Abstract
Background
Most plasmids replicate only within a particular genus or family.
Methodology/Principal Findings
Here we describe an engineered high copy number expression vector, pBAV1K-T5, that produces varying quantities of active reporter proteins in Escherichia coli, Acinetobacter baylyi ADP1, Agrobacterium tumefaciens, (all Gram-negative), Streptococcus pneumoniae, Leifsonia shinshuensis, Peanibacillus sp. S18-36 and Bacillus subtilis (Gram-positive).
Conclusions/Significance
Our results demonstrate the efficiency of pBAV1K-T5 replication in different bacterial species, thereby facilitating the study of proteins that don't fold well in E. coli and pathogens not amenable to existing genetic tools.
Introduction
The laboratory work horse, E. coli, cannot efficiently translate or fold many foreign proteins. Most prokaryotic plasmids replicate in a particular eubacterial genus or family. The employment of different bacterial species as expression systems therefore necessitates the acquisition or development of new expression vectors [1], [2]. Broad host range plasmids based on RK2 [3], IncaP [4] or rolling circle replication (RCR) [5] origins have been developed for the production of proteins, or the study of poorly characterized bacterial pathogens, but most are limited in their host range, genetic stability, size or capacity to accept large inserts [2], [6].
pWV01 is a cryptic plasmid originally purified from Streptococcus cremoris [7]. Its RCR origin has been used to create over 20 cloning vectors [5]. Among them, pGK12 is most widely used by other researchers. It replicates in Bacillus subtilis, Lactococcus lactis, E. coli, Borrelia burgdorferi, and numerous Lactobacilli (namely reuteri, fementum, casei, acidophilus, pentosus and helveticus) [6]. Unfortunately, pGK12 is unstable and does not replicate to high copy number in these species. Its performance in E. coli is particularly poor, so it was never widely adopted by researchers [6].
Little was known about RCR when pGK12 was first constructed [5]. Three decades of subsequent study [6], [8], [9] have laid the foundation for the rational design of better plasmid origins. RCR plasmids exist in eubacteria (Gram-positive and Gram-negative) and archaeabacteria [6]. Replication begins when the Rep protein, which is encoded on the plasmid (ORF A), recognizes a specific site on the plasmid (double-strand origin, or DSO) and catalyzes the nicking of one DNA strand. The Rep protein remains bound to the 5′ phosphate after the nicking action. The newly released 3′ hydroxyl on the opposite end serves as a primer for DNA synthesis. The host DNA polymerase uses the unnicked circular strand as a template, so that a single replication fork moves around a plasmid until it regenerates the DSO. A second copy of Rep protein catalyzes the cleavage of the newly formed DSO, effectively releasing a single stranded copy of the plasmid. In the absence of Rep, the replication fork continues to move around the template, forming a single stranded concatemer. The single strand origin (SSO), a non-coding element that forms extensive secondary structure, is required for synthesis of the lagging strand. SSO sequences vary considerably among different RCR plasmids, but are extremely important for robust replication of the plasmid in the cell [10]. Here we describe the engineering of the pWV01 RCR origin to create pBAV1K-T5, a very broad-host range expression vector.
Results and Discussion
We hypothesized that the RCR of pWV01-based plasmids in non-native hosts was inefficient. Cryptic plasmids by definition have no detectable effects on their hosts, so the copy number of pWV01 in its native host must be stringently controlled. If the copy number control mechanisms of a plasmid are more efficient than its RCR mechanisms in non-native hosts, the plamid would not be stable under non-selective conditions. The elimination of regulatory elements, particularly those not widely conserved among RCR plasmids, should allow the altered plasmid to replicate more freely. We sought to create a minimal plasmid origin that included only pWV01 ori elements that were shown to be necessary by other researchers [11]. Runaway replication is toxic to host cells, of course, but we speculated that the inefficiency of RCR in non-native hosts would moderate this risk.
We therefore sought to delete copy number control mechanisms from the pWV01 origin. Two inverted repeats (IRI and IRII), out of the six within the pWV01 origin, are sufficient for the conversion of single-stranded DNA to the double-stranded form [12]. The ORF D protein may play a role in copy number regulation, but is not essential for replication [7]. Three different inverted repeats (IRIV, IRV and IRVI), which may serve as an alternative SSO, and ORF D were deleted (Fig. 1). The ORF C protein is a negative regulator of P1 promoter that regulates the expression of repA [7]; we chose to retain it to moderate the risk of runaway plasmid replication. In addition, the T1 and t0 transcription terminators [13] were inserted on opposite sides of the origin of replication to prevent RNA read-through to the plasmid origin part. The terminators should prevent possible antisense interference by the wild-type RNA transcript with repA expression and SSO function.
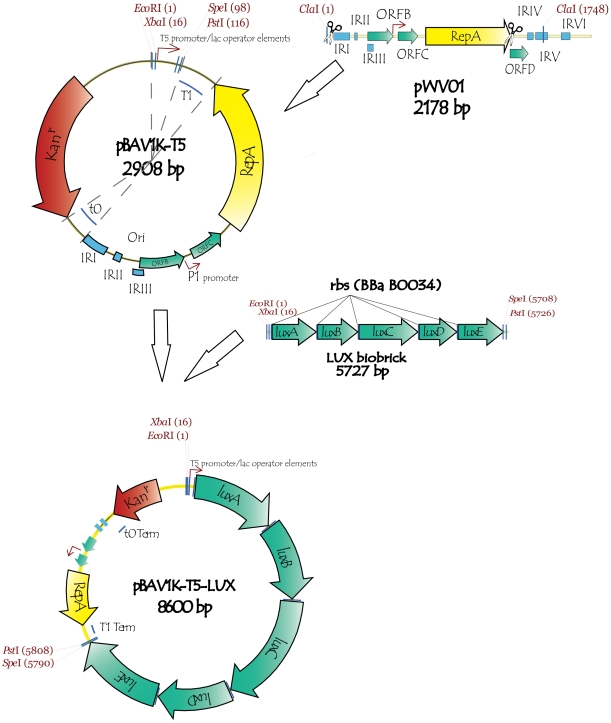
Figure 1. Construction of pBAV1K-T5-lux, a very broad host range expression vector.
