Abstract
To investigate effects of visual experience versus preprogrammed mechanisms on visual development, we used multiple regression analysis to determine the extent to which a variety of variables (that differ in the extent to which they are tied to visual experience) predict luminance and chromatic (red/green) contrast sensitivity (CS), which are mediated by the magnocellular (M) and parvocellular (P) subcortical pathways, respectively. Our variables included gestational length (GL), birth weight (BW), gender, postnatal age (PNA), and birth order (BO). Two-month-olds (n = 60) and 6-month-olds (n = 122) were tested. Results revealed that (1) at 2 months, infants with longer GL have higher luminance CS; (2) at both ages, CS significantly increases over a ~21-day range of PNA, but this effect is stronger in 2- than 6-month-olds and stronger for chromatic than luminance CS; (3) at 2 months, boys have higher luminance CS than girls; and (4) at 2 months, firstborn infants have higher CS, while at 6 months, non-firstborn infants have higher CS. The results for PNA/GL are consistent with the possibility that P pathway development is more influenced by variables tied to visual experience (PNA), while M pathway development is more influenced by variables unrelated to visual experience (GL). Other variables, including prenatal environment, are also discussed.
Keywords: infant vision, contrast sensitivity, magnocellular/parvocellular, magnocellular, parvocellular, visual experience
Introduction
The extent to which development is governed by “nature” versus “nurture” is a topic that has been discussed since the time of Aristotle. The nature argument proposes that development is dictated by genetic factors that are unaffected by an animal’s environment, which we refer to as “preprogrammed” development. The nurture argument proposes that experience plays a prominent role in development. With the advance of neuroscience in the last century, much empirical progress has been made on the nature/nurture debate, with clear evidence for both effects (sometimes referred to as “experience-independent” and “experience-dependent” effects; e.g., Crair, 1999). Perhaps the most progress has been made in the field of visual development due to the relative ease of manipulating the visual environment of a developing animal. Vision studies have been conducted in both human and animals, although the bulk of the data is from animals, and we generally restrict the discussion to monkeys as their visual systems are known to be very similar, in both structure and function, to that of humans (e.g., De Valois, Morgan, Polson, Mead, & Hull, 1974; Golomb, Andersen, Nakayama, MacLeod, & Wong, 1985; Jacobs, 2008; Newsome, Britten, & Movshon, 1989; Newsome & Paré, 1988).
To date, most studies investigating whether visual development is governed more by preprogrammed mechanisms versus visual experience have asked whether visual experience is necessary, with the assumption that if it is not, preprogrammed mechanisms can solely guide development. Obviously, one way this can be addressed is to determine whether the newborn’s visual system is adultlike. Although common sense suggests otherwise, it is not unreasonable to speculate that there may be some aspects of visual development that are completed in utero (and thus do not require visual experience). In support of this possibility, cell mitosis and differentiation in the anterior segment, retina, and optic nerves are completed by 30 weeks of gestation in humans (for a review, see Birch & Bosworth, 2004; Birch & O’Connor, 2001), and cortical ocular dominance columns are in place before birth (Horton & Hocking, 1996; Rakic, 1976). Still, these relatively complete aspects of visual development that occur in utero pale in comparison to the multitude of other aspects of visual development that take months or years to become adultlike, such as myelination of axons (Stiles, 2008).
Given that the newborn’s visual system is clearly not adultlike, the most common way the necessity of visual experience has been investigated involves asking whether postnatal development proceeds normally (and reaches the adultlike state) when the system is deprived of visual input. [The same question of necessity cannot easily be asked for preprogrammed development as this would require inactivating all the relevant genes, although the knockout mice model promises to provide insight into the question (for a review, see Welzl, D’Adamo, Wolfer, & Lipp, 2006).] In animals, this has been accomplished by dark rearing (Hendrickson & Boothe, 1976; Regal, Boothe, Teller, & Sackett, 1976) or lid suture (Blakemore & Vital-Durand, 1983; Harwerth, Smith, Boltz, Crawford, & von Noorden, 1983) after birth. Another variation of this experiment is to ask whether retinal input is necessary, by silencing retinal neurons using action potential blockers, such as tetrodotoxin (e.g., Shatz & Stryker, 1988). In humans, study of individuals who were visually deprived at birth due to congenital cataracts allows the necessity question to be addressed. Although a full review of the results from such studies is outside the scope of this Introduction, in brief, deprivation studies reveal different degrees of abnormal development depending on the function studied and the length of deprivation (for a review, see Boothe, Dobson, & Teller, 1985). Most relevant to the current study, contrast sensitivity is quite abnormal, particularly for medium to high spatial frequencies, in humans who experience early visual deprivation (e.g., Birch, Stager, Leffler, & Weakley, 1998; Ellemberg, Lewis, Maurer, & Brent, 2000; Ellemberg, Lewis, Maurer, Lui, & Brent, 1999; Maurer & Lewis, 1993; Tytla, Maurer, Lewis, & Brent, 1988). Because contrast sensitivity is thought to be mediated at or before the level of primary visual cortex (V1) (Boynton, Demb, Glover, & Heeger, 1999; Hawken & Parker, 1990; Palmer, Cheng, & Seidemann, 2007; although this may not be the case early in development, see Stavros & Kiorpes, 2008), this suggests that areas at or before the level of V1 are prone to the effects of visual deprivation. In sum, the results of deprivation studies demonstrate that visual experience is necessary, and thus by logical extension, preprogrammed mechanisms are not sufficient, for normal postnatal development of most aspects of vision.
Obviously, the most parsimonious description of visual development is that both preprogrammed mechanisms and visual experience are important. And, of course, it is now well accepted that the two forces do not act in isolation but rather interact. Specifically, the environment can affect gene expression, and genes can predispose an organism to seek out certain environments (for reviews, see Gottlieb, 1998; Stiles, 2008). Although this concept of gene/environment interaction is typically discussed outside the domain of vision, there is a very similar discourse in the vision field regarding how visual experience may guide biological development (for reviews, see Crair, 1999; Feller & Scanziani, 2005; Kiorpes & Movshon, 2004; Movshon & Van Sluyters, 1981). The nature of this guidance has been conjectured to take one of two forms. First, visual experience may permit biological development, either by triggering biological events (such as gene expression) or by allowing them to proceed in their preprogrammed fashion. Second, visual experience may instruct biological development, sculpting it to the statistics of the environment. Evidence for an instructive role has been shown in studies that rear animals in an environment with selective visual input, for example, with only one eye open (e.g., Horton & Hocking, 1997; for a review, see Boothe et al., 1985) or with both eyes receiving a selective set of visual cues (orientation: Hirsch & Spinelli, 1970; Muir & Mitchell, 1975; Movshon & Van Sluyters, 1981; motion: Cynader & Cmerneko, 1976; Kennedy & Orban, 1983; Pasternak, Schumer, Gizzi, & Movshon, 1985; color: Brenner, Cornelissen, & Nuboer, 1990; Sugita, 2004). An example of a naturally occurring type of visual instruction is the “oblique effect,” i.e., acuity and contrast sensitivity are better for vertical and horizontal orientations than for oblique orientations (adults: Appelle, 1972; Campbell, Kulikowski, & Levinson, 1966; Mitchell, Freeman, & Westheimer, 1967; infants: Sokol, Moskowitz, & Hansen, 1987, 1989; Teller, Morse, Borton, & Regal, 1974), which is thought to be driven by there being greater prevalence of, and thus greater experience with, cardinal orientations in the environment (Baddeley & Hancock, 1991; Bosworth, Bartlett, & Dobkins, 2006; Coppola, Purves, McCoy, & Purves, 1998; Keil & Cristobal, 2000; Switkes, Mayer, & Sloan, 1978; van der Schaaf & van Hateren, 1996).
