Abstract
Obese mice have increased responses to acute ozone (O3) exposure. T-cadherin is a binding protein for the high–molecular weight isoforms of adiponectin, an anti-inflammatory hormone that declines in obesity. The objective of the present study was to determine whether adiponectin affects pulmonary responses to O3, and whether these effects are mediated through T-cadherin. We performed bronchoalveolar lavage (BAL) and measured pulmonary responsiveness to methacholine after acute air or O3 exposure (2 ppm for 3 h) in adiponectin-deficient (Adipo−/−) or T-cadherin–deficient (T-Cad−/−) mice. O3 increased pulmonary responses to methacholine and increased BAL neutrophils and protein to a greater extent in wild-type than in Adipo−/− mice, whereas T-cadherin deficiency had no effect. O3-induced increases in BAL IL-6 and keratinocyte-derived chemokine (KC), which contribute to O3-induced pulmonary neutrophilia, were also greater in wild-type than in Adipo−/− mice. In contrast, responses to O3 were not altered by transgenic overexpression of adiponectin. To determine which adiponectin isoforms are present in the lung, Western blotting was performed. The hexameric isoform of adiponectin dominated in serum, whereas BAL was dominated by the high–molecular weight isoform of adiponectin. Interestingly, serum adiponectin was greater in T-Cad−/− versus wild-type mice, whereas BAL adiponectin was lower in T-Cad−/− versus wild-type mice, suggesting that T-cadherin may be important for transit of high–molecular weight adiponectin from the blood to the lung. Our results indicate that adiponectin deficiency inhibits pulmonary inflammation induced by acute O3 exposure, and that T-cadherin does not mediate the effects of adiponectin responsible for these events.
Keywords: T-cadherin, neutrophil, inflammation, airway responsiveness, bronchoalveolar lavage
CLINICAL RELEVANCE.
Obesity increases the pulmonary responses to ozone (O3), an air pollutant that can trigger asthma. Adiponectin is an adipose tissue–derived hormone that declines in obesity. Our results indicate that adiponectin deficiency attenuates pulmonary responses to acute O3 exposure in mice. Our results also suggest that T-cadherin, an adiponectin-binding protein, may be important for transit of adiponectin from the blood into the lung.
Obesity is a risk factor for asthma. Obesity increases the prevalence and incidence of asthma, and worsens asthma control, whereas weight loss in obese individuals with asthma results in fewer asthma symptoms, increased airflow rates, and better asthma control (see recent reviews in Refs. 1 and 2). Obese subjects also experience greater decrements in pulmonary function after exposure to ozone (O3) (3, 4), a common air pollutant that can act as a trigger for asthma (5, 6). Greater O3-induced pulmonary injury and inflammation and augmented O3-induced airway hyperresponsiveness (AHR) are also observed in obese versus lean mice (7–10). The mechanistic basis for these enhanced responses to O3 remains to be established.
Adiponectin is an insulin-sensitizing hormone synthesized in adipose tissue, which paradoxically declines with obesity (11, 12). Adiponectin is found in serum as three distinct oligomers: a trimer, a hexamer, and an even higher molecular weight (HMW) species. These isoforms differ in their bioactivity and degradation (13, 14), and HMW adiponectin correlates better than total adiponectin with body mass index and with measures of insulin sensitivity (15). Importantly, adiponectin can have anti-inflammatory effects. It inhibits expression of certain inflammatory genes, including TNF-α, while augmenting expression of other anti-inflammatory genes, such as IL-10, and inhibiting extracellular signal–regulated kinase activation (16–18). The anti-inflammatory effects of adiponectin include effects that impact the lungs, as we have shown that, in mice, exogenous administration of adiponectin results in an almost complete suppression of allergen-induced AHR, airway inflammation, and Th2 cytokine expression in the lung (19). Similarly, others have recently reported that adiponectin deficiency augments allergic airways inflammation in mice (20). Hence, it is possible that reductions in the anti-inflammatory effects of adiponectin in obesity result in augmented O3-induced inflammation.
Three adiponectin-binding proteins have been cloned: AdipoR1, AdipoR2, and T-cadherin (21, 22). T-cadherin, a member of the cadherin family of cell–cell adhesion molecules, is a glycosylphosphatidylinositol-anchored extracellular protein, lacking an intracellular domain (23). It is highly expressed on endothelial and smooth muscle cells, in the nervous system (23, 24), and has also been reported in type 2 alveolar epithelial cells (25). Hug and colleagues (22) have previously reported that T-cadherin acts as a receptor for the HMW forms of adiponectin. Importantly, adiponectin associates with vascular endothelial cells in wild-type mice (24), including pulmonary vascular endothelial cells (26), but this adiponectin binding is absent in mice deficient in T-cadherin (24).
The purpose of this study was to determine whether the absence of adiponectin alters the pulmonary effects of O3, and to determine whether T-cadherin mediates the pulmonary effects of adiponectin. Contrary to our expectations, we found that adiponectin deficiency reduced the pulmonary responses to acute O3 exposure. The effects of adiponectin did not appear to be mediated via adiponectin binding to T-cadherin, at least under these conditions. However, T-cadherin was required for uptake of HMW isoforms of adiponectin from the blood into the lung.
MATERIALS AND METHODS
Animals
These studies were approved by the Harvard Medical Area Standing Committee on Animals. Wild-type mice and mice genetically deficient in adiponectin (Adipo−/−) or T-cadherin (T-Cad−/−) were used. Adipo−/− mice were obtained from Dr. Yuji Matsuzawa (27). Because these mice were on a C57BL/6J background, C57BL/6 mice from Jackson Laboratory (Bar Harbor, ME) were used as wild-types for comparison. T-Cad−/− mice were generated as described previously (24). As these mice were generated on a C57BL/6 Taconic background, C57BL/6 mice from Taconic Farms (Germantown, NY) were used for comparison. WT, Adipo−/−, and T-Cad−/− mice used for functional studies were all male. Mice used for lung histology were mixed male and female. Adiponectin transgenic (Adipo Tg) mice were generated as previously described (28). Adipo Tg mice were bred with C57BL/6J mice. Adipo Tg mice from these matings were exposed to O3 along with their transgene negative siblings that were used as wild-type controls. Adipo Tg mice and their controls were female. All mice were used between 8 and 13 weeks of age.
