnormal distributed data were log10 transformed to achieve a closer approximation of a normal distribution.
To determine the effect of age and treatment with MV and LPS+MV on the physiology of the mice, we used two-way ANOVA with the post hoc Bonferroni adjustment for P < 0.01 (Prism 4.0; GraphPad Software, Inc., La Jolla, CA) to compare temperature, respiratory rate, heart rate, and systolic blood pressure in the juvenile and adult mice at 0, 0.5, 1, 2, and 3 hours. To determine the effect of age and treatment with MV and LPS+MV on cardiorespiratory physiology, we used one-way ANOVA with the post hoc Bonferroni adjustment for P < 0.01 to compare normally distributed data (pH) and the Kruskal-Wallis test with Dunn's multiple comparisons on nonnormally distributed data (PmvCO2, SmvO2, and lactate).
Microarray Data Analysis
The complete details of experimental design, hybridization procedures, and raw microarray data in compliance with the Minimum Information About a Microarray Experiment have been deposited at the Gene Expression Omnibus website (www.ncbi.nlm.nih.gov/geo, query GSE18341). Briefly, the raw gene expression array data were normalized using the Robust Multichip Average algorithm (24). To determine the variability of gene expression in the individual samples, a principal component analysis (PCA) was performed on the entire array dataset (MeV 4.0) (25, 26). Pairwise comparisons were performed using Bayesian t tests with a false discovery rate (FDR) less than 0.1%, using the Benjamini and Hochberg method (27, 28). To identify genes likely to be biologically significant for the acquisition of synergistic responses to LPS combined with MV, we first used two-way ANOVA (adjusted P = 0.01) to identify genes with an interaction effect between age and treatment (MeV 4.0) (25). K-median consensus (KMC) clustering using the Pearson's correlation with a maximum of 50 iterations was used on the genes with a significant interaction effect between age and treatment to identify major expression patterns across the experimental conditions (MeV 4.0) (25). Functional annotation and functional clustering were used to explore enriched biological modules in the KMC clusters of interest using the Database for Annotation, Visualization and Integration Discovery. Multiple hypothesis testing was addressed by applying a FDR cutoff of less than 5% (29).
Interaction networks were created from gene members of KMC clusters of interest using Ingenuity System's software and knowledge base (details provided in the online supplement) (30). For each cluster, an interaction network, or interactome, was built around genes with the highest connectivity (seeds) using an iterative algorithm that systematically connects additional nodes to the initial seed.
Quantitative PCR
To confirm the expression array results, quantitative real-time PCR was used to measure the expression of select candidate genes (details provided in the online supplement). The RNA was reverse transcribed to cDNA using a high-capacity cDNA reverse transcription kit (#4374966; ABI, Foster City, CA), and real-time PCR was performed on each sample in triplicate using assay-on-demand probe and primer sets. Analysis of the amplification was performed using the delta-delta threshold cycle method with 18 s rRNA (Hs99999901_s1; ABI) as the endogenous control.
RESULTS
Effect of LPS and MV on Lung Inflammation and Permeability
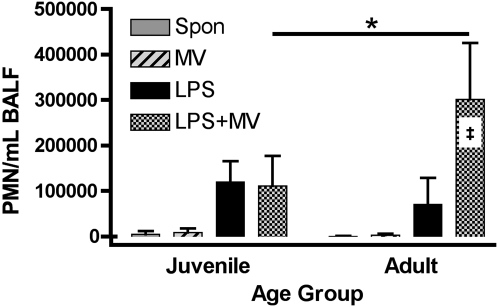
To determine whether age modifies the inflammatory responses in the lungs, adult and juvenile mice were treated with MV alone, with inhaled LPS alone, or with a combination of LPS and MV (LPS+MV) (n ≥ 12 per group). Treatment with LPS+MV resulted in a synergistic increase in the number of neutrophils in the BALF of adult but not juvenile mice (P = 2.11 × 10−14), and treatment with LPS+MV resulted in greater numbers of neutrophils in the BALF of adult as compared with juvenile mice (P = 8.15 × 10−10) (Figure 1). LPS resulted in significant increases in the number of neutrophils in juvenile and adult mice (P = 1.98 × 10−10 and 3.27 × 10−4, respectively), and there was no effect of age on the recruitment of neutrophils into the lungs of mice treated with LPS (P = 0.073). There were no significant differences in the number of neutrophils in the BALF of adult or juvenile mice treated with MV alone, and age did not modify the response to MV in the protocol that we used. The numbers of macrophages in the BALF of adult and juvenile mice were similar in each treatment group (data not shown).
Figure 1.
Effect of lipopolysaccharide (LPS) + mechanical ventilation (MV) on polymorphonuclear neutrophils (PMNs) in bronchoalveolar lavage fluid. PMNs were measured in the BALF from adult and juvenile mice treated with inhaled LPS, MV, and LPS+MV. Values are the mean ± SD (n ≥ 12 per group). ‡ Synergy between LPS and MV in adult mice (P = 2.1 × 10−14), and the horizontal bar indicates the effect of age on the PMN responses to LPS+MV (*P = 8.2 × 10−10).
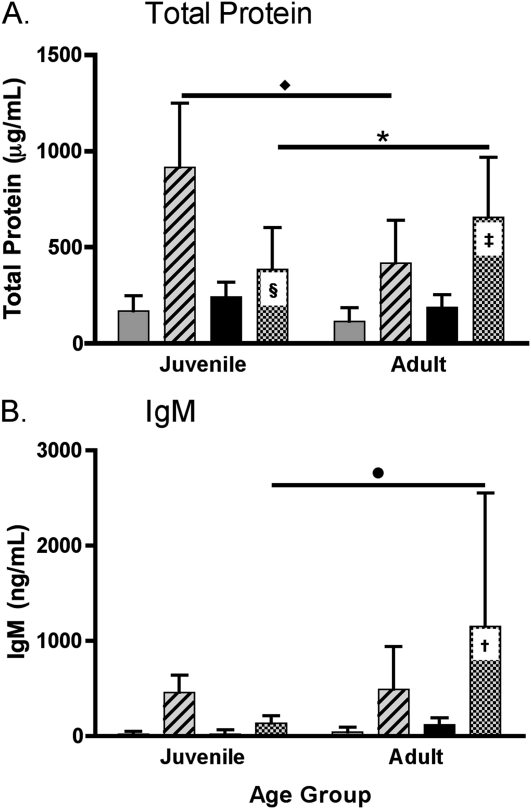
To determine the effect of age on the alveolar permeability responses in the lungs, total protein and IgM were measured in the BALF (n = 10 per group). Treatment with LPS combined with MV did not result in a synergistic increase in the concentration of total protein in the BALF in the lungs of adult mice (P = 0.132) (Figure 2A). However, treatment with LPS+MV resulted in a significant decrease in the amount of total protein in the BALF of juvenile mice (P = 5.29 × 10−9) (Figure 2A). Age modified the changes in the concentration of total protein in response to MV (P = 1.10 × 10−5) and LPS+MV (P = 6.01 × 10−7) (Figure 2A). Treatment with LPS+MV resulted in a synergistic increase in the concentration of IgM in the BALF in adult mice as compared with either treatment alone (P = 0.030), but this synergism was not seen in the juvenile mice, which had much higher total protein concentrations with MV alone. Age modified the change in the concentration of IgM in the BALF in response to LPS+MV; a synergistic effect was seen in the adult but not the juvenile animals (P = 0.011) (Figure 2B).
