Abstract
Voltage-dependent anion channel (VDAC) has been suggested to be a mediator of mitochondrial-dependent cell death induced by Ca2+ overload, oxidative stress and Bax-Bid activation. To confirm this hypothesis in vivo, we generated and characterized Drosophila VDAC (porin) mutants and found that Porin is not required for mitochondrial apoptosis, which is consistent with the previous mouse studies. We also reported a novel physiological role of Porin. Loss of porin resulted in locomotive defects and male sterility. Intriguingly, porin mutants exhibited elongated mitochondria in indirect flight muscle, whereas Porin overexpression produced fragmented mitochondria. Through genetic analysis with the components of mitochondrial fission and fusion, we found that the elongated mitochondria phenotype in porin mutants were suppressed by increased mitochondrial fission, but enhanced by increased mitochondrial fusion. Furthermore, increased mitochondrial fission by Drp1 expression suppressed the flight defects in the porin mutants. Collectively, our study showed that loss of Drosophila Porin results in mitochondrial morphological defects and suggested that the defective mitochondrial function by Porin deficiency affects the mitochondrial remodeling process.
Introduction
Mitochondria undergo mitochondrial membrane permeability transition (MPT) after opening of a channel called mitochondrial permeability transition pore (PTP) when cells succumb to apoptosis [1]. PTP is a site for cytochrome c release, which then leads to caspase activation and cell death. PTP is minimally composed of three proteins, VDAC (in the outer membrane), the adenine nucleotide translocase (ANT, in the inner membrane) and cyclophilin D (CypD, in the matrix). These PTP components have been initially proposed to be critically involved in mitochondrial cell death induced by Ca2+ overload, oxidative stress and Bax-Bid activation. However, recent studies have demonstrated that VDAC and ANT are not essential for MPT in cell death but only cyclophilin D required [2]–[4]. Therefore, the physiological role of VDAC and ANT remains questionable.
There are three VDAC isoforms in mammals, but in Drosophila four isoforms have been identified homologous to VDAC [5]–[6]. Among them, porin (CG6647) and porin2 (CG17137) show a higher similarity to mammalian VDAC (porin, about 58% identity; porin2, about 34% identity), whereas the two others, CG17139 and CG17149, show about 24% identity and 21% identity, respectively. Previous Drosophila Porin protein studies have demonstrated that Porin and Porin2 have similar functions because both proteins rescued the conditional lethal phenotype of yeast VDAC mutants [5]. Interestingly, gene expression studies have shown that Porin is ubiquitous in all body segments, whereas Porin2 is detected prominently in sperm tissue [6]–[8]. Therefore, Porin may play a general role in many different tissues.
Mitochondria constantly move to specific subcellular locations where high energy is demanded by undergoing morphological changes through mitochondrial fusion and fission [9]. These processes have been first identified in yeast, and several genes have been found conserved in mammals; mitofusin 1 (mfn1) and mitofusion 2 (mfn2), both encoding GTPases required for the outer membrane fusion, optic atrophy 1 (opa1), a GTPase for the inner membrane fusion, and dynamin-related protein 1 (drp1), a GTPase for mitochondrial fission. In Drosophila, the two homologs of mfn are fuzzy onion (fzo) and mitochondrial assembly regulatory factor (marf). fzo mutants show defects in mitochondrial fusion during spermatogenesis and knockdown of Marf induces mitochondrial fission in indirect flight muscle [10]–[11]. Recent studies have also demonstrated that Drosophila opa1 and drp1 mutants show defects in mitochondrial morphology [12]–[13].
To investigate the physiological role of Porin, we generated porin null mutants and characterized them in Drosophila. Our studies showed that loss of porin results in elongated mitochondria and Porin overexpression induces small mitochondria. Through genetic interaction analysis between Porin and mitochondrial fusion and fission regulators, we revealed that loss of porin leads to defective mitochondrial remodeling processes in Drosophila.
