Abstract
Objectives. We estimated the collective burden of mortality from autoimmune diseases among females in the United Kingdom and the effects of death certificate coding changes on this estimate.
Methods. We analyzed 1993–2003 England and Wales death certificate data for 3 150 267 females aged 1 year or older. We identified death certificates that listed autoimmune conditions as underlying or contributory causes of death. The percentages of all female deaths attributed to autoimmune disorders and to UK official mortality categories were ranked to determine the leading causes of death.
Results. In 2003, autoimmune diseases were the sixth or seventh most frequent underlying cause of death among females in all age groups below 75 years. Results were similar when both underlying and contributory causes of death were considered. The proportion of females dying with an autoimmune disorder remained relatively constant from 1993 to 2003. Analyses indicated that death counts for specific autoimmune diseases had been underestimated.
Conclusions. Autoimmune diseases are a leading cause of death among females in England and Wales, but their collective impact remains hidden in current disease classification systems. Grouping these disorders together may help promote research needed to identify common determinants and future prevention strategies.
Autoimmune disorders are a group of disabling conditions caused by pathological immune responses directed against host antigens.1 Autoimmune disorders occur more frequently among women and result in chronic ill health, poor quality of life, and major health care costs.1–3 However, because of the relative lack of population-based studies, remarkably little is known about the collective disease burden associated with autoimmune disorders or their shared environmental triggers.4
Many national and international organizations assess the burden of disease among a population by ranking the leading causes of death5–8 These rankings provide important information for health policymakers and those responsible for delivering health care, and the rankings highlight areas for further research on prevention or treatment of specific conditions. In ranking lists, deaths from specific causes are grouped into broader mortality categories, typically based on the International Classification of Diseases (ICD) system used to code death certificates.9,10
In the ICD classification, conditions are grouped according to pathogenic mechanism (e.g., neoplasms), organ system (e.g., diseases of the circulatory system), or etiology (e.g., external causes of death). Grouping causes of death according to pathogenesis is useful because grouping allows estimation of the collective burden of disease resulting from a specific pathological process and the overall impact of risk factors for the pathological process across all organ systems. However, autoimmune disorders are neglected in the ICD classification, because they are listed for the most part individually under separate organ systems. The combined burden of mortality from autoimmune disorders is therefore hidden.
The World Health Organization (WHO) recently developed a list of recommended disease categories to use when grouping causes of death in mortality rankings; this list has been adapted by the UK Office for National Statistics to classify local disease patterns in England and Wales.11,12 The recommended categories continue to be based on pathogenesis, organ systems, and etiology, together with individual conditions of public health importance (e.g., influenza and pneumonia). Regrettably, autoimmune disorders remain neglected in these newer classifications.
Walsh and Rau attempted to address this problem by investigating the combined burden of mortality among women attributed to 24 conditions that fulfilled Rose and Bona's criteria for autoimmune disorders.13,14 The Walsh and Rau study, in which 1995 US death certificate data were used, showed that autoimmune diseases as a group were among the top 10 causes of death in US women younger than 65 years.13 Their findings have been cited widely as evidence that autoimmune disorders are an important but neglected group of diseases that affect women's health.3,15,16
However, the Walsh and Rau study involved limitations. First, the version of the ICD used to code deaths in their study (ICD-9) did not include specific codes for some of the autoimmune diseases of interest, allowing only rough estimates of mortality.9 Second, only underlying cause of death data were assessed; other conditions listed on death certificates as contributory causes of death were excluded. Third, the combined burden of mortality attributable to autoimmune diseases was underestimated because the analyses were restricted to 24 disorders; many additional conditions have been identified in recent years that conform to Rose and Bona's criteria for autoimmune pathogenesis.1,14
To date, no collective burden of UK mortality estimates for autoimmune disorders have been published. The primary objective of our study was to estimate the burden of autoimmune diseases among females in the United Kingdom by analyzing 2003 death certificate data for England and Wales. The ICD-10 was used in coding these data, allowing more specific identification of autoimmune disorders. Information on both underlying and contributory causes of death listed on death certificates was available; in addition, data were available for a larger number of autoimmune disorders than those considered by Walsh and Rau, enabling a fuller analysis of the contribution of autoimmune disorders to female mortality. We also used an earlier (1993–2003) data set that included both ICD-9- and ICD-10–coded female deaths to assess the extent to which the number of deaths resulting from certain autoimmune disorders may have been overestimated in ICD-9–coded data and to examine the effects of other changes in coding rules on autoimmune mortality estimates.