Figure 2.
Effect of LPS+MV on alveolar permeability. (A) Total protein and (B) IgM were measured in the bronchoalveolar lavage fluid from adult and juvenile mice treated with inhaled LPS, MV, and LPS+MV. Values are mean ± SD (n = 10 per group). ‡Additive effect of LPS and MV in adult mice (P = 0.13). §Significant decrease in protein in juvenile mice treated with LPS+MV as compared with the effects of LPS or MV alone (P = 5.3 × 10−9). Asterisk and diamond indicate the effects of age on the responses to MV and LPS+MV, respectively (P = 6.0 × 10−7 and P = 1.1 × 10−5). †Synergism between LPS and MV in adult mice (P = 0.030). The circle indicates the effect of age on the response to LPS+MV (P = 0.011).
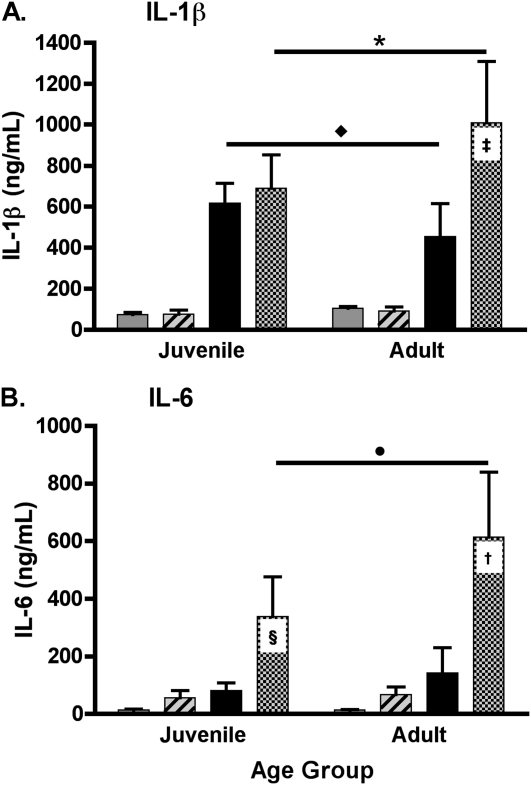
Regarding cytokine production, treatment with LPS+MV resulted in a synergistic increase in the amount of IL-1β in the lungs of adult but not juvenile mice (P = 9.44 × 10−10) (Figure 3A). Although treatment with LPS alone resulted in significantly higher concentrations of IL-1β in the lungs of juvenile as compared with adult mice (P = 9.22 × 10−6), treatment with LPS+MV resulted in significantly higher concentrations of IL-1β in the adult mice as compared with the juvenile mice (P = 2.18 × 10−5) (Figure 3A).
Figure 3.
Effect of LPS+MV on cytokines in the lungs. (A) IL-1β and (B) IL-6 were measured in whole lung homogenates of adult and juvenile mice treated with inhaled LPS, MV, and LPS+MV. Values are the mean ± SD (n = 10 per group). † and ‡, Synergy between LPS and MV in adult mice (P = 9.4 × 10−10 and P = 4.0 × 10−9). §Synergy between LPS and MV in juvenile mice (P = 9.0 × 10−4). Asterisk and circle indicate the effects of age on the responses to LPS+MV (P = 2.2 × 10−5 and P = 0.023). Diamond indicates the effect of age on the response to LPS (P = 9.2 × 10−6).
There were synergistic increases in the amount of IL-6 in the lungs of adult and juvenile mice treated with LPS plus MV (P = 3.97 × 10−9 and P = 9.00 × 10−4, respectively) (Figure 3B). However, age modified this response, with significantly lower amounts of IL-6 in the lungs of juvenile as compared with adult mice (P = 0.023) (Figure 3B). The amounts of TNF-α, KC, MIP-2, MCP-1, and the growth factor GM-CSF measured in the lungs of the juvenile and adult mice differed, but the differences were not consistently related to the observed neutrophil and protein leak in the lungs of the different groups of animals. The amounts of IL-2, IL-12p70, and IL-17 were below the detectable limit of the protein assay in all treatment groups of adult and juvenile mice.
The amounts of cytokines and growth factors measured in whole-lung homogenates after 3 hours of treatment with LPS and MV were compared with the expression of mRNA after 2 hours of treatment of juvenile and adult mice. The mRNA expression of IL-1β, IL-6, TNF-α, KC/CXCL1, MIP-2/CXCL2, MCP-1/CCL2, and the growth factor GM-CSF/Csf2 were similar to the pattern of protein expression in the lungs (see Figures E1–E4 in the online supplement).
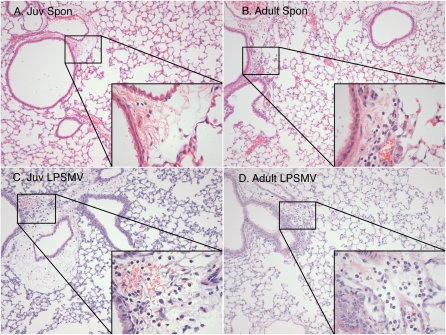
Histologic comparisons did not reveal differences in alveolar structure or edema among any of the age or treatment groups (Figure 4). There were small increases in the perivascular neutrophil infiltration in the adult mice treated with LPS+MV, but these increases were not seen in the juvenile mice.
Figure 4.
Effect of LPS+MV on lung histopathology of juvenile and adult mice. Photomicrographs were taken at ×20 and ×60 (inset) of untreated (A) juvenile and (B) adult mice and compared with (C) juvenile and (D) adult mice treated with LPS+MV (n = 5 per group).
