Abstract
Objectives. We examined the relationship between obstetrical intervention and preterm birth in the United States between 1991 and 2006.
Methods. We assessed changes in preterm birth, cesarean delivery, labor induction, and associated risks. Logistic regression modeled the odds of preterm obstetrical intervention after risk adjustment.
Results. From 1991 to 2006, the percentage of singleton preterm births increased 13%. The cesarean delivery rate for singleton preterm births increased 47%, and the rate of induced labor doubled. In 2006, 51% of singleton preterm births were spontaneous vaginal deliveries, compared with 69% in 1991. After adjustment for demographic and medical risks, the mother of a preterm infant was 88% (95% confidence interval [CI] = 1.87, 1.90) more likely to have an obstetrical intervention in 2006 than in 1991. Using new birth certificate data from 19 states, we estimated that 42% of singleton preterm infants were delivered via induction or cesarean birth without spontaneous onset of labor.
Conclusions. Obstetrical interventions were related to the increase in the US preterm birth rate between 1991 and 2006. The public health community can play a central role in reducing medically unnecessary interventions.
During the past 15 years, rates of obstetrical interventions have been rising in the United States.1,2 The percentage of births with induced labor more than doubled between 1991 and 2006, from 10.5% to 22.5%.1,2 After a decline in the early 1990s, the cesarean delivery rate increased by 50%, from 20.7% in 1996 to an all-time high of 31.1% in 2006.1 Large increases occurred for both primary and repeat cesarean deliveries and among mothers with no known medical risk factors or indications for cesarean delivery (such as diabetes, hypertension, or premature rupture of membranes).1,3,4 Recent studies have shown that changing primary cesarean rates did not correspond to shifts in mothers’ medical risk profiles but, rather, appeared to be related to increased use of cesarean delivery with all medical conditions.4–6
From 1991 to 2006, the preterm (less than 37 weeks of gestation) birth rate increased by 19%, from 10.8% to 12.8% of all births1; the preterm rate increased by 13% for singletons and by 22% for multiple births. An increase in the preterm birth rate is of concern because rates of death and disability are higher among preterm infants than among infants born at term (37–41 weeks).7–9 Although rates of death and disability are highest among infants born very preterm (less than 32 weeks), mortality rates among moderately preterm (32–33 weeks) and late preterm (34–36 weeks) infants are 7 and 3 times, respectively, the mortality rates for term infants.7
We examined the relationship between changes in the use of obstetrical intervention and changes in the preterm birth rate in the United States between 1991–2006. Specifically, we explored trends in singleton preterm births, delivery methods (cesarean or vaginal), and induction of labor.
METHODS
We obtained our data from the 1991–2006 National Center for Health Statistics natality data files.10 These files contain detailed information on each of the approximately 4 million births in the United States each year. We selected 1991 as the base year because it was the first year in which all relevant variables were reported by all states. Trends in cesarean delivery and induction of labor were examined by gestational age. In addition to gestational age, trends in preterm delivery were examined by maternal age, race/ethnicity, birth order, diabetes, chronic hypertension, and pregnancy-associated hypertension.
As a result of delays by states in implementing the 2003 revision of the US standard birth certificate, the revised birth certificate was used for only half of the births in the United States in 2006 (49%); the unrevised (1989) birth certificate was used for the remainder.1 Because some variables were measured differently in the 1989 and 2003 versions, we limited the variables we examined to those that were comparable between the 2 versions.1 The only exception was a subgroup analysis (described subsequently) of a new “trial of labor” variable available only on the 2003 birth certificate. A 2-proportion z test was used to assess the statistical significance of trends (in the Results section, mention of a given figure being higher or lower than another indicates a significant difference).
We measured gestational age as the interval between the first day of the mother's most recent menstrual period and the date of birth, except when gestational age was inconsistent with birthweight and plurality, in which case the clinical or obstetric estimate of gestation was used. These methods have been described in detail elsewhere.1 We defined preterm births as those occurring before 37 completed weeks of gestation.
Logistic regression was used to examine the relative odds of preterm obstetrical interventions (i.e., induction of labor, cesarean delivery, or both) among all singleton US births during 1991, 1996, 2001, and 2006, with 1991 as the reference year. Three models were run. The first model was unadjusted; the second controlled for maternal age (younger than 20 years, 20–34 years, 35 years or older), maternal race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic American Indian, non-Hispanic Asian or Pacific Islander), birth order (1, 2 or above), and gestational age (less than 32 weeks, 32–33 weeks, 34–36 weeks, 37 weeks or more); and the third also controlled for diabetes (yes, no), chronic hypertension (yes, no), and pregnancy-associated hypertension (yes, no). Records with missing values (which accounted for less than 3% of the data) were excluded from the models.
