Abstract
Highly ordered sphingolipid-enriched lipid raft microdomains (LRMs) within plasma membranes purportedly function as specialized signaling platforms. Leukocyte migration is believed to entail LRM redistribution, but progress in studying LRMs in situ during cell movement has been limited. By using an improved method for imaging the spectral shift of the environmentally sensitive probe, laurdan (expressed as a generalized polarization function), the plasma membrane order (i.e., tight packing of membrane bilayer lipids) of human polymorphonuclear neutrophils (PMNs) was mapped in real time during migration. Morphologically polarized PMNs exhibited prominent LRM clusters at the uropod, where in every instance membrane order was found to oscillate with mean periodicities of 37.0 ± 1.46 and 149.9 ± 9.0 seconds (P < 0.01). LRM aggregates were also demonstrated in punctate and clustered distributions of nonpolarized cells and transiently at the lamellipodia of polarized PMNs. Cellular polarization was not accompanied by an overall increase in membrane order. LRM disorganization with methyl-β-cyclodextrin had small negative effects on cell velocity, but it abrogated directionally biased migration toward chemotactic gradients of FMLP or leukotriene B4. LRMs disruption also caused redistribution of Rac 1/2 GTPase and GM3 ganglioside away from the lamellipodium, as well as extension of multiple pseudopods simultaneously or in rapid succession, rather than formation of a defined leading edge. Thus, we demonstrate that the plasma membrane order of migrating PMNs changes dynamically, with prominent oscillations consistently seen at the uropod. These findings solidify the existence of rapidly reorganizing LRMs in situ and support a role for LRMs in chemotaxin responsiveness.
Keywords: neutrophils, chemotaxis, lipid rafts, inflammation
CLINICAL RELEVANCE.
This study describes new mechanisms by which polymorphonuclear neutrophils respond appropriately to chemotactic stimuli. This information may lead to novel strategies for limiting the deleterious effects of acute inflammation and augmenting host responses to infectious pathogens. This study also describes an improved method for rapid imaging of lipid raft microdomains, which should be valuable to investigators studying plasma membrane dynamics.
Inflammation mediated by polymorphonuclear neutrophils (PMNs) requires the cells to recognize and migrate toward chemotactic signals. Coupled with morphologic polarization, there must be complex spatial redistribution of signaling molecules, cytoskeletal structures, and membrane constituents for directionally biased movement (1–4). Although the distribution of proteins has been examined extensively, there is growing appreciation for the importance of compartmentalized membrane lipids and lipid-mediated signaling (2, 4–6). Earlier models have depicted a uniform lipid bilayer in the plasma membrane, but considerable evidence indicates that it is highly heterogeneous, containing small phase-separated regions often designated as lipid raft microdomains (LRMs) (reviewed in Refs. 2, 7–9). These microdomains are rigid, gel-like regions enriched in tightly packed sphingolipids, resulting in a liquid-ordered (Lo) state that is stabilized by cholesterol. It is believed that LRMs act as platforms where multiple constituents colocalize to form competent signaling complexes. Cell fractionation experiments have been used to isolate detergent-resistant/low-density membrane fractions containing an array of receptor proteins and signaling molecules. These fractions have been viewed as in vitro analogs of LRMs, but artifacts created by detergents and other fractionation procedures have raised questions regarding the structure, composition, and existence of LRMs in situ. These concerns have been difficult to resolve, largely because the putative size of LRMs, though highly disputed, is thought to be well below the resolution limits of conventional microscopy (10). Compounding this problem, LRMs are heterogeneous in their protein and lipid content, so it is problematic to use individual LRM-associated constituents to draw general conclusions about LRM structure or function. The addition to living cells of bulky markers, such as antibodies and fluorescent labels, which are generally more massive than the supporting lipids of the LRMs, may have been another source of artifacts in this area of research. LRMs are clearly enriched in many receptors and downstream signaling intermediates important for cell movement (CD87, CD59, leukotriene B4 receptor-1 [BLT-1], Rac1, phosphatidylinositol bisphosphate, phosphatidylinositol 3,4,5-trisphosphate [PIP3], and transient receptor potential channel 1) as well as membrane proteins that link to the cytoskeleton (talin, ezrin/radixin/moesin, and focal adhesion kinase) (2, 11–16). Moreover, LRM markers polarize in motile PMNs, forming aggregates near the uropod and the lamellipodium (2, 13). It has not been shown whether this redistribution is static or fluid over time, so it remains to be resolved whether this LRM reorganization is a stable feature of cellular polarity or dynamically changing in motile cells.
To better understand membrane reorganization during cell migration, improved methods are necessary to examine LRM behavior in living cells. Laurdan (6-dodecanoyl-2-dimethylaminonaphthalene) was developed as an environmentally sensitive lipid probe (17, 18). Laurdan distributes uniformly within plasma membranes, where its fluorescence emission exhibits a red shift in a polar environment and a blue shift when located among nonpolar, highly ordered lipids (18). Although the ability to assess membrane order directly has great appeal, the utility of laurdan-based imaging techniques has been limited by its low extinction coefficient and its susceptibility to photobleaching. Two-photon laser scanning microscopy to image laurdan spectral shifts has demonstrated highly ordered domains at filopodia and focal adhesions, although the long image acquisition times of 20 to 30 seconds or more would prevent serial imaging of live cells (11, 17, 18). Because signaling events and cytoskeletal reorganization occur in precise locations and time frames during cell movement, one can expect that defining membrane reorganization with greater spatiotemporal detail would help illuminate the role of LRM in chemotaxis. In this study, we have applied an improved system using conventional fluorescence microscopy to simultaneously acquire images through two separate filter sets for quantifying laurdan spectral shifts. The greatly enhanced sensitivity, low background noise, and minimal photobleaching achieved with this system enabled us to track the distribution of highly ordered regions of plasma membrane with time-lapse imaging sequences of rapidly moving PMNs. High levels of membrane order were visualized most prominently at the uropods of polarized PMNs, and Eigen analysis indicates that membrane order in this region is highly dynamic, consistently oscillating with mean periodicities (i.e., cycling intervals) of approximately 37 and 150 seconds. To address the relevance of varying membrane order on PMN movement, cell tracking experiments demonstrated that LRM disorganization selectively disturbs biased migration toward chemotactic gradients with minimal effect on locomotion, suggesting a role for LRM organization in the direction-sensing component of chemotactic responsiveness.