METHODS
In our analyses, we used UK Office for National Statistics mortality files for all deaths registered in England and Wales between 1993 and 2003. From these files, we selected deaths among females aged 1 year or older registered during the study period. The ICD-9 was used in coding data from 1993 to 2000, and the ICD-10 (with 4-character codes) was used from 2001 to 2003. All records indicated the year of death registration, the age of the deceased, and multiple causes of death, including the designated underlying cause of death. Consistent with previous ranking analyses, we excluded infant deaths given that causes of death in this age group tend to be different than causes in other age groups; a different hierarchical system is used to group causes of death among infants in England and Wales, and neonatal deaths are not assigned an underlying cause.17,18
Autoimmune Diseases Examined
We examined the 24 autoimmune disorders described in the Walsh and Rau study: Addison's disease, autoimmune hemolytic anemia, autoimmune vasculitis (represented by Goodpasture's syndrome), bullous autoimmune disease (represented by pemphigus), chronic active hepatitis (CAH), glomerulonephritis, Graves’ disease, idiopathic thrombocytopenic purpura, multiple sclerosis, myasthenia gravis, myocarditis, pernicious anemia, polymyositis–dermatomyositis, primary biliary cirrhosis, relapsing polychondritis, rheumatic fever–heart disease, rheumatoid arthritis, Sjogren's disease, systemic lupus erythematosus, systemic sclerosis, thyroiditis, type 1 (insulin-dependent) diabetes mellitus (DM), uveitis, and vitiligo.13 In addition, we assessed 8 other conditions with good evidence of autoimmune pathogenesis: ankylosing spondylitis, celiac disease, Guillain-Barré disease, idiopathic fibrosing alveolitis (IFA), inflammatory bowel disease (ulcerative colitis and Crohn's disease), juvenile idiopathic arthritis, polyendocrine syndrome, and psoriasis.1
We also increased the number of conditions included under 2 of Walsh and Rau's autoimmune disorder categories. First, we added acquired epidermolysis bullosa, dermatitis herpetiformis, pemphigoid, and subcorneal pustular dermatitis to bullous autoimmune disease. Second, we added Behcet's disease, Churg–Strauss syndrome, Henoch–Schonlein purpura, Kawasaki's disease, microscopic polyangiitis, polyangiitis overlap syndrome, polyarteritis nodosa, Takayasu disease, and Wegener's granulomatosis to autoimmune vasculitis.1 Finally, we included an autoimmune disease general category for deaths attributed to an unspecified autoimmune disorder.
Identification of Autoimmune Deaths
One of the authors (S. L. T.) drew up ICD-9 and ICD-10 code lists for each autoimmune disorder of interest (e-Table 1, available as an online supplement to this article at http://www.ajph.org); agreement on these lists was then reached with 2 of the other authors (L. S. and A. J. H.). Each death for which at least 1 autoimmune code was listed on the death certificate was categorized as a specific or nonspecific autoimmune death depending on whether the code was specific for the autoimmune disorder of interest or represented both the autoimmune disorder and other (nonautoimmune) diseases. For example, there is no specific ICD-9 code for Addison's disease, so deaths with the code 255.4 (corticoadrenal insufficiency) were classified as nonspecific Addison's disease deaths.
TABLE 1.
Numbers of Deaths Associated With Autoimmune Diseases as an Underlying Cause or an Underlying or Contributory Cause (Any Mention) of Death Among Females Aged 1 Year or Older: England and Wales, 2003
| Aged 1–14 Years |
Aged 15–34 Years |
Aged 35–54 Years |
Aged 55–74 Years |
Aged ≥ 75 Years |
Overalla |
|||||||
| Underlying | Any Mention | Underlying | Any Mention | Underlying | Any Mention | Underlying | Any Mention | Underlying | Any Mention | Underlying | Any Mention | |
| Addison's disease | 0 | 0 | 0 | 1 | 3 | 10 | 2 | 26 | 11 | 52 | 16 | 89 |
| Ankylosing spondylitis | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 8 | 0 | 2 | 2 | 10 |
| Autoimmune hemolytic anemia | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 17 | 17 | 50 | 21 | 68 |
| Autoimmune thrombocytopenic purpura | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 8 | 11 | 35 | 15 | 44 |
| Bullous autoimmune disease | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 9 | 31 | 113 | 36 | 123 |
| Celiac disease | 0 | 0 | 0 | 1 | 0 | 4 | 4 | 18 | 12 | 37 | 16 | 60 |
| Chronic active hepatitis | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 3 | 1 | 1 | 4 | 5 |
| Dermatomyositis/polymyositis | 0 | 0 | 0 | 0 | 1 | 2 | 4 | 15 | 15 | 24 | 20 | 41 |
| Glomerulonephritis | 0 | 1 | 2 | 3 | 4 | 15 | 19 | 57 | 43 | 86 | 68 | 162 |
| Goodpasture's syndrome | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 4 | 5 | 5 | 8 | 10 |
| Graves’ disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 3 |
| Guillain–Barré syndrome | 0 | 0 | 0 | 0 | 1 | 3 | 4 | 6 | 15 | 25 | 20 | 34 |
| Idiopathic fibrosing alveolitis | 0 | 1 | 3 | 6 | 22 | 39 | 275 | 487 | 617 | 1035 | 917 | 1568 |
| Inflammatory bowel disease | 0 | 0 | 7 | 8 | 16 | 35 | 67 | 144 | 174 | 334 | 264 | 521 |
| Insulin-dependent diabetes mellitus | 2 | 2 | 22 | 44 | 24 | 61 | 79 | 260 | 111 | 414 | 238 | 781 |
| Juvenile idiopathic arthritis | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 |
| Lambert–Eaton myasthenic syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Multiple sclerosis | 0 | 0 | 8 | 14 | 199 | 268 | 319 | 495 | 138 | 235 | 664 | 1012 |