Finally, we conducted an exploratory analysis using the trial of labor variable from the 2003 revision of the birth certificate. The question added to the revised birth certificate was “If cesarean, was a trial of labor attempted?” (yes or no).11 The instructions were to respond yes if there was labor before the cesarean birth.12
In 2006, 19 states reported this variable (California, Delaware, Florida, Idaho, Kansas, Kentucky, Nebraska, New Hampshire, New York State [excluding New York City], North Dakota, Ohio, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Vermont, Washington, and Wyoming).1,11 Although these 19 states accounted for almost half (49%) of all US births in 2006, results for the 19 states may not be generalizable to the US population as a whole.11 For example, because California and Texas, which have substantial Mexican American populations, are among the 19 reporting states, the percentages of Mexican American births in these states are higher than that of the United States as a whole.11
The purpose of our trial of labor analysis was to estimate the percentage of singleton preterm births in which obstetrical interventions may have affected gestational age at delivery. If a woman already in labor has a preterm cesarean delivery, it is unlikely to substantially affect the infant's gestational age, given that she would probably have delivered in a short time. However, if a woman not in labor has a preterm cesarean delivery, it may affect gestational age at delivery because it is unknown how much longer the pregnancy might have continued without the intervention.13,14
To yield the most conservative estimate, we used the trial of labor variable in conjunction with other variables indicating labor on the 2003 revised birth certificate (precipitous, prolonged, induction of, or augmentation of labor; fetal intolerance of labor; use of forceps or vacuum). Cesarean births with no reported labor were defined as births in which no labor indications were reported. We combined this cesarean-without-labor category with inductions to yield an unadjusted estimate of the percentage of singleton preterm births in the 19 states where obstetrical interventions may have affected gestational age at delivery.
RESULTS
The singleton preterm birth rate increased by 13% between 1991–2006, from 9.8% to 11.1% (Table 1). Virtually all of this increase occurred among late preterm births; rates for very preterm and moderately preterm births remained relatively stable throughout the period.
TABLE 1.
Percentages of Singleton Preterm Births, Singleton Births With Cesarean Delivery, and Singleton Births With Induction of Labor, by Gestational Age: United States, 1991, 1996, 2001, and 2006
| Gestational Age | 1991 (n = 4 017 127), % | 1996 (n = 3 788 111), % | 2001 (n = 3 902 691), % | 2006 (n = 4 129 440), % | % Change 1991–2006 |
| Preterm births | |||||
| <37 wk | 9.8 | 9.7 | 10.4 | 11.1 | 13.3* |
| <32 wk | 1.7 | 1.6 | 1.6 | 1.7 | 0.0 |
| 32–33 wk | 1.3 | 1.2 | 1.2 | 1.3 | 0.0 |
| 34–36 wk | 6.9 | 6.9 | 7.6 | 8.1 | 17.4* |
| Cesarean deliveries | |||||
| ≥37 wk | 21.4 | 19.1 | 22.4 | 28.7 | 34.1* |
| <37 wk | 25.1 | 25.6 | 29.9 | 36.9 | 47.0* |
| <32 wk | 30.8 | 34.9 | 40.7 | 46.6 | 51.3* |
| 32–33 wk | 27.7 | 29.4 | 34.5 | 41.5 | 49.8* |
| 34–36 wk | 23.3 | 22.8 | 26.9 | 34.3 | 47.2* |
| Induction of labor | |||||
| ≥37 wk | 10.9 | 17.7 | 21.6 | 24.0 | 120.2* |
| <37 wk | 7.6 | 12.3 | 14.7 | 15.6 | 105.3* |
| <32 wk | 5.4 | 8.1 | 8.9 | 8.9 | 64.8* |
| 32–33 wk | 6.9 | 11.2 | 12.8 | 13.4 | 94.2* |
| 34–36 wk | 8.2 | 13.5 | 16.2 | 17.3 | 111.0* |
Note. Missing data were excluded from percentage computations. Data were derived from the natality detail files of the National Center for Health Statistics.
*P < .001.
