Abstract
Objectives. We explored whether the introduction of 3 lifesaving innovations introduced between 1989 and 1996 increased, decreased, or had no effect on disparities in Black–White mortality in the United States through 2006.
Methods. Centers for Disease Control and Prevention data were used to assess disease-, age-, gender-, and race-specific changes in mortality after the introduction of highly active anti-retroviral therapy (HAART) for treatment of HIV, surfactants for neonatal respiratory distress syndrome, and Medicare reimbursement of mammography screening for breast cancer.
Results. Disparities in Black–White mortality from HIV significantly increased after the introduction of HAART, surfactant therapy, and reimbursement for screening mammography. Between 1989 and 2006, these circumstances may have accounted for an estimated 22 441 potentially avoidable deaths among Blacks.
Conclusions. These descriptive data contribute to the formulation of the hypothesis that federal laws promote increased disparities in Black–White mortality by inadvertently favoring Whites with respect to access to lifesaving innovations. Failure of legislation to address known social factors is a plausible explanation, at least in part, for the observed findings. Further research is necessary to test this hypothesis, including analytic epidemiological studies designed a priori to do so.
Elimination of Black–White disparities in mortality is a continuing goal of the US Healthy People program. The introduction of lifesaving innovations would be expected to decrease disparities if there were equal dissemination of these innovations between Blacks and Whites but to increase disparities if there were unequal dissemination. For example, the release of the Sabin polio vaccine, a lifesaving innovation, was associated with increased Black–White disparity in poliomyelitis during the 1950s and 1960s.1,2 In the 1970s, Landrigan3 showed that geographic disparities in measles were related to social factors as opposed to biological differences in vaccine effectiveness. More recently, Black–White disparities in mortality increased after the release of lifesaving innovations for treating HIV4 and for treating breast cancer among the elderly.5 Here we describe changes in Black–White mortality disparities in the United States before and after the introduction of surfactant therapy for highly active anti-retroviral treatment (HAART) for treating HIV, treating respiratory distress syndrome in newborns, and Medicare reimbursement for breast cancer screening.
METHODS
We calculated yearly age-, race-, and gender-specific mortality rates before and after authorization of surfactants to treat newborn respiratory distress syndrome in 1989,6 licensure of HAART to treat HIV in 1995–1996,4 and Medicare reimbursement for screening mammography in 1991.5 We grouped each of these innovations into 3 before–after periods: the preinnovation period (period 1 throughout), the near-innovation period (period 2), and the postinnovation period (period 3). For HAART, period 1 was 1987 through 1991, period 2 was 1993 through 1995, and period 3 was 1997 through 2005. For screening mammography, period 1 was 1984 through 1990 (the year before initiation of Medicare reimbursement), period 2 was 1992 (the year after initiation of Medicare reimbursement) through 1998 (reflecting the variable period of time needed to achieve a reduction in mortality in screening studies7–9), and period 3 was 1999 through 2005. Finally, for respiratory distress syndrome, period 1 was 1979 through 1987, period 2 was 1988 through 1989, and period 3 included 1990 through 1998 and 1999 through 2005.
We used 3 measures of association between introduction of a lifesaving innovation and Black–White mortality rates. Relative risk, or the mortality rate ratio (MRR), is the mortality rate among Blacks divided by the corresponding rate among Whites. The MRR indicates the likelihood of dying among Blacks relative to the likelihood among Whites. The risk difference (a measure of the absolute excess risk of dying among Blacks as compared with the risk among Whites) is the mortality rate among Blacks subtracted by the corresponding rate among Whites. We also defined excess mortality among Blacks as the number of deaths that actually occurred subtracted by the number that might have occurred if 10-year age-specific Black–White MRRs had remained unchanged from the year after the implementation of a lifesaving innovation up until 2006.
All of the measures just described were based on national and age-, race-, and gender-specific mortality rate data obtained from the US Centers for Disease Control and Prevention's public Web site. On this site, the International Classification of Diseases (ICD) codes used for HIV through 1998 were O42 through O44 (ICD-9); from 1999 onward, the codes used were B20 through B24 (ICD-10). The corresponding codes for breast cancer were 174 and C50, and the corresponding codes for respiratory distress syndrome were 769 and P22.0.10,11
We calculated 95% confidence intervals (CIs) for each MRR and risk difference.12–14 We defined statistical significance as lack of overlap in the 95% CIs from one period to the next. We also compared Black and White age-adjusted mortality rates and Black–White MRRs before and after the introduction of each innovation in US counties with data classified by the Centers for Disease Control and Prevention as reliable. In the case of HIV among men, we focused on the period 3 years before (1990–1993) and the 3 years after (1999–2002) the release of HAART. A wider range was used for HIV among women (1987–1993 and 1999–2004) and for respiratory distress syndrome (1980–1988 and 1990–1998) so that more reliable county-level data could be obtained. For screening mammography, we focused on 2 periods of 5 years each after the onset of Medicare reimbursement (1992–1996 and 2000–2004). We included all counties with reliable mortality rate data in both periods.