MATERIALS AND METHODS
PMN Purification
PMNs were isolated from peripheral blood obtained from healthy volunteers in compliance with the University of Michigan Institutional Review Board for Human Subject Research. Briefly, citrate-anticoagulated blood was sedimented with 6% dextran/0.9% NaCl. Erythrocytes were removed by hypotonic lysis, and PMNs were isolated to greater than 95% purity by density gradient centrifugation on 10% Ficoll-Hypaque.
Laurdan Spectral Shift Microscopy
PMNs suspended in Hanks' balanced salt solution (HBSS) were incubated with 5 μM laurdan (Invitrogen, Carlsbad, CA) in 0.1% DMSO for 30 minutes at 4°C and washed. Images of laurdan-labeled PMNs were taken at 37°C using a Nikon Eclipse TE-2000U microscope (Nikon Instruments, Melville, NY) configured with a Dual-View image splitter (Optical Insights, Santa Fe, NM). A 355HT15 filter and a 390LP dichroic mirror were used for excitation (Chroma Technology Corp., Rockingham, VT). An Optical Insights slider containing a 460 dcxr dichroic mirror, D425/60 m and D510/80M filters, and a 0.3 neutral density filter (Chroma Technology Corp.) were used for emission. The system included a Princeton Instruments (Trenton, NJ) PI-MAX2 camera (Gen3 1Kx1K chip with extended blue sensitivity) with a S-133 controller, a Perkin-Elmer FX-4400 flash lamp with an optical water filter (Newport-Oriel Corp., Stratford, CT), and Win-Spec software (Princeton Instruments). Ten 6-microsecond flashes with camera gating set to 150 microseconds were acquired within 2.5 milliseconds and averaged with Metamorph 7.1.2.0 software (Molecular Devices, Downingtown, PA) for each image. To map laurdan spectral shifts, images acquired at 395 to 455 nm and 470 to 550 nm were processed to calculate the generalized polarization function (GP = [I395–455 − I470–550]/[I395–455 + I470–550]) (17, 18). The images were processed in Metamorph and ImageJ v. 1.38x (US National Institutes of Health, Bethesda MD). Adobe Photoshop CS2 v. 9.0.2 (Adobe, San Jose, CA) was used for background masking (examples of the original images are shown in Figure E2 in the online supplement without masking or background adjustment). To examine the dynamics of LRM distribution, PMNs were examined while exposed to FMLP gradients in Dunn chemotaxis chambers (Hawksley, Lancing Sussex, UK). FMLP (5 × 10−7 M) (Sigma, St. Louis MO) was injected into the outer ring of the chamber to establish a chemotactic gradient in the viewing area. Serial images were taken at 10-second intervals for 6 to 10 minutes, and the GP images were assembled into video clips.
Analysis of Temporal Variations in GP
To quantitatively characterize variations in GP intensity at the uropod, mathematical tools were used. To evaluate our data, we used Eigen analysis and Fourier analysis, which provide frequency determination of a time-varying data set using different mathematical approaches. Eigen analysis was performed with an order of 24 and Eigen values of 6 to 13, with the values adjusted for varying signal-to-noise ratios. Fourier-fast transform (FFT), using Best Exact N analysis with a Nmin = 60 and a threshold of 99%, was used for independent confirmation of the Eigen analysis. Both analyses were performed using AutoSignal v1.7 (Seasolve, San Jose, CA).
Differential Interference Contrast Microscopy
PMNs adhered to glass coverslips in HBSS with 1% BSA were viewed in Dunn chambers using 5 × 10−7 M FMLP to establish a chemotactic gradient. Images were taken with a QImaging Retiga 1×300 camera (Surrey, BC, Canada) with a 90-millisecond exposure at 1,000 every 5 seconds for 10 minutes.
Immunostaining
PMNs were adhered to MatTek dishes (MatTek Corp., Ashland, MA) and stimulated with FMLP (5 × 10−7 M in HBSS) for 30 minutes at 37°C in 5% CO2. Cells were then washed with warm HBSS and treated with methyl-β-cyclodextrin (MβCD) (5 mM, 5 min, 37°C) or left untreated (HBSS, 5 min, 37°C). Cells were then fixed with 25% glutaraldehyde for 30 minutes, washed with HBSS, and permeabilized with 0.01% saponin for 10 minutes, blocked with 10% serum, and stained with Alexa Fluor 555 (Invitrogen)-conjugated anti-Rac 1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). GM3 gangliosides were labeled with Alexa Fluor 488–conjugated mouse antihuman GM3 (Glycotech, Gaithersburg, MD). Nuclei were counterstained with Hoechst 33342 (Invitrogen). Cells were imaged immediately after immunostaining.
