Abstract
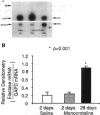
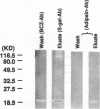
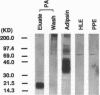
We showed previously a cause and effect relationship between increased activity of an endogenous vascular elastase (EVE) and experimentally induced pulmonary hypertension in rats. We now report the isolation and characterization of EVE. Degenerate oligonucleotides synthesized to homologous sequences in serine elastases were used in a PCR with rat pulmonary artery (PA) cDNA. The PCR product hybridized to a 1.2-kb mRNA and the intensity of hybridization was threefold increased in RNA from rat hypertensive PA at a timepoint when EVE activity was increased. The PCR product was used to screen a cDNA library and sequences obtained encoded rat adipsin. We then used immunoaffinity to purify EVE. An antibody to the elastin-binding protein was used to remove this competitor of elastase from the PA extract and the elastolytic activity increased 100-fold. The enzyme was purified using an antibody that recognizes NH2-terminal sequences of serine proteinases and the eluate was further purified using an antibody raised against recombinant adipsin. A single band at 20 kD immunoreactive with the adipsin antibody was resolved as an active enzyme on an elastin substrate gel. Immunogold labeling with an antibody to an adipsin peptide sequence localized EVE to PA smooth muscle cells. This is the first isolation of EVE; it appears to be a novel enzyme related to the serine proteinase adipsin originally found in adipose tissue.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldo A., Sniderman A. D., St-Luce S., Avramoglu R. K., Maslowska M., Hoang B., Monge J. C., Bell A., Mulay S., Cianflone K. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J Clin Invest. 1993 Sep;92(3):1543–1547. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cook K. S., Min H. Y., Johnson D., Chaplinsky R. J., Flier J. S., Hunt C. R., Spiegelman B. M. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987 Jul 24;237(4813):402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- Dempsey E. C., Stenmark K. R., McMurtry I. F., O'Brien R. F., Voelkel N. F., Badesch D. B. Insulin-like growth factor I and protein kinase C activation stimulate pulmonary artery smooth muscle cell proliferation through separate but synergistic pathways. J Cell Physiol. 1990 Jul;144(1):159–165. doi: 10.1002/jcp.1041440121. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Cook K. S., Usher P., Spiegelman B. M. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987 Jul 24;237(4813):405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Thömmes P., Weiser T., Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990 May;4(8):2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Hinek A., Mecham R. P., Keeley F., Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest. 1991 Dec;88(6):2083–2094. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Rabinovitch M. 67-kD elastin-binding protein is a protective "companion" of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol. 1994 Jul;126(2):563–574. doi: 10.1083/jcb.126.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Rabinovitch M., Keeley F., Okamura-Oho Y., Callahan J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest. 1993 Mar;91(3):1198–1205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Wrenn D. S., Mecham R. P., Barondes S. H. The elastin receptor: a galactoside-binding protein. Science. 1988 Mar 25;239(4847):1539–1541. doi: 10.1126/science.2832941. [DOI] [PubMed] [Google Scholar]
- Ilkiw R., Todorovich-Hunter L., Maruyama K., Shin J., Rabinovitch M. SC-39026, a serine elastase inhibitor, prevents muscularization of peripheral arteries, suggesting a mechanism of monocrotaline-induced pulmonary hypertension in rats. Circ Res. 1989 Apr;64(4):814–825. doi: 10.1161/01.res.64.4.814. [DOI] [PubMed] [Google Scholar]