In the current study, we investigated effects of visual experience versus preprogrammed development, not by removing or manipulating visual experience as other studies have done but by asking whether a variety of variables (that differ in the extent to which they are tied to visual experience) predict variance across subjects in visual performance. Using a multiple regression analysis (MRA), our predictor variables were gestational length, birth weight, gender, postnatal age, and birth order, which we chose because they have all been shown to predict visual and non-visual behaviors (see Discussion). Our visual performance measures were luminance (light/dark) and chromatic (red/green) CS, which are thought to be mediated by the magnocellular and parvocellular subcortical pathways, respectively (Lee, Pokorny, Smith, Martin, & Valberg, 1990; Shapley, 1990; Smith, Pokorny, Davis, & Yeh, 1995; for an opposing point of view, see Lennie & D’Zmura, 1988; for the possibility that CS is mediated by the M and P representations at the level of visual cortex, see Discussion section). These pathways make up the bulk of the projections from the retina to area V1. There has been some evidence that P pathway development is more dependent on visual experience (i.e., in general, early visual deprivation leads to greater deficits on P than on M pathway tasks: Bradley & Freeman, 1981; Davis et al., 2006; Demirci et al., 2002; Hess & Howell, 1977; Levi & Harwerth, 1977; but see Zele, Pokorny, Lee, & Ireland, 2007), and that M pathway development may be more susceptible in genetic-based developmental disorders (for a review, see Braddick, Atkinson, & Wattam-Bell, 2003). Thus, we hypothesized that predictor variables likely tied to visual experience (e.g., postnatal age) would account for more variance in our P than our M pathway measure, while predictor variables not tied to visual experience, and thus more likely tied to preprogrammed development (e.g., birth weight or gestational length), would account for more variance in our M than our P pathway measure. We also entertained a third possibility, which is that prenatal environment could contribute to the effects observed in the current study. This is based on a large literature showing that prenatal environmental factors (e.g., maternal nutrition, immune response, smoking/drug use, teratogens, etc.) affect development in many domains (for reviews, see Blanchard, 2001; Dalby, 1978; Khera, 1981; Leader, Wong, & Deitel, 1981).
Methods
Subjects
Subjects were recruited via mass mailings of 3,000 to 4,000 letters sent each month to new parents residing in San Diego County. Interested recipients of letters called the laboratory to enroll (which typically occurred when the infant was between 2 and 6 weeks old). Infants with impairments (neurological, ocular, visual, or hearing), illnesses, or pregnancy/labor complications, based upon parent reports, were not tested. Because we employed red/ green stimuli, also excluded were infants with a greater than 50% chance of colorblindness; for example, male infants whose paternal grandfather was known to be colorblind. A total of 182 infants participated and fell into one of two age ranges: 2-month-olds (n = 60) or 6-month-olds (n = 122). Data from the 2-month-old group were comprised of infants who served as control infants in a study investigating contrast sensitivity in premature infants (Bosworth & Dobkins, 2008). Data from the 6-month-old group were comprised of infants who served as control infants in a study investigating contrast sensitivity in infants at risk for autism spectrum disorders (see Supplementary materials of McCleery, Allman, Carver, & Dobkins, 2007). At the time of enrollment, parents provided the following information about their infant: gender, birth order (BO), birth weight (BW), and due date. Parent report has been shown to be quite accurate for BW and due date when the information is obtained soon after birth (Seidman, Slater, Ever-Hadani, & Gale, 1987), which is the case in the current study. With regard to obtaining BO information, we confirmed that non-firstborn infants had older siblings who lived in the same household, and that firstborn infants did not have older step- or half-siblings who lived in the same household.
Gestational length (GL) and postnatal age (PNA) on the first day of testing were restricted by criteria employed by our laboratory, which was ~±15 days. For PNA, ±15 refers to scheduling the first day of testing for an infant within ±15 days from the desired month-birthday. For GL (which was calculated, in part, from due date, see below), ±15 refers to including infants who were born ±15 days from their due date. In both our 2- and 6-month-old samples, both GL and PNA turned out to be normally distributed,1 and PNA and GL also ended up with roughly equal variances (see below). GL was calculated from the difference between an infant’s birth date and due date. For example, if an infant was born 3 days “late,” we considered their GL to be 266 days (which is an estimate of the “standard” GL for infants, based on a 38-week, post-conception, gestation period) + 3 days = 269 days. Note that while the value we chose for the “standard” GL (i.e., 266 days) could be debated, it is inconsequential for our statistical analyses. Also, we are of course aware that some variability in our GL measure will be due to error in predicted due date, the latter derived based on ultrasound dating, typically within the first trimester (86% of our sample) or last menstrual period, LMP (13% of our sample). We model the effect this error might have had on our results in Appendix A.
In our final samples, for 2-month-olds, PNA ranged from 54 to 77 days (mean = 63.3 days; SD = 5.1 days; range = 23 days), GL ranged from 251 to 283 days (mean = 264 days, SD = 6.4 days; range = 32 days), and mean birth weight was 7.8 lb (SD = 0.88 lb). For 6-month-olds, PNA ranged from 171 to 191 days (mean = 181.9 days; SD = 4.4 days; range = 20 days), GL ranged from 252 to 281 days (mean = 264 days, SD = 6.8 days; range = 29 days), and mean birth weight was 7.9 lb (SD = 0.91 lb). As would be expected, the 2- and 6-month-olds did not differ in GL (F(1,179) = 0.1, p = 0.75) or birth weight (F(1,179) = 0.35; p = 0.55). With regard to comparisons between ages, Levene’s statistical test of equal variance revealed no difference between 2- and 6-month-olds in PNA variance (F(1,180) = 0.54; p = 0.46) or GL variance (F(1,180) = 1.12; p = 0.29). With regard to comparisons between PNA and GL variances (which cannot be done statistically, but can be done by inspection of their standard deviations, see above), there is roughly equal variance between the two predictor variables in the 2-month-old group. In the 6-month-old group, there was a greater difference between PNA and GL variance, but we think this difference highly unlikely to account for our results since we found the opposite of what might be predicted from this difference; the variable with less variance (PNA) predicted contrast sensitivity, while the variable with more variance (GL) did not.
On a final note, for all of our statistical analyses, GL and PNA values were entered as linear days, but we also analyzed the data using logged values. The results were nearly identical, and thus we only present the results for linear days. In addition, using linear days is preferred because it makes slopes of regression lines easier to interpret.
Apparatus and stimuli
Luminance (light/dark) and chromatic (red/green) stimuli were presented on an Iiyama Vision Master Pro 510 monitor (1024 × 768 pixels, 100 Hz) powered by a Dell Dimension computer and viewed at a distance of 38 cm. Stimuli were horizontally oriented sinusoidal gratings (moving upward or downward) with a spatial frequency of 0.27 cycles/degree and a temporal frequency of 4.2 Hz. These parameters were chosen because they are near the peak of the contrast sensitivity functions for young infants (e.g., Atkinson, Braddick, & Moar, 1977; Banks & Salapatek, 1978; Dobkins, Anderson, & Lia, 1999; Hartmann & Banks, 1992; Rasengane, Allen, & Manny, 1997).2 The stimuli subtended 11° × 11° and were centered 15° to the left or right of the middle of the video monitor. The mean chromaticity of the gratings and the background was CIE = 0.486, 0.442. The mean luminance of gratings and the background was 20 cd/m2 for 2-month-olds and 13 cd/m2 for 6-month-olds; the luminance difference between ages being due to the fact that the data from 2- and 6-month-olds were obtained during the course of different studies (see above). Contrast of stimuli is described in terms of cone contrast, i.e., the amount of response modulation produced in the long-wavelength-selective (L) and medium-wavelength-selective (M) cones in the eye (for methodological details, see Dobkins et al., 1999; Gunther & Dobkins, 2002).
Determining red/green isoluminance
The red/green chromatic stimulus was presented at the mean isoluminance value obtained from 22 adults, using standard motion photometry (Dobkins & Teller, 1996b; Moreland, 1982; Teller & Lindsey, 1993). In this task, adults fixated on a small dot in the center of a moving red/green grating and adjusted the luminance contrast in the grating until the percept of motion was least salient. Each adult subject’s isoluminance point was determined from the mean of 25 trials. The stimulus conditions for the motion photometry procedure were identical to those employed in the main experiments (i.e., same size, orientation, spatiotemporal frequency). As previously discussed (e.g., Dobkins & Teller, 1996b), the justification for using the adult mean isoluminance value in our infant experiments is based on previous experiments demonstrating that infant and adult mean isoluminance points are highly similar for red/green stimuli (Bieber, Volbrecht, & Werner, 1995; Brown, Lindsey, McSweeney, & Walters, 1995; Dobkins, Anderson, & Kelly, 2001; Maurer, Lewis, Cavanagh, & Anstis, 1989; Morrone, Burr, & Fiorentini, 1993; Pereverzeva, Hui-Lin Chien, Palmer, & Teller, 2002; Teller & Lindsey, 1989). Moreover, Brown and colleagues argue quantitatively that the variability of isoluminance points across infant subjects is comparable to the variability across adult subjects, when measurement error is taken into account. In previous studies, we have calculated that the amount of luminance error likely to exist in our red/green stimuli is below luminance contrast threshold for infants (see Dobkins & Teller, 1996b).