Protocol
Several series of experiments were performed. In the first, wild-type and Adipo−/− mice were anesthetized and instrumented for the measurement of pulmonary mechanics and responsiveness 24 hours after O3 (2 ppm for 3 h) or room air exposure. We chose 24 hours after exposure because it is when O3-induced AHR is commonly observed in C57BL/6 mice (29). Pressure–volume (PV) curves were also obtained in these mice before administration of methacholine. In addition, blood was collected and bronchoalveolar lavage (BAL) was performed. In a second cohort of wild-type and Adipo−/− mice, we performed BAL 4 hours rather than 24 hours after O3 or air exposure to assess the effects of adiponectin deficiency on O3-induced changes in BAL chemokines and cytokines that have been shown to be required for O3-induced neutrophil recruitment to the lung. We chose 4 hours after exposure for these studies because we have previously shown that, whereas O3-induced changes in BAL protein and neutrophils continue to increase through 24 hours after exposure, changes in many O3-induced cytokines and chemokines peak earlier and then decline to near-basal levels by 24 hours (8, 9, 30). We also examined lung histology in a third cohort of air- and O3-exposed wild-type and Adipo−/− mice. In a fourth series of experiments, we examined Adipo Tg mice and their wild-type controls 4 hours after exposure to O3 (2 ppm for 3 h), and examined O3-induced changes in BAL chemokines and cytokines. Finally, O3-induced AHR and neutrophil recruitment were studied 24 hours after air or O3 exposure (2 ppm for 3 h) in wild-type and T-Cad−/− mice.
O3 Exposure
For O3 exposure, conscious mice were placed into individual wire mesh cages inside a stainless steel and Plexiglas exposure chamber and exposed for 3 hours to 2 ppm O3. Mice were then returned to their home cages, where they had access to food and water ad libitum for 24 hours until being examined. For room air exposures, a separate but identical exposure chamber was used. Details of the O3 exposure and monitoring system have been described previously (7).
Pulmonary Mechanics Measurements
At 24 hours after exposure to O3 or room air, mice were anesthetized with xylazine (7 mg/kg) and pentobarbital sodium (50 mg/kg). The trachea was cannulated with a tubing adaptor, and the tail vein was cannulated for the delivery of acetyl-β-methylcholine chloride (Sigma-Aldrich Co., St. Louis, MO). The mice were artificially ventilated (flexiVent; SCIREQ Inc., Montreal, PQ, Canada) at 150 breaths/min with a tidal volume of 0.3 ml. A wide incision in the chest wall was made bilaterally to expose the lungs to atmospheric pressure, and a positive end-expiratory pressure was applied by placing the expiratory line under 3 cm of water. Lungs were inflated to three times tidal volume to standardize volume history before each maneuver. Because differences in lung elastance (H) of Adipo−/− mice have been reported (31), we obtained quasistatic PV curves of the lung by introducing successive increments in volume, approximately 0.11 ml, from end-expiratory volume. Airway opening pressure was measured after each increment in volume was held for 1 second. Baseline pulmonary mechanics and responses to intravenous methacholine were then obtained by the forced oscillation technique, as previously described (9). After PBS (1 μl/g) or methacholine was injected, we measured pulmonary resistance (with a sinusoidal forcing function at a frequency of 2.5 Hz every eighth breath) until resistance peaked. At that point, measurements of lung impedance (ZL) were obtained by forced oscillation with a sinusoidal forcing function containing frequencies ranging from 0.25 to 19.63 Hz. A parameter estimation model (32) was used to partition ZL into components representing airway resistance (Raw), the coefficients of lung tissue damping (G) and H, and lung tissue hysteresivity, eta. We and others have previously reported that real and imaginary parts of ZL as a function of frequency conform well to this model (33, 34).
BAL
Once measurements of pulmonary mechanics were complete, the lungs were lavaged twice with 1 ml PBS/0.6 mM EDTA. The BAL was centrifuged, and total BAL cells and differentials were assessed as previously described (9, 35). The BAL supernatant was frozen at −80°C. Total BAL protein was subsequently determined spectrophotometrically according to the Bradford protein assay procedure (Bio-Rad, Hercules, CA). BAL and serum adiponectin were analyzed by ELISA (R&D Systems, Minneapolis, MN) and by Western blotting (see subsequent text). For the 4 hours after O3 experiments, we also measured keratinocyte-derived chemokine (KC) and IL-6 in the BAL fluid by ELISA or Duoset ELISA development systems (R&D Systems), as these substances have been shown to be required for neutrophil recruitment in this model (36, 37). We also measured BAL monocyte chemotactic protein 1 (MCP-1) and eotaxin by ELISA or Duoset ELISA (R&D Systems). MCP-1 has been reported to play a role in monocytic cell recruitment after O3 exposure (38), but is also a neutrophil chemotactic factor. In the case of the Adipo Tg mice, we also measured macrophage inflammatory protein-2 (MIP-2), another neutrophil chemotactic agent, by a Duoset ELISA development system (R&D Systems).
Blood Collection
Blood was collected from the heart via puncture of the right ventricle. Serum was isolated and stored at −20°C until being analyzed.
Histology
Lungs of some mice were fixed for histological assessment as follows. After mice were killed, the trachea was cannulated and attached to a system with a syringe containing 4% buffered paraformaldehyde and connected to a manometer to monitor the insufflation pressure. Lungs were slowly insufflated with paraformaldehyde until 20 cm H2O of pressure was achieved. The trachea was occluded with a suture silk, and the lungs were removed from the chest cavity en bloc and submerged into the fixative solution for up to 24 hours, followed by washing with PBS and storage in 70% ethanol. Two sections were made in the left lobe: one mid-saggital, and the other transversally at mid position. Lung slices were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Lungs used for histology did not undergo BAL or measurements of pulmonary mechanics.
In lungs of air-exposed wild-type, Adipo−/−, and T-Cad−/− mice, mean linear intercepts (MLIs) were obtained by counting the number of times the parenchymal wall intercepted a straight line (180 μm) using a graticule attached to a light microscope eyepiece, whereas sections were observed under 400× magnification (39, 40). MLI was calculated as MLI = L/I, where I is the number of intercepts and L is the length of line. We counted 5 lines per field and 10 fields per mouse. An average value was computed for each mouse from those 10 fields, and statistical analysis was performed with those average values.
Based on the data of others who reported age-related pulmonary vascular inflammation in Adipo−/− mice (26), we also prepared H&E-stained histological sections of lungs from O3-exposed wild-type and Adipo−/− mice to determine whether there were differences in the impact of O3 on the pulmonary vasculature. For these experiments, mice were exposed to O3 (2 ppm) for 3 hours and killed 4 hours after cessation of exposure.