Results
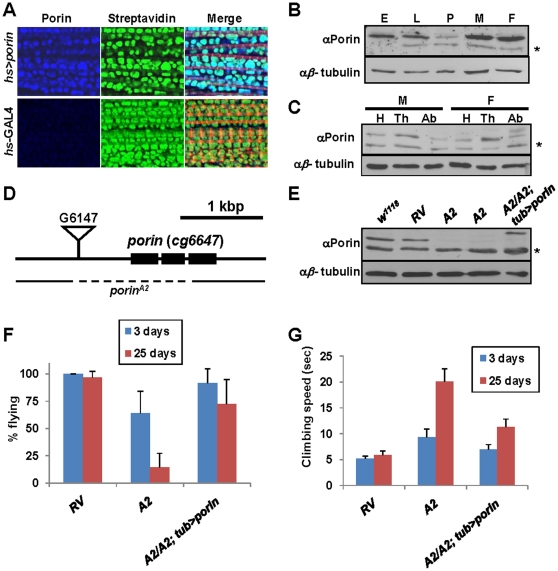
We first examined whether Porin protein localizes to the mitochondria. Through immunohistochemistry with indirect flight muscles of flies, Porin was mostly detected in the mitochondria (Figure 1A). We then examined the developmental and spatial expression patterns for Porin by performing immunoblot analysis. Porin protein was detected in all developmental stages (Figure 1B). Its expression in adults was ubiquitous throughout all segments, head, thorax and abdomen (Figure 1C). These results are consistent with the previous studies [6].
Figure 1. Characterization of Drosophila porin loss-of-function mutants.
(A) Mitochondrial localization of Porin. Subcellular localization of Flag-tagged Porin (blue) in indirect flight muscle. Streptavidin (green), and phalloidin (red) were used to mark mitochondria, and muscle fiber respectively. (B) Developmental expression pattern of Drosophila Porin. Immunoblot analysis of Porin from different developmental stages of wild type flies. Anti-Porin rabbit antibody binds specifically to the wild type samples, but not to porin null mutants (shown in Fig. 1E). E, embryo; L, larva; P, pupa; M, male; F, female. n = 3. * indicates a non-specific band. (C) Porin expression in head (H), thorax (Th) and abdomen (Ab) of adult flies of each sex (M, male; F, female). n = 3. (D) Genomic region of porin locus. The exons of porin gene are depicted as black boxes. The inverted triangle in front of porin gene is the P element used for imprecise excision. The deleted region for porinA2 allele is indicated. (E) Verification of Drosophila porin mutants through immunoblot analysis. Porin protein is absent in porin null mutants (A2). (F) Comparison of the flight ability. n = 10. S.D. for three independent experiments. (G) Comparison of the climbing rate. n = 10. S.D. for three independent experiments.
To investigate the in vivo function of Porin in Drosophila, we determined to generate porin loss-of-function mutants by mobilizing the P-element G6147 inserted near the porin locus (Figure 1D). Through P-element imprecise excision, a porin-deleted allele was isolated and named porinA2 (A2, Figure 1D). This allele has a 1,381bp-deletion, removing the start codon of translation and the first two exons, confirmed by genomic DNA-PCR analysis and DNA sequencing (data not shown). We also generated porin revertant line (porinRV, RV) to use as a control. To further assess whether Porin expression is completely eliminated in the porin homozygous mutants, immunoblot analysis was performed and confirmed that the allele is null for porin (Figure 1E).
The homozygous porin mutants were viable and the adults seemed normal after eclosion, which is consistent with previous studies [14]. However, they started to show defects in their behaviors such as slow climbing ability against geotaxis and flying disability at the age of 20 days and then died within 30 days (Figure 1F, 1G, and Figure S1, respectively).