Cell Tracking Analysis
PMNs were adhered to coverslips and treated with MβCD (5 mM, 5 min) (Sigma), which disorganizes LRMs by extracting cholesterol, thereby destabilizing the Lo state of packed sphingolipids (13, 19). For negative controls, cells were pretreated in parallel with HBSS or α-cyclodextrin, which is structurally similar to MβCD but has a low affinity for cholesterol. The coverslips were then applied to Dunn chemotaxis chambers and loaded with FMLP (5 × 10−7 M) or LTB4 (10−7 M) (Biomol International, Plymouth Meeting, PA). When LTB4 was used, arachidonic acid (1 mM) (Biomol International) was added to the buffer to minimize LTB4 binding to albumin (20). Serial images of a field of cells maintained at 37°C were collected every 30 seconds for 30 minutes. Cell coordinates were plotted manually at each frame, and the image stacks were processed with Metamorph and Microsoft Excel to analyze movement of individual cells. A PMN was considered motile if it moved 30 μm or more from its original coordinates at any point, analogous to a threshold used previously for shorter viewing periods (21). Among the motile cells, the mean cell velocity (μm/min) was calculated from the total distance traveled over 30 minutes. To assess the directional bias of movement, the net displacement along the axis of the gradient over 30 minutes was measured (μm) and expressed as a chemotactic index (displacement along the axis of the gradient as % of total path distance) (22). Each data point represents the mean values obtained from 60 or more cell tracks in each experiment, and n represents the number of independent experiments, each with a unique donor.
Statistics
Group means were compared with Student's t test. Multiple comparisons were performed with a one-way ANOVA. GP distributions were analyzed by nonlinear regression (GraphPad Prism version 5.00 for Windows; GraphPad Software, San Diego, CA).
RESULTS
Laurdan Spectral Shift Imaging of PMNs
Laurdan-labeled live PMNs were imaged, expressing membrane order by the GP function (17, 18). The GP calculation adjusts for variations in signal intensity caused by regional differences in laurdan labeling, cell shape, or membrane convolutions. The diameter of LRMs, putatively 25 to 75 nm, is well below the approximately 200-nm resolution of fluorescence microscopy, so pixels with high GP values are taken to represent the combined effects of the abundance of LRMs within the pixel area and degree of membrane order. The pixel diameter was approximately 125 nm, so image resolution did not further limit that of the microscopy system.
Elongated, Migating PMNs
Elongated PMNs exhibited polarized distributions of high-GP regions (Figure 1A). The most consistent region of high GP was found at the uropod. This is seen most clearly when GP images were averaged over 30, 50, and 200 seconds, approximating the types of GP images generated over long acquisition times by two-photon laser confocal microscopy (11, 17, 18). However, signal averaging leads to considerable loss of detail in the images, as is evident by comparing Figures 1A and 1B, due to transient variation in GP level or rapid redistribution of LRMs. Previous studies have demonstrated front–back polarization of LRMs in elongated PMNs, but none has suggested that high-GP regions are highly dynamic over short time intervals. To examine this possibility, rapid sequential imaging of laurdan-labeled PMNs was examined (Figure 1B and Figure E1). Large foci of persistently high GP were found at the uropod. In many cells, high-GP areas were also found near the leading edge (images 13–18), in keeping with earlier findings demonstrating concentrated LRM markers at lamellipodia (2, 13, 23). Lower GP values were generally found over the body of the cells, suggesting a predominance of non-LRM membrane, but transient foci of high GP were identified here as well. In all these regions, GP varied over surprisingly short time frames, suggesting dynamic variation in membrane order or relocation of stable LRMs. This observation prompted a formal analysis of temporal variations in GP.
Figure 1.
Laurdan spectral shift microscopy of moving polymorphonuclear neutrophils (PMNs). A representative laurdan-labeled PMN exposed to FMLP (5 × 10−7 M) was imaged at 10-second intervals for over 10 minutes and processed to display generalized polarization function (GP) values, as described in Materials and Methods. A scale bar referencing the pseudocolor images to GP values is provided in the upper right. (A) GP composite images were averaged over long time intervals (as indicated), revealing relatively uniform GP values over most the cell, except for a persistent high-GP region at the uropod. (B) Rapid sequential images of the same PMN. Flash lamp images were generated at 10-second intervals by collecting and averaging 10 6-microsecond exposures over 2.5 milliseconds. The rapid sequence reveals a dynamically changing high-GP region at the uropod. High-GP regions are also identified at the leading edge and in clusters over the cell body. Full video clips of this and two other PMNs are shown in Figure E1.
Analysis of Temporal Variations in GP
Just as signal processing tools can be used to investigate the time-varying behavior of electronic circuits, we have used AutoSignal v1.7 (Seasolve) to evaluate temporal changes in membrane order as reflected by the GP intensity. We used two mathematically distinct approaches, Eigen analysis and FFT analysis, to evaluate the frequency components of our time-varying data. The uropod was selected for this analysis because the GP variations were most prominent (Figure 1), and this region of interest could be contained within a well defined, round, 256-pixel area. The 256-pixel region of interest was chosen in preliminary experiments by sampling high-GP spots at uropods, which were consistent in size. A plot of uropod GP over time taken from a representative migrating PMN is shown in Figure 2. Ten cells from different donors (including the cell in Figure 2) were chosen for analysis because they remained in the field of view as they migrated steadily for long time intervals without contacting other PMNs. Analyses of all 10 cells demonstrated regular variations in GP, with periodicities of 37.0 ± 1.46 and 149.0 ± 9.0 seconds (mean ± SEM; both P < 0.01) (Table 1). Both of these periodicities were confirmed independently with FFT analysis (not shown). The magnitude of the GP variation at the uropod was substantial because the difference between the GPmax and GPmin was 0.271 ± 0.025 units, which comprises 63% of the range between the 5th to 95th percentiles of pooled GP values of 10 elongated PMNs (Figure 3C). Even in the face of this variation, the uropod is a region of high membrane order because the minimum GP values were more than 1 SD above the mean GP for entire cells (Table 1; Figure 3C). Figure 1 also shows that GP varies rapidly at the leading edge of the cell and within clusters over the cell body, but these areas could not be subjected to Eigen analysis because their size and shape were too variable to demarcate reliably.