In our analyses of HIV among men, we included 140 counties (80 in the South, 34 in the Northeast, 12 in the Midwest, 14 in the West) accounting for 17 137 of 21 170 Black deaths nationwide (81%) in the period reviewed.10,11 In analyses focusing on women with HIV, we included 52 counties (16 in the South, 23 in the Northeast, 3 in the Midwest, 10 in the West) accounting for 9819 of 17 184 Black deaths nationwide (55%) in the same period.10,11
In analyses of respiratory distress syndrome, we included 42 counties (22 in the South, 9 in the Northeast, 9 in the Midwest, 2 in the West) accounting for 2915 of 5649 Black deaths nationwide (52%).10,11 Finally, in analyses of breast cancer, we included 83 counties (40 South, 18 Northeast, 19 Midwest, 5 West) accounting for 6362 of 11 829 Black deaths nationwide (54%).10,11 We conducted zero-corrected, negative binomial regression analyses to assess HIV, respiratory distress syndrome, and breast cancer mortality rates among Blacks in each postinnovation period after adjustment for potential confounding factors, including socioeconomic status (SES), residential segregation, percentage of military veterans, and percentage of individuals speaking little or no English.15,16
To assess preimplementaton–postimplementation changes, we used a 4-quadrant scattergram depicting percentage changes in Black mortality after each innovation and corresponding changes in the Black–White MRR. The 4 quadrants were as follows: negative–negative (lower Black mortality rate and Black–White MRR), negative–positive (lower Black mortality rate and higher MRR), positive–positive (higher Black mortality rate and MRR), and positive–negative (higher Black mortality rate and lower MRR). We then constructed disease-specific multinomial regression models17 to identify demographic characteristics that might be associated with the counties falling in each quadrant. We used as the referent the quadrant defined by lower Black mortality rates and higher MRRs, the most commonly occurring pattern in the study counties.
We used odds ratios to estimate the magnitude of association between demographic characteristics (demographic data were derived from the 2000 US Census) in nonreference and reference counties.18 Selection of factors for modeling was guided by diffusion of innovation theory, which emphasizes SES19,20; by social determinants theory, which emphasizes social factors as fundamental determinants of mortality21; and by additional review of geographic variations in Black mortality.22
Each model comprised 5 factors, including 2 factors previously shown to be associated with increased Black mortality: low SES,23,24 represented by a composite index, and Black–White income inequality,25,26 represented as the Black–White poverty rate ratio. We also included 2 factors variously associated with both increased and decreased mortality among Blacks and other vulnerable populations, namely residential segregation22,26–28 (spatial proximity index29,30) and immigrant status31–33 (percentage of Blacks speaking little or no English). Finally, we included gender-specific percentages of civilian military veterans (male veterans aged 18–64 years for HIV among men, female veterans aged 18–64 years for HIV among women and for respiratory distress syndrome, and female veterans aged 65 years or older for breast cancer) given that military service and health care benefits available to veterans may be associated with different patterns of HIV,34 cancer,35,36 and infant mortality37,38 than are those found in the general population.
Because the percentage of Blacks with annual incomes below the poverty line, the percentage of Blacks aged 25 years or older without high school diplomas, and Black per capita income were highly correlated, we constructed a composite SES index that was the simple arithmetic average of these 3 components.39,40 Because the US Census Bureau provides race- and gender-specific percentages of individuals aged 25 years or older with less than a high school education, we used the corresponding percentages in grouping Black men (for HIV), Black women (for HIV and breast cancer), and the overall Black population (for respiratory distress syndrome).
Using SAS version 9.1 software (SAS Institute, Cary, NC) and data from the 2000 US Census, we ranked all US counties and county equivalents in ascending order for each index component.23 We then summed each county's rank with respect to the percentage of Black women with breast cancer, the percentage of Black residents overall with HIV and respiratory distress syndrome, the percentage of Blacks aged 25 years or older without a high school education, the percentage of Blacks with annual incomes below the poverty line, and (because a higher rank indicated a higher income) the complement of rank for per capita income among Blacks.
RESULTS
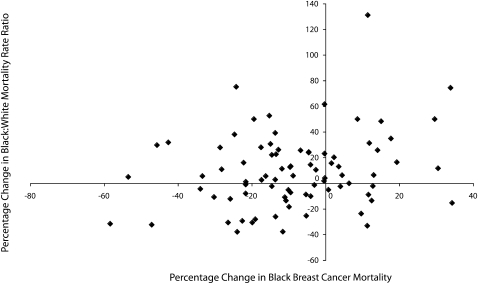