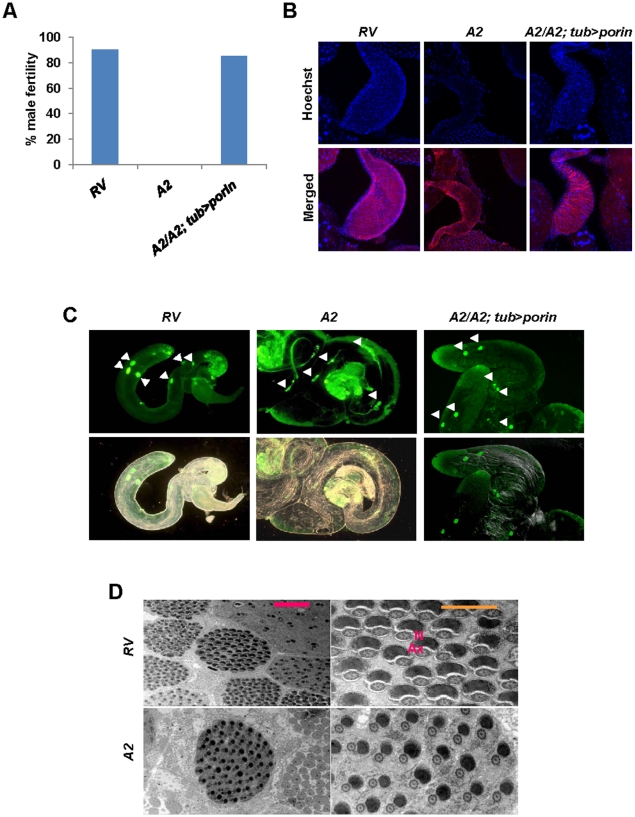
Homozygous female porin mutants were fertile, but male mutants were sterile (data not shown and Figure 2A). We observed the testis of porin mutants (A2), and the revertants (RV) by co-staining with Hoechst to mark sperms and phalloidin for the overall organ. As a result, we could not detect any mature sperms in the pouch of porin mutants called vas deferens, which is connected to the anterior ejaculatory duct, and this phenotype was rescued by ectopic porin expression (Figure 2B). To further investigate the cause of sterility of porin mutants, we assessed each stage of spermatogenesis development. In Drosophila testis, stem cells on the tip of the testis undergo differentiation and then go into rounds of mitotic division followed by meiosis, thereby producing syncytial cysts of 64 spermatids [15]. All these early developmental events seemed normal in porin mutants (Figure S2A). Therefore, we looked for defects by further observing the stages after post-meiosis; the interconnected 64 spermatids must undergo axoneme assembly, spermatid elongation, individualization, and coiling [15]. Through phase-contrast microscopy, we were able to find elongated spermatids (data not shown). However, immunostaining analysis showed that porin mutants have defects in spermatid individualization (Figures 2C and S2B). To understand the phenotype, we performed transmission electron microscopy (TEM) analysis with the spermatids. Interestingly, the mitochondria (N, nebenkern) in the wild type were fully condensed near axnome (Ax), but the mitochondria in the mutants were less condensed (Figure 2D). These results suggested that the defects in sperm individualization of porin mutants are related to mitochondrial dysfunction.
Figure 2. Sperm individualization defects in porin mutants.
(A) Comparison of fertility of males of indicated genotypes. n = 30. (B) Mature sperms determined by staining Hoechst33258 in the pouch vas deferens in 3-day-old male testis. Co-immunostaining with phalloidin (red) was performed to show the overall morphology of the pouch. porin mutants do not produce mature sperms whereas the control flies show linear morphology of nucleus of mature sperms. (C) Defects in the morphology of cystic bulges (CBs, white arrows) and waste bags (WBs) in porin mutants determined by acridine orange (AO) staining of testes. The control and the ectopic porin expression in porin mutants show intact CBs/WBs, but porin mutants show long-tailed signals. (D) TEM analysis of syncytial cyst of 64-spermatids. Red scale bar, 2 µm; orange scale bar, 1 µm. Ax, axoneme; N, nebenkern.
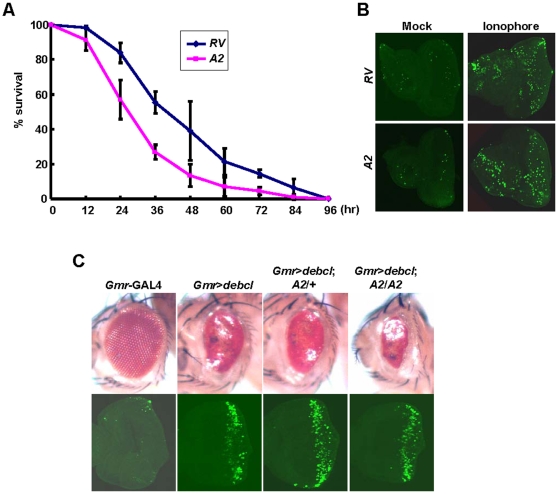
Since Porin was previously considered as an essential factor for MPT in cell death, we first tested this hypothesis using our Drosophila porin mutants. If Porin plays a role in promoting cell death induced by oxidative stress, Ca2+ overload and Bax activation, porin null mutants should be resistant to apoptosis following these toxic treatments. However, we found that Porin has no role in this process in vivo as we have obtained the following results. We investigated the effects of oxidative stress using paraquat on the survival of porin mutants. Flies were reared in 20 mM paraquat agar-contained vial and their survival was checked every 12 hrs. We found that porin mutants (A2) die at a rate similar to that of the revertants (RV) or a little faster (Figure 3A), suggesting that porin mutants are not resistant to oxidative stress. We further investigated whether porin null mutants are resistant to ionophore-induced cell death. The eye discs of porin mutants were incubated in A23187-containing M3 media for 4 hrs and observed for apoptosis by performing TUNEL assay. We found that the eye discs of porin mutants (A2) showed apoptosis as much as that of the revertants (RV) (Figure 3B), suggesting that Porin is not required for cell death mechanism induced by this stimulation. It has been also proposed that pro-apoptotic members of the Bcl-2 protein family such as Bax could promote MPT-induced cell death by binding to VDAC [16]. Overexpression of Debcl (Drosophila Bax) induced smaller eye phenotypes (Figure 3C). However, this phenotype was not rescued under heterozygous nor homozygous porin null background (Figure 3C), suggesting that Porin is not required for Bax-induced cell death. We also performed TUNEL assay to assess apoptosis in the eye discs of the mutants and found that apoptosis induced by Debcl overexpression was not inhibited by porin deletions (Figure 3C). Collectively, our results demonstrated that Porin is dispensable for cell death induced by oxidative stress, Ca2+ overload or Bax activation. These results are fully consistent with the previous VDAC mouse studies [2].