| Myasthenia gravis | 1 | 1 | 0 | 3 | 2 | 4 | 4 | 7 | 29 | 45 | 36 | 60 |
| Myocarditis | 11 | 12 | 16 | 20 | 25 | 53 | 30 | 127 | 40 | 307 | 122 | 519 |
| Pernicious anemia | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 10 | 24 | 108 | 25 | 121 |
| Polyendocrine syndromes | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Primary biliary cirrhosis | 0 | 0 | 0 | 1 | 7 | 9 | 47 | 81 | 57 | 90 | 111 | 181 |
| Psoriasis | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 | 9 | 26 | 11 | 32 |
| Relapsing polychondritis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Rheumatic fever/heart disease | 0 | 0 | 4 | 5 | 27 | 48 | 252 | 445 | 552 | 1207 | 835 | 1705 |
| Rheumatoid arthritis | 0 | 0 | 1 | 2 | 14 | 34 | 197 | 636 | 489 | 1310 | 701 | 1982 |
| Scleroderma/systemic sclerosis | 1 | 1 | 3 | 4 | 8 | 14 | 60 | 91 | 34 | 62 | 106 | 172 |
| Sjögren's disease | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 11 | 2 | 9 | 5 | 21 |
| Systemic lupus erythematosus | 2 | 2 | 11 | 14 | 19 | 31 | 29 | 53 | 11 | 23 | 72 | 123 |
| Thyroiditis | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | 1 | 5 |
| Uveitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vasculitis | 0 | 0 | 2 | 2 | 4 | 5 | 24 | 42 | 31 | 49 | 61 | 98 |
| Vitiligo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unspecified autoimmune disease | 0 | 0 | 1 | 3 | 6 | 11 | 7 | 12 | 7 | 14 | 21 | 40 |
| Total deathsb | 17 | 20 | 81 | 128 | 385 | 625 | 1450 | 2938 | 2487 | 5560 | 4420 | 9271 |
Seven females aged ≤ 1 year and 3 females with no International Classification of Diseases codes on their death certificates were excluded from the analyses.
In total, 316 females had 2 autoimmune disorders listed on their death certificates, and 7 females had 3 disorders listed; hence, column totals are less than the sum of the counts for individual autoimmune disorders.
We thus identified the number of specific and nonspecific autoimmune deaths among females aged 1 year or older for each year of the study (1993–2003), stratified by age. We obtained counts for autoimmune disorders both as the underlying cause of death and as any mention on the death certificate (underlying or contributory cause).
A more detailed definition was used to classify deaths resulting from type 1 DM. There is no specific ICD-9 4-digit code for type 1 DM. In contrast, the ICD-10 includes specific codes for type 1 and type 2 (nonautoimmune) DM as well as nonspecific DM codes; nonspecific DM codes are used in classifying the majority of DM deaths because death certifiers do not specify the type of diabetes. Given that type 2 DM affected mainly older individuals during the study period, we adopted Walsh and Rau's definition of ICD-9–coded type 1 DM deaths as deaths among females younger than 35 years with an ICD-9 DM code (a nonspecific autoimmune death) on their death certificate. For ICD-10–coded deaths, we included females of all ages with a type 1 DM code (a specific autoimmune death) on their death certificate together with females younger than 35 years with a nonspecific DM code (a nonspecific autoimmune death).
Official Categorization of All Deaths
We used the England and Wales 2005 modification of the WHO recommended disease categories for ranking causes of death, adopting the 46-category version in which cancers and accidents are each grouped into single categories. The Office for National Statistics code lists were used to assign approximately 9500 ICD-10 codes to these 46 categories.8 We then applied the ICD-10 list to 2003 England and Wales female mortality data to assign each death to a specific official cause of death category.
We used the UK official mortality categories and the new autoimmune disorder category to revise rankings of leading causes of death (both underlying causes and underlying or contributory causes) in 2003 for each age group. In each age group, we calculated the percentage of all deaths attributed to each mortality category and ranked the results to obtain the 10 leading causes of death. In calculating the official leading causes of death (in which autoimmune disorders as a group are not considered), we did not exclude autoimmune deaths from the other leading causes; instead, we determined how many deaths from autoimmune disorders fell into each of these official categories.
We examined trends in mortality related to autoimmune disorders by calculating, for each study year, the proportion of total female deaths for which an autoimmune disorder was mentioned on the death certificate. We then investigated how estimates of the number of autoimmune deaths were affected by use of nonspecific and specific ICD codes for individual autoimmune diseases and by the change from ICD-9 to ICD-10 coding of death certificates. We did so in 3 ways.
First, for each autoimmune disorder, we compared the mean number of female deaths in 1999 and 2000 (the final 2 years in which the ICD-9 was used in coding deaths) with the mean number of female deaths in 2001 and 2002 (the first 2 years in which the ICD-10 was used). Second, we used a bridge-coded data set of underlying-cause deaths for 1999 in which deaths had been coded independently to both the ICD-9 and the ICD-10.19 These data indicated where deaths coded for each autoimmune disorder in 1 ICD revision were coded in the other. Finally, we estimated the impact on autoimmune mortality estimates of widening the case definitions of certain autoimmune disorders to include less specific codes that were not used in our original case definitions but could also represent deaths from these disorders.