Table 1 presents MRR and risk difference data (Table S1, available as an online supplement to this article at http://www.ajph.org, presents data on excess mortality). Figure 1, a scattergram, shows county breast cancer distributions. With the exception of breast cancer data, all final models (Tables 2 and 3) included only statistically significant variables.
TABLE 1.
Black–White Disparities in Mortality Associated With HIV, Breast Cancer, and Respiratory Distress Syndrome: United States, 1979–2005
| Age Group and Perioda | Mortality Rate Ratio (95% CI) | Risk Difference (95% CI) |
| HIV among men | ||
| 25–34 y | ||
| 1987–1991 | 2.98 (2.91, 3.05) | 4.90 (4.75, 5.05) |
| 1993–1995 | 3.40 (3.32, 3.49) | 8.73 (8.48, 8.98) |
| 1997–2005 | 6.28 (6.08, 6.49) | 25.11 (21.47, 25.82) |
| 35–44 y | ||
| 1987–1991 | 3.21 (3.14, 3.29) | 7.80 (7.58, 8.02) |
| 1993–1995 | 3.94 (3.87, 4.02) | 16.53 (16.18, 16.89) |
| 1997–2005 | 6.20 (6.07, 6.32) | 6.24 (6.13, 6.35) |
| 45–54 y | ||
| 1987–1991 | 2.84 (2.73, 2.95) | 3.97 (3.77, 4.18) |
| 1993–1995 | 4.51 (4.38, 4.61) | 11.90 (11.52, 12.27) |
| 1997–2005 | 8.36 (8.17, 8.56) | 70.43 (69.13, 71.73) |
| 55–64 y | ||
| 1987–1991 | 3.17 (2.97, 3.38) | 18.81 (17.24, 20.38) |
| 1993–1995 | 5.05 (4.78, 5.34) | 5.49 (5.18, 5.80) |
| 1997–2005 | 11.08 (10.65, 11.52) | 47.88 (46.48, 49.29) |
| 65–74 y | ||
| 1987–1991 | 3.49 (3.06, 3.97) | 6.74 (5.64, 7.84) |
| 1994–1995 | 7.36 (6.49, 8.35) | 26.12 (23.13, 29.11) |
| 1997–2005 | 12.29 (11.41, 13.25) | 21.72 (20.51, 22.93) |
| HIV among women | ||
| 25–34 y | ||
| 1987–1991 | 8.85 (8.35, 9.39) | 16.70 (15.90, 17.42) |
| 1993–1995 | 8.13 (7.18, 8.55) | 34.71 (33.39, 36.04) |
| 1997–2005 | 14.02 (13.28, 14.81) | 16.53 (16.02, 17.05) |
| 35–44 y | ||
| 1987–1991 | 11.21 (10.50, 11.97) | 19.15 (18.29, 20.01) |
| 1993–1995 | 9.94 (9.49, 10.41) | 50.72 (49.07, 52.37) |
| 1997–2005 | 13.25 (12.76, 13.76) | 29.76 (29.09, 30.44) |
| 45–54 y | ||
| 1987–1991 | 9.28 (8.23, 10.45) | 76.13 (69.23, 83.04) |
| 1993–1995 | 10.76 (9.95, 11.64) | 26.67 (25.17, 28.16) |
| 1997–2005 | 15.69 (14.95, 16.46) | 23.87 (23.19, 24.55) |
| 55–64 y | ||
| 1987–1991 | 6.42 (5.39, 7.65) | 32.38 (27.15, 37.41) |
| 1993–1995 | 10.81 (9.40, 12.44) | 11.72 (10.53, 12.91) |
| 1997–2005 | 15.18 (13.90, 16.57) | 104.60 (98.86, 110.40) |
| Breast cancer | ||
| 45–54 y | ||
| 1984–1990 | 1.34 (1.31, 1.39) | 1.55 (1.39, 1.72) |
| 1992–1998 | 1.54 (1.50, 1.58) | 2.05 (1.91, 2.19) |
| 1999–2005 | 1.72 (1.69, 1.77) | 2.10 (2.00, 2.21) |
| 55–64 y | ||
| 1984–1990 | 1.10 (1.07, 1.12) | 0.81 (0.59, 1.03) |
| 1992–1998 | 1.29 (1.26, 1.13) | 1.92 (1.72, 2.19) |
| 1999–2005 | 1.45 (1.41, 1.48) | 2.47 (2.29, 2.65) |
| 65–74 | ||
| 1985–1990 | 0.81 (0.79, 0.83) | −0.25 (−0.28, −0.22) |
| 1992–1998 | 1.12 (1.09, 1.15) | 0.12 (0.09, 0.15) |
| 1999–2005 | 1.19 (1.06, 1.13) | 1.58 (1.34, 1.83) |
| 75–84 y | ||
| 1985–1990 | 0.90 (0.88, 0.93) | −0.14 (−0.17, −0.11) |
| 1992–1998 | 1.05 (1.02, 1.07) | 0.06 (0.02, 0.10) |
| 1999–2005 | 1.10 (1.07, 1.13) | 0.12 (0.09, 0.16) |
| ≥85 y | ||
| 1984–1990 | 0.94 (0.89, 0.99) | −0.11 (−0.20, −0.03) |
| 1992–1998 | 1.00 (0.96, 1.04) | −0.00 (−0.08, 0.08) |
| 1999–2005 | 1.10 (1.07, 1.14) | 0.19 (−0.12, 0.27) |
| Newborn respiratory distress syndrome (infancy) | ||
| 1979–1987 | 1.80 (1.76, 1.85) | 7.97 (7.59, 8.31) |
| 1988–1989 | 2.24 (2.13, 2.34) | 8.93 (8.21, 9.65) |
| 1990–1998 | 2.72 (2.64, 2.81) | 6.23 (5.96, 6.49) |
| 1999–2005a | 2.79 (2.65, 2.93) | 3.00 (2.80, 3.19) |
Note. CI = confidence interval.
For HIV, time periods were preinnovation, near innovation, and postinnovation, respectively. Two postinnovation periods, 1992–1998 and 1999–2005, are noted for breast cancer, and 2 postintervention periods, 1990–1998 and 1999–2005 are noted for respiratory distress syndrome.
FIGURE 1.
Scattergram of age-adjusted percentage changes in Black breast cancer mortality rates and Black–White mortality rate ratios for breast cancer: 82 US counties in 1992–1996 and 2000–2004.
TABLE 2.
Results of Negative Binomial Models Assessing Variations in Postinnovation Black Mortality Rates for Selected Diseases: US Counties with Reliable Rates, 1999–2005
| Disease and Measure | Wald χ21 | P | Mortality Rate (95% CI) |
| HIV among men | |||
| Socioeconomic indexa | 9.84 | .002 | 0.03 (0.01, 0.04) |
| Spatial proximity indexa | 7.08 | .008 | −1.17 (−2.02, −0.31) |
| % of Black male military veterans aged 18–64 ya | 22.60 | .002 | −4.04 (−5.70, −2.37) |
| HIV among women | |||
| Socioeconomic indexa | 763.72 | <.001 | 0.056 (0.02, 0.059) |
| % of Blacks who speak little or no English | 11.17 | <.001 | 15.09 (6.24, 23.94) |
| Breast cancer: % of Blacks who speak little or no English | 2.78 | .096 | −2.12 (−4.61, 0.37) |
| RDS: Socioeconomic indexa | 8.33 | .004 | 0.04 (0.01, 0.07) |
Note. CI = confidence interval; RDS = respiratory distress syndrome.
1-unit increase.
TABLE 3.