Western Blotting
Qualitative analysis of serum and BAL adiponectin isoforms from wild-type, Adipo−/−, and T-Cad−/− mice were performed by Western blot. Serum (1 μl) or 30 μl BAL fluid was added to an appropriate volume of 2× SDS sample buffer without reducing agents (Invitrogen, Carlsbad, CA), mixed, incubated at room temperature for 5 minutes, separated on 1.5 mm 4–12% PAGE in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (Invitrogen) at 30 V for 10 hours, and transferred to nitrocellulose membrane (12 V for 6 h; Idea Scientific blotter; Idea Scientific, Minneapolis, MN) with 10% methanol supplemented NuPage transfer buffer (Invitrogen). The membrane was blocked in 0.1% Tween-20/PBS supplemented with 5% milk and 2% donkey serum at 4°C overnight. Anti-globular adiponectin antiserum, generated by immunizing a rabbit with bacterially expressed globular mouse adiponectin with no additional fusion protein tag, was applied at a final concentration of 1:2,000 in blocking solution and incubated with shaking for 2 hours at room temperature. The blot was washed for 15 minutes three times in 0.1% Tween-20/PBS at room temperature. Secondary antibody (1:2,000 donkey anti-rabbit horseradish peroxidase conjugated; Amersham, Piscataway, NJ) was applied in blocking solution for 1 hour at room temperature, and the blot was again washed. The membrane was developed with West Pico chemiluminescent reagent (Pierce, Rockford, IL) and exposed for varying lengths of time to X-ray film. The relative migration of molecular weight standards (SeeBlue2; Invitrogen) was used to identify the trimer, hexamer, and HMW species.
Quantitative Real-Time RT-PCR
Once BAL was completed, the lungs were harvested and the right lung was immersed in RNAlater solution (QIAGEN, Valencia, CA) and stored at 4°C for 24 hours, then transferred to another clean tube and stored at −80°C. RNA was extracted with a rotor–stator homogenizer in lysis buffer (supplied in the kit) with 2-mercaptoethanol and purified (RNeasy RNA extraction kit; QIAGEN) after DNase I digestion of genomic DNA. Total RNA was eluted with 50 μl of water and quantified (Nanodrop; ThermoScientific, Waltham, MA). Complementary DNA was prepared from 1 μg of total RNA by using SuperScript III First Strand kit with random hexamer primers (Invitrogen). Quantitative real-time PCR was performed to evaluate the mRNA expression of AdipoR1 (5′-CTTCTACTGCTCCCCACAGC-3′ and 5′-TCCCAGGAACACTCCTGCTC-3′) and AdipoR2 (5′-CGGTGTACTGCCACTCAGAA-3′ and 5′-CATGTCCCACTGAGAGACGA-3′) with an RT-PCR thermocycler (ABI 3700; Applied Biosystems, Foster City, CA) and detected with SYBR Green (Bio-Rad). AdipoR1 and AdipoR2 mRNA expression values were normalized to 18S RNA expression (primers: 5′-CTAACCCGTTGAACCCATT-3′ and 5′-CCATCCAATCGGTAGTAGCG-3′) by the ΔΔCt method (41).
Statistical Analysis
Comparisons of pulmonary mechanics and BAL and serum parameters were assessed by factorial ANOVA, with genotype and exposure as the main effects. Fisher's least significant difference test was used as a follow-up to determine the significance of differences between individual groups. STATISTICA software (StatSoft, Tulsa, OK) was used to perform all statistical analyses. The results are expressed as means (±SE), except where noted. Comparison of AdipoR1 and AdipoR2 expression was by unpaired t test. A P value less than 0.05 was considered significant.
RESULTS
No genotype-related differences in body weight were observed in air-exposed mice. There was a small decrease in body weight in all groups of O3-exposed mice compared with their air-matched controls, which reached significance in one wild-type group (Table 1). This O3-induced decline in body weight has been previously reported (42, 43): mice substantially reduce their activity level after acute O3 exposure, including food seeking.
TABLE 1.
BODY WEIGHT AND BASELINE PULMONARY MECHANICS OF WILD-TYPE, ADIPONECTIN-DEFICIENT, AND T-CADHERIN–DEFICIENT MICE EXPOSED TO ROOM AIR OR OZONE
| Genotype | Weight (g) | Raw (cm H2O/ml/s) | G (cm H2O/ml) | H (cm H2O/ml) | Estat (cm H2O/ml) |
|---|---|---|---|---|---|
| Wild-type, Air | 28.4 ± 1.9 | 0.23 ± 0.02 | 2.94 ± 0.24 | 18.9 ± 1.0 | 14.8 ± 0.5 |
| Adipo−/−, Air | 31.0 ± 1.1 | 0.18 ± 0.02 | 2.25 ± 0.15* | 15.6 ± 0.7 | 12.4 ± 0.5* |
| Wild-type, O3 | 27.0 ± 1.0 | 0.24 ± 0.02 | 3.31 ± 0.12 | 20.4 ± 1.0 | 14.4 ± 0.5 |
| Adipo−/−, O3 | 28.6 ± 1.0 | 0.24 ± 0.01† | 2.65 ± 0.19* | 18.0 ± 1.7 | 13.2 ± 0.6 |
| Wild-type, Air | 29.8 ± 1.0 | 0.24 ± 0.01 | 3.17 ± 0.21 | 20.8 ± 0.6 | 15.9 ± 0.3 |
| T-Cad−/−, Air | 30.3 ± 0.7 | 0.23 ± 0.01 | 3.00 ± 0.25 | 19.9 ± 0.8 | 15.5 ± 0.5 |
| Wild-type, O3 | 24.8 ± 0.8† | 0.30 ± 0.01† | 3.39 ± 0.11 | 21.6 ± 0.9 | 16.1 ± 0.3 |
| T-Cad−/−, O3 | 29.2 ± 0.8* | 0.27 ± 0.02† | 3.21 ± 0.15 | 24.2 ± 0.7†* | 17.1 ± 0.4† |
Definition of abbreviations: Adipo−/−, adiponectin deficient; Estat, static elastance of the lung; G, coefficient of lung tissue damping; H, coefficient of lung tissue elastance; O3, ozone; Raw, airway resistance; T-Cad−/−, T-cadherin–deficient.
Results are means (±SE) of data from 6–10 mice in each group. Measurements were made 24 hours after exposure to room air or to O3 (2 ppm for 3 h).
P < 0.05 versus wild-type mice with the same exposure.
P < 0.05 versus air exposed mice of the same genotype.
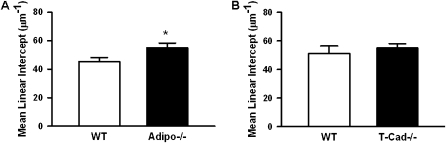
In air-exposed mice, quasistatic lung elastance (Estat) measured over the deflation portion of the PV curve from functional residual capacity to functional residual capacity plus 0.3 ml was approximately 15% lower in Adipo−/− mice than in wild-type mice (Table 1). Measures of pulmonary parenchymal oscillation mechanics (G and H) were also lower in Adipo−/− versus wild-type mice, although this only reached statistical significance for G, but not H. The reduction in Estat is consistent with reports by Summer and colleagues (31). This group also reported an increase in MLI on lung histological sections, consistent with an emphysema-like condition. To confirm these observations, we measured MLI in histological sections of wild-type and Adipo−/− mice exposed to air. Our results indicate a significant increase in MLI in Adipo−/− versus wild-type mice (Figure 1A).
