Abstract
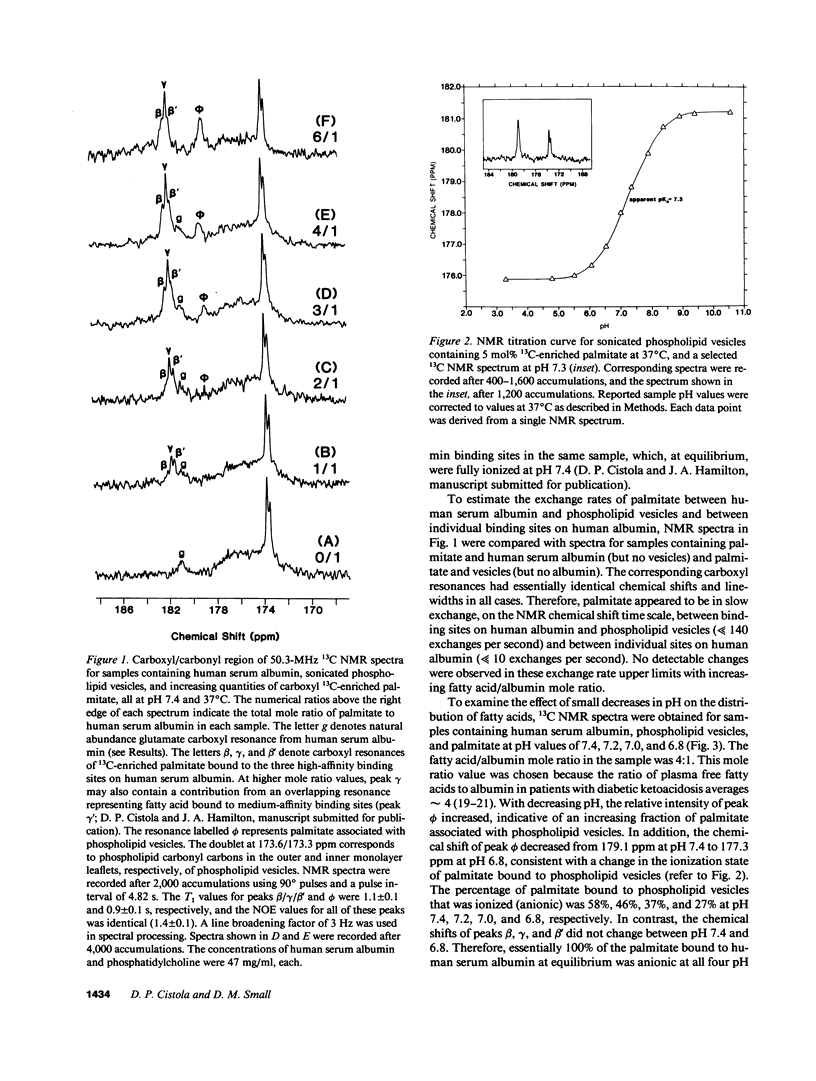
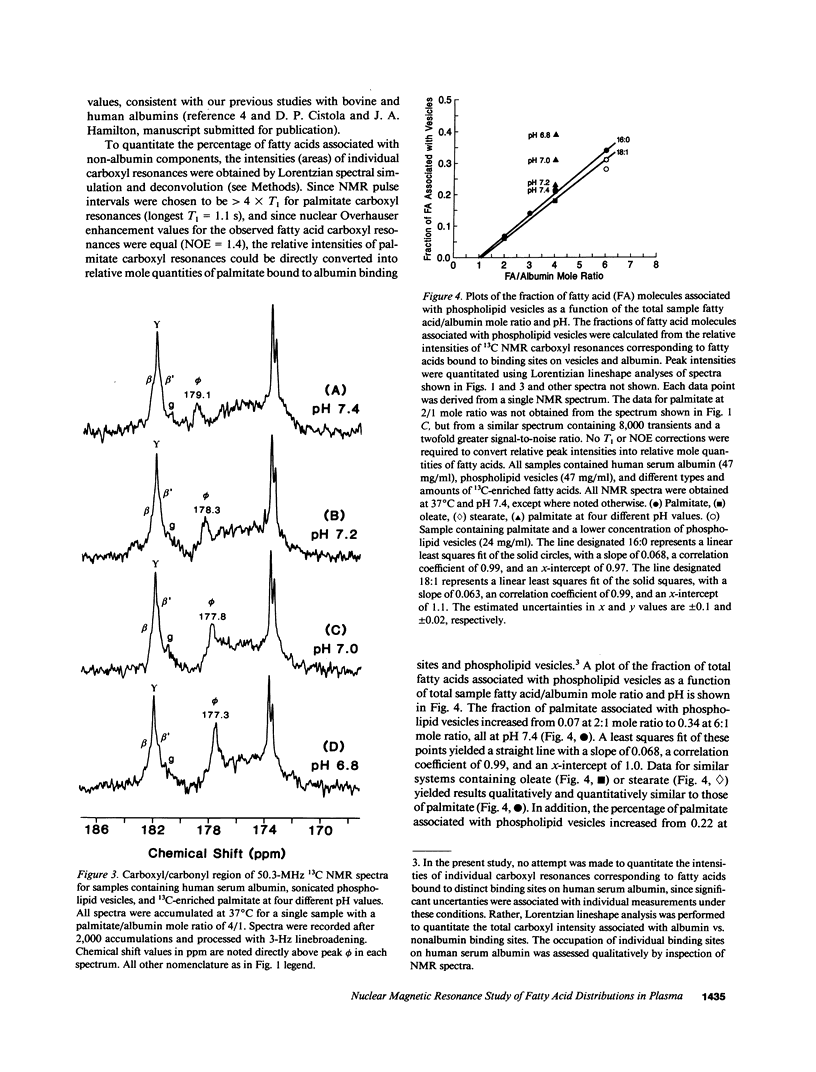
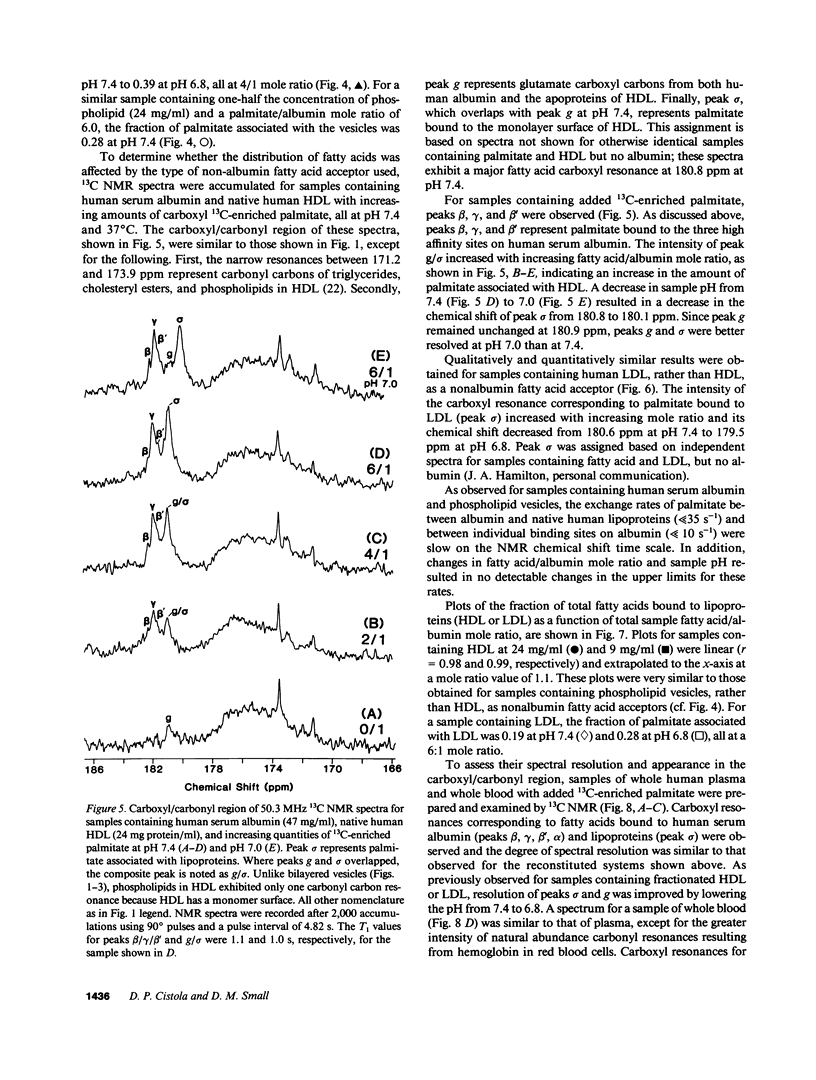
A nonperturbing 13C nuclear magnetic resonance (NMR) method was used to monitor the equilibrium distribution of carboxyl 13C-enriched fatty acids (FA) between distinct binding sites on human serum albumin, native human lipoproteins, and/or phospholipid model membranes, under conditions that mimic the normal and diabetic human circulation. Two variables pertinent to the diabetic circulation were examined: FA/albumin mole ratio (as elevated in insulin deficiency and/or nephrosis) and pH (as decreased in acidosis). 13C NMR spectra for samples containing carboxyl 13C-enriched palmitate, human serum albumin, and phospholipid vesicles or native lipoproteins (all samples at pH 7.4, 37 degrees C) exhibited up to six carboxyl NMR resonances corresponding to FA bound to distinct binding sites on albumin and nonalbumin components. When the sample FA/albumin mole ratio was 1, three FA carboxyl resonances were observed (182.2, 181.8, and 181.6 ppm; designated peaks beta, gamma, and beta', respectively). These resonances corresponded to FA bound to three distinct high-affinity binding sites on human serum albumin. When the sample mole ratio value exceeded 1, additional carboxyl resonances corresponding to FA bound to phospholipid vesicles (179.0 ppm, peak phi), lipoproteins (180.7 ppm, peak sigma), and lower affinity sites on albumin (183.8 ppm, peak alpha; 181.9 ppm, peak gamma'), were observed. The intensity of peaks phi and sigma increased with increasing mole ratio or decreasing pH. Using Lorentzian lineshape analysis, the relative mole quantities of FA bound to albumin and nonalbumin binding sites were determined. Plots of the fraction of FA associated with nonalbumin components as a function of FA/albumin mole ratio were linear and extrapolated to the abscissa at a mole ratio value of 1. This pattern of FA distribution was observed regardless of the type of nonalbumin acceptor used (phospholipid vesicles, human high- or low-density lipoproteins) or the type of FA used (palmitate, oleate, or stearate), and provided evidence for