The cryptic plasmid, pWV01, exhibits broad host range but is unstable in many species. The ORF D, and inverted repeats IV, V and VI were deleted from its plasmid origin; terminators t0 and T1 were inserted on opposite ends of the shortened origin (upper right). The selectable marker, the Enterococcus 3′,5″-aminoglycoside phosphotransferase type III, and a T5 promoter within a BioBrick multiple cloning site were cloned into the plasmid (top circle). The lux genes of Photorhabdus luminescens were individually PCR amplified, cloned, assembled with ribosome binding sites (middle) and cloned into the plasmid to create pBAV1k-T5-luxABCDE (bottom circle).
We completed our expression vector by cloning a selectable marker, a multiple cloning site, a promoter and reporter genes into the plasmid (Fig. 1). Each element was selected for its ability to function in the widest variety of hosts. The 3′,5″-aminoglycoside phosphotransferase type III, under its own promoter, confers kanamycin resistance upon many bacterial species [14]. We introduced a BioBrick multiple cloning site (EcoRI-NotI-XbaI-insert-SpeI-NotI-PstI) so that our vector, pBAV1K-T5, which does not otherwise contain those restriction sites, would be compatible with this standard [15]. We subsequently cloned a BioBrick containing a T5 promoter and two lacO operators [16] into the multiple cloning site (pBAV1K-T5). The gfp (0.8 kb), gusA (1.8 kb) and lacZ (3 kb) reporter genes, and the luxABCDE reporter operon (5 kb), were separately cloned downstream of the promoter (pBAV1K-T5-gfp, pBAV1K-T5-gusA, pBAV1K-T5-lacZ and pBAV1K-T5-lux).
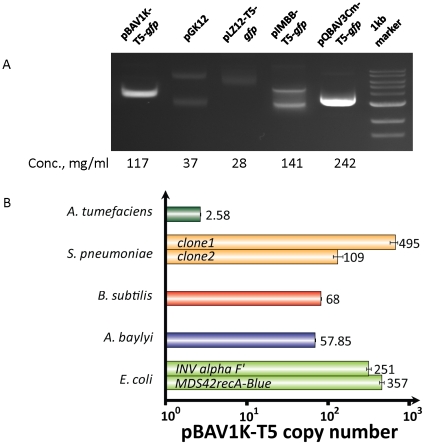
Like most other molecular biologists, we use E. coli as a cloning vehicle, even if the final construct intended to be used in other species. We transformed E. coli with pBAV1K-T5-gfp, or control RCR plasmids pGK12 (derived from pWV01) and pLZ12-T5-gfp (derived from pSH71[17] and has high level of homology to pWV01) or ColE1 plasmids (pQBAV, or pIMBB). The transformants were propagated in liquid LB cultures and lysed. The yield of plasmid from the pBAV1K-T5-gfp transformant was comparable to those of the ColE1-derived plasmids (Fig. 2a). The visibly higher amount of purified vector for pBAV1K-T5-gfp, relative to parental plasmids pGK12 and pLZ12-T5-gfp, was confirmed by Real-Time qPCR analysis(Fig. 2b). pBAV1K-T5-gfp replicates to 357 copies per cell in MDS42recA-Blue and 251 copies per cell in INV alpha F′. In contrast, pGK12 replicated only to 60 copies/cell in E. coli [5].
Figure 2. Plasmid pBAV1K-T5-gfp replicates to high copy number in Escherichia coli.
(A) E. coli was transformed with plasmids pBAV1K-T5-gfp, pLZ12-T5-gfp, pGK12 (two other RCR plasmids), pQBAV3Cm-T5-gfp or pIMBB-T5-gfp (two ColE1 derived plasmids). The transformants were propagated in liquid LB cultures supplemented with the appropriate antibiotics. The plasmids were purified, and 2 microliters of each were analyzed on a 0.8% agarose gel. The higher yield and faster mobility of the pBAV1k relative to the larger pWV01 derivatives indicates supercoiling. (B) Five different species of bacteria (namely Agrobacterium tumefaciens, Streptococcus pneumoniae, Bacillus subtilis, Acinetobacter baylyi ADP1 and E. coli) were transformed with pBAV1K-T5-luxABCDE (Table 3). The APH(3′)-IIIa gene present on the plasmid was used as a target to estimate the copy number in reference to the chromosomal relA/spoT gene (or its homolog) by quantitative real-time PCR. Each bar represents the average of three replicates. Error bars represent standard error.
We were concerned about the effect of high plasmid copy number (runaway replication) on cell fitness, so we used flow cytometry to assess the genetic stability of the plasmid of the transformed cells. We created T5-lacO-lacO-gfp versions of our vectors (pBAV1K-T5-gfp, pLZ12-T5-gfp, pIMBB-T5-gfp, and pQE30-T5-gfp) in parallel, using overlap extension PCR cloning (Materials and Methods). It proved far easier to clone genes into pBAV1K-T5 than into the other RCR-replicating plasmids (pLZ12 and pGK12). When E. coli were transformed with the in vitro recombination reactions, 172 pBAV1K-T5-gfp transformant colonies formed. In contrast, a single pLZ12-T5-gfp transformant colony grew on the second try, and no pGK12-T5-gfp transformants grew in four attempts. We hypothesized that the poor performance of pLZ12 and pGK12 as cloning vectors reflected their inability to replicate stably in E. coli. Indeed, flow cytometry analysis of mid-log (OD600 = 0.8) showed that populations of E. coli cells transformed with pBAV1K-T5-gfp had much lower proportions of non-fluorescent (and potentially dead) cells than populations of isogenic pLZ12-T5-gfp transformants (Fig. 3).