RESULTS
In 2003, according to the Office for National Statistics, the estimated England and Wales midyear population of females aged 1 year or older was 26 658 346, among whom there were 283 236 registered deaths. Among these 283 236 deaths, an autoimmune disorder was mentioned on the death certificate in 9271 cases and was listed as the underlying cause of death in 4420 cases. The conditions mentioned most frequently were rheumatoid arthritis, rheumatic heart disease, idiopathic fibrosing alveolitis, and multiple sclerosis (Table 1). In 316 cases 2 separate autoimmune disorders were listed on the death certificate, and in 7 cases 3 disorders were listed.
Tables 2 and 3 show the 2003 rankings of underlying causes and underlying or contributory causes of death among all females aged 1 year and older, and rankings stratified by age for females younger than 75 years. Autoimmune disorders were the 10th most common underlying cause of death among all females aged 1 year or older, and autoimmune disorders ranked as the 6th or 7th leading cause for females in each age group up to 75 years (Table 2). Autoimmune disorders were the 12th most frequent underlying or contributory cause of death among all females aged 1 year or older and then appeared in the top 15 causes of death for females in each age group up to 75 years; autoimmune disorders were the 6th or 7th most common cause of death among females aged 15 to 54 years (Table 3). Among women aged 75 years or older, autoimmune disorders were the 12th most common underlying cause of death and the 16th most common underlying or contributory cause of death (e-Table 2, available as an online supplement to this article at http://www.ajph.org).
TABLE 2.
Leading Underlying Causes of Mortality Among Females Aged 1 Year or Older: England and Wales, 2003
| Mortality Rankinga | Deaths, No. (%) |
| All females aged ≥ 1 y | |
| 1. Malignant neoplasms | 65 156 (23.0) |
| 2. Ischemic heart disease | 45 012 (15.9) |
| 3. Cerebrovascular diseases | 35 839 (12.7) |
| 4. Influenza and pneumonia | 21 252 (7.5) |
| 5. Dementia and Alzheimer's disease | 13 311 (4.7) |
| 6. Chronic lower respiratory diseases | 13 248 (4.7) |
| 7. Heart failure and complications of ill-defined heart disease (n = 97) | 8 375 (3.0) |
| 8. Diseases of the urinary system (n = 68) | 5 164 (1.8) |
| 9. Accidents | 5 019 (1.8) |
| 10. Autoimmune diseases | 4 420 (1.6) |
| All causes of deathb | 283 236 (100.0) |
| Females aged 1–14 y | |
| 1. Malignant neoplasms | 129 (20.0) |
| 2. Accidents | 87 (13.5) |
| 3. Congenital malformations | 76 (11.8) |
| 4. Homicide and probable homicide | 28 (4.3) |
| 5. Cerebral palsy and other paralytic syndromes | 25 (3.9) |
| 6. Meningitis | 18 (2.8) |
| 7. Autoimmune diseases | 17 (2.6) |
| 7. Epilepsy and status epilepticus | 17 (2.6) |
| 8. Influenza and pneumonia | 15 (2.3) |
| 8. Cardiomyopathy | 15 (2.3) |
| All causes of death | 646 (100.0) |
| Females aged 15–34 y | |
| 1. Malignant neoplasms | 559 (22.4) |
| 2. Accidents | 372 (14.9) |
| 3. Suicide and injury/poisoning of undetermined intent | 291 (11.7) |
| 4. Homicide and probable homicide | 110 (4.4) |
| 5. Congenital malformations | 95 (3.8) |
| 6. Mental/behavioral disorders due to psychoactive substance use | 93 (3.7) |
| 7. Autoimmune diseases | 81 (3.3) |
| 8. Cirrhosis and other diseases of the liver (n = 1) | 74 (3.0) |
| 8. Cerebrovascular diseases | 74 (3.0) |
| 9. Epilepsy and status epilepticus | 62 (2.5) |
| All causes of death | 2 491 (100.0) |
| Females aged 35–54 y | |
| 1. Malignant neoplasms | 5 791 (46.6) |
| 2. Cirrhosis and other diseases of the liver (n = 7) | 866 (7.0) |
| 3. Ischemic heart disease | 760 (6.1) |
| 4. Cerebrovascular diseases | 706 (5.9) |
| 5. Suicide and injury/poisoning of undetermined intent | 486 (3.9) |
| 6. Accidents | 459 (3.7) |
| 7. Autoimmune diseases | 385 (3.1) |
| 8. Chronic lower respiratory diseases | 295 (2.4) |
| 9. Influenza and pneumonia | 240 (1.9) |
| 10. Epilepsy and status epilepticus | 125 (1.0) |
| All causes of death | 12 438 (100.0) |
| Females aged 55–74 y | |
| 1. Malignant neoplasms | 24 449 (42.9) |
| 2. Ischemic heart disease | 8 143 (14.3) |
| 3. Cerebrovascular diseases | 4 202 (7.4) |
| 4. Chronic lower respiratory diseases | 3 950 (6.9) |
| 5. Influenza and pneumonia | 1 587 (2.8) |
| 6. Autoimmune diseases | 1 450 (2.6) |
| 7. Cirrhosis and other diseases of the liver (n = 49) | 1 072 (1.9) |
| 8. Diabetes (n = 79) | 776 (1.4) |
| 9. Dementia and Alzheimer's disease | 683 (1.2) |
| 10. Aortic aneurysm and dissection | 665 (1.2) |
| All causes of death | 57 001 (100.0) |
Numbers in parentheses are the number of autoimmune deaths included in the specific cause-of-death category.