negative cooperativity for human serum albumin upon binding of 1 mol of FA per mole albumin. These in vitro NMR results suggest that the threshold FA/albumin mole ratio value for alterations in FA distributions in the human circulation may be 1, rather than 3, as previously held. The pathophysiological implications of these findings are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson E. O., Green F. A., Laurell S. Branching and hydrophobic bonding. Partition equilibria and serum albumin binding of palmitic and phytanic acids. J Biol Chem. 1971 Sep 10;246(17):5373–5379. [PubMed] [Google Scholar]
- BIERMAN E. L., DOLE V. P., ROBERTS T. N. An abnormality of nonesterified fatty acid metabolism in diabetes mellitus. Diabetes. 1957 Nov-Dec;6(6):475–479. doi: 10.2337/diab.6.6.475. [DOI] [PubMed] [Google Scholar]
- BOGDONOFF M. D., ESTES E. H., Jr, TROUT D. Acute effect of psychologic stimuli upon plasma non-esterified fatty acid level. Proc Soc Exp Biol Med. 1959 Mar;100(3):503–504. doi: 10.3181/00379727-100-24676. [DOI] [PubMed] [Google Scholar]
- Baker L., Barcai A., Kaye R., Haque N. Beta adrenergic blockade and juvenile diabetes: acute studies and long-term therapeutic trial. Evidence for the role of catecholamines in mediating diabetic decompensation following emotional arousal. J Pediatr. 1969 Jul;75(1):19–29. doi: 10.1016/s0022-3476(69)80097-1. [DOI] [PubMed] [Google Scholar]
- Bihain B. E., Deckelbaum R. J., Yen F. T., Gleeson A. M., Carpentier Y. A., Witte L. D. Unesterified fatty acids inhibit the binding of low density lipoproteins to the human fibroblast low density lipoprotein receptor. J Biol Chem. 1989 Oct 15;264(29):17316–17321. [PubMed] [Google Scholar]
- Cistola D. P., Atkinson D., Hamilton J. A., Small D. M. Phase behavior and bilayer properties of fatty acids: hydrated 1:1 acid-soaps. Biochemistry. 1986 May 20;25(10):2804–2812. doi: 10.1021/bi00358a011. [DOI] [PubMed] [Google Scholar]
- Cistola D. P., Hamilton J. A., Jackson D., Small D. M. Ionization and phase behavior of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 1988 Mar 22;27(6):1881–1888. doi: 10.1021/bi00406a013. [DOI] [PubMed] [Google Scholar]