Figure 3. Porin is not resistant to oxidative stress, ionophore or Bax-induced cell death.
(A) Graph of % survival of 20mM paraquat-fed flies counted every 12 hr. n = 100. S.D. for three independent experiments. (B) TUNEL staining of the eye discs of indicated genotypes after incubation with ionophore. At least three independent experiments were performed. (C) Microscopy images of the eyes (upper panels) and TUNEL staining of the eye discs (lower panels) of indicated genotypes. Details of all indicated genotypes are described in Supporting Information File S1.
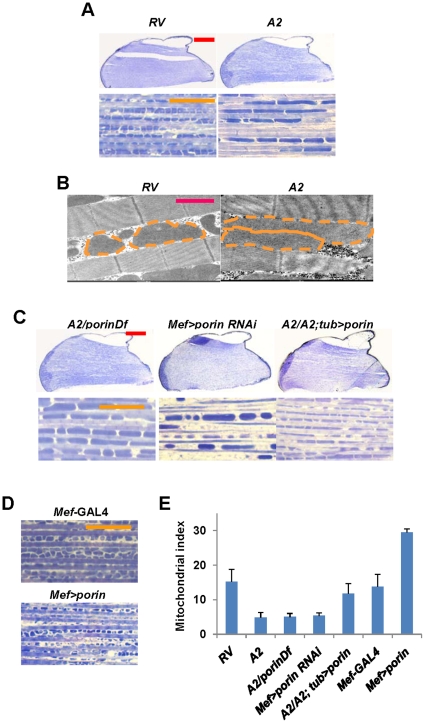
To reveal a novel role of Porin in vivo, we further characterized porin mutants. Because mitochondrial dysfunction was evident in the sperm of porin mutants, we assessed the mitochondrial morphology in another tissue, indirect flight muscle, where mitochondria could be easily observed. Through muscle sections of the thoraces embedded in Spurr's resin followed by coloring with toluidine blue, dark blue mitochondria were visible between the muscle fiber bands in light blue color in control flies (Figures 4A and 4E). However, it was surprising to see that the sections of porin mutants showed elongated tube-like mitochondria between the muscle fibers (Figures 4A and 4E). This result was further supported by examining the indirect flight muscle of porin mutants using TEM analysis. Indeed, porin mutants showed electron-dense elongated mitochondria in between the muscle fibers (Figure 4B). Moreover, porinA2 (A2) allele crossed to a porin-deficiency line (porinDf) showed the identical phenotype (Figures 4C and 4E). We further confirmed this phenotype by generating a porin RNAi line, and overexpression of porin RNAi by muscle specific mef-GAL4 driver led to similar phenotypes (Figures 4C and 4E). In addition, expression of Porin under tubulin-GAL4 (tub-GAL4) significantly rescued the porin mutant phenotype (Figures 4C and 4E), suggesting that the defects in mitochondrial morphology were caused by loss of porin gene. We then investigated whether overexpression of Porin produces the opposite phenotype. As expected, we found smaller mitochondria in between the muscle fibers in Porin-overexpressed indirect flight muscles (Figures 4D, 4E, and 5A). Collectively, these results suggest that Porin is important for maintaining mitochondrial morphology.
Figure 4. Porin mutants show defects in mitochondrial morphology.