Only the top 10 causes of death are listed, so the individual percentages will not total to 100%.
TABLE 3.
Leading Underlying or Contributory Causes of Mortality Among Females Aged 1 Year or Older: England and Wales, 2003
| Mortality Rankinga | Deaths, No. (%) |
| All females aged ≥ 1 y | |
| 1. Malignant neoplasms | 71 213 (25.1) |
| 2. Influenza and pneumonia | 62 788 (22.2) |
| 3. Ischemic heart disease | 60 693 (21.4) |
| 4. Cerebrovascular diseases | 46 555 (16.4) |
| 5. Heart failure and complications of ill-defined heart disease (n = 483) | 35 359 (12.5) |
| 6. Dementia and Alzheimer's disease | 26 214 (9.2) |
| 7. Chronic lower respiratory diseases | 20 455 (7.2) |
| 8. Diseases of the urinary system (n = 162) | 17 982 (6.4) |
| 9. Hypertensive diseases | 14 353 (5.1) |
| 10. Diabetes (n = 781) | 13 497 (4.8) |
| 11. Septicemia | 11 686 (4.1) |
| 12. Autoimmune diseases | 9 271 (3.4) |
| Females aged 1–14 y | |
| 1. Malignant neoplasms | 137 (21.2) |
| 2. Congenital malformations | 103 (15.9) |
| 3. Accidents | 100 (15.5) |
| 4. Influenza and pneumonia | 89 (13.8) |
| 5. Cerebral palsy and other paralytic syndromes | 44 (6.8) |
| 6. Septicemia | 39 (6.0) |
| 7. Epilepsy and status epilepticus | 38 (5.9) |
| 8. Respiratory failure | 34 (5.3) |
| 9. Homicide and probable homicide | 28 (4.3) |
| 10. Heart failure and complications of ill-defined heart disease (n = 6) | 25 (3.9) |
| 11. Cerebrovascular diseases | 24 (3.7) |
| 12. Meningitis | 21 (3.1) |
| 13. Autoimmune diseases | 20 (3.1) |
| Females aged 15–34 y | |
| 1. Malignant neoplasms | 569 (22.8) |
| 2. Accidents | 471 (18.9) |
| 3. Suicide | 292 (11.7) |
| 4. Influenza and pneumonia | 198 (8.0) |
| 5. Mental and behavioral disorders due to psychoactive substance use | 144 (5.8) |
| 6. Congenital malformations | 135 (5.4) |
| 7. Autoimmune diseases | 128 (5.2) |
| 8. Septicemia | 122 (4.9) |
| 9. Homicide and probable homicide | 115 (4.6) |
| 10. Cirrhosis and other diseases of the liver (n = 2) | 112 (4.5) |
| Females aged 35–54 y | |
| 1. Malignant neoplasms | 5 958 (47.9) |
| 2. Cirrhosis and other diseases of the liver (n = 9) | 1 196 (9.6) |
| 3. Influenza and pneumonia | 1 171 (9.4) |
| 4. Ischemic heart disease | 932 (7.5) |
| 5. Cerebrovascular diseases | 876 (7.0) |
| 6. Autoimmune diseases | 625 (5.0) |
| 7. Accidents | 579 (4.7) |
| 8. Septicemia | 528 (4.3) |
| 9. Suicide | 486 (3.9) |
| 9. Heart failure and complications of ill-defined heart disease (n = 39) | 486 (3.9) |
| Females aged 55–74 y | |
| 1. Malignant neoplasms | 25 785 (45.2) |
| 2. Ischemic heart disease | 10 970 (19.3) |
| 3. Influenza and pneumonia | 7 769 (13.6) |
| 4. Chronic lower respiratory diseases | 5 986 (10.5) |
| 5. Cerebrovascular diseases | 5 862 (10.3) |
| 6. Heart failure and complications of ill-defined heart disease (n = 119) | 4 748 (8.3) |
| 7. Diabetes (n = 260) | 3 364 (5.9) |
| 8. Diseases of the urinary system (n = 57) | 3 291 (5.8) |
| 9. Autoimmune diseases | 2 938 (5.2) |
| 10. Hypertensive diseases | 2 757 (4.8) |
Numbers in parentheses are the number of autoimmune deaths included in the specific cause-of-death category.
In total, 3.2% (n = 101 703) of all females aged 1 year or older who died between 1993 and 2003 had an autoimmune disorder mentioned on their death certificate (e-Table 3, available as an online supplement to this article at http://www.ajph.org). Age-specific percentages were lowest among women aged 75 years or older (2.4%–2.8% of deaths) and highest among females aged 15 to 34 years (4.6%–5.7% of deaths). The change from ICD-9 to ICD-10 coding in 2001 limited interpretation of the trend in proportions across the full study period, but in general age-specific proportions remained relatively constant.