Figure 3. Heterologous GFP expression from pBAV1K-T5-gfp, pLZ12-T5-gfp and pIMBB-T5-gfp.
E. coli was transformed with pIMBB-T5-gfp, pLZ12-T5-gfp, or pBAV1k-T5-gfp. A. baylyi was transformed with pBAV1kT5-gfp only. The transformants were propagated in liquid LB supplemented with the appropriate antibiotic and diluted to 5×105 cells per ml with M9 minimal media before flow cytometric analysis. The major peaks indicate differences in GFP expression; the minor peaks (left) indicate cells that have don't express GFP, possibly due to plasmid instability or cell death.
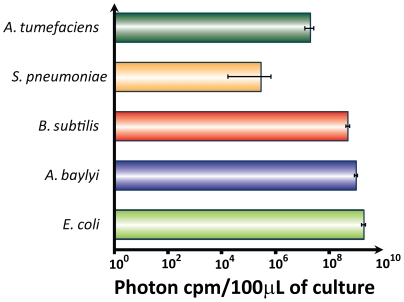
pBAV1K-T5-luxABCDE plasmid DNA purified from E. coli was used to transform E. coli, A. baylyi, S. pneumoniae, A. tumefaciens and B. subtilis (underlined in Fig. 4). Every species we tested was successfully transformed, except Deinococcus radiodurans, possibly because this species harbors its own incompatible cryptic plasmid [18]. The transformants were propagated in rich media, and the luminescence of 100 microliters of liquid culture was measured. Each species produced light, confirming the broad host specificity of the promoters, ribosome binding sites and selectable markers in our vector (Fig. 5). Light production is a function of many factors, including the plasmid copy number, codon bias, protein folding and the metabolic network of each host species, so the variations between species are difficult to rationalize. Plasmid DNA copy number in E. coli, A. baylyi, B. subtilis and A. tumefaciens was measured by real-time quantitative PCR using whole DNA purified from the bacterial cells; a relative quantification was used for copy number calculation (Fig. 2b). In B. subtilis, pBAV1K-T5-luxABCDE replicates to 10 times the reported copy number of pGK12 in the same species [5].
Figure 4. Dendrogram of bacterial species.
Dendrogram depicting the phylogenetic relationships among eubacteria adapted from phylogenetic trees inferred by Jun et al. [25] Cheng et al. [26] and Brown et al. [27]. Branch lengths do not represent evolutionary distance. Only species related to the study are shown; those that that have demonstrated the capacity to harbor the pBAV1K-T5-gfp and/or pBAV1k-T5-luxABCDE plasmid are underlined.
Figure 5. Heterologous expression of lux operon from pBAV1K-T5-luxABCDE.
Five bacterial species (Fig. 2) were transformed with pBAV1K-T5-lux. The transformants were propagated in liquid culture (Table 3). The luminescence from equal volumes of culture (100 microliters) was measured in a luminometer. The values (plotted on a log scale) represent the averages of three independent experiments.
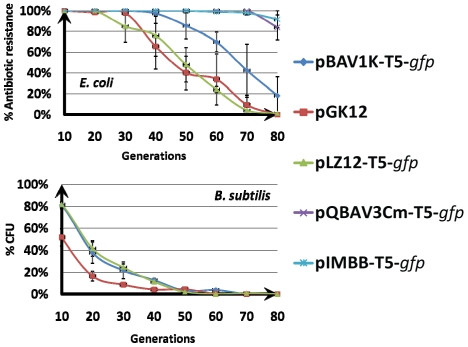
All bacterial cells transformed with pBAV1K-T5-gfp retain the plasmid when propagated under selective conditions (i.e. in media containing kanamycin). To determine whether the vector could stably replicate under non-selective conditions, E. coli, and B. subtilis were separately transformed with pBAV1K-T5-gfp, pGK12, or pLZ12-T5-gfp. E. coli cells were also separately transformed with pIMBB-T5-gfp or pQBAV3Cm-T5-gfp in parallel. The transformants were separately grown in non-selective broth for 80 generations by serial dilution and agitation, and the percentage of antibiotic-resistant colonies in the total viable count was determined. In E. coli, plasmids pBAV1K-T5-gfp (approximately 18% kanamycin-resistant colonies after 80 generations) exhibited higher stability than either pLZ12-T5-gfp or pGK12 (0% chloramphenicol-resistant colonies after 80 generations, Fig. 6). The specialized ColE1 based plasmids were, however, more stable than pBAV1K-T5-gfp in E. coli (80% and 90% ampicillin-resistant colonies after 80 generation for pQBAV3Cm-T5-gfp and pIMBB-T5-gfp respectively). When B. subtilis transformants were cultured for 80 generations in non-selective broth, pBAV1K-T5-gfp and pLZ12-T5-gfp exhibited greater stability than pGK12 (Fig. 6b). It is possible that the expression of the erythromycin resistance marker from pGK12 plasmid increased the cost of the plasmid in nonselective conditions, resulting in the earlier elimination of the plasmid from the bacterial population.
Figure 6. Plasmid stability assays.
(A) Plasmids pBAV1K-T5-gfp, pGK12, pLZ12-T5-gfp, pQBAV3Cm-T5-gfp and pIMBB-T5-gfp were separately propagated in E. coli for 80 generations without antibiotic selection. Plasmid stability was determined by replica plating onto selective media and presented as a percentage of cells that retain antibiotic resistance. (B) Plasmids pBAV1K-T5-gfp, pGK12, pLZ12-T5-gfp, were propagated in B. subtilis for 80 generations without antibiotic selection. Plasmid stability was determined by the plasmid content comparison in the total DNA pools between different generations. Error bars represent standard error.