The percentage of singleton preterm infants born via cesarean delivery increased by 47% during the study period, from 25.1% in 1991 to 36.9% in 2006 (Table 1), a larger increase than that occurring among full-term births (34%). The increase was slight from 1991 to 1996 but accelerated at a rapid pace thereafter, with a rise of 17% from 1996 to 2001 and a further 23% increase from 2001 to 2006. Cesarean delivery rates were highest at the lower gestational ages. By 2006, nearly half (46.6%) of very preterm infants were delivered via cesarean birth, compared with 41.5% of moderately preterm and 34.3% of late preterm infants.
The percentage of singleton preterm births with induced labor more than doubled from 1991 (7.6%) to 2006 (15.6%). Induction rates for preterm births were lower and increased at a slower pace than did the rates for term births. Among preterm births, the increase in induction rates was largest for late preterm births (110%), followed by moderately preterm (96%) and very preterm (65%) births.
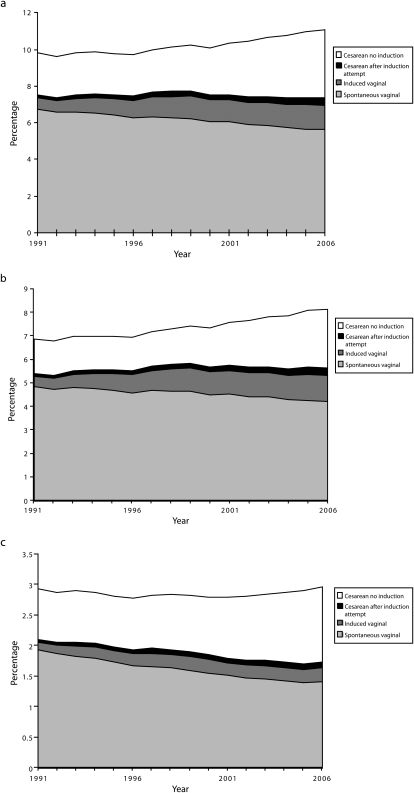
In Figure 1a, the overall percentage of singleton preterm births is divided into subgroups defined according to method of delivery: spontaneous vaginal, induced vaginal, cesarean delivery with no induction attempt, and cesarean delivery after an induction attempt. From 1991–2006, the percentage of spontaneous vaginal preterm births declined by 19%, from 6.8% to 5.7% of all births. By contrast, the percentage of induced vaginal preterm births more than doubled during the study period, from 0.6% to 1.3%. The percentage of preterm cesarean deliveries after an induction attempt also doubled from 1991 (0.2%) to 2006 (0.4%). Finally, the percentage of preterm cesarean deliveries without an induction attempt increased by 61% during the study period, from 2.3% to 3.7%.
FIGURE 1.
Percentages of singleton births, by method of delivery, for (a) preterm births (less than 37 weeks), (b) late preterm births (34–36 weeks), and (c) early preterm births (less than 34 weeks): United States, 1991–2006.
Note. Births lacking information on method of delivery and whether labor was induced are excluded.
An examination of singleton preterm births as a percentage of all preterm births showed that only half (50.9%) of singleton preterm births in 2006 were spontaneous vaginal deliveries, compared with 69.1% in 1991. From 1991–2006, the percentage of all singleton preterm births that were induced vaginal deliveries increased from 5.9% to 12.1%, and the percentage that were cesarean deliveries (with or without an induction attempt) increased from 25.1% to 37.0%.
Patterns for late preterm births and early preterm births were similar (Figure 1b and Figure 1c). During the study period, the percentage of singleton late preterm births that were spontaneous vaginal deliveries declined from 70% to 52%, and the percentage of early preterm births that were spontaneous vaginal deliveries declined from 66% to 48%.
Table 2 shows percentages of preterm singleton births, by maternal characteristics, for 1991, 1996, 2001, and 2006. Although the preterm birth rate among adolescent mothers was the highest of any of the age groups assessed, this rate did not increase from 1991 to 2006. By contrast, the preterm birth rate increased among mothers aged 20 to 34 years (15%) and mothers 35 years or older (17%). In the different race/ethnicity groups studied, the preterm birth rate increased most rapidly among non-Hispanic White women and declined among non-Hispanic Black women. Also, the preterm birth rate increased more rapidly among women with diabetes (26%), chronic hypertension (23%), and pregnancy-associated hypertension (46%) than among women without these conditions.
TABLE 2.