Results of Multinomial Models Comparing Patterns of Change in Black Mortality in 4 County Categories Before and After Implementation of Lifesaving Health Innovations: US Counties with Reliable Rates, 1999–2005
| Disease, Measure, and Comparison | Waldχ21 | P | Odds Ratio (95% CI) |
| HIV among men | |||
| Black–White poverty rate ratio: Positive–positive vs negative–positive | 8.24 | .004 | 2.18 (1.28, 3.70) |
| HIV among women | |||
| Spatial proximity index: Negative–negative vs negative–positive | 5.73 | .017 | 1.09 (1.02, 1.17) |
| % of Black female military veterans aged 18–64 y | |||
| Positive–positive vs negative–positive | 6.06 | .01 | 0.20 (0.00, 0.40) |
| Positive–negative vs negative–positive | 6.67 | .001 | 0.01 (<0.001, 0.31) |
| Breast cancer | |||
| % of Black female military veterans aged ≥ 65 y: Negative–negative vs negative–positive | 11.22 | <.001 | 0.98 (0.98, 0.99) |
| Respiratory distress syndrome | |||
| Socioeconomic index | |||
| Negative–negative vs negative–positive | 12.75 | <.001 | 0.98 (0.96, 0.99) |
| Positive–negative vs negative–positive | 14.47 | <.001 | 0.95 (0.93, 0.98) |
Note. CI = confidence interval; MRR = mortality rate ratio; SES = socioeconomic status. The 4 county categories were defined according to mortality rates in the preimplementation and postimplementation as follows: negative–negative (lower Black mortality rate and Black–White MRR), negative–positive (lower Black mortality rate and higher MRR), positive–positive (higher Black mortality rate and MRR), and positive–negative (higher Black mortality rate and lower MRR).
HIV
Men. There was no overlap in MRR CIs between periods 1 and 2 or between periods 2 and 3 for any of the age groups. Risk difference results were similar for men aged 25 to 34 years and for men aged 45 to 54 years, but there were significant declines from period 2 to period 3 among men aged 35 to 44 years and aged 65 to 74 years and from period 1 to period 2 among men aged 55–64 years; the latter group showed a significant risk difference increase from period 2 to period 3. From 1997 through 2006 (Table S1, available as an online supplement to this article at http://www.ajph.org), total numbers of excess deaths among Black men across age groups were as follows: 3559 (25–34 years), 3761 (35–44 years), 4322 (45–54 years), 1414 (55–64 years), and 280 (65–74 years).
Table 2 shows a significant positive trend between the SES index and the mortality rate among Blacks. By contrast, there were significant negative trends between the Black mortality rate and both the percentage of Black men aged 18–64 years who were civilian military veterans and the spatial proximity index. Table 3 shows that there was no statistically significant association between any of the county sociodemographic factors and the ideal outcome (i.e., a decline in both Black mortality and the Black–White MRR). The association with the Black–White poverty rate ratio was stronger, however, in counties where both the Black mortality rate and the MRR increased than it was in counties in which the Black mortality rate declined and the MRR increased.
Women. Unlike that among men, there was no MRR CI separation from period 1 to period 2 among women aged 25 to 54 years. As was true for men, however, there was no overlap in MRR CIs from period 2 to period 3. Risk differences increased among women aged 25 to 44 years and decreased among women aged 45 to 64 years between periods 1 and 2; also, risk differences decreased among women aged 25 to 64 years between periods 2 and 3. There were a total of 4662 excess deaths among Black women (Table S1, available as an online supplement to this article at http://www.ajph.org) from 1997–2006.
Table 2 shows a statistically significant positive trend between the Black mortality rate and both the SES index and the percentage of Blacks speaking little or no English. Table 3 indicates significantly greater residential segregation (as measured via the spatial proximity index) in counties with declines in both mortality rates and MRRs than in counties with declining mortality rates and increasing MRRs.
Breast Cancer
Figure 1 shows that the predominant preimplementation–postimplementation pattern was declining mortality and increasing MRR; the same pattern was also observed for HIV among men and women and for respiratory distress syndrome. Through age 74 years, there was no overlap in MRR CIs between period 1 and period 2. Risk difference increased significantly between periods 2 and 3 for ages 55 to 74 years. There were no disparities in MRRs among women aged 65 years or older until period 2, when no overlap in 95% CIs was evident for those aged 65–74 years and aged 75 to 84 years (Table 1); changes in the younger age groups reflected ongoing widening of MRRs that began before 1991, the year in which Medicare reimbursement was initiated (data not shown). Risk differences increased from period 1 to period 2 for each age group through age 74 years but were more variable between periods 2 and 3. There were a total of 3353 excess deaths among elderly Black women from 1992 through 2006 (Table S1, available as an online supplement to this article at http://www.ajph.org).
No statistically significant associations were found with respect to mortality among elderly Black women in period 3 (Table 2). The percentage of Black female civilian military veterans was significantly lower in counties with period 3 declines in Black mortality rates and MRRs than in counties with declining Black mortality rates and increasing MRRs (Table 3).
Respiratory Distress Syndrome
There was no overlap in the MRR CIs from period 1 to period 2 or from period 2 to period 3. Risk differences peaked in period 2 and declined thereafter (Table 1). There were an estimated 1070 premature deaths among Blacks (Table S1, available as an online supplement to this article at http://www.ajph.org). A statistically significant positive trend was observed between the SES index and the period 3 Black mortality rate (Table 2). The SES index was significantly lower in counties with declines in both Black mortality rates and MRRs from period 1 to period 3 than in counties with declines in Black mortality rates and increases in MRRs from period 1 to period 3 (Table 3).
DISCUSSION
Our descriptive data show that disparities in Black–White mortality increased after the introduction of 3 lifesaving innovations, each of which affected a different segment of the population and was mandated by US federal legislation. Our data indicate that federal laws may have contributed to the increased disparities in Black–White mortality observed. Specifically, the failure of key laws to account for recognized social determinants may have inadvertently favored Whites with respect to access to those lifesaving innovations. Existing societal efforts to equalize medical innovations across race, gender, and age through law may therefore do little to deliver equal access to lifesaving treatments.
Although research has linked many social determinants to increased racial disparities in mortality,21,22 these determinants have not generally been disease specific. By contrast, our data show disease-specific, asynchronous increases that are temporally linked to federally mandated lifesaving innovations. To our knowledge, there was no corresponding general increase in such disparities. At least 2 plausible hypotheses are consistent with our descriptive results. One hypothesis is that federal legislation inadvertently promoted the observed racial disparities, and the other is that federal legislation impeded the identification of these problems.