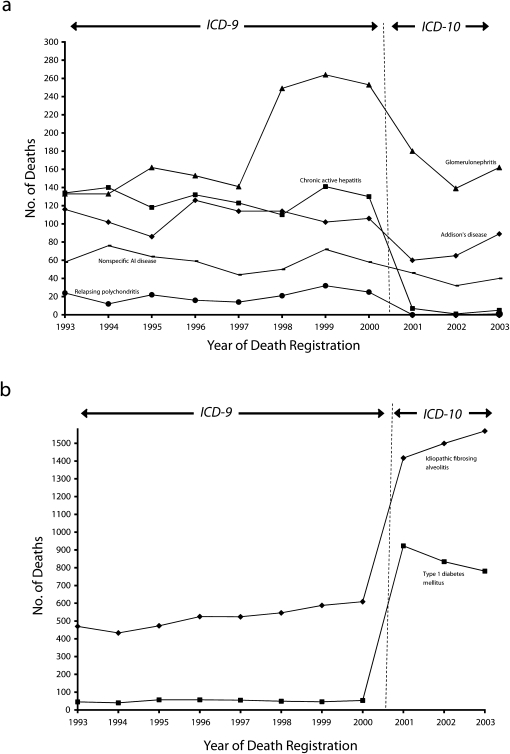
The change from ICD-9 to ICD-10 coding resulted in more than a 25% reduction in the number of underlying or contributory cause deaths for 5 autoimmune disorders (Addison's disease, CAH, glomerulonephritis, relapsing polychondritis, and unspecified autoimmune disease) involving 10 or more female deaths in the year 2000. Death counts for these disorders throughout the study period are summarized in Figure 1a. After being assigned nonspecific codes in the ICD-9, CAH and relapsing polychondritis were assigned specific codes in the ICD-10 in 2001, and each disorder showed a marked drop in number of deaths; death counts were then maintained at a lower level. Addison's disease, classified with a more specific code in the ICD-10 (primary adrenocortical insufficiency) than in the ICD-9 (corticoadrenal insufficiency), also showed a drop in death counts in 2001, but this decrease was not maintained.
FIGURE 1.
Trends in numbers of deaths associated with autoimmune disorders (as an underlying or contributory cause) among females aged 1 year or older by (a) decreases related to the change from ICD-9 to ICD-10 coding and (b) increases related to the change from ICD-9 to ICD-10 coding: England and Wales 1993–2003.
Note. ICD = International Classification of Diseases.
Unspecified autoimmune disease and glomerulonephritis were represented by specific or near-specific codes in both the ICD-9 and the ICD-10; deaths attributed to the former declined gradually throughout the study period, whereas there was a marked increase in glomerulonephritis-coded deaths in 1998 and a corresponding fall in 2001. We investigated ICD-9–coded glomerulonephritis deaths further and found that the 1998 increase resulted from a coding change whereby 3 conditions (reflux nephropathy, membranous nephropathy, and mesangiocapillary glomerulonephritis) were newly indexed to a glomerulonephritis code (582.9). In 2001, these 3 conditions were recoded to a nonglomerulonephritis ICD-10 code (N18.9, chronic renal failure, unspecified).
By contrast, the change from ICD-9 to ICD-10 coding was associated with marked increases in deaths attributed to type 1 DM and IFA (Figure 1b). Only 53 of the 12 018 ICD-9 DM-coded deaths in 2000 occurred among females aged younger than 35 years and were thus classified as type 1 DM deaths, in comparison with 923 female deaths in 2001 that fulfilled the ICD-10 case definition. The increase was attributable to the large number of deaths in 2001 (865) with specific ICD-10 type 1 DM codes among women aged 35 years or older. We examined the age distribution of DM-coded deaths in 2003 to assess the extent to which we may have continued to underestimate type 1 DM deaths in the ICD-10 data (e-Table 4, available as an online supplement to this article at http://www.ajph.org); 9546 (71%) DM-coded deaths among women 35 years or older involved a nonspecific DM code and were thus excluded from type 1 DM death counts. Consistent with our case definition, no type 2 DM-coded deaths occurred among females aged younger than 35 years.
Interestingly, the number of IFA-coded deaths increased from 609 deaths in 2000 to 1417 in 2001. There was a specific IFA code in the ICD-9 but a slightly less specific code in the ICD-10 that included IFA and diffuse pulmonary fibrosis but excluded interstitial lung diseases from other causes. In the 1999 bridge-coded data, 58% of the 742 ICD-10 IFA-coded female deaths were assigned the specific ICD-9 IFA code and 41% were assigned the nonspecific ICD-9 code 515, which included fibrosis of the lung, chronic or unspecified. In 2003, there were 1568 female IFA-coded deaths, 66% of which occurred among women aged 75 years or older. Reducing the calculation of 2003 IFA deaths to 58% of the total (the percentage of IFA ICD-10–coded deaths assigned the specific ICD-9 code in the bridge-coded data) did not alter any of the age-specific ranking positions of autoimmune disorders (Tables 2 and 3) with the exception of underlying or contributory cause deaths among women aged 55 to 74 years, which changed from the 9th to the 10th most frequent cause (data not shown).