Percentages of Singleton Births That Were Preterm Births, by Selected Maternal Characteristics: United States, 1991, 1996, 2001, and 2006
| Maternal Characteristic | 1991 (n = 4 017 127), % | 1996 (n = 3 788 111), % | 2001 (n = 3 902 691), % | 2006 (n = 4 129 440), % | % Change 1991–2006 |
| Age, y | |||||
| <20 | 13.9 | 12.9 | 13.3 | 13.9 | 0.0 |
| 20–34 | 9.1 | 9.1 | 9.8 | 10.5 | 15.4* |
| ≥35 | 10.2 | 10.3 | 11.1 | 11.9 | 16.7* |
| Race/ethnicity | |||||
| Hispanic | 10.3 | 10.1 | 10.5 | 11.1 | 7.8* |
| Non-Hispanic White | 7.7 | 8.1 | 9.0 | 9.7 | 26.0* |
| Non-Hispanic Black | 17.9 | 16.3 | 16.0 | 16.6 | −7.3* |
| Non-Hispanic American Indian | 11.2 | 11.0 | 12.2 | 13.1 | 17.0* |
| Non-Hispanic Asian/Pacific Islander | 9.5 | 9.2 | 9.2 | 9.5 | 0.0 |
| Total birth order | |||||
| 1 | 9.8 | 9.8 | 10.3 | 10.8 | 10.2* |
| ≥2 | 9.9 | 9.7 | 10.4 | 11.2 | 13.1* |
| Diabetes | |||||
| Yes | 12.0 | 13.4 | 14.3 | 15.1 | 25.8* |
| No | 9.7 | 9.6 | 10.2 | 10.9 | 12.4* |
| Chronic hypertension | |||||
| Yes | 19.7 | 21.4 | 23.7 | 24.3 | 23.4* |
| No | 9.7 | 9.6 | 10.3 | 10.9 | 12.4* |
| Pregnancy-associated hypertension | |||||
| Yes | 16.2 | 18.7 | 21.4 | 23.7 | 46.3* |
| No | 9.5 | 9.4 | 10.0 | 10.6 | 11.6* |
Note. Missing data were excluded from percentage computations. Data were derived from the natality detail files of the National Center for Health Statistics.
*P < .001.
We used logistic regression analyses to compute adjusted odds ratios for preterm obstetrical intervention (cesarean section or induction of labor) from 1991 to 2006 (Table 3). When 1991 data were used as the reference and after control for maternal demographic and medical characteristics (model 3), the adjusted odds ratio for preterm obstetrical intervention increased over time to 1.88 (95% confidence interval [CI] = 1.87, 1.90) in 2006. Thus, an infant born preterm in 2006 had 88% higher odds of having an obstetrical intervention (cesarean or induction of labor) than did an infant born in 1991 after control for maternal demographic and medical characteristics.
TABLE 3.
Odds Ratios for Preterm Obstetrical Intervention (Cesarean Delivery or Induction of Labor): United States, 1991, 1996, 2001, and 2006
| Year of Birth | Model 1,a OR (95% CI) | Model 2,b OR (95% CI) | Model 3,c OR (95% CI) |
| 2006 | 1.84* (1.82, 1.85) | 1.97* (1.96, 1.99) | 1.88* (1.87, 1.90) |
| 2001 | 1.45* (1.43, 1.46) | 1.52* (1.51, 1.54) | 1.48* (1.47, 1.49) |
| 1996 | 1.14* (1.13, 1.15) | 1.18* (1.17, 1.19) | 1.15* (1.14, 1.16) |
| 1991 (Ref) | 1.00 | 1.00 | 1.00 |
Note. OR = odds ratio; CI = confidence interval. Data were derived from the natality detail files of the National Center for Health Statistics.
Unadjusted.
Adjusted for year of birth, maternal age, maternal race/ethnicity, total birth order, very preterm status, and moderately preterm status.
Adjusted for all of the variables included in model 2 along with diabetes, chronic hypertension, and pregnancy-associated hypertension.
*P < .001.
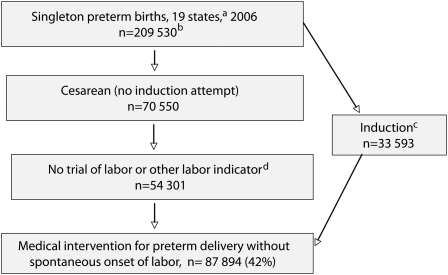
We used the new trial of labor variable, available for 19 states in 2006,11 to estimate the unadjusted percentage of preterm cesareans in which obstetrical interventions had the potential to change gestational age at delivery (Figure 2). The states reporting this item covered all US regions, and rates of induction of labor, cesarean delivery, and preterm birth in the 19 states were similar to those of the United States as a whole.
FIGURE 2.