- Cistola D. P., Sacchettini J. C., Banaszak L. J., Walsh M. T., Gordon J. I. Fatty acid interactions with rat intestinal and liver fatty acid-binding proteins expressed in Escherichia coli. A comparative 13C NMR study. J Biol Chem. 1989 Feb 15;264(5):2700–2710. [PubMed] [Google Scholar]
- Cistola D. P., Small D. M., Hamilton J. A. Carbon 13 NMR studies of saturated fatty acids bound to bovine serum albumin. I. The filling of individual fatty acid binding sites. J Biol Chem. 1987 Aug 15;262(23):10971–10979. [PubMed] [Google Scholar]
- Cistola D. P., Small D. M., Hamilton J. A. Carbon 13 NMR studies of saturated fatty acids bound to bovine serum albumin. II. Electrostatic interactions in individual fatty acid binding sites. J Biol Chem. 1987 Aug 15;262(23):10980–10985. [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. S. The interaction of human erythrocytes with sodium palmitate. J Clin Invest. 1958 Dec;37(12):1729–1735. doi: 10.1172/JCI103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Martin M. M., Recant L. Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Diabetes. 1971 Apr;20(4):228–238. doi: 10.2337/diab.20.4.228. [DOI] [PubMed] [Google Scholar]
- Gjesdal K., Nordoy A., Wang H., Berntsen H., Mjos O. D. Effects of fasting on plasma and platelet-free fatty acids and platelet function in healthy males. Thromb Haemost. 1976 Nov 30;36(2):325–333. [PubMed] [Google Scholar]
- Gurd F. R., Keim P. The prospects for carbon-13 nuclear magnetic resonance studies in enzymology. Methods Enzymol. 1973;27:836–911. doi: 10.1016/s0076-6879(73)27037-4. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., NAIMARK A., BORCHGREVINK C. F. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-C14. J Clin Invest. 1963 Jul;42:1054–1063. doi: 10.1172/JCI104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Cistola D. P. Transfer of oleic acid between albumin and phospholipid vesicles. Proc Natl Acad Sci U S A. 1986 Jan;83(1):82–86. doi: 10.1073/pnas.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Small D. M. Solubilization and localization of triolein in phosphatidylcholine bilayers: a 13C NMR study. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6878–6882. doi: 10.1073/pnas.78.11.