There were minor differences in physiologic parameters among the treatment groups, but these differences could not explain the observed differences between the adult and juvenile animals treated with LPS+MV (Figure E8). Temperature and heart rate differed only at time = 0 hours, and systolic blood pressure did not differ significantly among the groups at any time. The respiratory rates were approximately 2 breaths per minute higher at 1 and 2 hours in the juvenile as compared with adult animals. The peak inspiratory pressures were higher at 3 hours in juvenile mice treated with LPS+MV as compared with all other groups (Figure E8). Blood gas tensions measured in mixed venous blood at the end of the experiment showed that the juvenile animals had higher PmvCO2 than the adults. The juvenile animals were slightly more acidemic than the adults treated with MV alone, but there were no significant differences in mixed venous pH, lactate, or oxygen saturations between the juvenile and adult animals treated with LPS+MV (Figure E8).
Effects of LPS and MV on Transcriptional Responses in the Lungs of Adult and Juvenile Mice
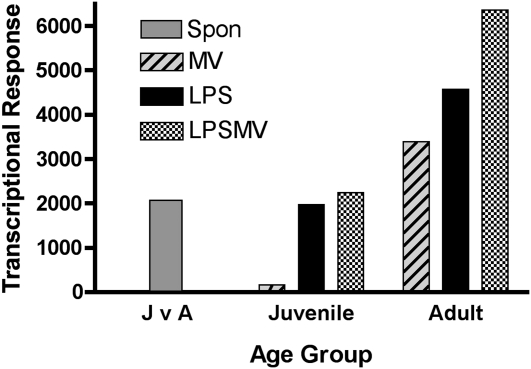
To determine whether age affects the transcriptional responses to LPS combined with MV, adult and juvenile mice were treated with MV, LPS, and LPS combined with MV, and total lung RNA was obtained at 2 hours and analyzed using the microarray method. A total of 2,069 genes were differentially expressed at baseline between adult and juvenile mice (Figure 5). The transcriptional response to MV was higher in the adult as compared with the juvenile mice (3,392 versus 167 genes). The transcriptional response of the adult mice treated with LPS (4,573 genes) and LPS+MV (6,362 genes) was much higher than the responses of the juvenile mice (1,970 and 2,246 genes, respectively) (Figure 5).
Figure 5.
Effect of LPS+MV on global gene expression in mouse lungs. J v A = the number of differentially expressed genes in lungs of untreated, spontaneously breathing (Spon) juvenile versus adult mice (shaded bar). The effects of MV (hatched bar), LPS (solid bar), and LPS+MV (checked bar) on gene expression in the lungs of juvenile and adult mice were compared with age-matched untreated control mice.
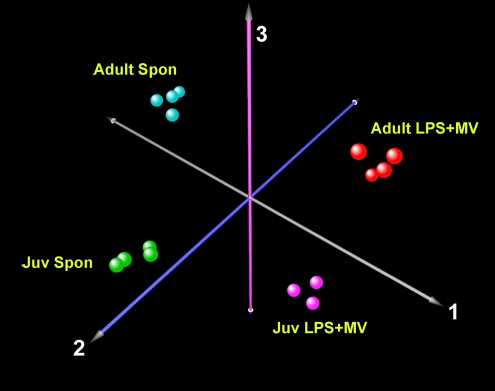
The PCA evaluates the variability within and between the different groups. The grouping of the samples is easiest to visualize when the PCA is limited to four groups (Figure 6). Spontaneously breathing, untreated adult and juvenile mice were compared with age-matched mice treated with LPS+MV. The PCA analysis showed that there was little variability among the individual animals in each group, confirming the reproducibility of the models, whereas most of the variability occurred between the different age and treatment groups.
Figure 6.
Principal component analysis of gene expression responses of adult and juvenile mice treated with LPS+MV. The first three principal components of gene expression in the lungs of juvenile and adult mice show the effect of age and treatment on the variability of gene expression. For clarity, only four age and treatment groups are shown. Each data point shows microarray data from one mouse.
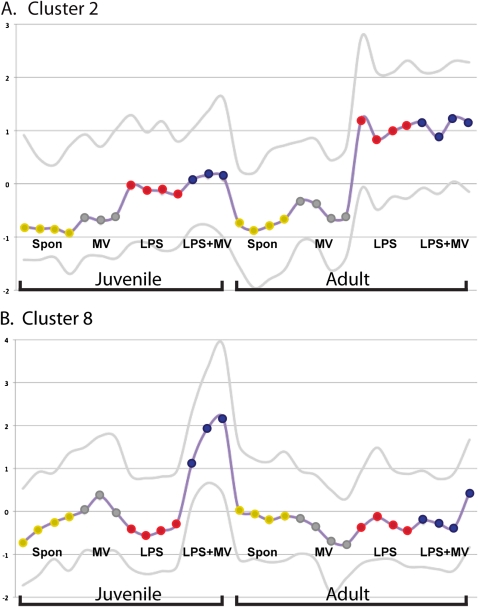
The differentially expressed genes in the juvenile and adult mice treated with LPS+MV were identified by using two-way ANOVA (25), which yielded 3,074 probesets with an interaction effect between age and treatment (post hoc Bonferroni; adjusted P < 0.1). KMC clustering was used to explore patterns of gene expression across the experimental conditions using the genes for which there was an interaction effect between age and treatment. Eight clusters showed discrete patterns of expression, and two interesting patterns were identified that could be important to the acquisition of synergistic interactions between LPS and MV (Figure 7). The second cluster contained 433 probe sets (387 genes), which increased expression only in adult mice treated with LPS and LPS+MV (Figure 7A). The eighth cluster contained 261 probe sets (248 genes) for which expression increased only in juvenile mice treated with LPS+MV (Figure 7B).
Figure 7.
K-median cluster analysis of the two-way ANOVA results in two different clusters. (A) Cluster 2, containing 387 genes that were increased in expression only in adult mice treated with LPS or LPS+MV. (B) Cluster 8, containing 248 genes that were increased in expression only in juvenile mice treated with LPS+MV. The horizontal axis represents individual mice (also shown as colored dots on the median line). The vertical axis is the median expression of the genes in the individual mice. The lower and upper lines show the 5th and 95th percentiles, respectively.