Estimate of the percentage of singleton preterm births in which obstetrical intervention affected gestational age at delivery: 19 US states, 2006.
aStates using the 2003 revision of the US standard birth certificate.
bBirths lacking data on method of delivery, whether or not labor was induced, and whether or not a trial of labor was attempted are excluded.
cInduction of labor (attempted or successful).
dNo reported precipitous, prolonged, or augmented labor; fetal intolerance of labor; or use of forceps or vacuum.
Of the 209 530 singleton preterm births occurring in the 19 states in 2006, there were 54 301 cesarean births with no reported trial of labor or any other labor indicators (precipitous, prolonged, induced, or augmented labor; fetal intolerance of labor; use of forceps or vacuum) reported on the birth certificate. An induction without a cesarean delivery was reported in another 33 593 cases, resulting in a total of 87 894 singleton preterm births with an obstetrical intervention and no spontaneous onset of labor (42% of all singleton preterm births; Figure 2).
DISCUSSION
Preterm birth has been identified as a major and growing health concern in the United States,13,15,16 with multiple efforts focusing on both prevention17,18 and treatment.19 Increases in obstetrical intervention during pregnancy have been linked to increases in the preterm birth rate in the United States13,20–22 as well as other countries.14 These findings are typified by a recent study that, although not examining induction or births involving a trial of labor, showed consistent increases in preterm cesarean delivery rates across different demographic groupings.21
We examined national trends in singleton preterm births and their relationship to cesarean deliveries and induction of labor during pregnancy. From 1991 to 2006, whereas the rate of singleton preterm births increased by 13%, the cesarean delivery rate for singleton preterm births increased by 47% and the rate of induced labor more than doubled. Given that both the relative and absolute proportion of spontaneous vaginal births declined from 1991 to 2006 for all gestational age groups, our data suggest that the increase in the preterm birth rate was related to increases in obstetrical interventions (Figure 1).
In a multivariate analysis, we found that a preterm infant born in 2006 had 88% higher odds of having an obstetrical intervention (cesarean delivery or induction of labor) than did a comparable infant born in 1991 after adjustment for maternal demographic and medical characteristics. In an exploratory analysis of new birth certificate data for 19 states, we estimated that obstetrical interventions had the potential to influence gestational age at delivery in 42% of singleton preterm births.
The strengths of this study include the comprehensive population-based nature of our birth certificate data, which included all births in the United States for a given year, together with many demographic and medical variables. Limitations include concerns about the accuracy of reporting of selected items on the birth certificate. Most demographic items and some medical items (including maternal age, race/ethnicity, live birth order, and method of delivery) are considered to be well reported.23–26 Induction of labor data have been found to be underreported in some studies27 and well reported in others.26 Underreporting, if present, would tend to understate the impact of obstetrical intervention on preterm birth rates.
Although gestational age data are generally considered to be reasonably well reported,23,24 the data are subject to error owing to imperfect maternal recall or misidentification of the date of the mother's most recent menstrual period.1 Improvements in the quality of gestational age data during the period covered by our study have been noted,28–30 in particular a lessening of gestational age overstatement among Black mothers of very preterm infants.28,30 For this reason, improvements in data quality may have led to a slight underestimate of the magnitude of the increase in preterm births from 1991 to 2006.30
Measures of medical risk (i.e., diabetes and hypertension) from birth certificates are generally found to be underreported relative to data from medical records, although data on diabetes and hypertension were often better reported than were other medical risk factors.23–25 The trial of labor variable is new to the birth certificate and thus has not been validated via comparisons with medical records or other sources. Any underreporting of this data item might lead to an overestimate of the percentage of births in which obstetrical intervention affects gestational age at delivery.