The pBAV1K-T5-gfp expression vector was also used to transform natural bacterial species in two arbitrarily collected soil samples. These experiments extended the known host-specificityof the plasmid and revealed naturally competent bacterial species. Gene transfer by natural transformation allows bacteria to adapt rapidly to changing environmental conditions. It probably occurs all the time, but is too infrequent to detect under natural conditions, particularly in soil [19], [20], [21]. The soil was mixed with the aqueous sample of the plasmid, agitated overnight at room temperature and allowed to grow in rich medium supplemented with kanamycin. Fluorescent colonies formed on the plates were collected and later identified by 16S RNA gene sequencing as Peanibacillus sp. S18-36 and YN14-0; Leifsonia sp.L89, WPCB149 and shinshuensis. The pBAV1K-T5 vector will allow researchers to study natural transformation, and evolution, of the soil bacteria. These species are unrelated to the others that we transformed (E. coli, A. baylyi, S. pneumoniae, A. tumefaciens, B. subtilis, B. burgdorferi, A. tumefacienes, Fig. 4). We have also shared the vector with collaborators, and have heard that it also replicates efficiently in A. baumannii, S. pyogenes and F. novicida (personal communication, Justin Gallivan, Julia Bugrysheva and David S. Weiss). We are therefore optimistic that pBAV1K-T5 will function in many other mesophilic eubacteriaThe genetic features of the natural occurring plasmids, including promoters, ribosomal binding sites, terminators, and codon usage, typically coincide with those of their hosts. The plasmid must serve as a template for host replication factors, namely DNA gyrase, DNA ligase, RNA polymerase, DNA polymerase I, and DNA polymerase III; the host ribosome must recognize the ribosome binding site of the repA transcript. Eubacterial genomes have been diverging from their last common ancestor for over three billion years. We were therefore surprised that pBAV1K-T5 replicated in so many species (Fig. 4), imparting kanamycin resistance and reporter protein production upon each. The factors that mediate transcription and translation in these Gram-negative and Gram-positive species must therefore be conserved to a heretofore underappreciated extent.
We intend to distribute pBAV1k-T5 without regard to intellectual property considerations. Microbiologists could employ it as genetic tool to elucidate the pathogenic mechanisms of poorly characterized bacteria. Protein engineers could use it to express their favorite genes in a variety of eubacteria. It is difficult to predict a priori which species will produce the highest yield of any particular protein, but we showed here that E. coli is not always the best choice. Synthetic biologists could better leverage the complex molecular machines that already exist within the vast domain of the eubacteria.
Materials and Methods
Materials
All primers used in the study (Table 1) were purchased from IDT (Coralville, IA). All plasmids (Table 2) and bacterial strains (Table 3) were obtained from commercial vendors or collaborators. Restriction enzymes and DNA modification enzymes were from NEB (Ipswich, MA), and reactions were carried out under the recommended conditions. All other chemicals used in the study were from Sigma-Aldrich (molecular biology grade). Plasmid DNA from E. coli was purified with the QIAprep spin miniprep kit as directed by the manufacturer (QIAGEN, Valencia, CA).
Table 1. Oligonucleotides and PCR primers used in the study.
| Primer | Sequence 5′-3′ | Reference |
| AVB 5 | TTCTTAGACGTCAAATTCTATCATAATTGTGGTTTCAAAATCGGCTCCGTCG | [28] |
| AVB6 | ACTGGATCTATCAACAGGAGTCCAAGCGAGCTCGGTACTAAAACAATTCATCCAGTAAA | [28] |
| AVB25 | ATAGAATTTGACGTCTAAGAAACCATTATTATCATGACATTAACC | This study |
| AVB26 | GACTGAGCCTTTCGTTTTATTTGATGCCTCTAGATTAATTAATTAAGCGGCCGCATCGATCG | This study |
| AVB29 | CGATTTTTTATTAAAACGTCTCAAAATCG | This study |
| AVB30 | GTCATTTTATTTCCCCCGTTTCAGCA | This study |
| AVB 33 | GGTTGCCGCCGGGCGTTTTTTATTGGTGAGAATCCAAGCACTAGGCGATTTTTTATTAAAACGTCTCAAAATCG | This study |
| AVB34 | TACCGAGCTCGCTTGGACTCCTGTTGATAGATCCAGTAATGACCTCAGAA | This study |
| AVB35 | CAACAAACTCTAGCGCCTTTAGATTATGGTTTGAGGGCAATTATCAGTGTGGATATAGAGCAAGTTATGCAAAGGTTCTTGA | This study |
| AVB36 | CCTACTCAGGAGAGCGTTCACCGACAAACAACAGATAAAACGAAAGGCCCAGTCTTTCGACTGAGCCTTTCGTTTTATTT | This study |
| AVB37 | TGTCGGTGAACGCTCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAGTGTCATTTTATTTCCCCCGTTTCAGCA | This study |
| AVB38 | ATTAATCTAGAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGAC | This study |
| AVB199 | GGATCCCTGCAGGCCTCAGGGCCCGATCGATGCCGCCGCTTAATTAATTAATCCAGAGGC | This study |
| AVB200 | CTAGAAGCGGCCGCGAATTCGACGTCAAATTCTATCATAATTGTGGTTTCAAAATCGGC | This study |
| AVB221 | AATTCGCGGCCGCTTCTAGAGGAAATCATAAAAAATTTATTTGCTTTGTGAGCGGATAACAATTATAATAGATTCAATTGTGAGCGGATAACAATTA | This study |
| AVB222 | CTAGTAATTGTTATCCGCTCACAATTGAATCTATTATAATTGTTATCCGCTCACAAAGCAAATAAATTTTTTATGATTTCCTCTAGAAGCGGCCGCG | This study |
| AVB264 | GGTCGTCAGACTGATGGGCCCCTGCATCAGGGCGATGGCCCACTACGTGG | This study |
| AVB265 | GGCACAGATGGTCATAACCTGAAGGAAGATCTGGGGCCTTTTGCTGGCCTTTTGCTCACATG | This study |
| AVB270 | GCCGATTTTGAAACCACAATTATGATAGAATTTGACGTCATCAGGGCGATGGCCCACTACGTGG | This study |
| AVB271 | AGCGGCGGCATCGATCGGGCCCTGAGGCGGCCTTTTGCTGGCCTTTTGCTCACATG | This study |
Table 2. Plasmids used in the study.