Figure 1.
Mean liner intercept of lung sections from wild-type and adiponectin-deficient (Adipo−/−) (A) or wild-type and T-cadherin–deficient (T-Cad−/−) (B) mice exposed to air. Results are mean (±SE) of data from 6–8 mice per group. *P < 0.05 versus wild-type mice.
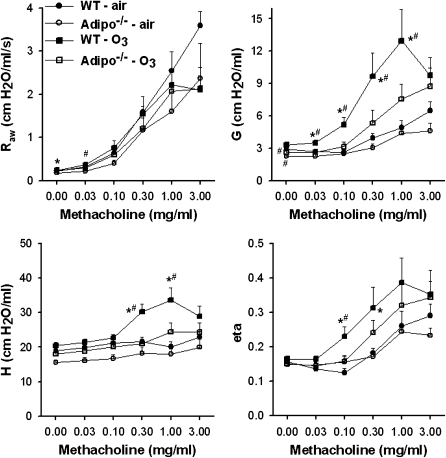
O3 exposure significantly increased Raw in Adipo−/− mice, but did not affect measures of pulmonary parenchymal mechanics. In wild-type mice, responsiveness to intravenous methacholine was greater in mice exposed to O3 (2 ppm for 3 h) versus room air (Figure 2). O3 augmented the changes in G, H, and eta (the ratio of G:H) that were induced by methacholine, whereas changes in Raw were not altered. O3-induced increases in responsiveness to methacholine, as measured by changes in G, H, and eta, were reduced in Adipo−/− versus wild-type mice (Figure 2).
Figure 2.
Changes in airway resistance (Raw), the coefficients of lung tissue damping (G) and lung elastance (H) and the ratio of G:H (eta) induced by intravenous methacholine in wild-type (WT) (C57BL/6) and Adipo−/− mice 24 hours after exposure to air or ozone (O3; 2 ppm for 3 h) (n = 6–11 mice for each group). *P < 0.05 compared with genotype-matched air-exposed controls; #P < 0.05 versus Adipo−/− mice with the same exposure.
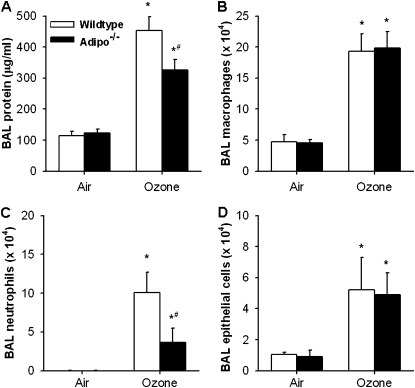
Compared with air exposure, BAL neutrophils, epithelial cells, and total BAL protein concentration, a measure of O3-induced lung injury, were increased 24 hours after O3 exposure (Figure 3). The O3-induced changes in both BAL protein and BAL neutrophils were lower in Adipo−/− versus wild-type mice, whereas O3-induced changes in macrophages and epithelial cells were not different in wild-type versus Adipo−/− mice.
Figure 3.
Total protein concentration (A) and total number of macrophages (B), neutrophils (C), and epithelial cells (D) in bronchoalveolar lavage (BAL) fluid of WT (C57BL/6) and Adipo−/− mice 24 hours after exposure to air or O3 (2 ppm for 3 h) (n = 6–11 mice for each group). *P < 0.05 compared with genotype-matched, air-exposed controls; #P < 0.05 versus WT mice with the same exposure.
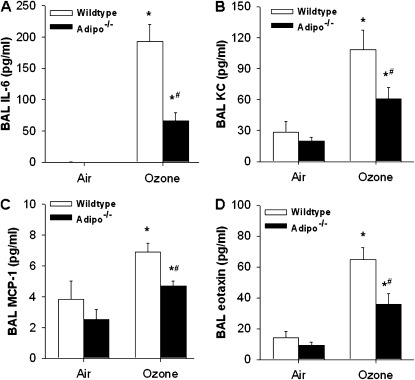
O3-induced changes in BAL neutrophils are a hallmark of O3-induced inflammation. To determine how adiponectin deficiency resulted in decreased recruitment of neutrophils by O3, we measured KC and IL-6 in BAL fluid, as each of these factors has been shown to be produced in the lungs after acute O3 exposure in mice, and to be involved in the O3-induced neutrophil influx induced by acute O3 exposure in mice (36, 37). KC and IL-6 were measured 4 hours, rather than 24 hours, after O3 exposure, because we have previously shown that they are induced early after O3 exposure and then decline to near-baseline (air-exposed) levels by 24 hours (8, 9, 30). Compared with air, O3 exposure resulted in an increase in BAL KC and IL-6 (Figures 4A and 4B), as previously reported (8, 9, 30). O3-induced changes in BAL KC and IL-6 were significantly lower in Adipo−/− versus wild-type mice. We also examined BAL MCP-1 and eotaxin, two other chemokines that are induced by acute O3 exposure (8, 9, 30). Similar to the results obtained with KC and IL-6, O3-induced increases in MCP-1 and eotaxin were reduced in Adipo−/− versus wild-type mice (Figures 4C and 4D).
Figure 4.
BAL concentrations of IL-6 (A), KC (B), MCP-1 (C), and eotaxin (D) in WT and Adipo−/− mice 4 hours after exposure to air or O3 (2 ppm for 3 h) (n = 5–7 mice in each group). *P < 0.05 compared with genotype-matched, air-exposed controls; #P < 0.05 versus WT mice with the same exposure.
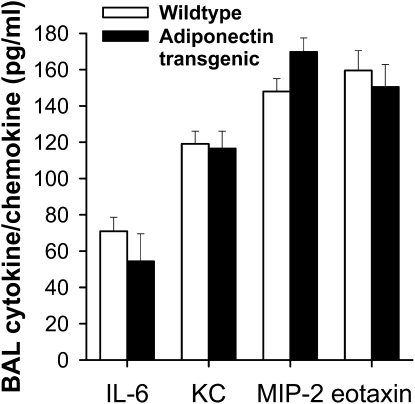
To determine whether adiponectin overexpression had opposing effects on responses to acute O3 exposure, we exposed Adipo Tg mice and littermate control animals to O3 (2 ppm for 3 h). BAL was performed 4 hours after exposure. Serum adiponectin levels were approximately 16 times higher in Adipo Tg versus wild-type mice (161.4 ± 5.6 versus 9.5 ± 0.2 ug/ml, respectively; P < 0.0001). Nevertheless, adiponectin overexpression had no effect on changes in cytokines and chemokines induced by acute O3 exposure (Figure 5). At 4 hours after cessation of exposure, BAL IL-6, KC, MIP-2, and eotaxin were similar in O3-exposed Adipo Tg mice versus O3-exposed wild-type control animals.