The staggered implementation of the 2003 revision of the birth certificate also created limitations in that only variables found to be comparable between the 2 versions of the birth certificate could be used in most of our analyses. For example, we were unable to include premature rupture of membranes (PROM) in our multivariate modeling because this item was not comparable across the 2 versions1; however, we did note that rates of preterm PROM declined from 1990 to 2002.10,31
Reasons for preterm obstetrical interventions can be grouped into 3 categories: emergency, strongly indicated, and marginally indicated. Cesarean deliveries can be lifesaving procedures in emergency situations such as placental abruption. However, these emergency situations are rare, and there is no clear indication that the incidence of such disorders has increased substantially during the past 15 years.32
Serious maternal conditions such as deteriorating severe preeclampsia and serious fetal compromise with ominous biophysical signs may be a strong indication for early cesarean delivery. With widespread use of ultrasonography and fetal Doppler velocimetry, fetal compromise may now be detected earlier than in previous years.33 Despite great variations in the diagnosis and management of fetal compromise, some obstetricians may be more inclined to deliver the fetus after steroids are given and the fetus has reached 34 weeks. Cesarean delivery may be chosen over induction as a result of breech presentation, extreme immaturity, an unfavorable cervix, previous cesarean section, or concerns regarding possible fetal intolerance of labor.34
With the significant recent advances in neonatal care, severe neonatal morbidity and mortality are relatively low after 34 weeks. This may foster a sense of safety with respect to late preterm delivery. Although the guidelines of the American College of Obstetricians and Gynecologists indicate that medically elective cesarean deliveries should not be performed before 39 weeks unless there is documentation of fetal lung maturity,35 there is evidence that these guidelines are not always followed.36,37
Among births with more marginal indications, there is evidence of a declining threshold before obstetrical interventions are deemed to be necessary. For example, Leitch and Walker, in a study of changes in indications for cesarean delivery over time, concluded that although indications for cesarean deliveries have not changed much over time, “there has been a lowering in the overall threshold concerning the decision to carry out a cesarean section.”38(p621)
Recent studies have also suggested a trend toward more aggressive management of various medical conditions during pregnancy, such as PROM,39,40 oligohydramnios,41 hypertension,42,43 and diabetes,42 and this trend is reflected as well in our data. For example, 16% of women with pregnancy-associated hypertension delivered a preterm infant in 1991, compared with 24% in 2006 (Table 2), a more rapid increase than that observed among nonhypertensive women. However, evidence is still scarce as to the exact circumstances in which the balance of benefits and risks favors cesarean delivery over expectant management (i.e., attempting to delay delivery, when possible, until the fetus is more mature).44
Some studies conducted in the 1990s revealed a positive relationship between increases in preterm obstetrical interventions and decreased fetal or perinatal mortality rates.14,31 The lack of decline in the US infant mortality rate from 2000 to 2005 related to increases in preterm birth and preterm-related causes of death7,45 and the lack of decline in the fetal mortality rate from 2003 to 200546 suggest the need for a reexamination of this issue with more recent data.
Also, studies that compare morbidity and mortality for births with obstetrical intervention with spontaneous deliveries at the same gestational age invite methodological difficulties. This is because it is impossible to know how much longer the pregnancy might have continued without the intervention, thus moving a preterm fetus into a lower-risk gestational age category. Of course, in the case of a medically complicated pregnancy, delays might also lead to increased risk. More detailed studies are needed to disentangle such complex relationships.
What is clear is that preterm birth is associated with increased rates of infant morbidity47,48 and mortality.7–9,47 Even among late preterm infants, mortality rates are 3 times those among term infants.7 Although the risk of severe morbidity and mortality for late preterm births is relatively low, such births contribute significantly to infant mortality in the United States because of their large numbers.9
The public health community can draw several lessons from this analysis. First, our findings reinforce the value of periodic revisions of vital statistics to incorporate new items, such as the trial of labor measure, that can allow more sensitive analyses of key trends, such as preterm birth. Second, publication by the public health community of case–mix-adjusted preterm rates and related interventions by hospital can provide the public and policymakers with critical information that can assist in efforts to reduce preterm birth rates.
Finally, preterm birth has been identified as a major factor in the poor performance of the United States in international comparisons of infant mortality.49 The public health community can lead the effort to ensure more judicious use of medically elective interventions, joining with advocacy groups (e.g., the March of Dimes) and provider groups. In 2009, for instance, the American College of Obstetricians and Gynecologists issued guidelines calling for no elective inductions before 39 weeks.50 The combining of clinical and public health research into the causes and consequences of preterm birth will enable us to begin to address this major maternal and child health challenge.
In conclusion, given the frequency of preterm birth after labor induction or cesarean section without a trial of labor, the 88% increased odds of preterm obstetrical intervention in 2006 relative to 1991, and the recent lack of decline in US infant and fetal mortality rates,7,46 further research is needed into the decision-making process surrounding obstetrical intervention in preterm births. Because of the increased risk of morbidity and mortality for preterm infants relative to term infants,7–9,51 it is important to ensure that all preterm obstetric interventions are truly medically necessary.
Human Participant Protection
Because a publicly available data set was used in this study, no protocol approval was needed.