| Plasmid | Description | Reference |
| pGK12 | pWV01 ori; Ermr Camr | [5] |
| pLZ12 | Modified pNZ12 vector; pSH71 ori (high level of similarity to pWV01); Camr | [29]. |
| pLZ12-T5-gfp | T5lacO; Camr | This study |
| pBAV1K-T5 | T5lacO; Kanr | This study |
| pBAV1K-T5-gfp | T5lacO; Kanr | This study |
| pBAV1K-T5-gus | T5lacO; Kanr | This study |
| pBAV1K-T5-lacZ | T5lacO; Kanr | This study |
| pBAV1K-T5-luxABCDE | T5lacO; Kanr | This study |
| pIMBB-T5-gfp | Custom biobrick accepting vector; T5lacO; ColE1 replicon; Ampr | [22] |
| pIMBB | Custom biobrick accepting vector; ColE1 replicon; Ampr | [22] |
| pQBAV3Cm-T5-gfp | Vector based on pQE30 (Qiagen) ; T5lacO; ColE1 replicon; Camr; | [22] |
| pQBAV3Cm-T5-gusA | Vector based on pQE30 (Qiagen); T5lacO; ColE1 replicon; Camr; | [22] |
| pQBAV3Cm-T5-lacZ | Vector based on pQE30 (Qiagen); T5lacO; ColE1 replicon; Camr; | [22] |
| pQBAV3Cm-T5-luxABCDE | Vector based on pQE30 (Qiagen); T5lacpo; ColE1 replicon; Camr; lux genes are from Photorhabdus luminescens (ATCC number 29999) | [22] |
Table 3. Bacterial strains and transformation procedures used in the study.
| Bacterial Strain | Description | Growth conditions | Transformation protocol | Kan [C, mkg/ml] for plasmid selection | Reference or source |
| E. coli | |||||
| MDS42 recA Blue | 15% reduced genome size | LB, 37°C | Chemically competent cells | 50 | Scarab Genomics [30]. |
| INV alpha F′ | F′ endA1 recA1 hsdR17 (rk−, mk+) supE44 thi-1 gyrA96 relA1 ϕ80lacZΔM15 Δ(lacZYA-argF)U169 λ- | LB, 37°C | Chemically competent cells | 50 | Invitrogen |
| B. burgdorferi | |||||
| B31 | high passage, non-pathogenic strain | BSK-II, 34°C | Electroporation [31] | 400 | [31] |
| A. tumefaciens | |||||
| C58 | LB, 28°C | Electroporationl [32]; | 50 | [33] | |
| B. subtilis | |||||
| JH642 | LB, 37°C | Naturally competent cells [34] | 20 | [35] | |
| S. pneumoniae | |||||
| R6 | non-encapsulated derivative of the serotype 2 strain D39 | Todd-Hewitt medium containing 0.5% yeast extract, 37°C, CO2 | Naturally competent [36] | 400 | [37] |
| A. baylyi | |||||
| ADP1 | LB, 30°C | Naturally competent cells [38] | 50 | [38] | |
| F. novicida | |||||
| U112 | Low virulence strain | Tryptic Soy Broth supplemented with 0.2% L-cysteine | Chemical transformation [39] | 30 | [39] |
Recombinant DNA
We used Overlap Extension PCR cloning [22] or restriction enzymes and ligase, to create recombinant plasmids; BioBricks were assembled as directed in the BioBrick Assembly Manual (NEB, Ipswich, MA). When necessary, DNA fragments were purified from agarose gels with QIAquick-gel extraction kits from QIAGEN (Valencia, CA). DNA fragments and PCR mixtures were analyzed on 0.8% Seakem LE agarose gels (Lonza Rockland, Rockland, ME) using the 1 kb DNA ladder (New England BioLabs, Ipswich, MA) a molecular size standard. DNA was sequenced by Macrogen (Rockville, MD).We propagated and transformed established laboratory bacteria according to published procedures (Table 3).
Transformation and identification of bacteria from soil samples
The soil samples (1 g each) were each mixed with water (100 microliters) and pBAV1K-T5-gfp (10 micrograms) and agitated overnight at room temperature. The sample was diluted with LB-kanamycin (1 mL, 100 microgram/mL), agitated for another two hours and spread on LB agar plates supplemented with kanamycin.. After several days of incubation at room temperature, transformed, fluorescent colonies were picked and propagated in liquid LB-kanamycin. Total DNA was prepared with the DNeasy kit (as directed by the Qiagen for gram-positive bacteria). The 16S RNA genes were PCR amplified (with the primers 27F and 1492R [23] ) and sequenced with the same primers and two others (946F and 518R [23]).