Obtaining contrast sensitivities
The dependent measures in this study were log luminance and log chromatic contrast sensitivities (CS), which were obtained using forced-choice preferential looking (Teller, 1979) with the method of constant stimuli, as described in detail previously (see Dobkins & Teller, 1996a, 1996b). Briefly, an adult experimenter held the infant 38 cm away from the front of the stimulus monitor in the view of a video camera aimed at the infant’s face. On each trial, a grating stimulus appeared on the left or right side of the video monitor (centered at 15° eccentricity), and the experimenter used cues such as the infant’s head turning and gazing behavior to judge the left versus right location of the stimulus. Chromatic or luminance gratings were presented randomly across trials, as was one of five contrast levels (luminance = 1.25%–80% cone contrast, chromatic = 1.25%–26% cone contrast). Stimuli remained present on the video monitor until the experimenter made the left/right judgment, which was typically less than two seconds. The experimenter’s answer was entered into the computer by pressing keys on the keyboard and computer beeps provided feedback as to whether the experimenter was correct. Our goal was to obtain 200 total trials per infant (100 trials × 2 conditions) over the course of 2 or 3 days within a 1-week period. The mean number of total trials obtained per infant was 158 (SD = 58) and 267 (SD = 40) for 2- and 6-month-olds, respectively.
For each infant, a psychometric curve was fit to chromatic and luminance data using Weibull functions and maximum likelihood analysis (Watson, 1979; Weibull, 1951). Threshold was defined as the contrast yielding 75% correct performance, and sensitivity was computed as the inverse of threshold × 100. Sensitivity was then logged since log, but not linear, sensitivity data conform to normal distributions (Graham, 1989). It is perhaps important to point out that there should be no measurement-imposed restriction in the variance of the CS measure (which if were the case, would lessen the chance of seeing effects of predictor variables on the CS measure). This is because there was a large range of possible CS values, and subjects’ CS values were neither at ceiling nor floor for our system (see scatter plots of data in Figure 1 and Figure 2). (This is to be contrasted with other sorts of dependent measures, e.g., “number of words produced at 10 months,” where the range/variance of the measure would clearly be restricted.)
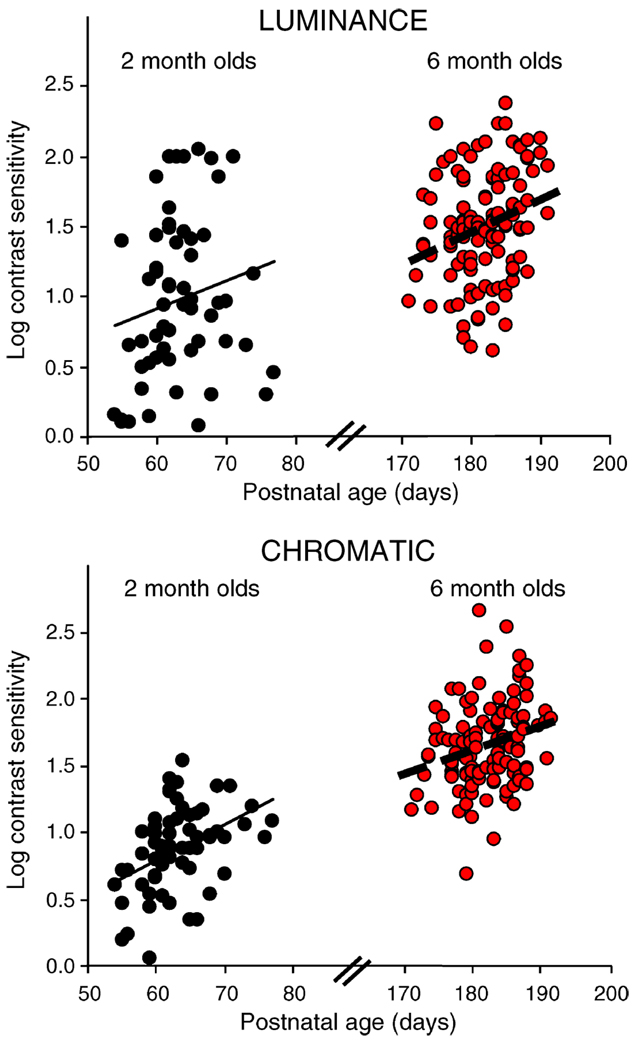
Figure 1.
Scatter plot of log contrast sensitivity versus postnatal age (PNA). Data are presented for luminance CS (top panel) and chromatic CS (bottom panel), with the two age groups, 2-month-olds (black circles, n = 60) and 6-month-olds (red circles, n = 122), plotted side by side. Simple linear regression lines are shown for each data set. These results show that for the range of PNA tested (~21 days), PNA predicts luminance and chromatic CS at both 2 and 6 months. Although the r value for luminance CS at 2 months was not significant (see Table 1), the effect for this condition was significant in the MRA (see Table 2).
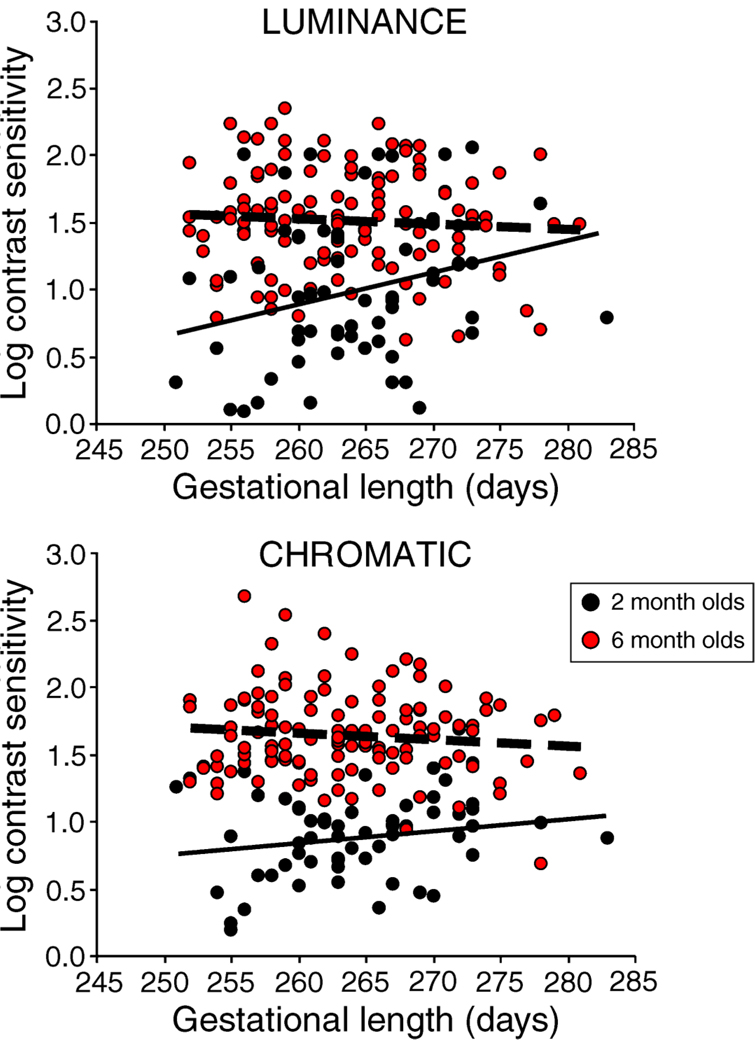
Figure 2.
Scatter plot of log contrast sensitivity versus gestational length (GL). Data are presented for luminance CS (top panel) and chromatic CS (bottom panel), with the two age groups, 2-month-olds (black circles, n = 60) and 6-month-olds (red circles, n = 122), plotted together. Simple linear regression lines are shown for each set of data. In both the MRA and linear regression lines, GL was found to be a significant predictor only for luminance CS in 2-month-olds; infants with longer GL exhibit higher luminance CS.
Multiple regression analyses (MRA)
Four separate MRAs were conducted, for two CS types (chromatic and luminance) and two age groups (2-month-olds and 6-month-olds). For each of the four MRAs, we tested the contribution of five different predictor variables. Three were continuous: gestational length (GL), postnatal age (PNA), and birth weight (BW). Two were categorical: gender (boy/girl) and birth order (BO, firstborn vs. non-firstborn).3 Note that we refer to these variables as “predictors” because we hypothesize that each might contribute to variance in CS (see Introduction). It is also for this reason that we simultaneously entered all predictors in our MRA (as opposed to using a hierarchical approach).
Before conducting the MRA, each variable was examined for acceptable skew, normality of residuals, and multicollinearity. Shapiro–Wilkes W tests on dependent measures (luminance and chromatic CS) and continuous predictor variables (GL, PNA, and BW) indicated that data were normally distributed. Pearson correlation coefficients (r values) between continuous variables tested for indications of overly high correlation among variables (for example, GL and BW are likely to be intercorrelated). Pearson correlation coefficients are often the first step in an MRA study, although unlike the MRA, they cannot control for possible covariates. Pearson r values are presented in Table 1. We also investigated whether continuous predictor variables (GL, PNA, and BW) differed between categorical predictor variables (gender: boy vs. girl; BO: firstborn vs. non-firstborn). The results revealed gender differences for BW, with boys being heavier as would be expected (p < 0.05), which is controlled for in the MRA. An unanticipated difference was seen for PNA in 2-month-olds, where the non-firstborn group was tested at a slightly, but significantly, older age than the firstborn group (p < 0.05). This is a random skew in the data set, which is non-meaningful and is controlled for in the MRA. All other variables were balanced across the categories.