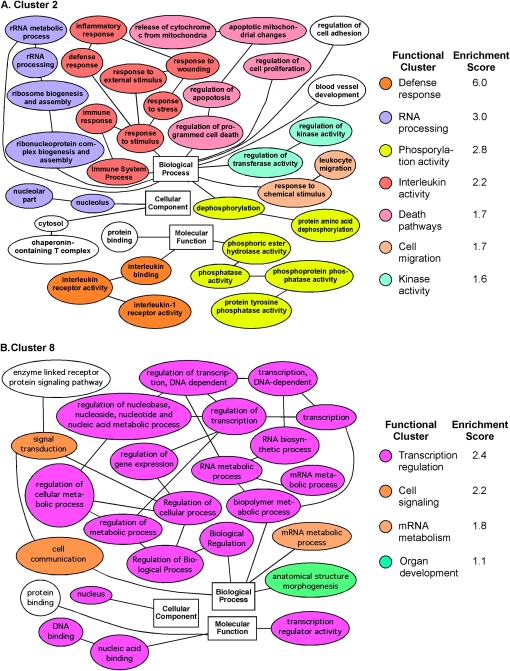
To determine the biological functions of the genes in clusters 2 (Figure 7A) and 8 (Figure 7B), the genes in each of the clusters were entered into the Database for Annotation, Visualization and Integration Discovery (29). Functional classification and clustering of the genes that were up-regulated only in adult mice treated with LPS or LPS+MV (cluster 2, Figure 7A) showed that defense responses (red) were the major biological themes (functional cluster enrichment score 6.05; FDR <5%) (Figure 8A). Functional classification and clustering of the genes that were up-regulated only in juvenile mice treated with LPS+MV (cluster 8, Figure 7B) showed that regulation of transcription (purple) were the major themes (functional cluster enrichment score 2.42; FDR <5%) (Figure 8B).
Figure 8.
Gene ontology relationships of functionally enriched genes in (A) cluster 2 and (B) cluster 8. In each panel, the color groups show functionally related groups of genes.
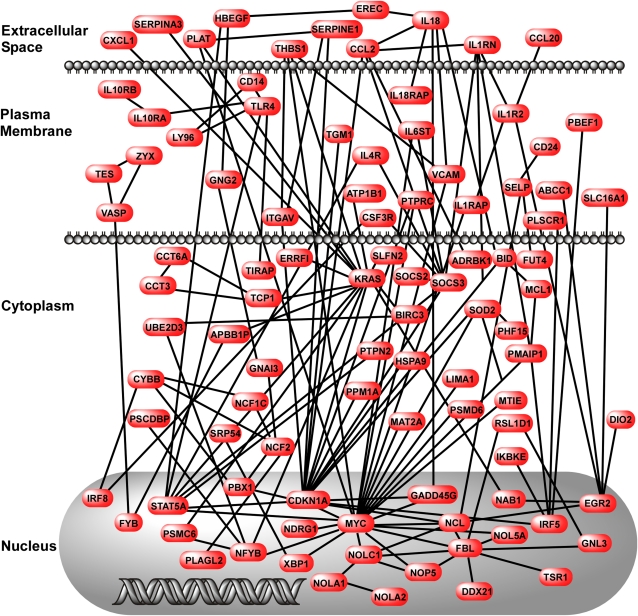
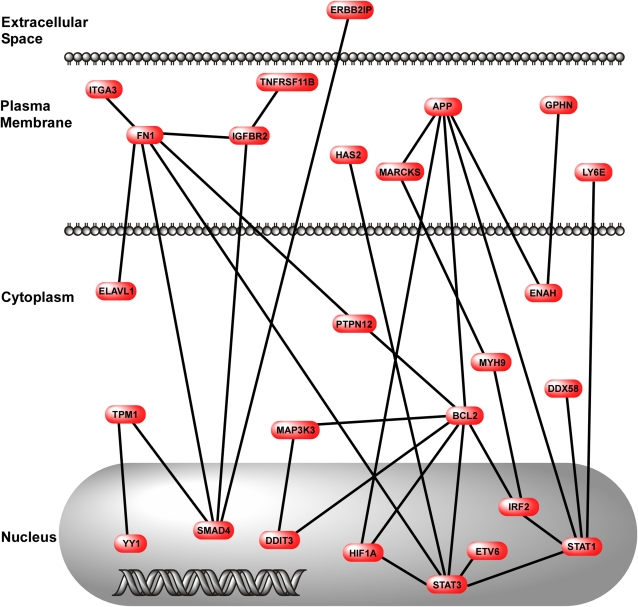
To determine the relationships between the up-regulated genes in clusters 2 and 8, we performed network analyses using the genes in each of these clusters. These data show that there are multiple interconnected pathways in cluster 2 (Figure 9). The most densely connected nodes were Kras (v-ki-ras2 Kirsten rat sarcoma viral oncogene homolog), Myc (myelocytomatosis oncogene), and Cdkn1a (cyclin-dependent kinase inhibitor 1A [p21]). Membrane components of the LPS-sensing pathway (CD14, TLR4, and TIRAP) were included in this network of up-regulated genes in adult mice. In contrast to cluster 2, there were fewer pathways in cluster 8, and the nodes were more sparsely interconnected (Figure 10). The nodes in cluster 8 were App (amyloid β-protein precursor), Bcl2 (B-cell leukemia/lymphoma 2), Fn1 (fibronectin 1), Stat1 (signal transducer and activator of transcription 1), and Smad4 (MAD homolog 4 [Drosophila]). Quantitative real-time PCR confirmed the results of Cd14, Bcl2, and Smad4 gene expression in adult and juvenile mice treated with LPS+MV (Figures E5–E7).
Figure 9.
Network map of genes in cluster 2. The map was created using genes identified in cluster 2 and published information about gene–gene interactions. The interactions were drawn with respect to the cellular location of the gene product.
Figure 10.
Network map of genes in cluster 8. The map was created using genes identified in cluster 8 and published information about gene–gene interactions. The interactions were drawn with respect to the cellular location of the gene product.
The expression array data suggest that type 1 IFN signaling through activation of Stat1 was strongly up-regulated only in the juvenile mice treated with LPS+MV (cluster 8). Because the type 1 IFN genes are regulated by the TRIF-dependent TLR4 pathway (lps2) (31, 32), these data led us to hypothesize that the phenotypic difference in inflammation between juvenile and adult mice could be explained in part by a relative increase in type 1 IFN responses to LPS in juvenile mice treated with LPS+MV. However, we treated juvenile TRIF−/− mice with LPS (n = 18) and LPS+MV (n = 11), and there was no difference (P = 0.4, unpaired t test; Prism 4.0) in the number of neutrophils in the BALF of juvenile TRIF−/− mice treated with LPS (52,832/ml BALF ± 31,427) versus LPS+MV (46,770 ± 38,578 per ml BALF).