Figure 5.
BAL concentrations of IL-6, KC, MIP-2, and eotaxin in adiponectin transgenic and WT mice 4 hours after exposure to O3 (2 ppm for 3 h) (n = 6 mice/group).
To determine whether the effects of adiponectin deficiency described previously here (Figures 1–4, Table 1) were mediated via adiponectin binding to T-cadherin, we examined the effect of acute O3 exposure on T-Cad−/− mice. In contrast to the reduction in pulmonary parenchyma mechanics observed in air-exposed Adipo−/− versus wild-type mice, we observed no difference in baseline Estat, G, or H in air-exposed T-Cad−/− versus wild-type mice (Table 1). Consistent with these observations, there was also no difference in MLI in wild-type versus T-Cad−/− mice (Figure 1B).
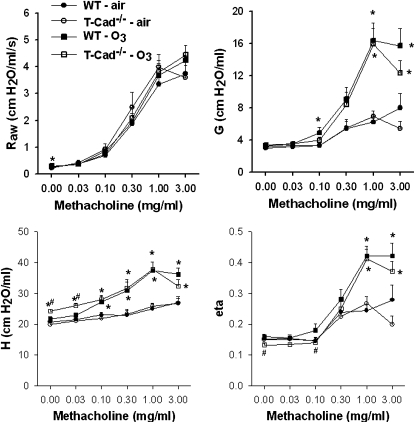
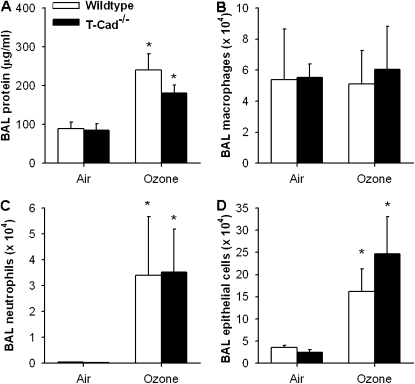
O3 exposure increased measures of lung stiffness (Estat and H) in T-Cad−/− mice, but not in wild-type mice. In contrast to the effect of adiponectin deficiency, which attenuated O3-induced hyperresponsiveness (Figure 2), O3-induced changes in responsiveness were not affected by T-cadherin deficiency (Figure 6). We also failed to observe any reduction in O3-induced changes in BAL protein and BAL neutrophils in T-Cad−/− versus wild-type mice (Figure 7), even though both BAL protein and neutrophils were lower in Adipo−/− mice versus wild-type mice (Figure 3). We considered the possibility that, because the mice were deficient in T-cadherin from birth, adaptive changes in other adiponectin-binding proteins could have occurred during development, but no difference in expression of either AdipoR1 or AdipoR2 mRNA, determined by quantitative RT-PCR, was observed between wild-type versus T-Cad−/− mice (data not shown).
Figure 6.
Changes in airway resistance (Raw), the G and H and eta induced by intravenous methacholine in WT (C57BL/6) and T-Cad−/− mice 24 hours after exposure to air or O3 (2 ppm for 3 h) (n = 7–10 mice for each group). *P < 0.05 compared with genotype-matched, air-exposed controls; #P < 0.05 versus WT mice with the same exposure.
Figure 7.
Total protein concentration (A) and total number of macrophages (B), neutrophils (C), and epithelial cells (D) in BAL fluid of WT (C57BL/6) and T-Cad−/− mice 24 hours after exposure to air or O3 (2 ppm for 3 h) (n = 5–10 mice for each group). *P < 0.05 compared with genotype-matched, air-exposed controls.
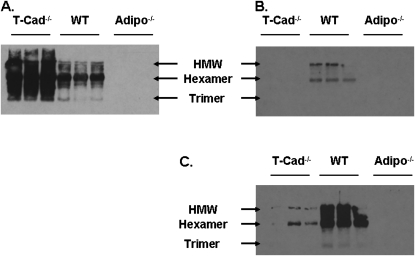
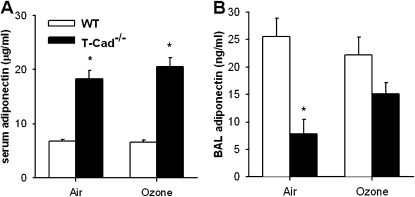
To determine which adiponectin isoforms are present in the lung, Western blotting for adiponectin was performed on BAL fluid from air-exposed lungs (Figure 8). We also examined serum for comparison. BAL and serum from Adipo−/− mice were used as negative controls. Serum of air-exposed wild-type mice contained mainly the hexameric form of adiponectin, with smaller amounts of the trimer and HMW forms (Figure 8A). However, the adiponectin isoform distribution in BAL fluid was different from that observed in serum (Figure 8B). Notably, HMW adiponectin dominated the BAL fluid with somewhat lesser amounts of hexamer. With longer blot exposure times (Figure 8C), a small amount of trimer was also observed. Because T-cadherin deficiency has been reported to increase serum adiponectin (24), we also examined BAL and serum from T-Cad−/− mice. We observed an increase in serum adiponectin in T-Cad−/− mice: all three adiponectin isoforms were increased (Figure 8A). However, T-cadherin deficiency resulted in a marked attenuation of adiponectin in BAL fluid (Figure 8B). With longer blot exposure times, we could observe some adiponectin in BAL of T-Cad−/− mice; however, the hexamer now dominated the HMW adiponectin (Figure 8C). These results were confirmed by ELISA (Figure 9). There was a marked increase in serum adiponectin in T-Cad−/− mice versus wild-type mice whether the mice were exposed to air or to O3 (Figure 9A). In contrast, BAL adiponectin was significantly lower in T-Cad−/− versus wild-type mice exposed to air, whereas, in mice exposed to O3, this difference was largely abolished, for the most part because of an increase in BAL adiponectin in the T-Cad−/− mice (Figure 9B).
Figure 8.
Adiponectin isoform distribution in serum (A) and BAL (B and C) of WT and T-Cad−/− mice. Adipo−/− mice were used as negative controls. HMW, high–molecular weight isoform. B and C are the same blot with C, having a longer exposure time on the film.
Figure 9.
Serum (A) and BAL (B) concentrations of adiponectin measured by ELISA in WT (C57BL/6) and T-Cad−/− mice 24 hours after exposure to air or O3 (2 ppm for 3 h) (n = 5–6 mice for each group). *P < 0.05 compared with WT mice with same exposure.