Table 1.
Pearson correlation coefficients (r values) for continuous variables.
| 2-month-olds (N = 60) |
6-month-olds (N = 122) |
|||||||
|---|---|---|---|---|---|---|---|---|
| PNA | BW | Luminance CS | Chromatic CS | PNA | BW | Luminance CS | Chromatic CS | |
| GL | 0.04 | 0.15 | 0.27* | 0.18 | −0.18 | 0.21* | −0.07 | −0.10 |
| PNA | 0.29* | 0.18 | 0.43* | −0.03 | 0.27* | 0.25* | ||
| BW | 0.18 | 0.27* | −0.08 | −0.15 | ||||
Note:
Significant values at p < 0.05. Note that r values for correlations of GL, PNA, and BW with both luminance CS and chromatic CS are presented within the same table even though the two types of CS were separated in the MRA.
Using SPSS, the four MRAs provided the following outputs:
The multiple R squared, R2, represents the percentage of variance in the dependent measure (luminance and chromatic CS) accounted for by all the predictor variables together.
The squared semipartial correlation coefficient, sr2, represents the percentage of variance in the dependent measure (luminance and chromatic CS) uniquely accounted for by a given predictor variable, once other variables have been taken into account. The sr2 also represents how much the R2 would decrease if that given variable was removed from the model. When the sr2 is statistically significant, that variable accounts for a significant portion of the variance in the regression model.
The unstandardized regression coefficient, B, represents the slope of a given variable’s effect while other variables are held constant.
The beta weight, β, is a standardized correlation coefficient measure, defined as the amount (in standard deviations) that the dependent measure (luminance and chromatic CS) changes for one standard deviation change in the predictor variable. β is often thought of as representing the relative contribution or importance of a given variable in the model, once other variables have been taken into account.
ANCOVA
As explained above, the MRAs were performed separately on the two CS types (luminance and chromatic CS) and also separately for the two age groups (2-month-olds and 6-month-olds). We therefore performed an analysis of covariance (ANCOVA) on all of the data together to investigate effects of age group (2- vs. 6-month-olds) and whether age group interacts with CS type (luminance and chromatic CS). The ANCOVA also allowed us to confirm/ disconfirm effects that were seen in the MRA (for example gender effects) or effects that were seen in the MRA that could not be tested statistically (for example, a reversal in the effect of BO between 2 and 6 months). The ANCOVA was also used to test specific interactions that test hypotheses based on results from previous studies (Dannemiller, 2004 reported an interaction between gender and BW on a visual performance measure in 2- to 5-month-olds; see Discussion). In the ANCOVA, CS type was the repeated-subjects variable, Age Group, Gender, and BO were between-subjects factors, and PNA, BW, and GL were included as covariates.
Results
Pearson correlation coefficients (r values) for continuous variables
Table 1 presents r for the two age groups (2-month-olds and 6-month-olds). In 2-month-olds, there was a significant correlation between GL and luminance CS and between PNA and chromatic CS. In 6-month-olds, there was a significant correlation between PNA and both luminance and chromatic CS. As might be expected, BW and GL were correlated (i.e., infants with shorter GL had lower BW), although this effect was only significant for our sample of 6-month-olds (we think the lack of an effect in 2-month-olds was due to insufficient power). Two unanticipated findings were seen in 2-month-olds. First, there was a correlation between BW and PNA. This is a random skew in the data set, which is non-meaningful and is controlled for in the MRA. Second, there was a correlation between BW and chromatic CS. This effect, which goes away in the MRA, is likely driven by a combination of the unanticipated (i.e., spurious) correlation between BW and PNA and a genuine correlation between PNA and chromatic CS.
Luminance and chromatic CS were found to be strongly correlated (2 months: r = 0.66, p < 0.0001; 6 months: r = 0.62, p < 0.0001). These correlations are driven by one or both of two factors. First, it is likely driven by the fact that PNA correlates positively with both types of CS (in other words, both types of CS are increasing with age). Second, it could be driven by a true interrelated-ness between luminance and chromatic CS, i.e., by a single source of variability for the two. Interestingly, while factor analysis studies in adults have demonstrated independent sources of variability for the two types of CS (Dobkins, Gunther, & Peterzell, 2000; Gunther & Dobkins, 2002, 2003; Peterzell & Teller, 2000), a previous study in 4-month-olds reported a single source of variability (Peterzell et al., 2000). However, as in the current study, it is possible that the Peterzell et al. (2000) finding could have been driven by small PNA variations in their sample. At the current time, we cannot distinguish between the two possibilities, PNA-driven correlation versus true intercorrelation between luminance and chromatic CS; however, we feel strongly that luminance and chromatic CS cannot be completely accounted for by a single source of variance since our MRA results (below) show differential effects of various variables on the two types of CS.
Multiple regression analyses (MRA)
Table 2 presents the results from four MRAs, for the two age groups (2-month-olds and 6-month-olds), and the two different CS types (luminance and chromatic CS). As described in the Methods section, the MRA computes the unique contribution of a given predictor variable, represented by sr2, taking all other variables into account. Controlling for the effects of other variables is especially important in cases where different predictor variables correlate with one another, either in an anticipated or unanticipated way (see Table 1). The MRA also computes an R2, which represents the percentage of variance in CS accounted for by all the predictor variables together (often referred to as the “full model”). Residual variance represents variance in the CS measure that is unaccounted for in the full model. Residual variance can be systematic (i.e., mediated by variable(s) that were not tested in our model) or unsystematic (i.e., due to noise in the measurement). R2 and residual variance values are shown at the bottom of each subtable in Table 2.
Table 2.
Results from multiple regression analysis (sr2, β, B, and p values).
| sr2 | β | B | p | |
|---|---|---|---|---|
| 2-month-olds | ||||
| Luminance | ||||
| PNA* | 9% | 0.35 | 0.04 | 0.015 |
| GL* | 7% | 0.29 | 0.03 | 0.03 |
| BW | 0% | 0.07 | 0.05 | 0.58 |
| Gender* | 6% | 0.28 | 0.31 | 0.045 |
| BO* | 7% | −0.30 | −0.36 | 0.03 |
| Full model R2 = 0.25, F(5,54) = 3.65, p = 0.006, RV = 0.49 | ||||
| Chromatic | ||||
| PNA* | 21% | 0.54 | 0.03 | 0.0001 |
| GL | 2% | 0.15 | 0.01 | 0.21 |
| BW | 2% | 0.17 | 0.06 | 0.21 |
| Gender | 2% | 0.14 | 0.09 | 0.27 |
| BO* | 6% | −0.28 | −0.19 | 0.03 |
| Full model R2 = 0.31, F(5,54) = 4.9, p = 0.001, RV = 0.27 | ||||
| 6-month-olds | ||||
| Luminance | ||||
| PNA* | 5% | 0.24 | 0.02 | 0.01 |
| GL | 0% | 0.01 | 0.001 | 0.89 |
| BW | 1% | −0.08 | −0.04 | 0.36 |
| Gender | 0% | 0.05 | 0.04 | 0.60 |
| BO (MS) | 2% | 0.15 | 0.12 | 0.10 |
| Full model R2 = 0.10, F(5,116) = 2.5, p = 0.03, RV = 0.36 | ||||
| Chromatic | ||||
| PNA* | 4% | 0.21 | 0.01 | 0.02 |
| GL | 0% | −0.004 | −0.005 | 0.97 |
| BW | 2% | −0.15 | −0.05 | 0.09 |
| Gender | 1% | 0.08 | 0.05 | 0.40 |
| BO* | 3% | 0.18 | 0.12 | 0.045 |
| Full model R2 = 0.12, F(5,116) = 3.5, p = 0.01, RV = 0.29 | ||||
Note:
Significant values at p < 0.05, MS denotes marginally significant values (p < 0.10). For continuous variables (PNA, GL, and BW), positive values indicate that a higher value of the variable predicts higher contrast sensitivity. For categorical variables (gender and birth order, BO), positive values indicate higher contrast sensitivity in boys than girls and higher contrast sensitivity in non-firstborn than firstborn infants. Also shown is the R2 for the full model and the residual variance (RV) that is unaccounted for by the full model.