DISCUSSION
The goal of this study was to determine whether age modifies the early inflammatory and injury responses to LPS+MV. Treatment with LPS+MV resulted in a synergistic increase in pulmonary inflammation in adult mice as compared with the effect of either treatment alone. In contrast, the synergism between LPS and MV was not present in the juvenile mice, suggesting that important interactions between innate immunity and responses to mechanical stretch in the lungs are acquired with age. These findings are consistent with previous studies in rodents showing that newborns have lesser inflammatory and injury responses to hypoxia, MV, and LPS as compared with adults (13, 18, 19, 33). These data are broadly consistent with clinical observations suggesting that children have a lower incidence and a better outcome from ALI than adults (1, 3).
The juvenile and adult animals had similar inflammatory responses to inhaled LPS, as reflected by lung PMN recruitment and cytokines, and the MV protocol did not cause inflammation in either age group (Figures 1 and 3). When treatment with LPS and MV were combined, there was a synergistic increase in PMN recruitment and release of IL-1β into the lungs of the adult but not the juvenile animals (Figures 1 and 3A). The PMN recruitment data show that MV and LPS interact to enhance the activation of innate immunity pathways in adult but not juvenile mice because PMN recruitment is an integrative signal that reflects the activation of many components of innate immunity.
In contrast to the PMN and IL-1β responses, the IL-6 response was synergistic in juveniles and in adults (Figure 3B). IL-6 production is influenced by a variety of stimuli, including TLR signaling, IL-1β, TNF-α, and others. These IL-6 data suggest that the differences between juveniles and adults are relative and not absolute. However, IL-1β is a direct product of LPS-dependent activation of the TLR4 pathway, and IL-1β gene expression has been identified as a common thread in multiple models of ventilator-induced lung injury. IL-1β causes neutrophil recruitment and injury in the lungs of hamsters (34, 35), and there is evidence that early inflammatory responses in the lungs of humans with ARDS are predominantly attributable to IL-1β and not to IL-6 or TNF-α (36, 37).
The MV protocol caused significant increases in the amounts of total protein and IgM (900,000 kD) in juvenile and adult mice (Figure 2). As with PMN recruitment, treatment with LPS+MV caused a significant increase in permeability to high-molecular-weight proteins, reflected by IgM concentrations in BALF, in the adult but not juvenile animals (Figure 2B). These data suggest that the juvenile animals were protected from the increases in protein permeability induced by the combined treatment of LPS+MV. Although MV alone caused greater increases in the total protein concentration in the juveniles than in the adults, age did not appear to modify the amount of IgM in the lungs of mechanically ventilated animals (Figure 2). Lung total protein concentration reflects protein movement from the plasma compartment into the lungs, protein shedding from the lung epithelium, and alveolar fluid clearance across the intact alveolar epithelium. It is possible that the juvenile animals treated with MV alone had increased protein sieving through the endothelial and epithelial barriers and increased alveolar fluid clearance without a significant change in global protein permeability.
The LPS inhalation protocol alone caused similar PMN recruitment and cytokine production in the lungs of the juvenile and adult mice. In this model of ALI, the concentration of LPS in the nebulizer, the minute ventilation of the animal, and the duration of exposure determine the dose of inhaled LPS. The adult and juvenile mice had similar resting minute ventilation. These observations suggest that the doses of LPS were similar, that the LPS exposure was sufficient to initiate an inflammatory response, and that the early pulmonary inflammatory responses of juvenile and adult mice exposed to LPS are similar. In addition, the early pulmonary responses to positive pressure MV alone using 15 ml/kg tidal volumes were similar in juvenile and adult mice. However, the lack of interaction between the two stimuli in the juvenile mice suggests that downstream signaling interactions between TLR4 and stretch-dependent pathways are acquired with age. The acquisition of synergy could be due to the development of positive interactions, loss of inhibitory interactions, or a combination of the two. The differences in response to LPS+MV between juvenile and adult mice also could not be explained by the minor physiologic differences in the animal groups.
To explore the differences in juvenile and adult lungs at a more fundamental level, we measured global gene expression in the lungs of animals in each group after 2 hours of treatment. Gene expression was much greater in the adult lungs in response to all three experimental conditions, suggesting that the global transcriptional responses are more active in the adult animals than in the juvenile animals (Figure 5).
The KMC cluster analysis was valuable in identifying differences in the patterns of gene expression between the juvenile and adult animals. Although Clusters 2 and 8 contained only up-regulated genes, there was a cluster that contained down-regulated genes. None of the experimental groups was unique in this cluster, suggesting that there were no differences between the adult and juvenile mice in the down-regulation of genes in response to any of the treatments. Cluster 2 contains genes that were uniquely up-regulated in adult animals treated with LPS and LPS+MV (Figure 7A), and these genes provide clues about mechanisms by which synergistic interactions between LPS and MV are acquired.
The most functionally enriched groups of genes in cluster 2 were related to stress responses, including innate immunity and inflammation (Figure 8A). Although these are expected responses to LPS and MV, these genes were not up-regulated in the juvenile mice treated with LPS+MV. CD14, TLR4, and TIRAP are important components of the LPS-sensing pathway, and up-regulation of expression of these proteins is a plausible mechanism by which MV could sensitize the lungs to LPS. Mechanical stretch has been shown to increase CD14 expression on lung cells and to increase the responsiveness of alveolar macrophages to LPS, and anti-TLR4 antibody therapy has been shown to reduce the synergistic inflammatory responses to LPS combined with MV in rabbits (38, 39). Previous studies have also shown that immature cells, rats, and human PMNs acquire age-dependent responsiveness to LPS in association with increased expression of CD14, MyD88 (myeloid differentiation primary response gene 88), and increased production of TNF-α (10–12, 40). Our results show that by 21 days of life, the juvenile and adult mice have similar responses to LPS alone but that LPS+MV results in greater up-regulation of key components of the TLR4 pathway in adult animals than in juvenile animals.
To identify other mechanisms by which the synergistic interactions between LPS and MV might be acquired with age, we compared the genes in cluster 2 with the results of previously published models of lung injury. Altemeier and colleagues performed a cross-species comparison of gene expression patterns of lung injury caused by MV and identified 32 candidate genes that could contribute to the synergistic interactions between LPS and MV (41). We compared the genes in Figure 7A with these 32 candidate genes and found eight common genes (Table 1). In addition, pre–B-cell colony-enhancing factor has recently been shown to be an important mediator and biomarker in ALI (42–44). Pre–B-cell colony-enhancing factor has been shown to be important to innate immunity and to have cytokine-like activity (42, 45–47).