(A) Longitudinally sectioned thorax images stained with toluidine blue. Red scale bar, 200 µm; orange scale bar, 20 µm. (B) TEM analysis of mitochondria in the indirect flight muscle of 3-day-old males. Red scale bar, 1 µm. Orange dotted line was drawn to show the mitochondrial size clearly. (C and D) Longitudinally sectioned thorax images stained with toluidine blue. Details of all indicated genotypes are described in Supporting Information File S1. (E) Quantification of the number of mitochondria within 50µm between two muscle fibers (mitochondrial index) shown in (A), (C), and (D). n>10.
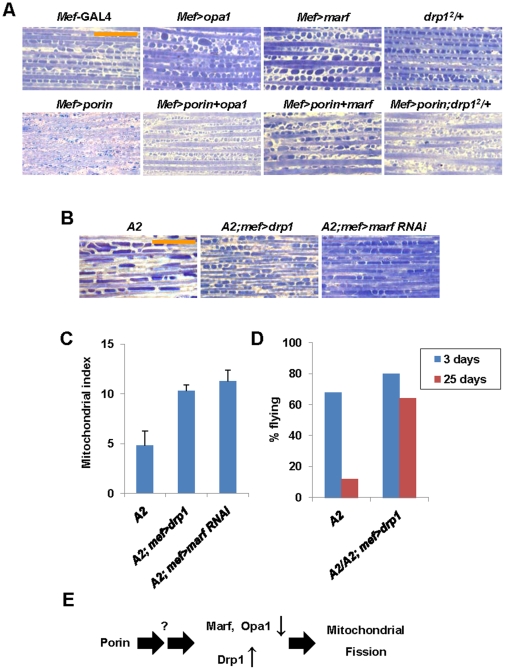
Figure 5. Genetic interaction analyses between Porin and the components involved in mitochondrial remodeling process.
(A and B) Longitudinally sectioned thorax images stained with toluidine blue. Orange scale bar, 20 µm. Details of all indicated genotypes are described in Supporting Information File S1. (C) Quantification of the number of mitochondria within 50µm between two muscle fibers (mitochondrial index) shown in (B). n>10. (D) Comparison of the flight ability shown in (B). n = 50. S.D. for three independent experiments. (E) A schematic illustration showing the function of Porin in mitochondrial fusion and fission proteins.
Because the changes in the level of Porin protein affect mitochondrial morphology in the indirect flight muscle, we thought that Porin might be involved in mitochondrial remodeling process. To further provide evidence to support our hypothesis, we conducted genetic interaction analysis between Porin and mitochondrial fusion and fission proteins. First, we tested whether the mitochondrial fission phenotype induced by Porin overexpression is altered by mitochondrial fusion and fission molecules. We therefore obtained several loss-of-function mutants or deficiency lines of drp1, opa1 and marf and generated UAS-drp1, UAS-opa1 and UAS-marf transgenic lines.
First, we tested the genetic interaction between Porin and Opa1. A heterozygous mutation of opa1 induced smaller mitochondria than those of wild type (Figures S3A and S4A), and overexpression of Opa1 results in larger mitochondria (Figures 5A and S4A). Coexpression of Opa1 significantly rescued the Porin overexpression phenotype (Figures 5A and S4A), but a heterozygotic mutation of opa1 further enhanced the phenotype induced by Porin expression (Figures S3A and S4A). Similarly, Marf overexpression resulted in a similar phenotype to that of Opa1 overexpression (Figures 5A and S4B), whereas flies with marf heterozygotic background showed mitochondrial size similar to, albeit smaller than, those of wild type flies (Figures S3B and S4B). The fragmented mitochondrial phenotype induced by Porin overexpression was suppressed by Marf overexpression (Figures 5A and S4B), but this phenotype was enhanced in a marf heterozygotic background (Figures S3B and S4B). In addition, coexpression of Drp1 and Porin showed fragmented mitochondria, whereas overexpression of Drp1 alone showed an almost normal mitochondrial morphology (Figures S3C and S4C). Overexpression of Porin with a heterozygotic mutation of drp1 produced smaller mitochondria or almost completely abolished the mitochondria (Figures 5A and S4C).
To further prove the role of Porin in mitochondria, we examined whether elongated mitochondria in porin null mutants are altered by increasing the level of mitochondrial fission genes. Expectedly, homozygous porinA2 (A2) phenotype was strongly suppressed by Drp1 overexpression or Marf knockdown (Figures 5B and 5C) and the flight disability in porin mutants was rescued by Drp1 overexpression (Figure 5D). These results suggested that Porin affects mitochondrial remodeling, and that the defective mitochondrial function by Porin deficiency appears to promote mitochondrial fusion or inhibit mitochondrial fission.