In terms of the unique contribution of the different variables, the significant effects were as follows. In 2-month-olds, both GL and PNA were significant predictors of luminance CS, accounting for 7% and 9% of the variance, respectively. However, only PNA (and not GL) was a significant predictor of chromatic CS, accounting for 21% of the variance. With regard to gender, boys had higher luminance CS than girls. With regard to BO, firstborn infants had higher CS (both luminance and chromatic) than non-firstborn infants. In 6-month-olds, like the results of 2-month-olds, PNA was a significant predictor of luminance and chromatic CS, accounting for 5% and 4% of the variance, respectively. And, with regard to BO, non-firstborn infants had higher CS than firstborn infants (the effect was significant for luminance CS and marginally significant for chromatic CS). Interestingly, this BO effect is opposite in 6-month-olds as compared to 2-month-olds, which is further supported by the results of the ANCOVA (below).
Since PNA was found to be a significant predictor of luminance and chromatic CS in both 2- and 6-month-olds, we investigated whether PNA was a stronger predictor in the younger versus older group. Using a formula for comparing two-sample regression coefficients provided by Clogg, Petkova, and Haritou (1995; and see Paternoster, Brame, Mazerolle, & Piquero, 1998), PNA was found to be a marginally stronger predictor of chromatic CS in 2- than 6-month-olds (β: 21% vs. 4%; z = 1.80; p = 0.07). Note that the variance of PNA did not differ between the two age groups (see Methods), so this alone cannot underlie the age-related difference in the effect of PNA on chromatic CS. In contrast to the results for chromatic CS, for luminance CS, PNA was found to be an equally strong predictor in 2- versus 6-month-olds (β: 9% vs. 5%; z = 1.06; p = NS). These findings suggest stronger PNA effects in 2- than 6-month-olds, selectively for chromatic CS. We also used Levene’s test of equal variance to ask whether the two age groups differed in residual variances (see Table 2). Residual variance did not differ between 2- and 6-month-olds, for chromatic CS (F(1,180) = 0.075; p = 0.78) or luminance CS (F(1,180) = 0.19, p = 0.89), which suggests that there are no differences between the two age groups in (1) the amount to which another untested variable accounts for variance and (2) the measurement error (this is despite the fact that 6-month-olds, on average, were tested with more total trials; see Methods).
Within each age group, we also inspected whether PNA was a stronger predictor of chromatic CS or luminance CS (and compared residual variance between the two CS types). For 2-month-olds, PNA was a stronger predictor of chromatic CS (accounting for 21% of the variance) than of luminance CS (accounting for 9% of the variance). By contrast, for 6-month-olds, PNA was an equal predictor of chromatic CS (accounting for 5% of the variance) and luminance CS (accounting for 4% of the variance). At both 2 and 6 months, residual variance was greater for luminance than for chromatic CS (see Table 2). This difference suggests that (1) there are other untested variables that account for more variance in luminance than in chromatic CS and/or (2) there is more measurement error for luminance than for chromatic CS (perhaps because infants find the luminance stimulus less interesting). Note, however, that the possibility that there is less measurement error for chromatic than for luminance CS should not be taken as the reason for why stronger PNA effects were found for chromatic CS (at least for 2 months) since for other variables (specifically, gender and GL), stronger effects were found for luminance CS at 2 months.
Scatter Plots
To visually demonstrate the effects of PNA and GL on contrast sensitivity revealed from the MRA, we present scatter plots of the data in Figure 1 (PNA) and Figure 2 (GL). However, note that these scatter plots and their regression lines cannot be derived directly from the outputs of the MRA but instead are derived from linear regression lines between two variables, as represented by the Pearson correlation coefficients (r values) for continuous variables in Table 1. Using the individual correlations as an approximation to results from the MRA is reasonable since, with one exception, the results of the individual correlations and the MRA are in line with one another. This exception is the effect of PNA on luminance CS in 2-month-olds, which was significant in the MRA (Table 2) but not in the individual correlation between PNA and luminance CS (Table 1).
ANCOVA
An ANCOVA was conducted to investigate effects that could not be studied directly with the MRAs. This includes investigating differences in contrast sensitivity (CS) between the two age groups, 2- versus 6-month-olds, and whether age group interacts with CS type (luminance and chromatic CS). In addition, the ANCOVA allowed us to confirm/disconfirm effects that were seen in the MRA (for example, gender effects) or effects that were seen in the MRA that could not be tested statistically (for example, a reversal in the effect of BO between 2 and 6 months). As would be expected, the results revealed a significant main effect of age group, with higher CS in 6-month-olds than 2-month-olds (F(1,171) = 12.7; p = 0.0005). This effect did not interact with CS type, suggesting comparable rates of luminance and chromatic CS development between 2 and 6 months. The ANCOVA also revealed a main effect of gender, with boys having higher CS than girls (F(1,171) = 4.7; p = 0.03). This result is generally in line with the results from all four MRAs, which revealed higher contrast sensitivity in boys than girls, although in the MRA this was only significant for luminance CS in 2-month-olds. We believe that gender became significant in the ANCOVA as a result of pooling all subject data. Finally, the ANCOVA revealed a significant interaction between age group and BO (F(1,171) = 7.04; p = 0.009), which did not create a three-way interaction with CS type. This is in line with the results from the MRAs, showing a reversal in the effect of BO between 2 months (firstborn infants had higher CS) and 6 months (non-firstborn infants had higher CS).
Discussion
The current study investigated whether luminance and chromatic contrast sensitivity (CS) are predicted by different variables that vary in the extent to which they are tied to visual experience. We chose to study luminance and chromatic CS because they are thought to be mediated by the magnocellular (M) and parvocellular (P) subcortical pathways, respectively (Lee et al., 1990; Shapley, 1990; Smith et al., 1995; for an opposing point of view, see Lennie & D’Zmura, 1988), and thus studying the development of these two types of CS should shed light on the development of the two pathways.4 In addition, studying the effects of these different variables on M and P pathway development may have clinical implications as the two pathways have been conjectured to be differentially affected by early abnormal visual experience as well as differentially susceptible in developmental disorders (see Introduction). Although we refer to our luminance and chromatic CS measures as tapping the subcortical M and P pathways, we should point out that CS measured at the behavioral level is unlikely to be controlled solely within subcortical (i.e., M and P) pathways. Rather, CS is likely mediated by a combination of contrast gain mechanisms at the cortical and subcortical levels. The fact that the current study reveals differential effects of various variables on our intended M and P pathway measures is consistent with a subcortical contribution, although it is certainly possible that the observed effects occur on M and P representations at the level of visual cortex.
In the remainder of the Discussion section, for each variable that was found to predict CS in the current study, we address the likelihood that this variable is tied to visual experience versus preprogrammed development. And, when applicable, we address the possibility that prenatal environment, although not providing visual experience, could contribute to our findings. This is based on a large literature showing that prenatal environmental factors (e.g., maternal nutrition, immune response, smoking/drug use, teratogens, etc.) affect many aspects of development (for reviews, see Blanchard, 2001; Dalby, 1978; Khera, 1981; Leader et al., 1981).
Effects of gestational length (GL)
The MRA of the current study revealed GL effects only for luminance CS at 2 months. We are not surprised by the finding of GL effects at 2 months, but not 6 months, because other studies in our laboratory have shown a difference in CS between “preterm” infants (with atypically short GL) and “full-term” infants at 2 months but not 6 months (Bosworth & Dobkins, 2008). That is, effects of GL on CS seem to disappear by 6 months of age. But why were effects found only for luminance CS, and not chromatic CS, at 2 months? Obviously, it cannot simply be due to restricted variance in GL since the amount of variance was sufficient to reveal significant effects on luminance CS. [In addition, note that the GL variance did not differ between 2- and 6-month-olds (see Methods), so this cannot account for a lack of GL effect at 6 months.] However, it is possible that we missed significant effects of GL on chromatic CS at 2 months due to noise in the data arising from error in the GL estimate. This type of noise would also make the effect of GL on luminance CS at 2 months appear weaker than it actually is. To address this, we conducted a simulation model using the known error in GL estimate (see Appendix A). The results of this simulation suggest that error in GL estimate is unlikely to account for finding a lack of effect of GL on chromatic CS at 2 months.