Laws that are relevant to HAART and surfactants include the Food, Drug, and Cosmetic Act (52 Stat 1040) and the Public Health Service Act (42 USC §§ 241 and 247, chapter 6a, subchapter 2). Laws related to reimbursement for screening mammography include the 2 aforementioned laws along with the Social Security Act (42 USC 7) and the Civil Rights Act (78 Stat 241).41–43 Laws relevant to postinnovation surveillance and control include Food and Drug Administration (FDA) legislation and the Anti-Lobbying Act (Pub L No. 80-772).44
The 1971 Kefauver–Harris amendments to the Food, Drug, and Cosmetic Act called for the FDA to be the gatekeeper for new medications. The Public Health Service Act acknowledges this gatekeeper role, and the FDA requires efficacy studies for licensure. FDA requirements are embedded in Title 18 of the Social Security Act, so Medicare may pay only for FDA-approved innovations. The Civil Rights Act of 1964 (42 USC § 2000d et seq) stipulates that federal funds cannot be allocated to racially segregated health care services. Collectively, these laws aim to guarantee safe and equal access to lifesaving health care innovations across race and gender. Medicare and the Civil Rights Act are credited with ending segregated health care in the southern United States.45,46
Unfortunately, the narrow definitions of drug efficacy and medical assistance in those laws may be biased against equitable diffusion of innovations. Medicare law defines medical assistance only in terms of payments to health care providers, whereas FDA law addresses only biological safety, efficacy, and effectiveness. By contrast, current theory suggests that the causes of racial disparities in health are fundamentally social, with health care and the biological characteristics of medications and procedures provided through the health care system being of secondary importance.21,47 Related evidence shows that lifesaving innovations may have adverse social effects.1–5
Medicare coverage of mammography was a positive step that benefited many women (both White and Black), but inequalities may have been exacerbated because the benefit was so narrowly focused on providing a payment for services that were already unequally distributed. The intent of the law is for both Black and White women to benefit from Medicare-funded mammograms, but White women may have been in a better position to do so, in part because they face fewer nonmonetary barriers. There is evidence that cost remains a barrier to predominantly Black low-income populations, even those with Medicare benefits.48,49 Moreover, Blacks more often identify noncash social barriers as deterrents to cancer screening.50,51
As noted by Clark et al.,50 Blacks are more likely than were Whites to face logistical barriers such as poor transportation and competing time demands, both of which may be augmented by fear, perceptions of risk, and interactions between such factors and negative attributes of providers and health systems (e.g., provider time pressures and fragmented care).50-56 If cash inadequacy and noncash barriers exert greater force among Blacks than among Whites, then Medicare's definitions of assistance could bias acquisition of screening mammography in favor of Whites even if health care delivery is not racially segregated. If our hypothesis is correct, future expansions of health care coverage will need to acknowledge this unintended consequence and create structural support for more equitable diffusion, distribution, and benefit.
The Food, Drug, and Cosmetic Act may exert bias by focusing exclusively on biological effects and excluding socially adverse effects from efficacy and effectiveness requirements. In previous research, FDA efficacy restrictions based on comorbidity have been associated with Black disparities in clinical trial participation,57,58 at least in part because comorbidity may be more likely to occur among sociodemographically disadvantaged groups. Our data may reflect this situation with respect to HAART (among men) and respiratory distress syndrome. The absence of this finding for HAART among women is also supportive given that women's clinical trial participation was low (although the clinical trial protocol did not specifically exclude women57). Exclusion of socially adverse effects from postinnovation surveillance may lead to bias in effectiveness assessments.
Our data also raise the alarming specter that 22 421 premature deaths from HIV, breast cancer, and respiratory distress syndrome among Blacks may have occurred from 1990 through 2006 as a result of unequal diffusion of lifesaving innovations. If the average number of premature deaths in each age group between 2002 and 2006 (the most recent year for which data are available) continued through 2009, an additional 6252 deaths may have occurred (28 673 deaths in all).
The US Public Health Service (PHS) identified the phenomenon of increased racial disparities following the introduction of a health care innovation1,2 and recognized that social factors may lead to variable population effects from the same biological agent.3 This phenomenon may have suggested a similar outcome when new innovations such as HAART, surfactants, and screening mammography were introduced. Specifically, as noted in the introduction, the PHS showed that introduction of Sabin vaccine led to increased racial disparities in poliomyelitis1,2 and that social factors influenced the effectiveness of the measles vaccine.3 By contrast, published PHS surveillance reports showing increased Black–White disparities after the introduction of HAART have included only general comments (e.g., “mortality rates vary by region and race”59[p3] and “mortality rates varied by region and race … and age group and race”60[p5]).
In addition, a National Cancer Institute report using PHS data on breast cancer mortality disparities between Black and White women did not specifically address women aged 65 years or older (women's age categories were 60–69 years, 70–79 years, and 80–89 years).61 A later report focused on overall age-adjusted rates.62 Many federally supported studies (e.g., Smith-Bindman et al.63) have noted Medicare's inability to prevent disparities in breast cancer mortality; however, to our knowledge, none of these studies have hypothesized that Medicare may promote disparities.
Regarding surfactants, a 10% deficit (not statistically significant) in the administration of surfactant therapy was observed among Black infants in 1 midwestern city even after potential confounders had been taken into account. This finding was reported in a leading scientific journal with the caveat that the results needed to be interpreted with caution.64 However, we have been unable to find the systematic national surveillance and control that might have indicated whether the cautious optimism about surfactant therapy was justified. We hypothesize that the paradoxical contrasts between PHS investigations of the Sabin1,2 and measles3 vaccines and PHS investigations into the public health impact of HAART, surfactants, and reimbursement for screening mammography reflect, in part, inadvertent impediments to public health surveillance that may appear in the Anti-Lobbying Act.