Figure 2.
Dynamic GP oscillations at the uropod. The GP values of a round 256-pixel area placed over the uropod of a representative linearly moving PMN was measured at 10-second intervals. Rapid and slower fluctuations in GP values are apparent. Eigen analysis of this and nine other cells is summarized in Table 1.
TABLE 1.
GENERALIZED POLARIZATION FUNCTION OSCILLATIONS AT POLYMORPHONUCLEAR NEUTROPHIL UROPODS
| Cell | Periodicity* (115–209 s) | Periodicity* (31–43 s) | GP (max) | GP (min) | GPΔ (max − min) |
|---|---|---|---|---|---|
| 1 | 164.5 | 42.6 | 0.500 | 0.258 | 0.242 |
| 2 | 115.5 | 34.8 | 0.430 | 0.273 | 0.157 |
| 3 | 130.1 | 36.3 | 0.656 | 0.375 | 0.281 |
| 4 | 208.8 | 35.3 | 0.523 | 0.313 | 0.210 |
| 5 | 190.8 | 40.5 | 0.617 | 0.320 | 0.297 |
| 6 | 139.0 | 38.1 | 0.594 | 0.313 | 0.281 |
| 7 | 138.4 | 40.8 | 0.625 | 0.328 | 0.297 |
| 8 | 144.4 | 36.3 | 0.664 | 0.429 | 0.235 |
| 9 | 146.5 | 38.6 | 0.703 | 0.227 | 0.476 |
| 10 | 120.9 | 31.1 | 0.453 | 0.219 | 0.234 |
| Mean ± SEM | 149.9 ± 9.0 | 37.0 ± 1.46 | 0.577 ± 0.028 | 0.306 ± 0.020 | 0.271 ± 0.025 |
Definition of abbreviation: GP = generalized polarization function.
Eigen analysis; P < 0.01.
Figure 3.
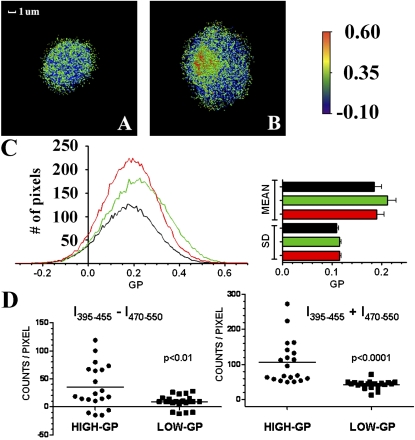
Laurdan spectral shift microscopy of round and elongated PMNs. Laurdan-labeled PMNs were rendered by calculating GP values, with images displayed in pseudocolor. Some round PMNs displayed uniformly distributed punctate regions of high-GP (A), but the majority also exhibited large high-GP aggregates (B). A scale bar for GP values is provided. (C) The corresponding distributions of GP values are displayed as histograms. Black: round/punctate and uniform. Green: round/focal aggregates. Red, elongated. The mean and SD of at least 10 cells of each category are shown. (D) To analyze the components of the GP calculation, 14 × 14 pixel regions of high GP and low GP were selected from 20 cells. The differences and sums of the emission intensities at 395 to 455 nm and 470 to 550 nm (the numerator and denominator of the GP calculation, respectively) were significantly larger in high-GP areas, indicating that high GP was not an artifact in areas with low total fluorescence intensity. Figure E2 includes examples of images at 395 to 455 nm and 470 to 550 nm without masking or background adjustment.
Round PMNs
Approximately one third of round PMNs exhibited punctate clusters of high GP randomly distributed over the cell surfaces, and two thirds also exhibited high-GP areas in large aggregates (Figures 3A and 3B). In separate experiments (not shown), more than 85% of PMNs fixed in suspension with 4% formalin immediately after purification exhibited randomly distributed punctate high-GP areas, suggesting that, in live cells that were allowed to adhere before GP imaging, the large aggregates were formed during adhesion. This observation is important because it indicates that LRM clustering is not a by-product of the transition to an elongated, migratory phenotype, and in some cells it may precede this transition.
Cellular activation may change the overall level of membrane order (24). To determine if this is the case for migrating PMNs, the distribution of GP values for at least 10 PMNs of each morphologic category (elongated, round/diffuse, and round/focal aggregates) were analyzed by nonlinear regression using a single Gaussian curve model (Figure 3C). The histograms generated from each category fit single Gaussian curves extremely well (all R2 > 0.95). The mean and SD of GP values were virtually identical among the morphologic categories, indicating that redistribution of high-GP areas was not associated with an overall change in membrane order. The means and ranges of GP values observed here are similar to those reported for other cell types (11).
Quantification of pixel intensities in regions of cells expressing high GP and low GP, as well as background controls, showed that differences in intensities associated with each wavelength were much greater than shot noise; typical integrated signal-to-shot noise ratios of approximately 188 are observed for high-GP areas, and similar ratios were found for low-GP areas. To assess the possibility of distortions generated by the GP calculation, 14 × 14 pixel high-GP areas (uropod) and low-GP areas (adjacent cell body) were selected from 20 cells, and the differences and sums of the emission intensities at the two wavelengths (the numerator and denominator of the GP calculation, respectively) were analyzed separately (Figure 3D). Both were significantly larger in high-GP areas (P < 0.01 and P < 0.0001, respectively), indicating that the GP reflects the magnitude of the spectral shift of laurdan, and high values were not simply artifacts produced in areas with low total fluorescence intensity.