Construction of pBAV1K-T5 and its derivatives
The BioBrick accepting vector pBAV1K-T5 was created by combining individual parts of the vector (Fig. 1) by overlap extension PCR [24]. First, the part of pWV01 origin of replication from IRI to the end of repA gene was PCR amplified using primers AVB29 and AVB30 (Table 1). The T1 and t0 E. coli transcription terminators were synthesized by overlap extension PCR reaction of primers AVB33, AVB34, AVB35 (t0) and AVB36, AVB37, AVB38 (T1). Second, the PCR products were then combined in another overlap extension PCR reaction that produced the shortened pWV01 origin of replication flanked by transcriptional terminators. Third, overlap extension PCR was used to combine obtained DNA fragment with the kanamycin resistance marker and the tetracycline promoter/multiple cloning site. The kanamycin resistance marker, the APH(3′) (5″)-IIIa gene from Enterococcus faecalis, was PCR amplified with its own native promoter using primers AVB5 and AVB6. To obtain the pBAV1K-T5 vector, the synthetic T5 promoter/lac operator [16] was created by overlap extension PCR of primers AVB221 and AVB222 and cloned into custom biobrick accepting vector pIMBB [22]. The plasmid intermediate (engineered origin plus APH(3′)(5″)-III a) was PCR amplified with the primers AVB199 and AVB200. The PCR product, and the T5 promoter/lac operator biobrick, were digested with EcoRI and PstI restriction endonucleases and ligated to each other.
Different custom biobricks luxABCDE operon, gfp, gusA, lacZ were when cloned into pBAV1K-T5 vector by standard methods [13] or overlap extension PCR cloning [22]. Primers AVB270 and 271 were used to transfer biobricks through overlap extension PCR cloning from pIMBB into pBAV1K-T5. The pBAV1KT5-gfp construct was sequenced. The annotated sequence was submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/; accession number HQ191434). We intend to distribute our expression vector pBAV1KT5-gfp through Addgene plasmid repository (addgene.org) and ATCC (http://www.atcc.org/). The pLZ12-T5-gfp was made from pLZ12 by overlap extension PCR cloning with primers AVB264 and AVB265. T5-gfp biobrick was transferred from pIMBB-T5-gfp to pLZ12 using primers AVB264 and AVB265.
Plasmid stability test
Cultures of E. coli cells, each harboring a plasmid (pBAV1K-T5-gfp, pGK12, pLZ12-T5-gfp, pQBAV3Cm-T5-gfp or pIMBB-T5-gfp, Fig. 6A) were propagated in LB medium containing the appropriate antibiotic at 37°C from a single colony. An aliquot (10 microliters) of each overnight culture was inoculated in 10 milliliters of fresh LB medium without antibiotic and grown for 24 hrs until the cells reached stationary phase. At this point the OD600 of the culture was typically between 2.0–2.4, which corresponds to a titer of ∼2×109 cells/milliliter. An aliquot (100 microliters) of this stationary phase culture was used to inoculate 100 milliliters of fresh medium and this process of sub-culturing was repeated for eight days. Since each inoculum was 0.1% (100 microliters in 100 ml) it represented a 1000 fold increase in the cell number. The OD and cfu/milliliter values were similar at the end of each round of sub-culturing. Thus every 24 hr period of growth represents 10 doublings and 10 generations. The fraction of untransformed cells emerging within each culture was calculated by plating appropriate dilutions of this culture onto LB plates (without antibiotics) to get isolated colonies. From each LB agar plate, 96 colonies were transferred onto 96-well plates containing LB plus antibiotic; the fractions of plasmid containing cells were calculated by counting the number of wells that had visible bacterial growth.
B. subtilis cells harboring the plasmid were similarly grown from a single colony in LB medium containing the appropriate antibiotic at 37°C. An aliquot of 10 microliters of this overnight culture was inoculated in 10 milliliters of fresh LB medium without antibiotic and grown for 24 hours until the cells reached stationary phase. At this point the OD600 of the culture was typically between 1.0–1.3. An aliquot (100 microliters) of this stationary phase culture was used to inoculate 100 milliliters of fresh medium and this process of sub-culturing was repeated for eight days. To avoid fails-positive results due to integration of the plasmid into the chromosome of B. subtilis, a method different from that used for E. coli was used to calculate the percentage of B. subtilis cells containing the plasmid. Total DNA was purified from 2 mL aliquots of each overnight saturated culture with Qiagen DNeasy kit. The eluate (1 microliters out of a 250 microliter elution volume) was used to transform chemically competent E. coli INV alpha F′ cells. The transformants were spread on LB plates supplemented with appropriate antibiotic; the colonies (corresponding to different generations of the B. subtilis culture) were counted. The number of the colonies obtained with the generation 0 culture was considered 100%. Test plasmid purifications and agarose gel electrophoresis were performed from several colonies to confirm plasmids identities.
Real-Time PCR assays
The copy numbers of the pBAV1K-T5-luxABCDE within different cell types were assessed by real-time PCR. Amplification and detection were carried out in LightCycler® 480 (Roche) using sequence specific fluorescent probes from “Universal ProbeLibrary®” (Roche). PCR primers were designed using Primer3 software located at the “Assay Design Center” of the Roche web-site. Total DNA from bacterial species was purified with Qiagen DNeasy kit (as directed by the manufacturer for gram-positive bacteria) and quantified in a ThermoFisher Nanodrop spectrophotometer. The ACIAD3326 (relA/spoT homolog), a single-copy gene on the chromosome of A. baylyi, and orthologues in other bacterial species, were used as a references (genes, primers and probes are listed in Table 4). A 59 bp fragment of the gene was amplified with the primers AVB 126 and AVB 127; probe #47 from Universal ProbLibrary (Roche) was used for detection of the product. The APH(3′)-IIIa gene was used as a target to estimate the copy number for the vector. A 59 bp fragment of the APH(3′)-IIIa gene was amplified with the primers AVB 128 and AVB 129; probe #48 from Universal ProbLibrary® (Roche) was used to detect the product. Both target and reference DNA standards were diluted in 8 to12 serial steps, each applied in duplicate. LightCycler® 480 Probes Master was used for the preparation of all samples. The PCR conditions included a single denaturation cycle of 95°C for 7 min, followed by 45 cycles of 95°C for 10 s, and combined annealing- elongation for 1min at 55°C. All real-time PCRs were done in triplicate and average results are reported.