DISCUSSION
Our results indicate that pulmonary responses to acute O3 exposure are reduced in Adipo−/− versus wild-type mice: O3-induced pulmonary responsiveness to intravenous methacholine, O3-induced lung injury (as assessed by increases in BAL protein), O3-induced inflammation (as assessed by increases in BAL neutrophils), and O3-induced increases in IL-6, KC, MCP-1, and eotaxin were all lower in Adipo−/− than in wild-type mice (Figures 2–4). In contrast to the effects of adiponectin deficiency, responses to acute O3 exposure were not different in wild-type versus T-Cad−/− mice (Figures 6 and 7). The results indicate that adiponectin deficiency inhibits responses to acute O3 in the lung, but indicate that the effects of adiponectin, the deficiency of which results in reduced responses to O3, are not mediated by signaling pathways involving T-cadherin, suggesting that other adiponectin binding proteins—perhaps AdipoR1 or AdipoR2—are involved instead. Nevertheless, our results showing decreased BAL adiponectin despite markedly increased blood adiponectin in T-Cad−/− versus wild-type mice (Figures 8 and 9), suggest that T-cadherin does have a role in adiponectin biology in the lung. In particular, T-cadherin may be involved in uptake of adiponectin from the blood into the lung.
In previous studies, we have shown that pulmonary responses to acute O3 exposure are increased in obese versus lean mice (7–10). Because adiponectin has some anti-inflammatory properties (12, 16–18), we hypothesized that reductions in adiponectin that occur in obesity (8) may be contributing to the augmented responses to O3 that are observed in obese mice (7–9) and obese humans (3, 4). However, in these lean mice, adiponectin deficiency reduced rather than augmented responses to O3 (Figures 2–4). The reduction in neutrophil recruitment in the Adipo−/− mice (Figure 3) is consistent with the reductions in BAL IL-6 and KC (Figure 4), as both IL-6, as well as chemokines, such as KC, that stimulate the CXCR2 receptor, are known to be required for the neutrophil influx that occurs after acute O3 exposure in mice (36, 37). Notably, lack of the receptor for KC (CXCR2) also attenuates O3-induced AHR (36). Thus, the reduction in KC (Figure 4) may also explain the reduction in AHR observed in the Adipo−/− mice (Figure 2).
We were surprised by the reduction in O3-induced inflammation in Adipo−/− mice, because many reports indicate that adiponectin has anti-inflammatory effects (12, 16–18). However, there are also reports of adiponectin having proinflammatory effects under certain circumstances (see Ref. 12 for review). For example, in some cell types, adiponectin has been shown to activate the proinflammatory transcription factors, NF-κB and activator protein (AP)-1 (13, 44). Adiponectin also causes IL-6 release from synovial fibroblasts and macrophages (45, 46), and increases production of the neutrophil chemotactic factors, IL-8 and/or MCP-1, from epithelial cells, including airway epithelial cells (47, 48). Consistent with these reports, we also observed a reduction in O3-induced IL-6, KC (murine equivalent of IL-8), and MCP-1 in BAL fluid from Adipo−/− versus wild-type mice (Figure 4). However, transgenic overexpression of adiponectin, resulting in substantial increases in serum adiponectin, did not augment responses to acute O3 (Figure 5). It is conceivable that, compared with adiponectin deficiency, normal endogenous levels of adiponectin are sufficient to produce maximal augmentation of proinflammatory effects of acute O3 exposure. In that case, further increasing adiponectin by transgenic overexpression would not be expected to have any additional effects, as observed (Figure 5). Alternatively, adiponectin deficiency may result in some lung developmental change that impacts the response to O3.
For example, it has been reported that Adipo−/− mice have evidence of pulmonary vascular infiltration with inflammatory cells, which increases progressively with age, but is present as early as 3 months of age (26)—the age of many of the mice in this study. Such basal inflammatory changes could increase the expression of antioxidants in the lung, leading to reduced responses to O3. However, routine histological evaluation of H&E-stained lung sections failed to demonstrate any differences in the pulmonary vasculature of Adipo−/− versus wild-type mice after air or O3 exposure (data not shown), although it is conceivable that, with more sensitive techniques, perivascular inflammation might have been observed. It has also been reported (31) that, even in the absence of any overt inflammatory stimulus, Adipo−/− mice have decreased H, an increase in the MLI, and evidence of alveolar macrophage activation, consistent with an emphysema-like condition. Others have argued that the phenotype more closely resembles disruption of alveolarization in the neonate resulting from chronic inflammation (49). We also observed an increase in MLI (Figure 1A), as well as decreases in Estat and in G and H (Table 1), two other indices of parenchymal mechanics, in Adipo−/− versus wild-type mice, although the change in Estat was smaller than that reported by Summer and colleagues (31). It is conceivable that lung architectural changes in Adipo−/− mice could lead to altered distribution and/or deposition of O3 in such a manner as to impact subsequent responses.
With respect to the parenchymal abnormalities in the Adipo−/− mice, it is interesting to note that the ability of O3 to cause AHR was related to its effects on the parenchymal parameters, G and H, whereas changes in Raw induced by methacholine were unaffected (Figures 2 and 6). Such exaggerated changes in G and H may reflect enhanced small airway closure (50, 51). This increased airway closure is unlikely to be the result of greater airway constriction, as Raw was unaffected (Figures 2A and 6A). Instead, increased airway closure may be a consequence of greater instability of peripheral airways, perhaps as a result of alterations in the airway liquid surface properties caused by O3 (52). It is conceivable that the parenchymal changes observed in Adipo−/− mice (Figure 1A) could reduce such peripheral airway instability, leading to reduced AHR (Figure 2).
Three adiponectin-binding proteins have been cloned: AdipoR1, AdipoR2, and T-cadherin (21, 22). Given that the HMW isoform of adiponectin dominated the lung lining fluid (Figure 8B), and that T-cadherin primarily binds the HMW isoforms of adiponectin (22), we examined the hypothesis that the effects of adiponectin responsible for reductions in responses to O3 exposure in Adipo−/− mice (Figures 2–4) might be mediated through adiponectin signaling pathways activated by binding to T-cadherin. Our results do not support this hypothesis, at least for this concentration of O3 at this time point. Whereas adiponectin deficiency resulted in reduced O3-induced AHR, inflammation, and injury (Figures 2–4), deficiency in T-cadherin did not (Figures 6 and 7). These results suggest that other adiponectin-binding proteins mediate the observed injurious effects of adiponectin (Figures 2–4). Nevertheless, we cannot rule out the possibility that, in the lung, other effects of T-cadherin may counter those related to adiponectin binding. For example, overexpression of T-cadherin decreases surfactant protein D expression in A549 cells (25), suggesting that T-Cad−/− mice may have increased pulmonary SP-D expression. Based on the effects of surfactant protein D deficiency (53), an increase in SP-D should result in a reduced inflammatory response to O3.