According to the Anti-Lobbying Act and associated code, it is a felony to use federal funds directly or indirectly to pay for any personal service, advertisement, printed matter, or other device intended to influence a member of Congress, a jurisdiction, or an official of any government to favor or oppose (by vote or otherwise) any legislation, ratification, policy, or appropriation unless Congress specifically requests such advice. To our knowledge, Congress has not authorized such funding and, despite its 1948 origin, there is evidence that the Anti-Lobbying Act retains considerable force at the PHS (I. Arias, Centers for Disease Control and Prevention, written communication, May 2008). By contrast, the present report was authorized through congressional approval for scientific peer-reviewed research on the scientific basis for US racial disparities.
Limitations
Several limitations should be mentioned. Because our data are descriptive, they contribute relevant information to the formulation of but not the testing of hypotheses.65 Also, our data were based on death certificate information and thus are subject to the usual limitations associated with such information.66 However, death certificates have been found to provide valid estimates of HIV mortality.4 Reports as to breast cancer validity are mixed, and concern has been expressed regarding validity of estimates among the elderly.67,68 Our multivariable analyses were limited by the relatively small number of counties with reliable mortality rate data, particularly for respiratory distress syndrome, and the results of our analyses should be interpreted with caution. The results may suggest, however, that factors associated with communities’ success in diffusion of innovations are not the simple opposite of those factors associated with postinnovation mortality.
Moreover, although our ecological data show associations between such factors as county-level SES and Black mortality, the data cannot address the question of whether the same is true at the individual level. Finally, the results have other plausible alternative explanations, including the possibility that the disparities observed depend on biological differences such as the earlier maturation of surfactants among Blacks64 and the possibility that environmental factors are associated with greater comorbidity among Blacks independent of SES. Therefore, although confirmation of the hypotheses proposed in this article might lead to calls for fundamental change, the present data are not a suitable basis for policy.
Analytic epidemiological studies comparing racial differences in diffusion of innovations and individual mortality would provide important and timely data to test hypotheses concerning inadvertent promotion of racial disparities in mortality by both Medicare (e.g., because the narrow definition of medical assistance as cash may favor acquisition of services by those for whom cash alone is the major barrier) and the Food, Drug, and Cosmetic Act (e.g., because failure to consider that the introduction of an innovation may have adverse social effects, as well as biological effects, may promote the persistence of social effects).
Such research would also provide a means for the following: (1) distinguishing the relative effects of cash and noncash barriers to diffusion of innovations among Blacks and Whites and delineating causal pathways for such barriers (should they exist); (2) estimating confounding attributable to social or biological factors; (3) explaining the extensive geographical variation observed in our data (including results consistent with Landrigan's finding that the same biological agent may produce significantly different health outcomes in biologically comparable communities3); and (4) explaining differences in factors predicting Black mortality and those predicting postinnovation reductions in both Black mortality and Black–White MRRs. Evaluation of our hypothesis concerning the anti-lobbying law would require more qualitative approaches, including surveys, focus groups, and interviews with key informants.
In our analyses, we used different measures of association to estimate Black–White disparities because each confers importantly relevant and complementary information. In the past, the risk difference measure has been preferred by some for policy decisions, partly because that measure does not reflect baseline values.68 It is not clear, however, whether risk difference would be appropriate for tracking disparities. For example, if mortality rates declined from 4 to 3 per 1000 population among Blacks and from 2 to 1 per 1000 population among Whites, this measure would show no difference, even though the percentage of decline among Blacks (25%) was only half that among Whites. The MRR, with an increase of 50% in this example, does reflect such inequities and also enables estimation of excess mortality. Although risk differences were less consistent than MRRs in our data, the specific ways in which such values relate to unequal diffusion of innovations will remain unknown until appropriate analytic studies are done.
Conclusions
In general, our data contribute to a growing body of evidence that US disparities in mortality are most strongly associated with diseases that are preventable or treatable.69 In other words, lifesaving breakthroughs in screening, diagnosis, and treatment may predictably intensify structural inequities in delivery or diffusion. Elimination of poverty would be a long-term solution.21 In the interim, however, our data are compatible with the possibility that conscious, targeted efforts, including possible modifications of federal law, may be needed to ensure more equitable distribution of innovations.
If our primary hypothesis is valid, then strategies designed to achieve equality in health care delivery, real-world effectiveness, and population-level health outcomes should become an explicit goal of all publicly funded programs at each stage, including approval, licensure, payment, and delivery. Going forward, public health may need to take a more assertive role in surveillance of and structural support for equitable diffusion and benefit of new innovations to ensure equity in health outcome improvements across all segments of the population. Further research is necessary to test this hypothesis, including analytic epidemiological studies designed a priori to do so.
Acknowledgments
This study was supported in part by the National Center on Minority Health and Health Disparities (grant 2P20MD000516-05A1) and the Meharry–State Farm Alliance.
Special thanks to the anonymous reviewers and to Maureen Sanderson and Paul Henkel of Meharry Medical College for their comments. Thanks to Linda Sparks of Meharry Medical College for editorial advice.
Note. The views expressed do not necessarily reflect the official policies of the US Department of Health and Human Services, nor does mention of trade names, commercial practices, or organizations imply endorsement by the US government. Also, the views expressed do not necessarily reflect the official views of Meharry Medical College or the State Farm Insurance Company.
Human Participant Protection
No protocol approval was needed because the information used is publicly available from the US Centers for Disease Control and Prevention and the US Census Bureau.