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Talkowski C., Williams E., Avila E. M., Allerhand A., Cordes E. H., Camejo G. Natural abundance carbon-13 nuclear magnetic resonance spectra of human serum lipoproteins. Science. 1973 Apr 15;180(4082):193–195. doi: 10.1126/science.180.4082.193. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Ekelund L. G., Holmgren A. Kinetic analysis of the oxidation of palmitate-1-14C in man during prolonged heavy muscular exercise. J Lipid Res. 1967 Jul;8(4):366–373. [PubMed] [Google Scholar]
- Hawley H. P., Gordon G. B. The effects of long chain free fatty acids on human neutrophil function and structure. Lab Invest. 1976 Feb;34(2):216–222. [PubMed] [Google Scholar]
- Hennig B., Shasby D. M., Fulton A. B., Spector A. A. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984 Sep-Oct;4(5):489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- Hoak J. C., Spector A. A., Fry G. L., Warner E. D. Effect of free fatty acids on ADP-induced platelet aggregation. Nature. 1970 Dec 26;228(5278):1330–1332. doi: 10.1038/2281330a0. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Adams P. W., Wynn V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia. 1976 Dec;12(6):563–571. doi: 10.1007/BF01220632. [DOI] [PubMed] [Google Scholar]
- Kurien V. A., Oliver M. F. Serum-free-fatty-acids after acute myocardial infarction and cerebral vascular occlusion. Lancet. 1966 Jul 16;2(7455):122–127. doi: 10.1016/s0140-6736(66)92420-2. [DOI] [PubMed] [Google Scholar]
- LAURELL S. Plasma free fatty acids in diabetic acidosis and starvation. Scand J Clin Lab Invest. 1956;8(1):81–82. doi: 10.3109/00365515609049249. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Pjura W. J., Kleinfeld A. M., Karnovsky M. J. Partition of fatty acids and fluorescent fatty acids into membranes. Biochemistry. 1984 Apr 24;23(9):2039–2043. doi: 10.1021/bi00304a024. [DOI] [PubMed] [Google Scholar]
- Reitsma W. D. The relationship between serum free fatty acids and blood sugar in non-obese and obese diabetics. Acta Med Scand. 1967 Sep;182(3):353–361. doi: 10.1111/j.0954-6820.1967.tb11535.x. [DOI] [PubMed] [Google Scholar]