TABLE 1.
CANDIDATE GENES THAT DIFFER IN JUVENILE AND ADULT LUNGS TREATED WITH LPS AND MECHANICAL VENTILATION
| Increased in Adults* | Increased in Juveniles† |
|---|---|
| Cd14 | Spry4 |
| Tlr4 | Fibp |
| Tirap | Bcl2 |
| Arg2 | Map3k3 |
| Cdkn1a | App |
| Gch1 | Fn1 |
| Il1r2 | Stat1 |
| S100a9 | Smad4 |
| Serpine1 | |
| Gadd45 | |
| Pbef | |
| Kras | |
| Myc |
Genes that increase signaling through inflammatory and injury pathways.
Genes that increase signaling through inhibitory pathways.
The network analysis of the genes in cluster 2 suggests that there are three highly interconnected mediators of the biological responses to LPS and MV. Kras is a small GTPase relevant to cell growth and differentiation, which when inhibited has been shown to trigger an antiinflammatory effect (48). Myc is a nuclear phosphoprotein related to cell cycle progression, apoptosis, and cellular transformation (49), and Myc has previously been identified as an early stress response gene in rodent models of ALI (50). Cdkn1a is an important inhibitory regulator of cell cycle progression in response to oxidative and genotoxic stresses and has been shown to be important to the proinflammatory responses to LPS in the lungs (41, 51). Up-regulation of these genes provides plausible mechanisms by which the synergistic interactions between LPS and MV are acquired by age.
In contrast to cluster 2, the genes in cluster 8 (Figure 7B) were uniquely up-regulated in juvenile animals treated with LPS+MV, and these are candidates for inhibitors of the interactions between LPS and MV. There were fewer functional groups of genes in cluster 8 as compared with cluster 2, and the functional importance of the genes in cluster 2 is much broader than those in cluster 8 (Figure 8B). Also, the network of gene interactions in cluster 8 was smaller than in cluster 2. We identified several candidate genes in cluster 8 that might inhibit inflammatory and injurious interactions between LPS and MV (Table 1). Sprouty homolog 4 (Spry4), fibronectin, fibroblast growth factor (acidic) intracellular binding protein (Fibp), signal transducer and activator of transcription 1 (Stat1), and MAD homolog 4 (Drosophila) (Smad4) are important in regulating fibroblast growth factor (FGF) and transforming growth factor (TGF)-β signaling in the lungs (14, 52–54). Upadhyay and colleagues have shown that mitogen-activated protein kinases mediate the protective effects of FGF-10 on DNA damage to alveolar epithelial cells treated with mechanical stretch (55). The FGF-FGFR-SPROUTY and the TGFβ/BMP-SMAD pathways have been identified as “potential therapeutic targets for rational therapy … in children and adults with intractable pulmonary insufficiency” (14).
We considered whether the absence of synergy in the juvenile mice treated with LPS+MV was dependent on TRIF signaling. We found that juvenile TRIF−/− mice did not develop synergy to LPS+MV. Therefore, if juvenile mice treated with LPS+MV up-regulate type 1 IFN responses via Stat1, these responses are likely to be dependent on IRF3 or IRF7 (56). Apoptosis is an important process in normal lung development as well as in lung injury and repair (57, 58). B-cell leukemia/lymphoma 2 (Bcl2) and mitogen-activated kinases (MAPK) have been shown to be important in mediating antiinflammatory and antiapoptotic responses to hypo- and hyperoxia, lipopolysaccharide, and mechanical stretch (33, 55, 59–61). Amyloid β-protein precursor (App) has been shown to suppress FE65 (a neural adaptor protein) mediated apoptosis in neurons (62), but the role of this pathway in the lungs is not known.
This study has some limitations that need to be considered. First, the tidal volume used in this model is larger than the current standard of care for patients with ALI. However, in a heterogeneously injured lung, a delivered tidal volume of 6 ml/kg results in low compliance regions of lung receiving smaller tidal volumes, whereas other regions of highly compliant lung receive higher tidal volumes. Although there has been a trend toward the use of lower tidal volumes in patients with ALI in the ARDSNet institutions, patients are still being treated with tidal volumes in excess of 12 ml/kg before and after ALI has been identified (63, 64).
The juvenile mice had a respiratory acidosis as compared with the adult mice. Hypercapnia has been shown to be immunomodulatory, but it is not clear whether it is pro- or antiinflammatory (65–67). The juvenile mice were intubated with the same size of catheter as the adult mice, and the peak inspiratory pressures tended to be higher in the juvenile as compared with the adult mice. Therefore, it is not likely that the juvenile mice were treated with a lower effective tidal volume than the adult mice. Although we have removed as much dead space as possible from the ventilator circuit, it is most likely that the ratio of physiologic dead space to tidal volume was higher in the juvenile mice. We do not have arterial blood gases to compare, so it is not possible to determine whether the delivered minute ventilation resulted in equivalent arterial blood gas tensions in the adult and juvenile mice. However, we do not believe that the degree of hypercapnia in the juvenile mice treated with LPS+MV as compared with the adult mice is sufficient to cause the significant differences in inflammation between the mice.
We used LPS as an agonist of the innate immune system because LPS is commonly present in the lungs of patients with ARDS even in the absence of clinical evidence of infection (5). Infections are the most common antecedent event for patients with ALI, and gram-negative pathogens continue to cause up to 60% of hospital-acquired infections in adults and children (68–70).
Finally, assays using whole-lung homogenates cannot differentiate between changes in gene expression versus a change in the population of cells in the lungs. Because we have shown that the numbers of neutrophils recruited into the lungs are different, the gene expression array analysis may, in part, show differences in the expression of genes by the neutrophils recruited to the lungs.
The data analysis identifies multiple pathways that are strongly supported in the literature. We have not directly tested whether the acquisition of synergy between LPS and MV is a consequence of the loss of an inhibitory function of the candidate genes in cluster 8 (Figure 7B) or whether it is due to gain of function by the up-regulation of genes in cluster 2 (Figure 7A). The gene expression data suggest that several interconnected pathways are likely to be important in the inflammatory and injury responses to the combination of infectious stimuli and MV. Inflammatory, injury, and repair mechanisms are highly interconnected and redundant, and these pathways overlap with the regulation of organ development. These data also provide a road map for investigating complex interactions between biological pathways important to the development of ALI in mechanically ventilated patients.