Effects of LRM Disruption on the Distribution of Rac 1/2
PMNs stimulated with FMLP were immunostained for Rac GTPases, which have been shown to distribute asymmetrically in polarized PMNs, partition to LRMs, and participate in orienting cells toward chemotactic gradients (16, 25). Rac 1/2 concentrates in the region of the lamellipodium, with considerably less expression toward the tail. LRM disruption with MβCD resulted in a uniform distribution along the entire cell, suggesting that Rac 1/2 associates with LRM aggregates at the lamellipodium or that LRM organization otherwise directs Rac 1/2 distribution (Figure 4). Similarly, the LRM-associated ganglioside GM3 concentrates at the leading edge, as described previously (2, 26), but it too distributed uniformly after MβCD treatment.
Figure 4.
Effects of lipid raft microdomain (LRM) disruption on localization of Rac 1/2 and GM3. PMNs were stimulated with FMLP (5 × 10−7 M) and immunostained as in Materials and Methods. In the absence of methyl-β-cyclodextrin (MβCD) pretreatment (NT), Rac 1/2 accumulation was seen at the leading edges of elongated PMNs, but LRM disruption with MβCD (5 mM, 5 min) rendered a uniform distribution. The LRM-associated ganglioside GM also concentrated at the leading edge and distributed uniformly in response to MβCD. Two representative PMNs are shown for each condition, and all images were oriented so the leading edges are facing upward.
Effect of LRM Disruption on PMN Migration
In these experiments, PMNs were pretreated with MβCD to disorganize LRMs so their effects on directional migration could be assessed. We previously demonstrated by microspectrofluorometry that this MβCD extraction protocol reduces membrane order of laurdan-labeled, polarized PMNs (13), even though it reduces membrane cholesterol content by only 17.4 ± 0.9% (P < 0.01). Analyzing the GP distributions of laurdan-labeled cells treated with MβCD (5 μM, 5 min) also revealed a substantial effect on membrane order. Using the highest 10 and 5% of GP values of untreated controls as threshold values, MβCD reduced the frequency of high-GP pixels by 57 and 51%, respectively (not shown). The uropod regions of MβCD-treated cells were not sufficiently demarcated to analyze as in Figure 2, but an effect on membrane order at the uropod is highly likely because 50 and 61% of high-GP pixels (top 10 and 5%, respectively) of the cells presented in Table 1 were localized to the uropod region. To determine the effects of MβCD on chemotaxis, we adhered PMNs to glass coverslips in HBSS/1% BSA to favor a high level of spontaneous cell movement, and cells were exposed to FMLP or LTB4 gradients for 30 minutes. Imaging was terminated at that point because the MβCD pretreatment was limited to 5 minutes (because of concerns over cellular toxicity), and our preliminary work indicated that membrane cholesterol levels recover within 30 to 40 minutes after MβCD is removed. Some of the observation period elapsed before the gradients were well established, but despite these limitations, MβCD had striking effects on FMLP-induced chemotaxis. There was no significant effect on % motile cells, but mean velocity decreased from 8.1 to 6.3 μm/min (P < 0.03) (Figure 5). The most dramatic effect of MβCD was to negate the directional bias of motile cells along the FMLP gradient. The mean distance traveled along the axis of the gradient was reduced from 27.4 to 2.6 μm (P < 0.002), and the chemotactic index was reduced from 11.2 to 0.95% (P < 0.01). Alpha-cyclodextrin, a negative control with low affinity for cholesterol, had no effects. To determine if a chemotactic gradient is necessary for the effects of MβCD, PMNs were stimulated with a uniform concentration of FMLP (5 × 10−7 M). MβCD did not affect % motile cells, but it reduced cell velocity (6.8 ± 0.09 versus 5.9 ± 0.28 μm/min; P < 0.03). Spontaneous migration of unstimulated PMNs was unaffected by MβCD (data not shown).
Figure 5.
Effects of LRM disruption on FMLP-induced PMN chemotaxis. PMNs were pretreated with buffer, MβCD (5 mM, 5 min), or α-CD (5 mM, 5 min), a negative control. The PMNs were then loaded onto Dunn chambers charged with FMLP (5 × 10−7 M). Cell tracks were assembled from serial images over 30 minutes. MβCD did not significantly affect % motile cells but did have a small suppressive effect on cell velocity. The displacement along the axis of the chemotactic gradient was negated, and the chemotactic index (displacement along the gradient axis as % of total path length) was reduced to near zero. Mean ± SEM (n = 7), at least 60 tracks per experiment. α-CD did not significantly affect any of these parameters.
Unlike formyl peptide receptor (FMLP receptor), BLT-1 (LTB4 receptor) partitions to LRMs, and its function is disrupted by MβCD (15), so we sought to determine if LRM disruption has unique effects on LTB4–induced chemotaxis. Therefore, the experiments were reproduced using LTB4 (10−7 M) (Figure 6). MβCD had no effect on % motile cells, caused a small decrease in cell velocity (P < 0.0003), and negated directional bias toward the chemotaxin (P < 0.002). α-CD did not affect these parameters (not shown).
Figure 6.
Effects of LRM disruption on LTB4–induced PMN chemotaxis. The protocol described in Figure 5 is repeated here, substituting LTB4 (10−7 M) as the chemotaxin. The data are expressed as in Figure 5. MβCD had no effect on % of motile cells and a small suppressive effect on velocity but negated directional bias along the axis of the gradient. n = 6; at least 60 tracks per experiment.
Effect of LRM Disruption on PMN Morphology
The striking effect of MβCD pretreatment on chemotaxis prompted us to examine the corresponding cellular morphology. PMNs migrating in FMLP gradients often elongated extensively with a single, well defined pseudopod extension at the leading edge (Figure 7). MβCD-pretreated PMNs were far less likely to extend into elongated forms, but most striking was the multiple pseudopod extensions appearing simultaneously or in rapid succession over different regions of the cell. After MβCD had been removed for 30 minutes, PMNs demonstrated single pseudopod extensions at the leading edge.