Fewer than 10 deaths in each year were assigned specific codes for Graves’ disease. Because most thyrotoxicosis among UK women is due to Graves’ disease, we investigated underascertainment of autoimmune deaths from this cause by identifying the number of female deaths in 2003 assigned the nonspecific thyrotoxicosis code E059 (thyrotoxicosis, unspecified).1 We identified an additional 251 potential Graves’ disease deaths, 82% of which occurred among women aged 75 years or older (thus not affecting the rankings in Tables 2 and 3).
DISCUSSION
Our results show that autoimmune disorders are a major cause of mortality among females in England and Wales, appearing among the 10 most frequent underlying causes of death for females in all age groups between 1 and 75 years. Our findings are consistent with those of Walsh and Rau,13 who identified autoimmune disorders as a leading underlying cause of death among US women in 1995. Unlike the Walsh and Rau study, however, we ranked autoimmune disorders not only as an underlying cause of death but also as an underlying or contributory cause. This ranking allowed us to more thoroughly assess the burden of mortality attributable to such disorders. This ranking also enabled us to estimate trends in the contribution of these disorders to mortality without having to adjust our data for changes over time in the coding rules used to choose the underlying cause of death from conditions listed on death certificates.20
Ranking analyses involving underlying or contributory cause data are affected by the inclusion on the death certificate of conditions that are part of the mechanism of death such as heart failure or respiratory failure, because these conditions compete for ranking positions. Nonetheless, autoimmune disorders remained in the 10 most frequent underlying or contributory causes of death among females aged 15 to 74 years. Analyses of mortality over an 11-year period showed no obvious decreases in the proportion of female deaths over time in which autoimmune disorders were underlying or contributory causes. Age-stratified counts demonstrated that although autoimmune disorders ranked lower among women aged 75 years or older, the numbers of women who had autoimmune disorders at the time of their death increased with age, and 60% of all underlying or contributory cause autoimmune deaths occurred among this age group in 2003.
It is likely that we underestimated the true burden of mortality associated with autoimmune disorders. More than 80 disorders are now thought to result from autoimmune responses, although the strength of evidence for autoimmune pathogenesis varies for different disorders; we restricted our analyses to 35 conditions with strong evidence.1,14 It is also likely that we excluded some autoimmune deaths assigned less specific ICD-10 codes. For example, although it is likely that up to 80% of thyrotoxicosis in females is due to Graves’ disease, only 3 of the 251 deaths coded as Graves’ disease or as nonspecific thyrotoxicosis had specific codes for Graves’ disease and were included in our analyses.21
Similarly, we excluded a large number of diabetes deaths among women aged 35 years or older because they were assigned nonspecific DM codes. It is not known how many of these diabetes deaths were type 1 DM deaths, but age-stratified analyses of ICD-10 data indicated that many type 1 DM deaths occur among older women. In addition, underestimation of the burden of mortality is likely to have occurred because some women who have an autoimmune disorder at the time of their death do not have the disorder documented on their death certificate.22,23
Conversely, we may have overestimated deaths for some disorders, particularly in the case of the ICD-9–coded data given that these data included a higher proportion of nonspecific codes. Our comparison of deaths coded with the ICD-9 and deaths coded with the ICD-10 indicated that we may have overestimated deaths related to Addison's disease prior to 2001. Our analyses also suggested that deaths assigned ICD-9 codes for CAH and for relapsing polychondritis may have included largely nonautoimmune conditions, in that both CAH and relapsing polychondritis are rare relative to the other disorders represented by these nonspecific codes. However, any overestimation will have had a limited impact on our ICD-9 analyses because the total number of deaths assigned these 2 nonspecific ICD-9 codes was small. In addition, the ICD-10 autoimmune CAH code (K75.4) was an update (as of 1999) and was rarely implemented in the first few years after the introduction of ICD-10 coding in England and Wales mortality data, resulting in underestimation of CAH deaths.
The marked increase in deaths attributed to glomerulonephritis between 1998 and 2000 was unexpected and was found to be the result of a 1998 indexing change in which additional conditions (some glomerulonephritis specific) were assigned to a glomerulonephritis code. This situation illustrates that minor alterations in death certificate coding can result in marked changes in mortality trend data for rare conditions.
An advantage of our ICD-10–coded data was that most conditions were assigned codes that were either specific or nearly specific for the autoimmune conditions of interest. Thus, the increase in underlying or contributory cause deaths associated with IFA after the change from ICD-9 coding to ICD-10 coding was surprising. Examination of 1999 bridge-coded data revealed that a proportion of ICD-10–coded IFA deaths were bridge coded to a nonspecific ICD-9 code for chronic lung fibrosis. Because the ICD-10 IFA codes excluded lung fibrosis from other causes (such as infections, drugs, and other external agents), the specific ICD-9 IFA code may not have captured all IFA deaths and thus may have led to underestimation of IFA mortality. Alternatively, the ICD-10 code may include some nonautoimmune cases of lung fibrosis. However, when we reduced ICD-10-coded IFA counts to the minimum percentage of cases likely to involve IFA (58%), there was almost no difference in the overall ranking of autoimmune diseases as a major cause of female mortality. We are unlikely to have greatly overestimated autoimmune deaths by including nonspecific diabetes deaths among females younger than 35 years, because such deaths in this group during the study period were likely to have been the result of type 1 diabetes.