In summary, our results support the conclusion that mechanical stretch and activation of innate immunity have synergistic effects on lung inflammation and permeability and suggest for the first time that these synergistic interactions are acquired during postnatal lung development. We have identified several plausible mechanistic pathways, including a gain of proinflammatory pathway activity in the lungs of adults and down-regulation of death pathways as well as FGF and TGF-β pathways in the lungs of juvenile mice. These findings are consistent with the clinical observation that ALI is less likely to occur and is less severe in children than in adults and suggest that this is explained in part by differences in the response of the juvenile lung to specific stimuli and not solely by the presence of other comorbidities in older patients.
Supplementary Material
Acknowledgments
Statistical modeling for the regression analysis was performed by Kristy Seidel, M.S., Director, Office of Biostatistical Services, Department of Children's Hospital and Regional Medical Center Research Institute. We graciously acknowledge the technical support of Venus A. Wong, John T. Ruzinski, and Steve M. Mongovin.
This work was supported by grants NIH/NHLBI HL081764, HL073996-01, HL072370, and HL074223, by the Medical Research Service of the Department of Veterans Affairs, and by the Lyford Family Endowment for Intensive Care.
This article contains an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0039OC on November 9, 2009
Author Disclosure: T.R.M. is a full-time employee of the Veterans' Administration and received a sponsored grant for more than $100,001. He also serves as a consultant for the National Institutes of Health, receiving $0 and a sponsored grant for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.ARDSNet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 3.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005;171:995–1001. [DOI] [PubMed] [Google Scholar]
- 4.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:293–301. [DOI] [PubMed] [Google Scholar]
- 5.Martin TR, Rubenfeld GD, Ruzinski JT, Goodman RB, Steinberg KP, Leturcq DJ, Moriarty AM, Raghu G, Baughman RP, Hudson LD. Relationship between soluble cd14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;155:937–944. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 2006;32:24–33. [DOI] [PubMed] [Google Scholar]
- 7.Burri PH, West JB, Crystal RG, Weibel ER, Barnes PJ. Postnatal development and growth. The lung: scientific foundations. Philadelphia, PA: Lippincott-Raven; 1997. pp. 1013–1026.
- 8.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 2000;92:55–81. [DOI] [PubMed] [Google Scholar]
- 9.Wohl MEB, Chernick V, Boat TF, Kendig EL. Developmental physiology of the respiratory system. In: Kendig's disorders of the respiratory tract in children. Philadelphia, PA: W.B. Saunders Company; 1998. pp. 19–27.
- 10.Martin TR, Ruzinski JT, Wilson CB, Skerrett SJ. Effects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory response. J Infect Dis 1995;171:134–144. [DOI] [PubMed] [Google Scholar]
- 11.Qing G, Rajaraman K, Bortolussi R. Diminished priming of neonatal polymorphonuclear leukocytes by lipopolysaccharide is associated with reduced cd14 expression. Infect Immun 1995;63:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of myd88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun 2004;72:1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. Nuclear factor-kb activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med 2007;175:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warburton D, Bellusci S. The molecular genetics of lung morphogenesis and injury repair. Paediatr Respir Rev 2004;5:S283–S287. [DOI] [PubMed] [Google Scholar]
- 15.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 2002;282:L924–L940. [DOI] [PubMed] [Google Scholar]
- 16.Whitsett JA. Intrinsic and innate defenses in the lung: Intersection of pathways regulating lung morphogenesis, host defense, and repair. J Clin Invest 2002;109:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitsett JA, Bachurski CJ, Barnes KC, Bunn PA Jr, Case LM, Cook DN, Crooks D, Duncan MW, Dwyer-Nield L, Elston RC, et al. Functional genomics of lung disease. Am J Respir Cell Mol Biol 2004;31:S1–S81. [DOI] [PubMed] [Google Scholar]
- 18.Copland IB, Martinez F, Kavanagh BP, Engelberts D, McKerlie C, Belik J, Post M. High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med 2004;169:739–748. [DOI] [PubMed] [Google Scholar]
- 19.Kornecki A, Tsuchida S, Ondiveeran HK, Engelberts D, Frndova H, Tanswell AK, Post M, McKerlie C, Belik J, Fox-Robichaud A, et al. Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med 2005;171:743–752. [DOI] [PubMed] [Google Scholar]
- 20.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 1977;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 21.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol 1999;276:L715–L727. [DOI] [PubMed] [Google Scholar]
- 22.Tankersley CG, Fitzgerald RS, Levitt RC, Mitzner WA, Ewart SL, Kleeberger SR. Genetic control of differential baseline breathing pattern. J Appl Physiol 1997;82:874–881. [DOI] [PubMed] [Google Scholar]
- 23.Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JHT. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L710–L717. [DOI] [PubMed] [Google Scholar]
- 24.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003;19:185–193. [DOI] [PubMed] [Google Scholar]
- 25.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. Tm4: a free, open-source system for microarray data management and analysis. Biotechniques 2003;34:374–378. [DOI] [PubMed] [Google Scholar]
- 26.Raychaudhuri S, Stuart JM, Altman RB. Principal components analysis to summarize microarray experiments: application to sporulation time series. Pac Symp Biocomput 2000;455–466. [DOI] [PMC free article] [PubMed]
- 27.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 2001;17:509–519. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 1995;1:289–300. [Google Scholar]
- 29.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. David: database for annotation, visualization, and integrated discovery. Genome Biol 2003;4:3. [PubMed] [Google Scholar]
- 30.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, et al. A network-based analysis of systemic inflammation in humans. Nature 2005;437:1032–1037. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel toll/il-1 receptor domain-containing adapter that preferentially activates the IFN-b promoter in the toll-like receptor signaling. J Immunol 2002;169:6668–6672. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the myd88-independent toll-like receptor signaling pathway. Science 2003;301:640–643. [DOI] [PubMed] [Google Scholar]