Table 4. Probes and Primers for Real Time PCR used in the study.
| Primer | Bacterial specie/Target gene | Sequence 5′-3′ | Probe |
| AVB 126 | A. baylyi/ACIAD3326 | CGCAGACCGCTATCATAACA | #47 |
| AVB 127 | A. baylyi/ACIAD3326 | CGTGCACGTTTGTCTGGT | #47 |
| AVB 128 | /(APH)(3′)(5″)-IIIa | GCGCGGATCTTTAAATGG | #48 |
| AVB 129 | /(APH)(3′)(5″)-IIIa | GATCTGGCCGATGTGGATT | #48 |
| AVB 283 | E. coli/spoT (ECBD_0075) | CTGGTAGCCACGGATATTACG | #138 |
| AVB 284 | E. coli/spoT (ECBD_0075) | AGCCCCGGTAAAGGTCTG | #138 |
| AVB 285 | B. subtilis/relA | CCCACTCTACCGGGATCAG | #117 |
| AVB 286 | B. subtilis/relA | CGAAGACTGTCCGAATGTCA | #117 |
| AVB 287 | A. tumefaciens/NP_354053.2 | GCATCGGCACTGACTTCTC | #42 |
| AVB 288 | A. tumefaciens/NP_354053.2 | TTCAGTTGCCGCAAATCC | #42 |
| AVB 289 | Streptococcus pneumoniae/spoT | GAAAGACAAGTCTTCTAATTCCCATT | #47 |
| AVB 290 | Streptococcus pneumoniae/spoT | GAAATCTATGCCCCACTTGC | #47 |
Bacterial detection GFP+/GFP- discrimination by flow cytometry
E. coli INV alpha F′ cells transformed with pBAV1K-T5-gfp, pLZ12-T5-gfp or pIMBB-T5-gfp were grown in LB at 30°C with aeration to the OD600 = 1.2. A. baylyi ADP1 cells were grown in LB at 30°C with aeration to the OD600 = 0.8. Cells were then washed twice with M9 minimal media, resuspended in M9, and analyzed by flow cytometry with a FACSCalibur flow cytometer (BD Biosciences; San Jose, California). Cell samples were diluted to approximately 5×105 cells per ml with M9 minimal media and delivered at the flow rate of 50 to 150 cells/sec. The FSC (Forward Scatter), SSC (Side Scatter), and fluorescence signal were measured. A band pass filter of 530 nm (515 to 545 nm) was used to collect the green fluorescence. All signals were collected by using logarithmic amplifications. A combination of FSC and SSC were used to discriminate bacteria from background. A total of 20,000 events for each sample were collected and analyzed with the CellQuest Pro software.
Luminescence assay
E. coli MDS42 recA, A. tumefaciens, B subtilis and A baylyi were transformed with pBAV1K-T5-luxABCDE vector. The transformed cells, and untransformed negative controls were grown in rich media to mid-exponential phase with aeration. S. pneumoniae cells were grown in Todd-Hewitt medium containing 0.5% yeast extract without aeration but were aerated for 2 hours at room temperature before measurement. After incubation, luminescence from 100 microliters of each bacterial culture was measured with a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices; Sunnyvale, CA). All the measurements were performed in triplicate independent experiments, each in octuplicate.
Acknowledgments
We thank Felipe Cabello, Julia Bugrysheva, Dorothea Zahner, Shana Topp, David S. Weiss, Connie Arthur and Justin Gallivan for their guidance, and for their invaluable assistance with bacterial species or techniques new to us.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work was supported by the National Institutes of Health grants 1 R01 GM074264 and 1 R01 GM086824 to IM. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huang HH, Camsund D, Lindblad P, Heidorn T. Design and characterization of molecular tools for a Synthetic Biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010;38:2577–2593. doi: 10.1093/nar/gkq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosse JT, Durham AL, Rycroft AN, Kroll JS, Langford PR. New plasmid tools for genetic analysis of Actinobacillus pleuropneumoniae and other pasteurellaceae. Appl Environ Microbiol. 2009;75:6124–6131. doi: 10.1128/AEM.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aakvik T, Degnes KF, Dahlsrud R, Schmidt F, Dam R, et al. A plasmid RK2-based broad-host-range cloning vector useful for transfer of metagenomic libraries to a variety of bacterial species. FEMS Microbiol Lett. 2009;296:149–158. doi: 10.1111/j.1574-6968.2009.01639.x. [DOI] [PubMed] [Google Scholar]
- 4.Dennis JJ. The evolution of IncP catabolic plasmids. Curr Opin Biotechnol. 2005;16:291–298. doi: 10.1016/j.copbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kok J, van der Vossen JM, Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984;48:726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leenhouts KJ, Tolner B, Bron S, Kok J, Venema G, et al. Nucleotide sequence and characterization of the broad-host-range lactococcal plasmid pWVO1. Plasmid. 1991;26:55–66. doi: 10.1016/0147-619x(91)90036-v. [DOI] [PubMed] [Google Scholar]
- 8.Boer DR, Ruiz-Maso JA, Lopez-Blanco JR, Blanco AG, Vives-Llacer M, et al. Plasmid replication initiator RepB forms a hexamer reminiscent of ring helicases and has mobile nuclease domains. EMBO J. 2009;28:1666–1678. doi: 10.1038/emboj.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Solar G, Giraldo R, Ruiz-Echevarria MJ, Espinosa M, Diaz-Orejas R. Replication and Control of Circular Bacterial Plasmids. Microbiology and Molecular Biology Reviews. 1998;62:434. doi: 10.1128/mmbr.62.2.434-464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SA. Plasmid rolling-circle replication: highlights of two decades of research. Plasmid. 2005;53:126–136. doi: 10.1016/j.plasmid.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seegers JF, Zhao AC, Meijer WJ, Khan SA, Venema G, et al. Structural and functional analysis of the single-strand origin of replication from the lactococcal plasmid pWV01. Mol Gen Genet. 1995;249:43–50. doi: 10.1007/BF00290234. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Russell DW. Molecular cloning : a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 14.Shaw KJ, Rather PN, Hare RS, Miller GH. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty RP, Endy D, Knight TF., Jr Engineering BioBrick vectors from BioBrick parts. J Biol Eng. 2008;2:5. doi: 10.1186/1754-1611-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bujard H, Gentz R, Lanzer M, Stueber D, Mueller M, et al. A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 1987;155:416–433. doi: 10.1016/0076-6879(87)55028-5. [DOI] [PubMed] [Google Scholar]