References
- 1.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2006. Natl Vital Stat Rep. January 7, 2009;57(7). [Google Scholar]
- 2.National Center for Health Statistics Advance report of maternal and infant health data from the birth certificate, 1991. Month Vital Stat Rep. May 11, 1994;42(suppl 11). [Google Scholar]
- 3.Menacker F. Trends in cesarean rates for first births and repeat cesarean rates for low-risk women: United States, 1990–2003. Natl Vital Stat Rep. September 22, 2005;54(4). [PubMed] [Google Scholar]
- 4.Declercq E, Menacker F, MacDorman MF. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health. 2006;96(5):867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailit JL, Love TE, Mercer B. Rising cesarean rates: are patients sicker? Am J Obstet Gynecol. 2004;191(3):800–803 [DOI] [PubMed] [Google Scholar]
- 6.Declercq E, Menacker F, MacDorman MF. Rise in “no indicated risk” primary cesareans in the United States, 1991–2001. BMJ. 2005;330(7482):71–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. July 30, 2008;57(2). [PubMed] [Google Scholar]
- 8.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41 [DOI] [PubMed] [Google Scholar]
- 9.Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;284(7):843–849 [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics US birth (natality) data sets, selected years. Available at: http://www.cdc.gov/nchs/data_access/VitalStatsOnline.htm. Accessed July 31, 2010
- 11.Osterman MJK, Martin JA, Menacker F. Expanded health data from the new birth certificate, 2006. Natl Vital Stat Rep. October 28, 2009;58(5). [PubMed] [Google Scholar]
- 12.Guide for Completing the Facility Worksheets for the Certificate of Live Birth and Report of Fetal Death (2003 Revision). Hyattsville, MD: National Center for Health Statistics; 2006 [Google Scholar]
- 13.Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among US singleton births: impact of rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30(1):8–15 [DOI] [PubMed] [Google Scholar]
- 14.Joseph KS, Demissie K, Kramer MS. Obstetric intervention, stillbirth, and preterm birth. Semin Perinatol. 2002;26(4):250–259 [DOI] [PubMed] [Google Scholar]
- 15.Green NS, Damus K, Simpson JL, et al. Research agenda for preterm birth: recommendation from the March of Dimes. Am J Obstet Gynecol. 2005;193(3):626–635 [DOI] [PubMed] [Google Scholar]
- 16.Behrman RE, Butler AS, Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007 [PubMed] [Google Scholar]
- 17.Hall RT. Prevention of premature birth: do pediatricians have a role? Pediatrics. 2000;105(5):1137–1140 [DOI] [PubMed] [Google Scholar]
- 18.Smith V, Devane D, Begley CM, Clarke M, Higgins S. A systematic review and quality assessment of systematic reviews of randomized trials of interventions for preventing and treating preterm birth. Eur J Obstet Gynecol. 2009;142(1):3–11 [DOI] [PubMed] [Google Scholar]
- 19.Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 2008;199(6):620.e1–620.e8 [DOI] [PubMed] [Google Scholar]
- 20.MacDorman MF, Mathews TJ, Martin JA, Malloy MH. Trends and characteristics of induced labour in the United States, 1989–98. Paediatr Perinat Epidemiol. 2002;16(3):263–273 [DOI] [PubMed] [Google Scholar]
- 21.Bettegowda VR, Dias T, Davidoff MJ, Damus K, Callaghan WM, Petrini JR. The relationship between cesarean delivery and gestational age among US singleton births. Clin Perinatol. 2008;35(2):309–324 [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roohan PJ, Josberger RE, Acar J, Dabir P, Feder HM, Gagliano PJ. Validation of birth certificate data in New York State. J Community Health. 2003;28(5):335–346 [DOI] [PubMed] [Google Scholar]
- 24.DiGiuseppe D, Aron DC, Ranbom L, Harper DL, Rosenthal GE. Reliability of birth certificate data: a multi-hospital comparison to medical records information. Matern Child Health J. 2002;6(3):169–179 [DOI] [PubMed] [Google Scholar]
- 25.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2008;13(5):660–666 [DOI] [PubMed] [Google Scholar]
- 26.Roberts CL, Bell JC, Ford JB, Morris JM. Monitoring the quality of maternity care: how well are labour and delivery events reported in population health data? Paediatr Perinat Epidemiol. 2009;23(2):144–152 [DOI] [PubMed] [Google Scholar]
- 27.Lydon-Rochelle MT, Holt VL, Nelson JC, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19(6):460–471 [DOI] [PubMed] [Google Scholar]