- 33.Molloy E, O'Neill AJ, Doyle B, Grantham J, Taylor C, Sheridan-Pereira M, Fitzpatrick J, Webb D, Watson RW. Effects of heat shock and hypoxia on neonatal neutrophil lipopolysaccharide responses: altered apoptosis, toll-like receptor-4 and cd11b expression compared with adults. Biol Neonate 2006;90:34–39. [DOI] [PubMed] [Google Scholar]
- 34.Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc 2007;4:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patton LM, Saggart BS, Ahmed NK, Leff JA, Repine JE. Interleukin-1 beta-induced neutrophil recruitment and acute lung injury in hamsters. Inflammation 1995;19:23–29. [DOI] [PubMed] [Google Scholar]
- 36.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F III, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:1896–1903. [DOI] [PubMed] [Google Scholar]
- 37.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med 1996;153:1850–1856. [DOI] [PubMed] [Google Scholar]
- 38.Smith LS, Kajikawa O, Elson G, Wick M, Mongovin S, Kosco-Vilbois M, Martin TR, Frevert CW. Effect of tlr4 blockade on pulmonary inflammation caused by mechanical ventilation and bacterial endotoxin. Exp Lung Res 2008;34:225–243. [DOI] [PubMed] [Google Scholar]
- 39.Moriyama K, Ishizaka A, Nakamura M, Kubo H, Kotani T, Yamamoto S, Ogawa EN, Kajikawa O, Frevert CW, Kotake Y, et al. Enhancement of the endotoxin recognition pathway by ventilation with a large tidal volume in rabbits. Am J Physiol Lung Cell Mol Physiol 2004;286:L1114–L1121. [DOI] [PubMed] [Google Scholar]
- 40.Martin TR, Mongovin SM, Tobias PS, Mathison JC, Moriarty AM, Leturcq DJ, Ulevitch RJ. The cd14 differentiation antigen mediates the development of endotoxin responsiveness during differentiation of mononuclear phagocytes. J Leukoc Biol 1994;56:1–9. [DOI] [PubMed] [Google Scholar]
- 41.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 2005;175:3369–3376. [DOI] [PubMed] [Google Scholar]
- 42.Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, Lavoie TL, Verin AD, Natarajan V, Garcia JG. Pre-b-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc Res 2005;70:142–151. [DOI] [PubMed] [Google Scholar]
- 43.Simon BA, Easley RB, Grigoryev DN, Ma S-F, Ye SQ, Lavoie T, Tuder RM, Garcia JGN. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L851–L861. [DOI] [PubMed] [Google Scholar]
- 44.Luk T, Malam Z, Marshall JC. Pre-b cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol 2008;83:804–816. [DOI] [PubMed] [Google Scholar]
- 45.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-b cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest 2004;113:1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ognjanovic S, Bryant-Greenwood GD. Pre-b-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol 2002;187:1051–1058. [DOI] [PubMed] [Google Scholar]
- 47.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 2007;178:1748–1758. [DOI] [PubMed] [Google Scholar]
- 48.Mor A, Keren G, Kloog Y, George J. N-ras or k-ras inhibition increases the number and enhances the function of foxp3 regulatory T cells. Eur J Immunol 2008;38:1493–1502. [DOI] [PubMed] [Google Scholar]
- 49.Pelengaris S, Khan M, Evan G. C-myc: more than just a matter of life and death. Nat Rev Cancer 2002;2:764–776. [DOI] [PubMed] [Google Scholar]
- 50.Ma SF, Grigoryev DN, Taylor AD, Nonas S, Sammani S, Ye SQ, Garcia JG. Bioinformatic identification of novel early stress response genes in rodent models of lung injury. Am J Physiol Lung Cell Mol Physiol 2005;289:L468–L477. [DOI] [PubMed] [Google Scholar]
- 51.Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O'Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and FMLP in mice. Am J Respir Cell Mol Biol 2008;39:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perl AK, Hokuto I, Impagnatiello MA, Christofori G, Whitsett JA. Temporal effects of sprouty on lung morphogenesis. Dev Biol 2003;258:154–168. [DOI] [PubMed] [Google Scholar]
- 53.Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem 2001;276:40247–40253. [DOI] [PubMed] [Google Scholar]
- 54.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 2004;125:754–765. [DOI] [PubMed] [Google Scholar]
- 55.Upadhyay D, Correa-Meyer E, Sznajder JI, Kamp DW. Fgf-10 prevents mechanical stretch-induced alveolar epithelial cell DNA damage via MAPK activation. Am J Physiol Lung Cell Mol Physiol 2003;284:L350–L359. [DOI] [PubMed] [Google Scholar]
- 56.Gale M Jr, Foy EM. Evasion of intracellular host defence by hepatitis c virus. Nature 2005;436:939–945. [DOI] [PubMed] [Google Scholar]
- 57.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol 1999;20:228–236. [DOI] [PubMed] [Google Scholar]
- 58.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med 2003;31(4, Suppl)S184–S188. [DOI] [PubMed] [Google Scholar]
- 59.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, et al. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 2005;115:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410:37–40. [DOI] [PubMed] [Google Scholar]
- 61.Dolinay T, Wu W, Kaminski N, Ifedigbo E, Kaynar A, Szilasi M, Watkins S, Ryter S, Hoetzel A, Choi A, et al. Mitogen-activated protein kinases regulate susceptibility to ventilator-induced lung injury. PLoS One 2008;3:e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakaya T, Kawai T, Suzuki T. Regulation of fe65 nuclear translocation and function by amyloid beta-protein precursor in osmotically stressed cells. J Biol Chem 2008;283:19119–19131. [DOI] [PubMed] [Google Scholar]
- 63.Checkley W, Brower R, Korpak A, Thompson BT, Investigators ARDSN. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med 2008;177:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs B. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med 2006;34:300–306. [DOI] [PubMed] [Google Scholar]
- 65.Kavanagh BP. Therapeutic hypercapnia: careful science, better trials. Am J Respir Crit Care Med 2005;171:96–97. [DOI] [PubMed] [Google Scholar]
- 66.Lang JD, Figueroa M, Sanders KD, Aslan M, Liu Y, Chumley P, Freeman BA. Hypercapnia via reduced rate and tidal volume contributes to lipopolysaccharide-induced lung injury. Am J Respir Crit Care Med 2005;171:147–157. [DOI] [PubMed] [Google Scholar]
- 67.Takeshita K, Suzuki Y, Nishio K, Takeuchi O, Toda K, Kudo H, Miyao N, Ishii M, Sato N, Naoki K, et al. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-[kappa]b activation. Am J Respir Cell Mol Biol 2003;29:124–132. [DOI] [PubMed] [Google Scholar]
- 68.Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol 2007;28:825–831. [DOI] [PubMed] [Google Scholar]
- 69.Raymond J, Aujard Y. Nosocomial infections in pediatric patients: A European, multicenter prospective study. European study group. Infect Control Hosp Epidemiol 2000;21:260–263. [DOI] [PubMed] [Google Scholar]
- 70.Grohskopf LA, Sinkowitz-Cochran RL, Garrett DO, Sohn AH, Levine GL, Siegel JD, Stover BH, Jarvis WR, Network PP. A national point-prevalence survey of pediatric intensive care unit-acquired infections in the united states. J Pediatr 2002;140:432–438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.