Discussion
Mitochondria are dynamic organelles, which constantly fuse and divide and move to specific subcellular locations where energy demands are high [17]. Controlling mitochondrial remodeling process is not only important for maintaining mitochondrial morphology but also for mitochondrial functions, which may determine the cellular activity and survival [17]. However, there is not much known about how this event is regulated or is related to apoptosis, especially MPT.
Recently, researchers studying the function of PTP components have questioned about the interaction between mitochondrial fusion and fission and the MPT components [18]. To address the question, we generated VDAC, one of the core components of PTP, null flies, but found that Porin is dispensable for oxidative stress-, Ca2+ overload- or Bax activation-induced cell death (Figure 3). Rather, we now suggest a novel role of VDAC in mitochondrial remodeling process. Overexpression of Drosophila Porin induced mitochondrial fission, whereas its loss-of-function resulted in mitochondrial fusion in muscle tissues (Figure 4). Furthermore, Drosophila Porin showed strong genetic interactions with known mitochondrial fusion and fission proteins. Particularly with expression of Drp1, not only we found a significant rescue of the mitochondrial morphology in the indirect flight muscle of porin mutants but also in their flight ability (Figures 5B and 5D). Similar mitochondrial phenotypes were also shown in the previous studies with VDAC1 null mice [3], which further support our results. Most recently, another group has published a paper on the fly mutant of the same gene, but generated differently from a different P-element mutant origin, and their mutants have the same phenotype as ours [19].
We also wanted to know whether genetic interaction between Porin and mitochondrial fusion/fission proteins is also conserved in different tissues, so we observed the rescue effect of mitochondrial fusion/fission genes on male infertility of porin mutants (data not shown). Interestingly, an increase in mitochondrial fission by overexpression of Drp1 or marf RNAi could not rescue the phenotype of porin mutants. We thought that it was because the spermatogenesis is highly complex event, so only inducing mitochondrial fission could not restore the defects or the timing of ectopic gene expression during spermatogenesis was not appropriate. Further studies are required to determine the etiology of this tissue-specific phenotype.
In addition, another PTP component ANT, located in the mitochondrial inner membrane, has been also demonstrated as a nonessential component for MPT [4]. We found that SesB, Drosophila ANT, genetically interacted with Porin in promoting mitochondrial fission because small mitochondria induced by Porin overexpression were markedly rescued by a heterozygotic mutation of sesB in fly muscle tissues (data not shown). These results strongly suggest that the functions of PTP or, at least, some components of PTP including VDAC and ANT control mitochondrial remodeling processes either directly or indirectly.
Although we showed that Drosophila Porin can be genetically involved in mitochondrial remodeling process, we cannot exclude the possibility that the morphological changes of mitochondria in porin mutants is an indirect effect or a mere consequence of unhealthy mitochondria induced by loss of Porin functions. Further genetic and biochemical analysis are required for detailed understanding of how PTP components affect mitochondrial remodeling processes.
In the present study, our Drosophila genetic data revealed that Porin may be related with mitochondrial remodeling process. We believe that our finding will further enhance the understanding of the molecular mechanism of mitochondrial remodeling and the physiological role of VDAC/Porin and PTP.
Materials and Methods
Fly stocks
We have generated UAS-drp1 (C-terminally HA-tagged), UAS-opa1 (C-terminally Flag-tagged) and UAS-marf (C-terminally Flag-tagged) transgenic lines, and their expression was confirmed by immunoblot analyses. UAS-debcl [20], opa1EP (opa1p{EPgy2}CG8479) [12] and drp12 [13] line were kindly provided by S. Kumar, A. Mcquibban, and H. J. Bellen, respectively. Mef-GAL4 was kindly provided by E. N. Olson [21]. Hs-GAL4, tub-GAL4, gmr-GAL4, porinDf (Df(2L)BSC143, BL#7142) and marfDf (Df(1)dx81, BL#5281) lines were obtained from the Bloomington Stock Centre (Bloomington, IN). marf RNAi line was obtained from the Vienna Drosophila Resource Centre.