Clearly, the observed effects of GL on luminance CS are not tied to visual experience, and thus we can say with certainty that GL is more likely tied to preprogrammed development than to visual experience. If preprogrammed mechanisms do, in fact, account for the effect of GL on luminance CS, this would mean that genetic/biological factors underlying maturation of luminance mechanisms in the M pathway are developing at a substantial rate in utero, with the result that an extra day in utero has implications for luminance CS measured postnatally. However, there is another possibility that must be entertained; perhaps effects of prenatal environment underlie variance in both GL and luminance CS at 2 months. One reasonable candidate is maternal nutrition, which has been shown to correlate with GL (Delgado, Martorell, Brineman, & Klein, 1982; Jacobson et al., 2008; Olsen et al., 2000; Olsen et al., 1992; Rayco-Solon, Fulford, & Prentice, 2005; Rush, Stein, & Susser, 1980; Smuts et al., 2003), as well as with BW (Godfrey, Robinson, Barker, Osmond, & Cox, 1996; Harding, 2001; Lou et al., 1994; Mora, Sanchez, de Paredes, & Herrera, 1981). [Note that, in general, studies report effects of maternal nutrition on either GL or BW effects, without attempting to investigate whether there are independent effects on BW and GL.] If maternal nutrition is also correlated with visual performance measured postnatally, then maternal nutrition could be the “third variable” (much cautioned against in correlational analyses), which drives the correlation between GL and CS observed in the current study. Although we know of no study that has investigated CS specifically, there is evidence that both prenatal maternal nutrition (e.g., Jacobson et al., 2008; although these results should be interpreted with caution since the study did not use randomized assignment) and postnatal infant nutrition (e.g., dietary supplementation of docosahexaenoic acid and arachidonic acid; Birch et al., 2007; Birch, Hoffman, Uauy, Birch, & Prestidge, 1998; Hoffman et al., 2004; Makrides, Neumann, Simmer, Pater, & Gibson, 1995; O’Connor et al., 2001) correlates with visual acuity in infants/children, making this maternal nutrition hypothesis a possible account of our data. Of course, this account must be considered in any study that finds a correlation between a measure of physiological maturity at birth (e.g., gestational length, weight, length, or head circumference) and an outcome measure. In fact, there is a sizeable literature showing that different aspects of physiological maturity at birth predict visual performance (visual orienting at 2 to 5 months: Dannemiller, 2004; attention to faces at 4 to 6 months: Camp, Jamieson-Darr, Hansen, & Schmidt, 1990; visual recognition memory from 5 to 12 months: Rose, 1994) and non-visual performance (language and gross movement at 4 years: Ounsted, Moar, & Scott, 1984; IQ in childhood: Churchill, 1965; Jefferis, Power, & Hertzman, 2002; Matte, Bresnahan, Begg, & Susser, 2001; Scarr, 1969). Analyses that control for prenatal environment factors will help determine whether correlations observed between physiological measures of maturity at birth and later visual performance (as in the current and previous studies) are driven by these factors.
In any event, the current study’s finding of GL effects on luminance, but not chromatic CS, indicates that M pathway development is more affected by GL than is P pathway development. This could reflect a faster rate of M than of P pathway preprogrammed development in utero. Alternatively, if the effect is tied to prenatal environment, it suggests that the M pathway may be more affected by factors like maternal nutrition, possibly because M neurons are larger than P neurons and therefore have greater metabolic demands (Glovinsky, Quigley, & Dunkelberger, 1991; Perry, Silveira, & Cowey, 1990; Quigley, Sanchez, Dunkelberger, L’Hernault, & Baginski, 1987; but cf. Crawford, Harwerth, Smith, Shen, & Carter-Dawson, 2000). At the current time, we cannot distinguish between the two possibilities.
Effects of PNA
The finding from our MRAs that PNA predicts chromatic and luminance contrast sensitivity (CS) in 2-and 6-month-olds is not terribly surprising since both types of CS obviously increase with age (FPL: Dobkins et al., 1999, 2001; Peterzell et al., 2000; visually evoked potentials (VEP): Allen, Banks, & Norcia, 1993; Crognale, 2002; Kelly, Borchert, & Teller, 1997; Morrone et al., 1993). Thus, given that the range of PNA in a sample is sufficiently large, a significant age effect will be revealed. In the current study, the range was 23 and 20 days for 2- and 6-month-olds, respectively, and thus our results show that significant improvement in luminance and chromatic CS occurs over this relatively short period of time (~3 weeks). In addition, the current study shows differences in PNA effects between 2- and 6-month-olds and between chromatic and luminance CS. Specifically, the MRA data showed that (1) PNA effects on chromatic CS were stronger in 2- than 6-month-olds, whereas PNA effects on luminance CS were statistically indistinguishable between 2- and 6-month-olds; and (2) PNA effects in 2-month-olds were stronger for chromatic than for luminance CS, while in 6-month-olds, PNA effects were nearly identical for chromatic and luminance CS.
If we believe that postnatal development of luminance and chromatic CS is preprogrammed, the PNA results of the current study could be explained by supposing that biological/genetic factors dictate a different developmental trajectory for the M (luminance) and P (chromatic) pathways. Specifically, the P pathway may be programmed to develop quickly early on (~2 months) followed by a slowing period (~6 months), while the M pathway may be programmed to develop at a more even pace. If this scenario were true, we would expect longitudinal data between 2 and 6 months to show a greater compressive non-linearity for chromatic than luminance CS. However, this possibility is not supported by data from our laboratory, which show that developmental trajectories for both chromatic and luminance CS are well described by log functions, and thus by definition, the two functions have the same compressive non-linearity (Bosworth & Dobkins, 2008). Nonetheless, we cannot rule out that preprogrammed development dictates a different (possibly subtle) trajectory for M versus P pathway development, which could underlie the differential effects of PNA observed in the current study.
Alternatively, if visual experience plays a role in postnatal development and given that a longer postnatal period affords greater visual experience, the PNA results of the current study could be explained by supposing that visual experience has differential impact on the development of the M and P pathways. Specifically, visual experience may exert stronger effects on the P pathway than on the M pathway early on (~2 months) but equivalent effects later on (~6 months). There are at least two ways this effect could be mediated. First, it could be that the P pathway is generally more amenable to being shaped by visual experience than is the M pathway. In line with this possibility are adult data from our laboratory showing greater perceptual learning on a P pathway (chromatic CS) than on an M pathway (luminance CS) task (Thurston & Dobkins, 2007, and see Heckman & Engel, 2006). Second, it could be that the P and M pathways are equally amenable to being shaped by visual experience, but that the statistics of the visual environment stimulate the P pathway more than the M pathway. For example, this could occur if visual scenes contain relatively more frequent or higher contrast, chromatic (red/green) versus luminance information, although this possibility is not supported by studies measuring the chromatic/ luminance statistics of natural scenes (MacLeod, 2003; Webster & Mollon, 1997). Still, it is possible that other visual properties that favor the P pathway, such as high spatial frequency/low temporal frequency, are particularly prominent in visual scenes (see Bex & Makous, 2002; Bosworth et al., 2006; Dakin, Mareschal, & Bex, 2005). In truth, it is more fitting to ask about the statistics of the infant’s visual environment, where the statistics of scenes that contain toys, mobiles, etc., may be biased to stimulate one visual pathway more than another (and may change as an infant starts to locomote and explore). Finding a relationship between the statistics of the infants’ visual environment and the strength of PNA effects on the different visual pathways would at least provide correlational evidence for effects of visual experience (of the sort used to account for the “oblique effect” being driven by a preponderance of cardinal orientations in the environment; see Introduction). Future studies that analyze the statistics of the infants’ visual environment will be required to help inform these possibilities.
In sum, if the PNA effects of the current study are, in fact, tied to visual experience, our results suggest that early in development (~2 months) visual experience exerts stronger effects on the P pathway than on the M pathway. This scenario is the flip-side of the asymmetry seen for GL (tied to either preprogrammed development or prenatal environment), where stronger effects were revealed for the M than for the P pathway measure (see above).
Gender effects
The current study revealed higher CS in boys than girls (ANCOVA results), which was driven mainly by higher luminance CS in boys at 2 months (MRA results). Previous studies investigating gender differences in visual abilities have yielded mixed results, depending on the visual function and age tested. In a study of luminance CS, it was reported that girls outperformed boys at 6 months (but not at 4 or 8 months; Peterzell et al., 1995), an effect that was not seen in the current study (and note that many previous studies of CS did not explicitly include gender as a variable for analysis; e.g., Banks & Salapatek, 1981; Dobkins et al., 1999; Hammarrenger et al., 2003). With respect to other visual abilities, an advantage for girls has been reported for vernier and stereoacuity (e.g., 3- to 6 months; for reviews, see Gwiazda, Bauer, & Held, 1989; Held, Thorn, Gwiazda, & Bauer, 1996), while an advantage for boys has been reported for visual accommodation (1 to 3 months; Horwood & Riddell, 2008) and mental rotation (5 months; Moore & Johnson, 2008). One possibility is that gender differences in visual performance arise from biological differences, either in preprogrammed development determined from genes on the sex chromosomes or in testosterone levels.5 Gender differences in visual performance have, in fact, been tied to differences in testosterone levels (monkeys: Bachevalier, Hagger, & Bercu, 1989; humans: Held et al., 1996). The other possibility is that gender differences in visual performance arise from differences in visual experience. It has been shown that by 3 months of age, parents’ socialization habits are different with boys versus girls (Donovan, Taylor, & Leavitt, 2007; Stern & Karraker, 1989), which could differentially affect their visual experiences.
