Abstract
Previous studies have established that in a variety of human glomerulopathies the reduced glomerular filtration rate (GFR) is due to a marked lowering of the ultrafiltration coefficient (Kf). To identify the factors which lower Kf, we measured the filtering surface area per glomerulus, filtration slit frequency, basement membrane thickness, and GFR and its determinants in patients with minimal change and membraneous nephropathies and in age-matched healthy controls. Overall values of Kf for the two kidneys were calculated from GFR, renal plasma flow rate, systemic colloid osmotic pressure, and three assumed values for the transcapillary pressure difference. "Experimental" values of the glomerular hydraulic permeability (kexp) were then calculated from Kf, glomerular filtering surface area, and estimates of the total number of nephrons of the two kidneys. Independent estimates of the glomerular hydraulic permeability (kmodel) were obtained using a recent mathematical model that is based on analyses of viscous flow through the various structural components of the glomerular capillary wall. Individual values of basement membrane thickness and filtration slit frequency were used as inputs in this model. The results indicate that the reductions of Kf in both nephropathies can be attributed entirely to reduced glomerular hydraulic permeability. The mean values of kexp and kmodel were very similar in both disorders and much smaller in the nephrotic groups than in healthy controls. There was good agreement between kexp and kmodel for any given group of subjects. It was shown that, in both groups of nephrotics, filtration slit frequency was a more important determinant of the water flow resistance than was basement membrane thickness. The decrease in filtration slit frequency observed in both disorders caused the average path length for the filtrate to increase, thereby explaining the decreased hydraulic permeability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Diamond J. R., Karnovsky M. J., Brenner B. M. Mechanisms underlying transition from acute glomerular injury to late glomerular sclerosis in a rat model of nephrotic syndrome. J Clin Invest. 1988 Nov;82(5):1757–1768. doi: 10.1172/JCI113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S. M., Lieberman J. S., Newton L. D., Mejia M., Peters W. A., Myers B. D. Slope of serial glomerular filtration rate and the progression of diabetic glomerular disease. J Am Soc Nephrol. 1993 Jan;3(7):1358–1370. doi: 10.1681/ASN.V371358. [DOI] [PubMed] [Google Scholar]
- Battilana C., Zhang H. P., Olshen R. A., Wexler L., Myers B. D. PAH extraction and estimation of plasma flow in diseased human kidneys. Am J Physiol. 1991 Oct;261(4 Pt 2):F726–F733. doi: 10.1152/ajprenal.1991.261.4.F726. [DOI] [PubMed] [Google Scholar]
- Bendtsen T. F., Nyengaard J. R. The number of glomeruli in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1992 Sep;35(9):844–850. doi: 10.1007/BF00399930. [DOI] [PubMed] [Google Scholar]
- Bendtsen T. F., Nyengaard J. R. Unbiased estimation of particle number using sections--an historical perspective with special reference to the stereology of glomeruli. J Microsc. 1989 Jan;153(Pt 1):93–102. doi: 10.1111/j.1365-2818.1989.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Bohman S. O., Jaremko G., Bohlin A. B., Berg U. Foot process fusion and glomerular filtration rate in minimal change nephrotic syndrome. Kidney Int. 1984 Apr;25(4):696–700. doi: 10.1038/ki.1984.76. [DOI] [PubMed] [Google Scholar]
- Canaan-Kühl S., Venkatraman E. S., Ernst S. I., Olshen R. A., Myers B. D. Relationships among protein and albumin concentrations and oncotic pressure in nephrotic plasma. Am J Physiol. 1993 Jun;264(6 Pt 2):F1052–F1059. doi: 10.1152/ajprenal.1993.264.6.F1052. [DOI] [PubMed] [Google Scholar]
- Daniels B. S., Deen W. M., Mayer G., Meyer T., Hostetter T. H. Glomerular permeability barrier in the rat. Functional assessment by in vitro methods. J Clin Invest. 1993 Aug;92(2):929–936. doi: 10.1172/JCI116668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B. S., Hauser E. B., Deen W. M., Hostetter T. H. Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol. 1992 Jun;262(6 Pt 2):F919–F926. doi: 10.1152/ajprenal.1992.262.6.F919. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Drumond M. C., Deen W. M. Stokes flow through a row of cylinders between parallel walls: model for the glomerular slit diaphragm. J Biomech Eng. 1994 May;116(2):184–189. doi: 10.1115/1.2895718. [DOI] [PubMed] [Google Scholar]
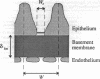