It is also important to note that, in contrast to adiponectin deficiency, T-cadherin deficiency did not alter either parenchymal mechanics or MLI (Table 1, Figure 1). The results indicate that the effects of adiponectin, the deficiency of which results in these parenchymal changes, are not mediated through T-cadherin deficiency.
Although the results in T-Cad−/− mice (Figures 6 and 7) suggest that other adiponectin-binding proteins, perhaps AdipoR1 or AdipoR2, mediate the observed effects of adiponectin, it is noteworthy that non–receptor-mediated functions of adiponectin have also been reported. For example, adiponectin interacts directly with platelet-derived, epidermal, and other growth factors, blocking their binding to their own cell membrane receptors, and thus suppressing their mitogenic effects on vascular smooth muscle cells (54). Adiponectin also interacts directly with certain chemokines, including stromal cell–derived factor–1, and interferes with their ability to bind heparin (55). Adiponectin also promotes phagocytosis of apoptotic cells by macrophages in an AdipoR1-, AdipoR2-, and T-cadherin–independent manner (56). Whether such functions contribute to the effects of adiponectin reported here remains to be established.
Adiponectin is produced primarily in adipose tissue (57), and is not synthesized to any meaningful extent in the lung (31). Consequently, the presence of adiponectin in the lung lining fluid requires its uptake from the blood. Indeed, others have noted that, after correction for the dilution that occurs during the BAL procedure, the concentrations of adiponectin in lung lining fluid are remarkably high, almost one-eighth the values in serum (31). Our data suggest that, at least under baseline conditions, the transit of adiponectin from the blood to lung is not a simple process of the protein diffusing through gaps between endothelial cells. If this were so, one would expect a greater predominance of the trimeric form of adiponectin in the BAL fluid, because it is smallest and should diffuse most easily. Instead, the HMW and hexameric isoforms dominated (Figure 8B), although we cannot rule out the possibility that the lower molecular weight isoforms of adiponectin are more easily degraded in the lung. In addition, if diffusion through paracellular pathways were involved, one would also have expected an increase in BAL adiponectin in T-Cad−/− mice, because serum levels of adiponectin were markedly elevated in those mice. Instead, BAL adiponectin was reduced in air-exposed T-Cad−/− mice (Figures 8B and 9B). One hypothesis consistent with these data is that binding of adiponectin to T-cadherin is required for uptake of this protein from the blood into the lung. The observation that T-cadherin binds primarily the HMW and hexameric isoforms of adiponectin (22), and that these were the dominant isoforms observed in the BAL fluid (Figure 8B), is consistent with this hypothesis. In the pulmonary microvasculature, even albumin, which has a molecular weight on the same order as the trimeric form of adiponectin, is transported, at least in part, by a vesicular transcytosis pathway that requires the albumin-binding protein gp60 (58). It is conceivable that T-cadherin serves a similar function for HMW adiponectin.
Given the very low concentrations of adiponectin in BAL fluid of T-Cad−/− mice, we were surprised that the phenotypes of the T-Cad−/− and Adipo−/− mice were so different. It may be that even the very low concentrations of adiponectin present in the T-Cad−/− mouse lung were sufficient to reproduce the effects of adiponectin observed in wild-type mice, but it is important to note that, whereas BAL adiponectin was markedly reduced in air-exposed T-Cad−/− versus wild-type mice, adiponectin substantially increased in BAL fluid of T-Cad−/− mice exposed to O3 (Figure 9B). O3 also induced a marked increase in total BAL protein (Figures 3 and 7), consistent with increased vascular permeability resulting from effects of inflammatory mediators acting on endothelial cells to reduce paracellular resistance. Under such circumstances, adiponectin might move between endothelial cells. Because the concentration gradient for adiponectin between blood and BAL fluid was much greater in T-Cad−/− than wild-type mice, one might expect a greater impact of such changes in permeability in the T-Cad−/− mice leading to the observed increase in BAL adiponectin after O3 exposure. Similarly, adiponectin has been shown to accumulate in heart tissue damaged by ischemia–reperfusion as a result of leakage from the vasculature (59).
There are two important pulmonary health implications of these findings. First, it is apparent that measurements of serum adiponectin cannot be used to predict lung adiponectin concentration or isomer distribution. Second, factors that affect T-cadherin expression can impact the concentration of adiponectin in the lung, as well as the concentration of adiponectin that circulates to and affects other organ systems in the body.
In summary, our results indicate that, in the setting of acute O3 exposure, adiponectin deficiency results in reduced lung injury, inflammation, and AHR. Although it is possible that these changes are the result of proinflammatory effects of adiponectin, changes in O3 deposition or distribution resulting from lung architectural changes in Adipo−/− mice may also contribute. Although these effects of adiponectin do not appear to be mediated by a signaling process requiring T-cadherin, our results suggest that T-cadherin may be important for transit of adiponectin from the blood into the lung.
Acknowledgments
The authors thank Dr. Lester Kobzik for assistance with routine histological examination of pulmonary vessels.