Figure 7.
Effects of LRM disruption on PMN morphology. Differential interference microscopy of MβCD-pretreated PMNs (5 mM, 5 min) in Dunn chambers charged with FMLP (5 × 10−7 M). (A) Control PMNs demonstrated well defined pseudopod extensions confined to the leading edge (arrows). (B) MβCD-pretreated cells often demonstrated multiple pseudopod extensions (arrows). (C) Once the MβCD effect wore off, the PMNs revert to normal morphology, with single pseudopod extensions at the leading edge.
DISCUSSION
We have demonstrated that PMN plasma membrane order is not only spatially heterogeneous but also varies dynamically during polarization and migration. The most persistent high-GP region of migrating cells is the uropod, which is also characterized by GM1 labeling (Figures 1 and 4). Because GM1 is a marker for a subset of LRM, these highly ordered regions likely represent the distribution of these LRM (2, 14). There is extensive evidence that many signaling intermediates involved in cellular migration, most notably PI-3 kinase, PIP3, G proteins, GTPases, and tyrosine kinases, reside at least transiently in LRMs (2, 11–15). LRMs are also enriched in cytoskeletal linker proteins and are therefore thought to provide a nexus between signaling and actin reorganization during cell movement. The distribution of high-GP regions indicates far more spatial heterogeneity than indicated by images of LRM markers (Figures 1 and 4). In part, this may reflect the correction for varying signal intensity caused by cell shape or membrane convolutions inherent in ratiometric GP calculations. Second, LRM associations are not absolute, so one may expect that small amounts of putative LRM constituents within nonraft membrane may compromise the resolution with which LRMs are localized. Another important consideration in reconciling marker imaging to direct visualization of membrane order relates to very nature of LRMs. For all the controversies regarding their content, the sine qua non of LRMs is that they are regions with highly ordered lipids, and membrane order itself is a potentially important determinant of plasma membrane function. The Lo phase confers greater membrane rigidity, which, if distributed heterogeneously, would be expected to affect the shape and motility of a cell (27). Furthermore, the function of some proteins is affected by their ability to bind sphingolipids, and the high lateral pressure within Lo domains could dramatically affect the conformation of imbedded proteins (27). Therefore, it may be important to look beyond the specific content of LRMs and consider the possibility that, in some instances, regional variations in membrane order could impose functional heterogeneity on otherwise uniformly distributed membrane proteins.
Rapid, sequential imaging of live PMNs revealed that GP at the uropod varies substantially and that GP values oscillate with periodicities of approximately 37 and 150 seconds (Table 1). This cyclic variation in plasma membrane organization is consistent with other features of PMNs that have been shown to fluctuate with regular spatiotemporal patterns, including oxidant release, Ca2+ signals, cell shape, membrane potential, and metabolism (28–30). Some of these fluctuations are similar in periodicity to those reported in this study. Previous work on the dynamics of LRM organization has led to conflicting conclusions. Although some researchers have conceptualized LRMs as being relatively stable structures, there is evidence to support the opposing view that LRMs must be extremely small and exchange proteins easily with non-LRM membrane (31–33). Indeed, LRMs may be as small as a few protein molecules and less than 100 lipid molecules (34). Nonetheless, LRMs are too large to laterally diffuse within membranes at the time scale necessary to account for the observed changes in GP levels. On the other hand, the rapid redistribution of LRMs could be mediated by tethers to a rapidly remodeling cytoskeleton.
One need not rely only on the movement of quasi-stable LRM structures to explain variations in membrane order. Because the rate of lipid diffusion in PMN membranes is much greater than that of proteins and protein clusters (35), the time scale of the changes in GP at the uropod may be explained by the reorganization of lipid molecules but is unlikely to be explained by the diffusion of membrane proteins. One ramification of this is that some LRM components may be relatively stationary in the membrane, whereas the membrane order varies substantially due to lipid association and dissociation. Additional factors that may influence the density of lipid packing are (1) changes in lipid composition by the exchange of lipids with the intracellular compartment or by the actions of lipid modifying enzymes, (2) in situ modifications of LRM proteins, (3) LRM coalescence induced by formation of multiprotein complexes, or (4) spatially localized signaling events such as [Ca2+] fluxes, which influence sphingolipid and phosphatidylserine packing and membrane fusogenicity (13, 34, 36, 37). Because the uropod is rich in oscillating Ca2+ signals (38), this could also be a contributor to enhanced membrane order in this region. All of these possibilities are areas for further investigation.
Despite the considerable evidence that many LRM constituents are necessary for PMN migration, there are comparatively little data describing how LRM disorganization perturbs PMN movement. Pierini and colleagues found that MβCD rendered PMNs virtually immobile over a short observation period (21). Similarly, Bodin and Welch demonstrated that cholesterol depletion of differentiated HL-60 cells suppressed migration predominantly by decreasing cell velocity, with little effect on cellular orientation (39). These studies support our findings that MβCD interferes with PMN chemotaxis, but there are important distinctions as well. First, we quantified directionally biased migration over a long observation period (30 min) by measuring net displacement of large populations of PMNs, as opposed to demonstrating orientation toward a chemotaxin at a single time point or measuring the proportion of morphologically polarized cells. Second, we did not adhere PMNs to matrix proteins, whereas other researchers used fibronectin-coated surfaces where the influence of LRMs on integrin function may have added importance (11, 21, 39, 40). It is likely that the effects of LRM disruption on PMN migration are specific to the precise experimental conditions, including the cell model, degree of cholesterol depletion, and adhesive surface. It is possible that MβCD has other unintended effects on PMN function, but a cyclodextrin effect unrelated to cholesterol extraction is unlikely because a similar compound, α-CD, had no effect on PMN migration. Because the only established methods for acutely disrupting LRM organization in live cells involve extraction or binding of cholesterol, we cannot exclude the possibility that loss of membrane cholesterol affects PMN migration by mechanisms other than LRM disruption. In addition, our data do not address the question of what constitutes LRM disruption. Although the demonstrable effect of MβCD is reduced cholesterol content and membrane order, it may be loss or dissipation of LRM-associated signaling molecules, or disassembly of signaling platforms, rather than changes in membrane order per se, that affected biased migration.