References
- 1.Chin TD, Marine WM. The changing pattern of poliomyelitis observed in two urban epidemics. Public Health Rep. 1961;76:553–563 [PMC free article] [PubMed] [Google Scholar]
- 2.Chin TDY, Marine WM, Hall EC, Gravelle CR, Speers JF. Poliomyelitis in Des Moines, Iowa, 1959: the influence of Salk vaccination on the epidemic pattern and the spread of the virus in the community. Am J Hyg. 1961;74:67–69 [DOI] [PubMed] [Google Scholar]
- 3.Landrigan PJ. Epidemic measles in a divided city. JAMA. 1972;221(6):567–570 [PubMed] [Google Scholar]
- 4.Levine RS, Briggs NC, Kilbourne BS, et al. Black:White mortality from HIV in the United States before and after introduction of highly active antiretroviral therapy in 1996. Am J Public Health. 2007;97(10):1884–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RS, Kilbourne BE, Baltrus PA, et al. Black-white disparities in elderly breast cancer mortality before and after implementation of Medicare benefits for screening mammography. J Health Care Poor Underserved. 2008;19(1):103–134 [DOI] [PubMed] [Google Scholar]
- 6.More help for preemies. FDA Consumer. February 1990:1 [Google Scholar]
- 7.Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld G, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomized trials. Lancet. 2002;359(9310):909–919 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher SW, Black W, Harris R, et al. Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst. 1993; 85(20):1644–1656 [DOI] [PubMed] [Google Scholar]
- 9.Canadian Cancer Society Progress against cancer in the past few decades. Available at: http://www.cancer.ca/sitecore/content/Home.aspx. Accessed July 20, 2010
- 10.Centers for Disease Control and Prevention Compressed mortality file 1979–1998. Available at: http://wonder.cdc.gov/cmf-icd9.html. Accessed July 20, 2010
- 11.Centers for Disease Control and Prevention Compressed mortality file 1999–2006. Available at: http://wonder.cdc.gov/cmf-icd10.html. Accessed July 20, 2010
- 12.Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health. Available at: http://www.OpenEpi.com. Accessed July 20, 2010
- 13.Martin D, Austin H. An efficient program for computing conditional maximum likelihood estimates and exact confidence limits for a common odds ratio. Epidemiology. 1991;2(5):359–362 [DOI] [PubMed] [Google Scholar]
- 14.Martin D, Austin H. Exact estimates for a rate ratio. Epidemiology. 1996;7(1):29–33 [DOI] [PubMed] [Google Scholar]
- 15.Slymen DJ, Ayala GX, Arredondo EM, Elder JP. A demonstration of modeling count data with an application to physical activity. Epidemiol Perspect Innov. 2006;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner W, Mulvey EP, Shaw EC. Regression analysis of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118(3):392–404 [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons Inc; 1989 [Google Scholar]
- 18.CensusCD Estimates (2003) and Projections (2008) Bellmawr, NJ: Easy Analytics Software; 2004 [Google Scholar]
- 19.Katz E. The social itinerary of technical change: two studies on the diffusion of innovation. Hum Organ. 1961;20(2):70–82 [Google Scholar]
- 20.Rogers E. Diffusion of Innovations. 5th ed New York, NY: Free Press; 2003 [Google Scholar]
- 21.Marmot M, Wilkinson DL, Social Determinants of Health. 2nd ed Oxford, England: Oxford University Press; 2006 [Google Scholar]
- 22.Levine RS, Briggs NC, Husaini BA, Hennekens CH. Geographic studies of black-white mortality. : Satcher D, Pamies RJ, Multicultural Medicine and Health Disparities. New York, NY: McGraw-Hill Medical Publishing Division; 2006:33–104 [Google Scholar]
- 23.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kindig DA, Seplaki CL, Libby DL. Death rate variation in US subpopulations. Bull World Health Organ. 2002;80(1):9–15 [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin DK, Stokes CS. Income inequality and mortality in US counties: does minority racial concentration matter? Am J Public Health. 2002;92(1):99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper RS, Kennelly JF, Durazo-Arvizu R, et al. Relationship between premature mortality and socioeconomic factors in black and white populations of US metropolitan areas. Public Health Rep. 2001;116(5):464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart KD, Kunitz SJ, Sell RR, Mukamel DB. Metropolitan governance, residential segregation, and mortality among African Americans. Am J Public Health. 1998;88(3):434–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polednak AP. Mortality among blacks living in census tracts with public housing projects in Hartford, Connecticut. Ethn Dis. 1998;8(1):36–42 [PubMed] [Google Scholar]
- 29.Massey D, Denton N. The dimensions of residential segregation. Soc Forces. 1988;67(2):281–315 [Google Scholar]
- 30.Arizona State University, GeoDa Center for Geospatial Analysis and Computation. Diversity and social segregation. Available at: http://geodacenter.asu.edu/node/131. Accessed July 20, 2010
- 31.Gany F, Trinh-Shevrin C, Aragones A. Cancer screening and Haitian immigrants: the primary care provider factor. J Immigr Minor Health. 2008;10(3):255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh GK, Siahpush M. Ethnic-immigrant differentials in health behaviors, morbidity, and cause-specific mortality in the United States: an analysis of two national data bases. Hum Biol. 2002;74(1):83–109 [DOI] [PubMed] [Google Scholar]
- 33.Page JB. The concept of culture: a core issue in health disparities. J Urban Health. 2005;82(suppl 3):iii35–iii43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the Veterans Aging Cohort 3-Site Study and VA administrative data. Am J Public Health. 2003;93(10):1728–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(1):25–31 [DOI] [PubMed] [Google Scholar]
- 36.Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kugler JP, Connell FA, Henley CE. Lack of difference in neonatal mortality between blacks and whites served by the same medical care system. J Fam Pract. 1990;30(3):281–287 [PubMed] [Google Scholar]
- 38.Alexander GR, Baruffi G, Mor JM, Kieffer EC, Hulsey TC. Multiethnic variations in the pregnancy outcomes of military dependents. Am J Public Health. 1993;83(12):1721–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackenbach JP, Kunst AE. Health inequalities in modern societies and beyond. Soc Sci Med. 1997;44(6):757–771 [DOI] [PubMed] [Google Scholar]
- 40.Symanski E, Stock TH, Tee PG, Chan W. Demographic, residential, and behavioral determinants of elevated exposures to benzene, toluene, ethylbenzene, and xylenes among the US population: results from 1999 to 2000 NHANES. J Toxicol Environ Health A. 2009;72(14):915–924 [DOI] [PubMed] [Google Scholar]
- 41.Federal Food, Drug, and Cosmetic Act. Available at: http://www.fda.gov/opacom/laws/fdact/fdctoc.htm. Accessed April 27, 2009. [PubMed]
- 42.Public Health Service Act on the Food and Drug Administration Web Site. Available at: http://www.fda.gov/RegulatoryInformation/Legislation/ucm148717.htm. Accessed November 16, 2009.