In addition to main effects of gender, it is also interesting to consider the possibility that gender might interact with other variables. Raising this possibility is motivated by reports that the relationship between BW and visual orienting in 2- to 5-month-old infants (Dannemiller, 2004)6 and between BW and IQ in 7-year-olds (Matte et al., 2001) is significantly stronger for boys than girls. Given that maternal nutrition has an effect on both BW and later visual/non-visual performance (see above), these larger effects of BW for boys might be accounted for by the fact that the link between maternal nutrition and BW has been shown to be greater for boys (Mora et al., 1981; possibly because boys grow faster, see discussion in Dannemiller, 2004). In the current study, the MRA did not reveal an effect of BW on our visual measure, i.e., contrast sensitivity. However, based on the results from Dannemiller (2004), we entertained the possibility that we could have washed out a BW effect by including both girls and boys in the MRA. To address this, we conducted a hypothesis-driven analysis in our ANCOVA, specifically looking for an interaction between BW × Gender. Given the close association between BW and GL and because we did find an effect of GL on our visual measure in the MRA, we also looked for an interaction between GL and gender. Both these interactions came up non-significant. The difference between the current result (no BW × Gender interaction for contrast sensitivity) and that of Dannemiller (significant interaction between BW × Gender for visual orienting) could be due to differences in the visual measure between the two studies or differences in power between the two studies. Clearly, more studies are needed to address this issue.
In sum, the current study observed gender effects, with 2-month-old boys having higher luminance (M pathway) CS than girls. Further studies will be needed to determine whether these differences are driven by gender differences in biological factors (testosterone/sex chromosome genes), postnatal experience, or effects of prenatal environment. It is interesting that the gender effect of the current study was seen in the same condition (i.e., luminance CS at 2 months) that revealed significant effects of GL. If there is, in fact, a common mechanism for the two effects, we could rule out postnatal experience as a possible mechanism (since postnatal experience is not a component of GL). In any event, whatever the mechanism, our results suggest that it affects the M more than the P pathway early in development.
Birth order (BO) effects
The current study revealed significantly higher CS (both luminance and chromatic) in 2-month-olds who were firstborn yet significantly higher CS (both luminance and chromatic) in 6-month-olds who were non-firstborn. Birth order effects have previously been addressed in the literature, with perhaps the most studied outcome being intelligence (higher birth order is associated with lower IQ score; Belmont & Marolla, 1973; Bjerkedal, Kristensen, Skjeret, & Brevik, 2006; Boomsma et al., 2008) and sexual orientation (higher fraternal birth order is associated with homosexuality; for a review, see Blanchard, 2008). In line with the notions raised earlier in the Discussion section, the IQ/sexual orientation literature discusses two factors that could contribute to the BO effect. The first is based on a proposed change in the prenatal environment. Specifically, with increasing parity comes an increase in the probability of maternal immune attack upon the fetal brain, a hypothesis referred to as the “maternal immune hypothesis” (see Blanchard, 2001, 2004; Blanchard & Bogaert, 1996; Blanchard & Klassen, 1997; Foster & Archer, 1979). The second factor that could contribute to BO effects is presence of siblings, i.e., higher birth order children have more siblings. There is, in fact, a literature that specifically addresses the influence of older siblings on younger siblings in the domains of social, cognitive, and language development. Some of these studies report a benefit of having an older sibling (Barr & Hayne, 2003; Barr, Hildreth, & Rovee-Collier, 2001; Brody, 2004; Hanna & Meltzoff, 1993; McHale & Updegraff, 2001; Oshima-Takane, Goodz, & Derevensky, 1996; Perner, Ruffman, & Leekham, 1994), which is attributed to the younger sibling interacting with (and learning from) the older sibling. Other studies report a detriment of having an older sibling (Wellen, 1985; Woollett, 1986), which is attributed to less interaction with parents whose resources are tapped by the older sibling. One way the two factors (prenatal environment vs. presence/absence of siblings) have been teased apart has been to study situations where parity dissociates from presence of siblings. In the field of sexual orientation, Bogaert (2006) reported that biological brothers increase the odds of homosexuality in later-born males, even if they were reared in different households, whereas stepbrothers or adoptive brothers have no effect on sexual orientation. Thus, the available evidence indicates that the BO effect on homosexuality results from prenatal environment.
Likewise, we must entertain that the effect of BO on CS observed in the current study could be due either to prenatal environment or presence/absence of an older sibling. Keeping in mind that we found a reversal of the BO effect between 2 and 6 months, if prenatal environment accounts for our results, we would need to suppose that the direction of this prenatal effect reverses over time, which seems unlikely. If presence versus absence of an older sibling accounts for our results, we would likewise need to suppose that the direction of the sibling effect reverses over time. This seems plausible, however. It may be that having an older sibling is a detriment for a 2-month-old, if parent resources are tapped by the older sibling (and sibling interaction is minimal), yet a benefit for a 6-month-old, if sibling interaction (which presumably enriches the infant’s visual experience) increases at this time. This latter effect could either override the “tapped parental resources” effect or could coincide with parents getting better at “juggling two kids” over time. A final possibility is that the reversal in BO effects between 2 and 6 months reflects a combination of the two factors. That is, prenatal environment effects may persist in both 2- and 6-month-olds, but at 6 months, this effect may be overridden by the benefits of experience with an older sibling. If the observed BO effects on visual contrast sensitivity are, in fact, tied to sibling experience, we acknowledge that the relevant experiential cue may not necessarily be visual in nature. Instead, the driving force could be some other factor, like amount of social experience, which by itself is a fascinating prospect.
Summary
In conclusion, the results of the current study suggest that the M and P pathways are differentially influenced by the different variables we tested. Given that these variables differ in the extent to which they could be tied to (1) preprogrammed (genetic) mechanisms, (2) postnatal visual experience, and (3) prenatal environment, the results shed light on the extent to which M and P pathway development in humans is “nature versus nurture.” In general, the results of the current study suggest that P pathway development is more tied to visual experience than is M pathway development, which is in line with results from studies of humans with early visual deprivation show greater detrimental effects on P pathway tasks (see Introduction). There are, of course, other ways to investigate the effects of nature versus nurture on visual development in humans. One way is to study twin pairs. Finding that monozygotic twins are more similar on a visual measure than are dizygotic twins (assuming both have similar environments during early infancy) is evidence for a role of nature. To date, there are only a handful of visual perceptual studies in twin pairs (all in adults), with the results supporting a genetic role in stereopsis (Wilmer & Backus, 2007), color matching (Bimler & Kirkland, 2004; Paramei, Bimler, & Mislavskaia, 2004), but not eye vergence (Wilmer & Backus, 2008). We are currently taking this same approach by studying luminance and chromatic CS in infant twins. Another way to study nature versus nurture in humans would be to carefully monitor and quantify the visual statistics of infants’ daily experience (which is now becoming possible given new head-mounted video techniques for recording an infant’s visual world, Aslin, 2009) and then determine if the visual experience instructs development, e.g., do infants raised in rooms with heavily saturated colors develop chromatic contrast sensitivity faster than those raised in rooms with desaturated colors? We hope to address these questions in future experiments. Regardless of the approach one takes, it is important to keep in mind that the study of nature versus nurture will always be complicated by what is now an accepted notion; genes (nature) and environment (nurture) interact. Future research should be guided in ways that address this interaction (see Gottlieb, 1998, 2007).
Acknowledgments
This work was supported by NIH grants R01-EY12153 (KRD) and EY19035 (RGB).
We would like to thank Elizabeth Allman, Fiona Yeh, and Marie Chuldzhyan for assistance with data collection and Vanitha Sampath and Jeff Judson for assistance in programming. We would also like to thank the anonymous reviewers, John Wixted, Nicholas Christenfeld, and Mark Appelbaum for many useful discussions about data analyses and James Dannemiller for helpful discussions about this project.
Appendix A
Simulating errors in GL estimate
Our GL estimate was derived from the difference between the due date of the infant, as reported to us by the parent, and the birth date, with the assumption that the due date is for a 266-day gestation period. However, there are several sources of error in this due date information. The most likely sources of error are (1) errors in estimate of last menstrual period and (2) measurement errors in ultrasound technology used to calculate the age of the fetus (i.e., “ultrasound dating”), both employed to predict the due date. (While errors in parent reports may also exist, we believe they are minimal, see Methods). Because the vast majority of our sample reported due dates based on ultrasound dating within the first trimester (see Methods), we restrict the discussion to this type of error. Errors in ultrasound dating have been well documented in obstetrical studies that compare the known gestational dates of infants conceived via in vitro fertilization (defined as day from oocyte retrieval in IVF pregnancies) versus the gestational dates using ultrasound technology (which is based on data from a large number of spontaneous pregnancies). These studies show that, in the first trimester, ultrasound dating is off from the actual gestational age within a range that has a standard deviation of ±2.30 to 2.45 days across studies (see Sladkevicius et al., 2005; Tunon et al., 2000, which reviewed several other studies, employing large samples of pregnant participants, using different dating formulae, all of which had fairly good agreement). Thus, error in ultrasound dating necessarily injects error into our GL estimate, which in turn will lower the strength of GL as a predictor in our MRA model.