- 28.Kirmeyer SE, Martin JA. Trends and differentials in higher-birthweight infants at 28–31 weeks of gestation, by race and Hispanic origin, United States, 1990–2002. Paediatr Perinat Epidemiol. 2007;21(suppl 2):31–40 [DOI] [PubMed] [Google Scholar]
- 29.Martin JA. United States vital statistics and the measurement of gestational age. Paediatr Perinat Epidemiol. 2007;21(suppl 2):13–21 [DOI] [PubMed] [Google Scholar]
- 30.Vahratian A, Buekens P, Alexander GR. State-specific trends in preterm delivery: are rates really declining among non-Hispanic African Americans across the United States? Matern Child Health J. 2006;10(1):27–32 [DOI] [PubMed] [Google Scholar]
- 31.Ananth CV, Joseph KS, Oyelese O, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol. 2005;105(5):1084–1091 [DOI] [PubMed] [Google Scholar]
- 32.Ananth CV, Oyelese Y, Yeo L, Pradhan A, Vintzileos AM. Placental abruption in the United States, 1979 through 2001: temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192(1):191–198 [DOI] [PubMed] [Google Scholar]
- 33.Mari G, Hanif F. Intrauterine growth restriction: how to manage and when to deliver. Clin Obstet Gynecol. 2007;50(2):497–509 [DOI] [PubMed] [Google Scholar]
- 34.Iams JD, Creasy RK. Preterm labor and delivery. : Creasy RK, Resnik R, Iams JD, Maternal-Fetal Medicine: Principles and Practice. 5th ed Philadelphia, PA: WB Saunders Co; 2004:651–654 [Google Scholar]
- 35.American College of Obstetricians and Gynecologists Cesarean delivery on maternal request. Obstet Gynecol. 2007;110(6):1501–1504 [DOI] [PubMed] [Google Scholar]
- 36.Laye MR, Dellinger EH. Timing of scheduled cesarean delivery in patients on a teaching versus private service: adherence to American College of Obstetricians and Gynecologists guidelines and neonatal outcomes. Am J Obstet Gynecol. 2006;195(2):577–584 [DOI] [PubMed] [Google Scholar]
- 37.Tita ATN, Landon MB, Spong CY, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitch CR, Walker JJ. The rise in caesarean section rate: the same indications but a lower threshold. BJOG. 1998;105(6):621–626 [DOI] [PubMed] [Google Scholar]
- 39.Naef RW, Allbert JR, Ross EL, Weber M, Martin RW, Morrison JC. Premature rupture of membranes at 34 to 37 weeks’ gestation: aggressive versus conservative management. Am J Obstet Gynecol. 1998;178(1):126–130 [DOI] [PubMed] [Google Scholar]
- 40.Grable IA. Cost-effectiveness of induction after preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187(5):1153–1158 [DOI] [PubMed] [Google Scholar]
- 41.Casey BM, McIntire DD, Bloom SL, et al. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks’ gestation. Am J Obstet Gynecol. 2000;182(4):909–912 [DOI] [PubMed] [Google Scholar]
- 42.Sibai BM, Caritis SN, Hauth JC, et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. Am J Obstet Gynecol. 2000;183(6):1520–1524 [DOI] [PubMed] [Google Scholar]
- 43.Hnat MD, Sibai BM, Caritis S, et al. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparas. Am J Obstet Gynecol. 2002;186(3):422–426 [DOI] [PubMed] [Google Scholar]
- 44.Grant A, Glazener CMA. Elective caesarean section versus expectant management for delivery of the small baby. Cochrane Database Syst Rev. 2001;(2):CD000078 [DOI] [PubMed] [Google Scholar]
- 45.MacDorman MF, Mathews TJ. Recent Trends in Infant Mortality in the United States. Hyattsville, MD: National Center for Health Statistics; 2008. NCHS data brief 9 [Google Scholar]
- 46.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. January 28, 2009;57(8). [PubMed] [Google Scholar]
- 47.Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B. Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr. 2003;143(2):186–191 [DOI] [PubMed] [Google Scholar]
- 48.Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009;154(2):169–176 [DOI] [PubMed] [Google Scholar]
- 49.MacDorman MF, Mathews TJ. Behind International Rankings of Infant Mortality: How the United States Compares With Europe. Hyattsville, MD: National Center for Health Statistics; 2009. NCHS data brief 23 [PubMed] [Google Scholar]
- 50.American College of Obstetricians and Gynecologists ACOG practice bulletin 107: induction of labor. Obstet Gynecol. 2009;114(2):386–397 [DOI] [PubMed] [Google Scholar]
- 51.Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr. 2007;151(11):450–456 [DOI] [PubMed] [Google Scholar]