- 43.Compilation of the Social Security Laws. Available at: http://www.ssa.gov/OP_Home/ssact/comp-ssa.htm. Accessed April 27, 2009.
- 44.Anti-Lobbying Act. Available at: http://codes.lp.findlaw.com/uscode/18/I/93/1913. Accessed November 16, 2009.
- 45.Height DI. Thirty years of Medicare: a personal reflection on Medicare's impact on black Americans. Health Care Financ Rev. 1996;18(2):87–90 [PMC free article] [PubMed] [Google Scholar]
- 46.Quadagno J. Promoting civil rights through the welfare state: how Medicare integrated southern hospitals. Soc Probl. 2000;47(1):68–89 [PubMed] [Google Scholar]
- 47.House JS, Schoeni FR, Kaplan GA, Pollack H. The health effects of social and economic policy: the promise and challenge for research and policy. : Schoeni RF, House JS, Kaplan GA, Pollack H, Making Americans Healthier: Social and Economic Policy as Health Policy. New York, NY: Russell Sage Foundation; 2008:3–26 [Google Scholar]
- 48.Kiefe CI, McKay SV, Halevy A, Brody BA. Is cost a barrier to screening mammography for low-income women receiving Medicare benefits? A randomized trial. Arch Intern Med. 1994;154(11):1217–1224 [PubMed] [Google Scholar]
- 49.McAlearney AS, Reeves KW, Tatum C, Paskett ED. Cost as a barrier to screening mammography among underserved women. Ethn Health. 2007;12(2):189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark CR, Baril N, Kunicki M, et al. Addressing social determinants of health to improve access to early breast cancer detection: results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women's Health Demonstration Project. J Womens Health (Larchmt). 2009;18(5):677–690 [DOI] [PubMed] [Google Scholar]
- 51.Moy B, Park ER, Feibelmann S, Chiang S, Weissman JS. Barriers to repeat mammography: cultural perspectives of African-American, Asian, and Hispanic women. Psychooncology. 2006;15(7):623–634 [DOI] [PubMed] [Google Scholar]
- 52.Peek ME, Han JH. Disparities in screening mammography: current status, interventions and implications. J Gen Intern Med. 2004;19(2):184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crane LA, Kaplan CP, Bastani R, Scrimshaw SC. Determinants of adherence among health department patients referred for a mammogram. Women Health. 1996;24(2):43–64 [DOI] [PubMed] [Google Scholar]
- 54.Oluwole SF, Ali AO, Adu A, et al. Impact of a cancer screening program on breast cancer stage at diagnosis in a medically underserved urban community. J Am Coll Surg. 2003;196(2):180–188 [DOI] [PubMed] [Google Scholar]
- 55.Purc-Stephenson RJ, Gorey KM. Lower adherence to screening mammography guidelines among ethnic minority women in America: a meta-analytic review. Prev Med. 2008;46(6):479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett K, Legg J. Demographic and health factors associated with mammography utilization. Am J Health Promot. 2005;19(6):401–405 [DOI] [PubMed] [Google Scholar]
- 57.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans into clinical treatment trials: study design barriers. J Clin Oncol. 2004;22(4):730–734 [DOI] [PubMed] [Google Scholar]
- 58.Struble KA, Tiogo TA, Behrman RE, Birnkrant DB, Kimberly A. Enrollment of women in HIV clinical trials. Paper presented at: IX International AIDS Conference, July 7–12 1996, Vancouver, British Columbia, Canada [Google Scholar]
- 59.Centers for Disease Control and Prevention Deaths among persons with AIDS through December 2000. HIV AIDS Surveill Rep. 2002;8(1):1–22 [Google Scholar]
- 60.Centers for Disease Control and Prevention Deaths among persons with AIDS through December 2006. HIV AIDS Surveill Rep. 2009;14(3):1–35 [Google Scholar]
- 61.Chu KC, Tarone RE, Brawley OW. Breast cancer trends of black women compared with white women. Arch Fam Med. 1999;8(6):521–528 [DOI] [PubMed] [Google Scholar]
- 62.Stewart SL, King JB, Thompson TD, Friedman C, Wingo PA. Cancer mortality surveillance—United States, 1990–2000. MMWR Surveill Summ. 2004;53(SS3):1–108 [PubMed] [Google Scholar]
- 63.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–553 [DOI] [PubMed] [Google Scholar]
- 64.Hamvas A, Wise PH, Yang RK, et al. The influence of the wider use of surfactant therapy on neonatal mortality among Blacks and Whites. N Engl J Med. 1996;334(25):1635–1640 [DOI] [PubMed] [Google Scholar]
- 65.Hennekens CH, Buring JE, Mayrent SL, Epidemiology in Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 1987 [Google Scholar]
- 66.Goldoni CA, Bonora K, Ciatto S, et al. Misclassification of breast cancer as cause of death in a service screening area. Cancer Causes Control. 2009;20(5):533–538 [DOI] [PubMed] [Google Scholar]
- 67.Grulich AE, Swerdlow AJ, dos Santos Silva I, Beral V. Is the apparent rise in cancer mortality in the elderly real? Analysis of changes in certification and coding of cause of death in England and Wales, 1970–1990. Int J Cancer. 1995;63(2):164–168 [DOI] [PubMed] [Google Scholar]
- 68.Carter-Pokras O, Baquet C. What is a “health disparity”? Public Health Rep. 2002;117(5):426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav. 2004;45(3):265–285 [DOI] [PubMed] [Google Scholar]