- SHAFRIR E., GATT S., KHASIS S. PARTITION OF FATTY ACIDS OF 20-24 CARBON ATOMS BETWEEN SERUM ALBUMIN AND LIPOPROTEINS. Biochim Biophys Acta. 1965 Apr 5;98:365–371. doi: 10.1016/0005-2760(65)90129-3. [DOI] [PubMed] [Google Scholar]
- SHAFRIR E. Partition of unesterified fatty acids in normal and nephrotic syndrome serum and its effect on serum electrophoretic pattern. J Clin Invest. 1958 Dec;37(12):1775–1782. doi: 10.1172/JCI103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]
- Schmidt C. F., Barenholz Y., Huang C., Thompson T. E. Phosphatidylcholine 13C-labeled carbonyls as a probe of bilayer structure. Biochemistry. 1977 Sep 6;16(18):3948–3954. doi: 10.1021/bi00637a002. [DOI] [PubMed] [Google Scholar]
- Shindo H., Cohen J. S. Observation of individual carboxyl groups in hen egg-white lysozyme by use of high field 13C-nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1979–1983. doi: 10.1073/pnas.73.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Barclay M., Barclay R. K., Fetzer V. A., Good J. J., Archibald F. M. Lipid composition of human serum lipoproteins. Biochem J. 1967 Aug;104(2):340–352. doi: 10.1042/bj1040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Ashbrook J. D., Santos E. C., Fletcher J. E. Quantitative analysis of uptake of free fatty acid by mammalian cells: lauric acid and human erythrocytes. J Lipid Res. 1972 Jul;13(4):445–451. [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Spector A. A. Influence of pH of the medium on free fatty acid utilization by isolated mammalian cells. J Lipid Res. 1969 Mar;10(2):207–215. [PubMed] [Google Scholar]
- Spector A. A. Influence of pH of the medium on free fatty acid utilization by isolated mammalian cells. J Lipid Res. 1969 Mar;10(2):207–215. [PubMed] [Google Scholar]
- Taggart P., Carruthers M. Endogenous hyperlipidaemia induced by emotional stress of racing driving. Lancet. 1971 Feb 20;1(7695):363–366. doi: 10.1016/s0140-6736(71)92207-0. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Bogardus C., Kennedy A., Howard B. V. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985 Aug;76(2):637–644. doi: 10.1172/JCI112016. [DOI] [PMC free article] [PubMed] [Google Scholar]