Generation of porin loss-of-function and overexpression flies
From the GenExel library, we isolated a P-element insertion line in the second exon of the gene. porinA2 allele was generated through imprecise excision of the P-element and was confirmed to be a loss-of-function mutant for porin. We also generated a revertant (RV) allele (porinRV) by precise excision. For generation of UAS-porin flies, Drosophila porin EST (clone #GH11331) was obtained from DGRC. The entire open reading frame (ORF) was subcloned into N-terminally Flag-tagged pUAST vector. porin RNAi line was generated through PCR using this primer pair: 5′-GCGGAATTCCTGGATGTGGGTGTACAGC-3′ and 5′-CGCGAATTCCAGCTCCAGACCCACACC-3′ and then cloned into pSymp vector. These generated constructs were subjected to DNA sequencing for validation and then microinjected into w1118 embryos for generation of transgenic flies.
Fly behavioral assays
Flight ability and climbing assays were performed as previously described [22] with 3- or 25-day-old males (n>30). Fly survival was examined as previously described [22].
Male fertility test
Individual 3-day-old males of RV, A2, and A2/A2; tub>porin were allowed to mate with two virgin w1118 females. The fully mated females were allowed to lay eggs for the same period (∼1 day) on the standard medium. After 15 days, the number of adult progenies was counted for fertility.
Mitochondrial staining in the thorax muscle
Fly thoraces were fixed with 4% paraformaldehyde for 10 min at 4°C and then were cut in half by dissecting vertically along the bristles in the middle of the thorax. They were then blocked in PBS-T with 2% BSA for 1 hr at room temperature and were incubated with Alexa 488-conjugated streptavidin (Invitrogen) for mitochondria visualization overnight at 4°C. After several rounds of washing with PBS-T, they were dehydrated in serial dilutions of ethanol and then were added with methylsalicylate (Sigma). They were mounted on a hollow glass slide (Matsunami, Japan) due to the thorax volume.
Paraquat-sensitivity assay
This assay was performed as previously described [23] but with some modifications. 1–3 day old flies were reared in 20 mM paraquat agar-contained vial and their survival was checked every 12 hrs.
Apoptosis assay by ionophore treatment
This experiment was conducted as previously described [24] with some modifications. The eye discs were incubated in A23187-containing M3 media for 4 hrs and then observed for apoptosis by performing TUNEL assay.
Immunostaining and TUNEL assay
Indirect flight muscle samples were stained with Mitotracker for 30 min and fixed with 4% paraformaldehyde and blocked in TBST with 2% BSA. For TUNEL assay, apoptosis in the eye discs of third instar larvae was detected using the in situ cell death detection kit (Roche).
Generation of porin antibody
The polyclonal antibody to Drosophila porin was generated in rabbit by injecting porin peptide (Anygen, Korea) and further purified.
Immunohistochemistry
To observe the sperms at the stage of spermatid individualization, immunohistochemistry was conducted as previous described [25].
Mitochondrial index
The number of mitochondria within 50 µm between two fibers in thorax from a single image frame were grouped and averaged; means from multiple thoraces were averaged to obtain a population mean and S.D.
Supporting Information
Supplementary Genotypes
(0.03 MB DOC)
Reduced lifespan of porin mutants. n = 100. S.D. for three independent experiments.
(0.07 MB TIF)
Examination of sperm. (A) No difference in the morphology of sperms between the onion stages (right panels) or earlier stages (left panels) of control and porin mutants. (B) Defects in spermatid individualization of porin mutants determined by anti-active Drice antibody (green) and phalloidin (red). Active Drice is detectable in CBs (white arrows) and WBs (red arrows). Porin mutants do not show CBs or WBs.
(0.44 MB TIF)
Genetic interaction analysis between Porin and the components involved in mitochondrial remodeling process. (A–C) Longitudinally sectioned thorax images stained with toluidine blue. Details of all indicated genotypes are described in Supplementary Information.
(0.35 MB TIF)
Quantification of the thorax mitochondria phenotype. (A–C) Measurement of the number of mitochondria within 50 um distance between two thorax muscle fibers (mitochondrial index) in each genotypes. Genetic interaction analysis between porin and opa1 (A), marf (B), and drp1 (C). n>10.