- 17.Gruss A, Ehrlich SD. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989;53:231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh K, Tu Z, Ohba H, Narumi I. Development of versatile shuttle vectors for Deinococcus grandis. Plasmid. 2009 doi: 10.1016/j.plasmid.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Romanowski G, Lorenz MG, Wackernagel W. Use of polymerase chain reaction and electroporation of Escherichia coli to monitor the persistence of extracellular plasmid DNA introduced into natural soils. Appl Environ Microbiol. 1993;59:3438–3446. doi: 10.1128/aem.59.10.3438-3446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trevors JT. DNA in soil: adsorption, genetic transformation, molecular evolution and genetic microchip. Antonie Van Leeuwenhoek. 1996;70:1–10. doi: 10.1007/BF00393564. [DOI] [PubMed] [Google Scholar]
- 21.Pietramellara G, Ceccherini MT, Ascher J, Nannipieri P. Persistence of transgenic and not transgenic extracellular DNA in soil and bacterial transformation. Riv Biol. 2006;99:37–68. [PubMed] [Google Scholar]
- 22.Bryksin AV, Matsumura I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. BioTechniques. 2010;48:305–307. doi: 10.2144/000113418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno C, Romero J, Espejo RT. Polymorphism in repeated 16S rRNA genes is a common property of type strains and environmental isolates of the genus Vibrio. Microbiology. 2002;148:1233–1239. doi: 10.1099/00221287-148-4-1233. [DOI] [PubMed] [Google Scholar]
- 24.Shevchuk NA, Bryksin AV. Construction of long DNA molecules from multiple fragments using PCR. In: Hughes S, Moody A, editors. PCR (Methods Express Series) London: Scion Publishing; 2007. pp. 197–216. [Google Scholar]
- 25.Jun SR, Sims GE, Wu GA, Kim SH. Whole-proteome phylogeny of prokaryotes by feature frequency profiles: An alignment-free method with optimal feature resolution. Proc Natl Acad Sci U S A. 2010;107:133–138. doi: 10.1073/pnas.0913033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng CH, Yang CH, Chiu HT, Lu CL. Reconstructing genome trees of prokaryotes using overlapping genes. BMC Bioinformatics. 2010;11:102. doi: 10.1186/1471-2105-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JR, Douady CJ, Italia MJ, Marshall WE, Stanhope MJ. Universal trees based on large combined protein sequence data sets. Nat Genet. 2001;28:281–285. doi: 10.1038/90129. [DOI] [PubMed] [Google Scholar]
- 28.Shevchuk NA, Bryksin AV, Nusinovich YA, Cabello FC, Sutherland M, et al. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 2004;32:e19. doi: 10.1093/nar/gnh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Casal J, Caparon MG, Scott JR. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posfai G, Plunkett G, 3rd, Feher T, Frisch D, Keil GM, et al. Emergent properties of reduced-genome Escherichia coli. Science. 2006;312:1044–1046. doi: 10.1126/science.1126439. [DOI] [PubMed] [Google Scholar]
- 31.Bugrysheva JV, Bryksin AV, Godfrey HP, Cabello FC. Borrelia burgdorferi rel is responsible for generation of guanosine-3′-diphosphate-5′-triphosphate and growth control. Infect Immun. 2005;73:4972–4981. doi: 10.1128/IAI.73.8.4972-4981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cangelosi GA, Best EA, Martinetti G, Nester EW. Genetic analysis of Agrobacterium. Methods Enzymol. 1991;204:384–397. doi: 10.1016/0076-6879(91)04020-o. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton RH, Fall MZ. The loss of tumor-initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia. 1971;27:229–230. doi: 10.1007/BF02145913. [DOI] [PubMed] [Google Scholar]
- 34.Harwood CR, Cutting SM. Molecular biological methods for Bacillus. Chichester ; New York: Wiley; 1990. p. xxxv, 581. [Google Scholar]
- 35.Brehm SP, Staal SP, Hoch JA. Phenotypes of pleiotropic-negative sporulation mutants of Bacillus subtilis. J Bacteriol. 1973;115:1063–1070. doi: 10.1128/jb.115.3.1063-1070.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alloing G, Martin B, Granadel C, Claverys JP. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol Microbiol. 1998;29:75–83. doi: 10.1046/j.1365-2958.1998.00904.x. [DOI] [PubMed] [Google Scholar]
- 37.Ottolenghi E, Hotchkiss RD. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J Exp Med. 1962;116:491–519. doi: 10.1084/jem.116.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaneechoutte M, Young DM, Ornston LN, De Baere T, Nemec A, et al. Naturally transformable Acinetobacter sp. strain ADP1 belongs to the newly described species Acinetobacter baylyi. Appl Environ Microbiol. 2006;72:932–936. doi: 10.1128/AEM.72.1.932-936.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]