- Drumond M. C., Deen W. M. Structural determinants of glomerular hydraulic permeability. Am J Physiol. 1994 Jan;266(1 Pt 2):F1–12. doi: 10.1152/ajprenal.1994.266.1.F1. [DOI] [PubMed] [Google Scholar]
- Dunnill M. S., Halley W. Some observations on the quantitative anatomy of the kidney. J Pathol. 1973 Jun;110(2):113–121. doi: 10.1002/path.1711100202. [DOI] [PubMed] [Google Scholar]
- Ellis E. N., Steffes M. W., Chavers B., Mauer S. M. Observations of glomerular epithelial cell structure in patients with type I diabetes mellitus. Kidney Int. 1987 Nov;32(5):736–741. doi: 10.1038/ki.1987.268. [DOI] [PubMed] [Google Scholar]
- Guasch A., Deen W. M., Myers B. D. Charge selectivity of the glomerular filtration barrier in healthy and nephrotic humans. J Clin Invest. 1993 Nov;92(5):2274–2282. doi: 10.1172/JCI116831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch A., Hashimoto H., Sibley R. K., Deen W. M., Myers B. D. Glomerular dysfunction in nephrotic humans with minimal changes or focal glomerulosclerosis. Am J Physiol. 1991 May;260(5 Pt 2):F728–F737. doi: 10.1152/ajprenal.1991.260.5.F728. [DOI] [PubMed] [Google Scholar]
- Guasch A., Myers B. D. Determinants of glomerular hypofiltration in nephrotic patients with minimal change nephropathy. J Am Soc Nephrol. 1994 Feb;4(8):1571–1581. doi: 10.1681/ASN.V481571. [DOI] [PubMed] [Google Scholar]
- Guasch A., Sibley R. K., Huie P., Myers B. D. Extent and course of glomerular injury in human membranous glomerulopathy. Am J Physiol. 1992 Dec;263(6 Pt 2):F1034–F1043. doi: 10.1152/ajprenal.1992.263.6.F1034. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Seefeldt T., Osterby R. Glomerular epithelial foot processes in normal man and rats. Distribution of true width and its intra- and inter-individual variation. Cell Tissue Res. 1980;205(1):147–155. doi: 10.1007/BF00234450. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Hoyer J. R., Seiler M. W., Brenner B. M. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982 Jan;69(1):185–198. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. B., Gundersen H. J., Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc. 1979 Jan;115(1):19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Lane P. H., Steffes M. W., Mauer S. M. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992 Apr;41(4):1085–1089. doi: 10.1038/ki.1992.165. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Ellis E. N., Sutherland D. E., Brown D. M., Goetz F. C. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984 Oct;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. D., Chagnac A., Golbetz H., Newton L., Strober S., Sibley R. K. Extent of glomerular injury in active and resolving lupus nephritis: a theoretical analysis. Am J Physiol. 1991 May;260(5 Pt 2):F717–F727. doi: 10.1152/ajprenal.1991.260.5.F717. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Peterson C., Molina C., Tomlanovich S. J., Newton L. D., Nitkin R., Sandler H., Murad F. Role of cardiac atria in the human renal response to changing plasma volume. Am J Physiol. 1988 Apr;254(4 Pt 2):F562–F573. doi: 10.1152/ajprenal.1988.254.4.F562. [DOI] [PubMed] [Google Scholar]
- Nyengaard J. R., Bendtsen T. F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992 Feb;232(2):194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- Osterby R., Gundersen H. J., Hørlyck A., Kroustrup J. P., Nyberg G., Westberg G. Diabetic glomerulopathy. Structural characteristics of the early and advanced stages. Diabetes. 1983 May;32 (Suppl 2):79–82. doi: 10.2337/diab.32.2.s79. [DOI] [PubMed] [Google Scholar]
- Rodewald R., Karnovsky M. J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974 Feb;60(2):423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandling J. D., Black V. M., Deen W. M., Myers B. D. Glomerular permselectivity in healthy and nephrotic humans. Adv Nephrol Necker Hosp. 1992;21:159–176. [PubMed] [Google Scholar]
- Scholey J. W., Miller P. L., Rennke H. G., Meyer T. W. Effect of converting enzyme inhibition on the course of adriamycin-induced nephropathy. Kidney Int. 1989 Nov;36(5):816–822. doi: 10.1038/ki.1989.267. [DOI] [PubMed] [Google Scholar]
- Shemesh O., Ross J. C., Deen W. M., Grant G. W., Myers B. D. Nature of the glomerular capillary injury in human membranous glomerulopathy. J Clin Invest. 1986 Mar;77(3):868–877. doi: 10.1172/JCI112384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBEL E. R., GOMEZ D. M. A principle for counting tissue structures on random sections. J Appl Physiol. 1962 Mar;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Rennke H. G., Salant D. J., Deen W. M., Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res. 1987 Oct;61(4):531–538. doi: 10.1161/01.res.61.4.531. [DOI] [PubMed] [Google Scholar]