This work was supported by National Institute of Health grants HL-084044, ES-013307, HD25938, HL-077499, and ES-00002. C.H. was supported by a Career Development Fellowship from Children's Hospital Boston.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0086OC on November 13, 2009
Author Disclosure: C.H. has a patent pending from Whitehead Institute for use of T-cadherin as a target. R.A.J. received a sponsored grant from the National Institutes of Health (NIH) for more than $100,001. B.R. received a sponsored grant from NIH for more than $100,001. S.A.S. serves on the advisory board of Schering Plough and receives $1,001–$5,000, and also received lecture fees from Merck Pharmaceutical for $1,001–$5,000 and Merck Frosst Canada for more than $1,000, as well as a sponsored grant from NIH for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 2006;110:83–102. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005;115:897–909, quiz 910. [DOI] [PubMed] [Google Scholar]
- 3.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: Effect modified by obesity and airways hyperresponsiveness in the VA Normative Aging Study. Chest 2007;132:1890–1897. [DOI] [PubMed] [Google Scholar]
- 4.Bennett WD, Hazucha MJ, Folinsbee LJ, Bromberg PA, Kissling GE, London SJ. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal Toxicol 2007;19:1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003;290:1859–1867. [DOI] [PubMed] [Google Scholar]
- 6.Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, Carlin BP, Klein M, Dorley J, Butler AJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol 2000;151:798–810. [DOI] [PubMed] [Google Scholar]
- 7.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 2003;95:938–945. [DOI] [PubMed] [Google Scholar]
- 8.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L856–L865. [DOI] [PubMed] [Google Scholar]
- 9.Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E–deficient mice. Am J Physiol Regul Integr Comp Physiol 2006;290:R126–R133. [DOI] [PubMed] [Google Scholar]
- 10.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 2008;104:1727–1735. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 2008;582:74–80. [DOI] [PubMed] [Google Scholar]
- 12.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol 2008;121:326–330. [DOI] [PubMed] [Google Scholar]
- 13.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state–dependent activation of NF-kappa B signaling pathway by adipocyte complement–related protein of 30 kDa (Acrp30). J Biol Chem 2002;277:29359–29362. [DOI] [PubMed] [Google Scholar]
- 14.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 2008;149:2270–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537–1545. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1Ra in human leukocytes. Biochem Biophys Res Commun 2004;323:630–635. [DOI] [PubMed] [Google Scholar]
- 17.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 2004;316:924–929. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol 2003;14:561–566. [DOI] [PubMed] [Google Scholar]
- 19.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389–395. [DOI] [PubMed] [Google Scholar]
- 20.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol 2009;41:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–769. [DOI] [PubMed] [Google Scholar]
- 22.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high–molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 2004;101:10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron 1991;7:391–402. [DOI] [PubMed] [Google Scholar]
- 24.Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res 2008;68:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi T, Misaki A, Fujita J, Sonobe H, Ohtsuki Y. T-cadherin (CDH13, H-cadherin) expression downregulated surfactant protein d in bronchioloalveolar cells. Virchows Arch 2001;438:370–375. [DOI] [PubMed] [Google Scholar]
- 26.Summer R, Fiack CA, Ikeda Y, Sato K, Dwyer D, Ouchi N, Fine A, Farber HW, Walsh K. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol 2009;297:L432–L438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/Acrp30. Nat Med 2002;8:731–737. [DOI] [PubMed] [Google Scholar]
- 28.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 2009;23:241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams AS, Issa R, Leung SY, Nath P, Ferguson GD, Bennett BL, Adcock IM, Chung KF. Attenuation of ozone-induced airway inflammation and hyper-responsiveness by c-Jun NH2 terminal kinase inhibitor SP600125. J Pharmacol Exp Ther 2007;322:351–359. [DOI] [PubMed] [Google Scholar]
- 30.Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol 2007;37:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L1035–L1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 33.Hjoberg J, Shore S, Kobzik L, Okinaga S, Hallock A, Vallone J, Subramaniam V, De Sanctis GT, Elias JA, Drazen JM, et al. Expression of nitric oxide synthase–2 in the lungs decreases airway resistance and responsiveness. J Appl Physiol 2004;97:249–259. [DOI] [PubMed] [Google Scholar]
- 34.Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-alpha increases lung tissue hysteresivity in transgenic mice. J Appl Physiol 2001;91:2730–2734. [DOI] [PubMed] [Google Scholar]
- 35.Lang JE, Williams ES, Mizgerd JP, Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol 2008;294:L1013–L1020. [DOI] [PubMed] [Google Scholar]
- 36.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol 2005;288:L61–L67. [DOI] [PubMed] [Google Scholar]
- 37.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 2005;288:L390–L397. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol 1998;274:L39–L46. [DOI] [PubMed] [Google Scholar]
- 39.Robbesom AA, Versteeg EM, Veerkamp JH, van Krieken JH, Bulten HJ, Smits HT, Willems LN, van Herwaarden CL, Dekhuijzen PN, van Kuppevelt TH. Morphological quantification of emphysema in small human lung specimens: comparison of methods and relation with clinical data. Mod Pathol 2003;16:1–7. [DOI] [PubMed] [Google Scholar]
- 40.Flo C, Lopes FD, Kasahara DI, Silva AC, Jesus RC, Rivero DH, Saldiva PH, Martins MA, Jacob-Filho W. Effects of exercise training on papain-induced pulmonary emphysema in Wistar rats. J Appl Physiol 2006;100:281–285. [DOI] [PubMed] [Google Scholar]
- 41.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription–polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 2000;285:194–204. [DOI] [PubMed] [Google Scholar]
- 42.Johnston RA, Theman TA, Terry RD, Williams ES, Shore SA. Pulmonary responses to acute ozone exposure in fasted mice: effect of leptin administration. J Appl Physiol 2007;102:149–156. [DOI] [PubMed] [Google Scholar]
- 43.Last JA, Gohil K, Mathrani VC, Kenyon NJ. Systemic responses to inhaled ozone in mice: cachexia and down-regulation of liver xenobiotic metabolizing genes. Toxicol Appl Pharmacol 2005;208:117–126. [DOI] [PubMed] [Google Scholar]
- 44.Hattori Y, Hattori S, Akimoto K, Nishikimi T, Suzuki K, Matsuoka H, Kasai K. Globular adiponectin activates nuclear factor-kappaB and activating protein–1 and enhances angiotensin II–induced proliferation in cardiac fibroblasts. Diabetes 2007;56:804–808. [DOI] [PubMed] [Google Scholar]
- 45.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, et al. The potential of adiponectin in driving arthritis. J Immunol 2006;176:4468–4478. [DOI] [PubMed] [Google Scholar]
- 46.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun 2005;335:1254–1263. [DOI] [PubMed] [Google Scholar]
- 47.Ogunwobi OO, Beales IL. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept 2006;134:105–113. [DOI] [PubMed] [Google Scholar]
- 48.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol 2009;182:684–691. [DOI] [PubMed] [Google Scholar]
- 49.Wert SE. Does adiponectin play a role in pulmonary emphysema? Am J Physiol Lung Cell Mol Physiol 2008;294:L1032–L1034. [DOI] [PubMed] [Google Scholar]
- 50.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol 2004;96:2019–2027. [DOI] [PubMed] [Google Scholar]
- 51.Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol 2007;102:221–230. [DOI] [PubMed] [Google Scholar]
- 52.Podgorski A, Sosnowski TR, Gradon L. Deactivation of the pulmonary surfactant dynamics by toxic aerosols and gases. J Aerosol Med 2001;14:455–466. [DOI] [PubMed] [Google Scholar]
- 53.Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, Beers MF, Salmon M, Panettieri RA Jr, Haczku A. Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D. Respir Res 2006;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization dependent manner. J Biol Chem 2005;280:18341–18347. [DOI] [PubMed] [Google Scholar]
- 55.Masaie H, Oritani K, Yokota T, Takahashi I, Shirogane T, Ujiie H, Ichii M, Saitoh N, Maeda T, Tanigawa R, et al. Adiponectin binds to chemokines via the globular head and modulates interactions between chemokines and heparan sulfates. Exp Hematol 2007;35:947–956. [DOI] [PubMed] [Google Scholar]
- 56.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 2007;117:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–26749. [DOI] [PubMed] [Google Scholar]
- 58.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006;86:279–367. [DOI] [PubMed] [Google Scholar]
- 59.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, Ouchi N, Walsh K. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia–reperfusion injury via leakage from the vascular compartment. Cardiovasc Res 2007;74:471–479. [DOI] [PubMed] [Google Scholar]