- Kislauskis E. H., Li Z., Singer R. H., Taneja K. L. Isoform-specific 3'-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J Cell Biol. 1993 Oct;123(1):165–172. doi: 10.1083/jcb.123.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J., Wigle D., Childs T., Zhu L., Keeley F. W., Rabinovitch M. Serum-induced vascular smooth muscle cell elastolytic activity through tyrosine kinase intracellular signalling. J Cell Physiol. 1994 Jul;160(1):121–131. doi: 10.1002/jcp.1041600115. [DOI] [PubMed] [Google Scholar]
- Krishnan B. R., Blakesley R. W., Berg D. E. Linear amplification DNA sequencing directly from single phage plaques and bacterial colonies. Nucleic Acids Res. 1991 Mar 11;19(5):1153–1153. doi: 10.1093/nar/19.5.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Ye C. L., Woo M., Venkatacharya H., Lines L. D., Silver M. M., Rabinovitch M. Chronic hypoxic pulmonary hypertension in rats and increased elastolytic activity. Am J Physiol. 1991 Dec;261(6 Pt 2):H1716–H1726. doi: 10.1152/ajpheart.1991.261.6.H1716. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol. 1980 Dec;101(3):527–542. [PMC free article] [PubMed] [Google Scholar]
- Min H. Y., Spiegelman B. M. Adipsin, the adipocyte serine protease: gene structure and control of expression by tumor necrosis factor. Nucleic Acids Res. 1986 Nov 25;14(22):8879–8892. doi: 10.1093/nar/14.22.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. P., Regan W. A study of elastic tissue and actinic radiation in "aging," temporal arteritis, polymyalgia rheumatica, and atherosclerosis. The actinic storm in the modern world. J Am Acad Dermatol. 1991 May;24(5 Pt 1):765–776. doi: 10.1016/0190-9622(91)70118-l. [DOI] [PubMed] [Google Scholar]
- Oho S., Rabinovitch M. Post-cardiac transplant arteriopathy in piglets is associated with fragmentation of elastin and increased activity of a serine elastase. Am J Pathol. 1994 Jul;145(1):202–210. [PMC free article] [PubMed] [Google Scholar]
- Perkett E. A., Badesch D. B., Roessler M. K., Stenmark K. R., Meyrick B. Insulin-like growth factor I and pulmonary hypertension induced by continuous air embolization in sheep. Am J Respir Cell Mol Biol. 1992 Jan;6(1):82–87. doi: 10.1165/ajrcmb/6.1.82. [DOI] [PubMed] [Google Scholar]
- Perkett E. A., Lyons R. M., Moses H. L., Brigham K. L., Meyrick B. Transforming growth factor-beta activity in sheep lung lymph during the development of pulmonary hypertension. J Clin Invest. 1990 Nov;86(5):1459–1464. doi: 10.1172/JCI114862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Bothwell T., Hayakawa B. N., Williams W. G., Trusler G. A., Rowe R. D., Olley P. M., Cutz E. Pulmonary artery endothelial abnormalities in patients with congenital heart defects and pulmonary hypertension. A correlation of light with scanning electron microscopy and transmission electron microscopy. Lab Invest. 1986 Dec;55(6):632–653. [PubMed] [Google Scholar]
- Rosen B. S., Cook K. S., Yaglom J., Groves D. L., Volanakis J. E., Damm D., White T., Spiegelman B. M. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989 Jun 23;244(4911):1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. C., Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol. 1988 Dec;255(6 Pt 2):H1484–H1491. doi: 10.1152/ajpheart.1988.255.6.H1484. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J., Koli K., Keski-Oja J. Release of transforming growth factor-beta 1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J Biol Chem. 1992 Dec 15;267(35):25378–25384. [PubMed] [Google Scholar]
- Todorovich-Hunter L., Dodo H., Ye C., McCready L., Keeley F. W., Rabinovitch M. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am Rev Respir Dis. 1992 Jul;146(1):213–223. doi: 10.1164/ajrccm/146.1.213. [DOI] [PubMed] [Google Scholar]
- Todorovich-Hunter L., Johnson D. J., Ranger P., Keeley F. W., Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest. 1988 Feb;58(2):184–195. [PubMed] [Google Scholar]
- Ye C. L., Rabinovitch M. Inhibition of elastolysis by SC-37698 reduces development and progression of monocrotaline pulmonary hypertension. Am J Physiol. 1991 Oct;261(4 Pt 2):H1255–H1267. doi: 10.1152/ajpheart.1991.261.4.H1255. [DOI] [PubMed] [Google Scholar]