Although we have yet to identify the specific mechanisms that account for the loss of directional bias, undoubtedly many will be found to influence such a complex process. Nonetheless, certain novel conclusions can be drawn. First, MβCD affected FMLP- and LTB4–induced chemotaxis in virtually identical fashion (Figures 5 and 6). We showed previously that formyl peptide receptor is a non-LRM protein and that FMLP-induced Ca2+ mobilization and ERK1/2 phosphorylation are unaffected by MβCD, whereas BLT-1 partitions to LRMs, and MβCD blocks the ability of LTB4 to trigger the same activation signals (15). This indicates that LRM disruption does not block directional migration by selectively interfering with chemotaxin receptors located within LRMs and suggests a mechanism further downstream. Also, the small effect on cell velocity argues that LRMs disruption primarily affects directionality of movement rather than a fundamental effect on motility. Many membrane constituents involved in adhesion and locomotion may be found in LRMs, but they are not sufficiently sensitive to cholesterol content or membrane order that MβCD rendered the PMNs immobile. These effects of MβCD were reversible; the cells regained normal polarized morphology at 30 minutes, when membrane cholesterol would be reconstituted. This model should be useful for studying chemotaxin gradient recognition because directional bias can be disrupted selectively.
The “compass” mechanisms known to enable PMN orientation to a chemotactic gradient can only be summarized briefly here, but this complex system involves a spatial strategy whereby weak asymmetries in external signal intensity are greatly amplified into steep internal signaling gradients (4, 25, 39, 41). Prominent among these internal signals is PIP3 enrichment at the leading edge, leading to asymmetrical activation of the GTPases Rac 1/2 and Rho, which locally regulate actin polymerization and myosin function. More recently, a cooperative effect of PIP3 and phosphatidic acid in localizing Dock2 at the leading edge has also been demonstrated (6). In some instances, selectively ablating individual signaling pathways has not disrupted chemotaxis, suggesting redundant signaling, multiple isoforms sharing functions within a pathway, or subtle defects in chemotaxis that have escaped detection (4, 41). It is also possible that the function of these pathways is highly specific to individual or classes of chemotaxins, steep versus shallow chemotactic gradients, or other signals from the cellular microenvironment. For the purposes of the present study, it is important to emphasize that PI-3 kinase, PIP3, and Rho family GTPases have been associated with LRMs (2, 12, 39). The ability of LRM disruption to abolish the spatial asymmetry of Rac and Rho GTPases (Figure 4 and Ref. 39) and PIP3 (2, 39) illustrate this point. Consistent with the general affinity of signaling lipids for LRMs, these findings suggest that the spatial distribution of LRMs contributes to the formation of internal signaling asymmetries. Furthermore, it is possible that the cyclic LRM reorganization demonstrated in the present study contributes to transient, repetitive signaling events that recalculate and maintain orientation along a gradient.
The morphologic changes induced by MβCD (Figure 7) were similar to those observed previously (2, 21, 39, 42). The most striking and consistent findings were the multiple pseudopods appearing simultaneously or in rapid succession and the reduced tendency to assume and maintain extensively elongated forms. The inability to sustain a single, defined leading edge seems adequate to explain the loss of directionally biased migration and suggests that normally organized LRMs function, in part, to focus and restrict membrane extension toward a chemotaxin.
In summary, we have adapted laurdan spectral-shift microscopy to demonstrate substantial regional heterogeneity of LRMs and rapid oscillations in membrane order at the uropods of migrating human PMNs. This work supports the existence of dynamically changing LRMs in situ and their contribution to morphologic polarization and directional movement toward chemotaxins.
Supplementary Material
This work was supported by NIH awards RO1 AI060983 (R.G.S.) and CA74120 (H.R.P.).