(0.08 MB TIF)
Acknowledgments
We are grateful to Drs. S. Kumar, A. Mcquibban, H. J. Bellen, H. Steller and E. N. Olson for providing us the fly stocks. We appreciate the Korea Basic Science Institute for the use of TEM electron microscope. We also would like to thank the Chung's lab members for helpful discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a grant from the Korean National Creative Research Initiatives Program (2010-0018291), funded by the Ministry of Education, Science and Technology. Y. Kim is supported by World Class Institute Program of National Research Foundation of Korea funded by the Ministry of Education, Science and Technology of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 2.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1−/− mitochondria. Biochem Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 5.Komarov AG, Graham BH, Craigen WJ, Colombini M. The physiological properties of a novel family of VDAC-like proteins from Drosophila melanogaster. Biophys J. 2004;86:152–162. doi: 10.1016/S0006-3495(04)74093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BH, Craigen WJ. Mitochondrial voltage-dependent anion channel gene family in Drosophila melanogaster: complex patterns of evolution, genomic organization, and developmental expression. Mol Genet Metab. 2005;85:308–317. doi: 10.1016/j.ymgme.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Oliva M, Messina A, Ragone G, Caggese C, De Pinto V. Sequence and expression pattern of the Drosophila melanogaster mitochondrial porin gene: evidence of a conserved protein domain between fly and mouse. FEBS Lett. 1998;430:327–332. doi: 10.1016/s0014-5793(98)00693-0. [DOI] [PubMed] [Google Scholar]
- 8.Guarino F, Specchia V, Zapparoli G, Messina A, Aiello R, et al. Expression and localization in spermatozoa of the mitochondrial porin isoform 2 in Drosophila melanogaster. Biochem Biophys Res Commun. 2006;346:665–670. doi: 10.1016/j.bbrc.2006.05.172. [DOI] [PubMed] [Google Scholar]
- 9.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 10.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQuibban GA, Lee JR, Zheng L, Juusola M, Freeman M. Normal mitochondrial dynamics requires rhomboid-7 and affects Drosophila lifespan and neuronal function. Curr Biol. 2006;16:982–989. doi: 10.1016/j.cub.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 13.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Leung HT, Kim E, Jang J, Lee E, et al. Effects of a mutation in the Drosophila porin gene encoding mitochondrial voltage-dependent anion channel protein on phototransduction. Dev Neurobiol. 2007;67:1533–1545. doi: 10.1002/dneu.20526. [DOI] [PubMed] [Google Scholar]
- 15.Fuller MT. Spermatogenesis. The Development of Drosophila Melanogaster: In: Martinez-Arias A, Bate M, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1993. pp. 71–147. [Google Scholar]
- 16.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;339:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 17.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 18.Gazaryan IG, Brown AM. Intersection between mitochondrial permeability pores and mitochondrial fusion/fission. Neurochem Res. 2007;32:917–929. doi: 10.1007/s11064-006-9252-2. [DOI] [PubMed] [Google Scholar]
- 19.Graham BH, Li Z, Alesii EP, Versteken P, Lee C, et al. Neurologic dysfunction and male infertility in Drosophila porin mutants: a new model for mitochondrial dysfunction and disease. J Biol Chem. 2010;285:11143–11153. doi: 10.1074/jbc.M109.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colussi PA, Quinn LM, Huang DC, Coombe M, Read SH, et al. Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. J Cell Biol. 2000;148:703–714. doi: 10.1083/jcb.148.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranganayakulu G, Shulz RA, Olson EN. Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev Biol. 1996;176:143–148. doi: 10.1006/dbio.1996.9987. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Lee SB, Lee S, Kim Y, Song S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 23.Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 24.Jang C, Lee G, Chung J. LKB1 induces apical trafficking of Silnoon, a monocarboxylate transporter, in Drosophila melanogaster. J Cell Bio. 2008;183:11–17. doi: 10.1083/jcb.200807052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Genotypes
(0.03 MB DOC)
Reduced lifespan of porin mutants. n = 100. S.D. for three independent experiments.
(0.07 MB TIF)
Examination of sperm. (A) No difference in the morphology of sperms between the onion stages (right panels) or earlier stages (left panels) of control and porin mutants. (B) Defects in spermatid individualization of porin mutants determined by anti-active Drice antibody (green) and phalloidin (red). Active Drice is detectable in CBs (white arrows) and WBs (red arrows). Porin mutants do not show CBs or WBs.
(0.44 MB TIF)
Genetic interaction analysis between Porin and the components involved in mitochondrial remodeling process. (A–C) Longitudinally sectioned thorax images stained with toluidine blue. Details of all indicated genotypes are described in Supplementary Information.
(0.35 MB TIF)
Quantification of the thorax mitochondria phenotype. (A–C) Measurement of the number of mitochondria within 50 um distance between two thorax muscle fibers (mitochondrial index) in each genotypes. Genetic interaction analysis between porin and opa1 (A), marf (B), and drp1 (C). n>10.
(0.08 MB TIF)