We grouped cancers and accidents into single categories in our ranking analyses so that we could examine deaths associated with different pathological processes. Such broad categories may be useful for setting global health priorities in prevention and control, although examination of more specific categories of disease is also important (e.g., because they may require different treatments).7
Irrespective of how nonautoimmune conditions are categorized, autoimmune disorders as a group remain neglected in ranking lists, and the collective impact of autoimmune disorders on female health has been masked. Autoimmune disorders predominantly affect females, who represent more than 80% of cases for some conditions.2 Our results demonstrate that autoimmune disorders are a major public health issue for females in the United Kingdom. Grouping these disorders together emphasizes their shared underlying pathogenesis and may help promote the research needed to identify common determinants and thus future preventative strategies.
Acknowledgments
This work was supported by a project grant from the Wellcome Trust (079482/Z/06/Z). Liam Smeeth is supported by a Wellcome Trust Senior Research Fellowship in Clinical Science.
Human Participant Protection
This study was approved by the institutional review boards of the London School of Hygiene and Tropical Medicine and the UK Office for National Statistics.
References
- 1.Rose NR, Mackay IR, The Autoimmune Diseases. 4th ed San Diego, CA: Elsevier Academic Press; 2006 [Google Scholar]
- 2.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–780 [DOI] [PubMed] [Google Scholar]
- 3.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–125 [DOI] [PubMed] [Google Scholar]
- 4.Autoimmune Disease Coordinating Committee. Autoimmune Diseases Research Plan. Bethesda, MD: National Institutes of Health; 2002 [Google Scholar]
- 5.World Health Organization The 10 leading causes of death by broad income group, 2004. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/index.html Accessed July 7, 2008
- 6.National Center for Health Statistics Deaths and mortality. Available at: http://www.cdc.gov/nchs/FASTATS/deaths.htm. Accessed July 28, 2010
- 7.Niederlaender E. What Are the Leading Causes of Death in the EU? Luxembourg: Eurostat; 2006 [Google Scholar]
- 8.United Kingdom Office for National Statistics Death registrations in England and Wales: 2006, causes. Available at: http://www.statistics.gov.uk/downloads/theme_health/Death_registration_2006.pdf. Accessed July 28, 2010
- 9.International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1980 [Google Scholar]
- 10.International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Geneva, Switzerland: World Health Organization; 1994 [Google Scholar]
- 11.Becker R, Silvi J, Ma Fat D, L'Hours A, Laurenti R. A method for deriving leading causes of death. Bull World Health Organ. 2006;84(4):297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths C, Rooney C, Brock A. Leading causes of death in England and Wales—how should we group causes? Health Stat Q. 2005;28:6–17 [PubMed] [Google Scholar]
- 13.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90(9):1463–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today. 1993;14(9):426–430 [DOI] [PubMed] [Google Scholar]
- 15.Pinn VW. Sex and gender factors in medical studies—implications for health and clinical practice. JAMA. 2003;289(4):397–400 [DOI] [PubMed] [Google Scholar]
- 16.Gold LS, Ward MH, Dosemeci M, De Roos AJ. Systemic autoimmune disease mortality and occupational exposures. Arthritis Rheum. 2007;56(10):3189–3201 [DOI] [PubMed] [Google Scholar]
- 17.Alberman E, Botting B, Blatchley N, Twidell A. A new hierarchical classification of causes of infant deaths in England and Wales. Arch Dis Child. 1994;70(5):403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dattani N, Rowan S. Causes of neonatal deaths and stillbirths: a new hierarchical classification in ICD-10. Health Stat Q. 2002;15:16–22 [Google Scholar]
- 19.Rooney C, Griffiths C, Cook L. The implementation of ICD-10 for cause of death coding—some preliminary results from the bridge coding study. Health Stat Q. 2002;13:31–41 [Google Scholar]
- 20.Rooney CI, Smith SK. Implementation of ICD-10 for mortality data in England and Wales from January 2001. Health Stat Q. 2000;8:41–49 [Google Scholar]
- 21.Brent GA. Graves’ disease. N Engl J Med. 2008;358(24):2594–2605 [DOI] [PubMed] [Google Scholar]
- 22.Calvo-Alen J, Alarcon GS, Campbell R, Jr., Fernandez M, Reveille JD, Cooper GS. Lack of recording of systemic lupus erythematosus in the death certificates of lupus patients. Rheumatology. 2005;44(9):1186–1189 [DOI] [PubMed] [Google Scholar]
- 23.Laakso M, Isomäki H, Mutru O, Koota K. Death certificate and mortality in rheumatoid arthritis. Scand J Rheumatol. 1986;15(2):129–133 [DOI] [PubMed] [Google Scholar]