Originally Published in Press as DOI: 10. 1165/rcmb.2009-0286OC on November 20, 2009
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol 2003;35:1619–1638. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Mouton C, Lacalle RA, Mira E, Jimenez-Baranda S, Barber DF, Carrera AC, Martinez AC, Manes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol 2004;164:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell 2005;9:19–34. [DOI] [PubMed] [Google Scholar]
- 4.Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol 2008;9:455–463. [DOI] [PubMed] [Google Scholar]
- 5.El Alwani M, Wu BX, Obeid LM, Hannun YA. Bioactive sphingolipids in the modulation of the inflammatory response. Pharmacol Ther 2006;112:171–183. [DOI] [PubMed] [Google Scholar]
- 6.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, et al. Sequential regulation of Dock2 dynamics by two phospholipids during neutrophil chemotaxis. Science 2009;324:384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykstra M, Cherukuri A, Pierce SK. Rafts and synapses in the spatial organization of immune cell signaling receptors. J Leukoc Biol 2001;70:699–707. [PubMed] [Google Scholar]
- 8.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 2003;44:655–667. [DOI] [PubMed] [Google Scholar]
- 9.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J 2004;378:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishitsuka R, Sato SB, Kobayashi T. Imaging lipid rafts. J Biochem 2005;137:249–254. [DOI] [PubMed] [Google Scholar]
- 11.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol 2006;174:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem 2002;277:43399–43409. [DOI] [PubMed] [Google Scholar]
- 13.Kindzelskii A, Sitrin R, Petty H. Cutting edge: optical micro-spectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of human neutrophils: apparent role in calcium signaling. J Immunol 2004;172:4681–4685. [DOI] [PubMed] [Google Scholar]
- 14.Sitrin RG, Johnson DR, Pan PM, Harsh DM, Huang J, Petty HR, Blackwood RA. Lipid raft compartmentalization of urokinase receptor signaling in human neutrophils. Am J Respir Cell Mol Biol 2004;30:233–241. [DOI] [PubMed] [Google Scholar]
- 15.Sitrin RG, Emery SL, Sassanella TM, Blackwood RA, Petty HR. Selective localization of recognition complexes for leukotriene B4 and formyl-met-leu-phe within lipid raft microdomains of human polymorphonuclear neutrophils. J Immunol 2006;177:8177–8184. [DOI] [PubMed] [Google Scholar]
- 16.Jaksits S, Bauer W, Kriehuber E, Zeyda M, Stulnig TM, Stingl G, Fiebiger E, Maurer D. Lipid raft-associated GTPase signaling controls morphology and CD8+ T cell stimulatory capacity of human dendritic cells. J Immunol 2004;173:1628–1639. [DOI] [PubMed] [Google Scholar]
- 17.Gaus K, Gratton E, Kable EP, Jones AS, Gelissen I, Kritharides L, Jessup W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci USA 2003;100:15554–15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parasassi T, Gratton E, Zajicek H, Levi M, Yu W. Detecting membrane lipid microdomains by two-photon fluorescence microscopy. IEEE Eng Med Biol Mag 1999;18:92–99. [DOI] [PubMed] [Google Scholar]
- 19.Fessler MB, Arndt PG, Frasch SC, Lieber JG, Johnson CA, Murphy RC, Nick JA, Bratton DL, Malcolm KC, Worthen GS. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. J Biol Chem 2004;279:39989–39998. [DOI] [PubMed] [Google Scholar]
- 20.Spector AA. Fatty acid binding to plasma albumin. J Lipid Res 1975;16:165–179. [PubMed] [Google Scholar]
- 21.Pierini LM, Eddy RJ, Fuortes M, Seveau S, Casulo C, Maxfield FR. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem 2003;278:10831–10841. [DOI] [PubMed] [Google Scholar]
- 22.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci 2005;118:5205–5220. [DOI] [PubMed] [Google Scholar]
- 23.Seveau S, Eddy RJ, Maxfield FR, Pierini LM. Cytoskeleton-dependent membrane domain segregation during neutrophil polarization. Mol Biol Cell 2001;12:3550–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorini R, Curatola G, Kantar A, Giorgi PL, Gratton E. Use of laurdan fluorescence in studying plasma membrane organization of polymorphonuclear leukocytes during the respiratory burst. Photochem Photobiol 1993;57:438–441. [DOI] [PubMed] [Google Scholar]
- 25.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood 2006;108:2814–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Mouton C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez AC. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA 2001;98:9642–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niemela PS, Ollila S, Hyvonen MT, Karttunen M, Vattulainen I. Assessing the nature of lipid raft membranes. PLOS Comput Biol 2007;3:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jäger U, Gruler H, Bultmann B. Morphological changes and membrane potential of human granulocytes under influence of chemotactic peptide and/or echo-virus, type 9. Klin Wochenschr 1988;66:434–436. [DOI] [PubMed] [Google Scholar]
- 29.Omann GM, Porasik MM, Sklar LA. Oscillating actin polymerization/depolymerization responses in human polymorphonuclear leukocytes. J Biol Chem 1989;264:16355–16358. [PubMed] [Google Scholar]
- 30.Wymann MP, Kernen P, Deranleau DA, Baggiolini M. Respiratory burst oscillations in human neutrophils and their correlation with fluctuations in apparent cell shape. J Biol Chem 1989;264:15829–15834. [PubMed] [Google Scholar]
- 31.Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol 2004;6:238–243. [DOI] [PubMed] [Google Scholar]
- 32.Glebov OO, Nichols BJ. Distribution of lipid raft markers in live cells. Biochem Soc Trans 2004;32:673–675. [DOI] [PubMed] [Google Scholar]
- 33.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol 2004;165:735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 2004;33:269–295. [DOI] [PubMed] [Google Scholar]
- 35.Petty HR, Smith LM, Fearon DT, McConnell HM. Lateral distribution and diffusion of the C3b receptor of complement, HLA antigens, and lipid probes in peripheral blood leukocytes. Proc Natl Acad Sci USA 1980;77:6587–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep 2007;8:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahmann H, Schifferer F, Beitinger H. Calcium-ganglioside interactions and synaptic plasticity: effect of calcium on specific ganglioside/peptide (valinomycin, gramicidin a)-complexes in mixed mono- and bilayers. Neurochem Int 1992;20:323–338. [DOI] [PubMed] [Google Scholar]
- 38.Clark AJ, Petty HR. Observation of calcium microdomains at the uropod of living morphologically polarized human neutrophils using flash lamp-based fluorescence microscopy. Cytometry A 2008;73:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodin S, Welch MD. Plasma membrane organization is essential for balancing competing pseudopod- and uropod-promoting signals during neutrophil polarization and migration. Mol Biol Cell 2005;16:5773–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marwali MR, Rey-Ladino J, Dreolini L, Shaw D, Takei F. Membrane cholesterol regulates LFA-1 function and lipid raft heterogeneity. Blood 2003;102:215–222. [DOI] [PubMed] [Google Scholar]
- 41.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HRA. Ptdinsp(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol 2002;4:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niggli V, Meszaros AV, Oppliger C, Tornay S. Impact of cholesterol depletion on shape changes, actin reorganization, and signal transduction in neutrophil-like HL-60 cells. Exp Cell Res 2004;296:358–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.