Although we could not remove the error from our GL estimates, we instead took an alternative approach of asking to what extent errors can disrupt a significant effect. To this end, we ran a simulation model in which we injected the amount of noise known to exist in the GL estimate (standard deviation of ~±2.4) into our PNA values (since PNA was found to have a significant and strong effect on CS in all conditions, see Table 2) and then measured the resulting decrease in PNA effects. Specifically, we randomly added a Gaussian distribution with mean of zero and a standard deviation of ±2.4 days to the PNA values in our subject sample, repeated 100 times, and for each sample, ran the MRA. Each of the 100 runs yielded sr2, β, B, and p values, although for the sake of brevity, we only report the β and p values here. As might be expected, the results of this simulation showed that the degree to which significant effects of PNA were retained (to a probability of ≤0.05) in the face of adding noise varied as a function of how strong the effect was without noise. For luminance and chromatic CS in 2-month-olds, the average β values (across 100 simulations) were 0.30 (66% of the simulations significant) and 0.46 (100% of the simulations significant) compared to the values obtained without noise (see Table 2), where β values were 0.35 and 0.54 and p values were 0.015 and 0.0001, respectively. For luminance and chromatic CS in 6-month-olds, the average β values were 0.21 (74% of the simulations significant) and 0.19 (60% of the simulations significant) compared to the values obtained without noise (see Table 2), where β values were 0.24 and 0.21 and p values were 0.01 and 0.02, respectively. On average, across the four separate MRAs (two CS types and two age groups), the decrease in β resulting from adding noise to the PNA value was 13%. If we now work backwards and increase the β obtained for the effects of GL by 13%, for chromatic CS at 2 months, which we found to have a non-significant β value of 0.15 in the original analyses (see Table 2), the β value would increase to 0.17, a value that is still far from significant. This simulation result suggests that the lack of a significant effect of GL on chromatic CS at 2 months in our original analyses was unlikely due to too much error in the GL estimate.
Footnotes
Commercial relationships: none.
Our GL values were normally distributed because the real-world distribution is normal (see Sladkevicius et al., 2005; Tunon, Eik-Nes, Grottum, Von During, & Kahn, 2000), and because we randomly sampled over a range (±15 days from the due date) that captures a large extent of the real-world range. We believe the PNA distribution was normal because our lab coordinator, who calls parents for the study, attempts to get an infant in right at the time of their month-birthday. If this is not possible for the parent (or if the day is a weekend), then the infant is scheduled for another day that is as close as possible to their month-birthday. The extent to which this “other day” differs from their month-birthday varies in a normal distribution.
We chose to use the same spatial frequency in 2- and 6-month-olds because collection of data from 6-month-olds (at 0.27 cycles/degree) had started first and we did not want to go to lower spatial frequency for 2-month-olds. This is because the low end of the contrast sensitivity function is pretty flat (e.g., Peterzell, Chang, & Teller, 2000; Peterzell, Kaplan, & Werner, 1995), making it difficult to determine which spatial frequency in 2-month-olds is equivalent to 0.27 cycles/degree in 6-month-olds. For this reason, we thought it safest to use the same spatial frequency at both ages. For temporal frequency, previous data on infant temporal contrast sensitivity functions (e.g., Dobkins et al., 1999; Dobkins, Lia, & Teller, 1997; Rasengane et al., 1997) likewise led us to think it best to use the same temporal frequency in 2- and 6-months-olds.
Note that because the vast majority of our infants who were not firstborn were secondborn (i.e., only a few were thirdborn or fourthborn), we made this variable categorical: firstborn versus non-firstborn. The number of firstborn versus non-firstborn infants was 41 and 19 for 2-month-olds and 73 and 49 for 6-month-olds.
There is also a third subcortical pathway, referred to as the koniocellular (K) pathway. A lot less is known about this pathway, although recent evidence suggests it is involved in processing blue/yellow information (for reviews, see Dobkins, 2000; Hendry & Reid, 2000). This pathway will not be discussed further here.
It is not clear whether testosterone should be thought of as being tied to genes or tied to environment. With regard to reproductive organs, testosterone is produced predominantly in the male testes, with only small amounts produced in the female ovaries. Because the testes develop in a preprogrammed way, based on the presence of the Y chromosome, one could argue that testosterone level is, in this respect, under the influence of genes. However, because testosterone is also produced in the adrenal glands (of both genders) and because its production and release are affected by environment (e.g., stress), testosterone levels are not tied entirely to genetic factors.
Dannemiller did not test the effects of gestational length directly; however, he noted that the birth weight effect is likely explained by gestational length as well.
Contributor Information
Karen R. Dobkins, Department of Psychology, University of California, San Diego, La Jolla, California, USA
Rain G. Bosworth, Department of Psychology, University of California, San Diego, La Jolla, California, USA
Joseph P. McCleery, Division of Developmental Medicine, Children’s Hospital Boston and Harvard Medical School, USA
References
- Allen D, Banks MS, Norcia AM. Does chromatic sensitivity develop more slowly than luminance sensitivity? Vision Research. 1993;33:2553–2562. doi: 10.1016/0042-6989(93)90134-i. [DOI] [PubMed] [Google Scholar]
- Appelle S. Perception and discrimination as a function of stimulus orientation: The “oblique effect” in man and animals. Psychology Bulletin. 1972;78:266–278. doi: 10.1037/h0033117. [DOI] [PubMed] [Google Scholar]
- Aslin RN. How infants view natural scenes gathered from a head-mounted camera. Optometry and Vision Science. 2009;86:561–565. doi: 10.1097/OPX.0b013e3181a76e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Moar K. Contrast sensitivity of the human infant for moving and static patterns. Vision Research. 1977;17:1045–1047. doi: 10.1016/0042-6989(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Hagger C, Bercu B. Gender differences in visual habit formation in 3-month-old rhesus monkeys. Development Psychobiology. 1989;22:585–599. doi: 10.1002/dev.420220605. [DOI] [PubMed] [Google Scholar]
- Baddeley RJ, Hancock PJ. A statistical analysis of natural images matches psychophysically derived orientation tuning curves. Proceedings of the Royal Society of London B: Biological Sciences. 1991;246:219–223. doi: 10.1098/rspb.1991.0147. [DOI] [PubMed] [Google Scholar]
- Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Investigative Ophthalmology & Visual Science. 1978;17:361–365. [PubMed] [Google Scholar]
- Banks MS, Salapatek P. Infant pattern vision: A new approach based on the contrast sensitivity function. Journal of Experimental Child Psychology. 1981;31:1–45. doi: 10.1016/0022-0965(81)90002-3. [DOI] [PubMed] [Google Scholar]
- Barr R, Hayne H. It’s not what you know, it’s who you know: Older siblings facilitate imitation during infancy. International Journal of Early Years Education. 2003;11:7–18. [Google Scholar]
- Barr R, Hildreth K, Rovee-Collier C. Making the train go: Infants learn from their siblings; Biennial meeting for the International Conference on Infant Studies; Brighton, UK: 2001. [Google Scholar]
- Belmont L, Marolla FA. Birth order, family size, and intelligence. Science. 1973;182:1096–1101. doi: 10.1126/science.182.4117.1096. [DOI] [PubMed] [Google Scholar]
- Bex PJ, Makous W. Spatial frequency, phase, and the contrast of natural images. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2002;19:1096–1106. doi: 10.1364/josaa.19.001096. [DOI] [PubMed] [Google Scholar]
- Bieber ML, Volbrecht VJ, Werner JS. Spectral efficiency measured by heterochromatic flicker photometry is similar in human infants and adults. Vision Research. 1995;35:1385–1392. doi: 10.1016/0042-6989(95)98718-o. [DOI] [PubMed] [Google Scholar]
- Bimler D, Kirkland J. Twins and odd-ones-out: A twin study of genetic contributions to variability in personal colour space. Clinical and Experimental Optometry. 2004;87:313–321. doi: 10.1111/j.1444-0938.2004.tb05060.x. [DOI] [PubMed] [Google Scholar]
- Birch EE, Bosworth RG. Visual evoked potentials in infants and children. In: Aminoff MJ, editor. Electrodiagnosis in Clinical Neurology. New York: Churchill-Livingstone; 2004. pp. 439–450. [Google Scholar]
- Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Human Development. 2007;83:279–284. doi: 10.1016/j.earlhumdev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatric Research. 1998;44:201–209. doi: 10.1203/00006450-199808000-00011. [DOI] [PubMed] [Google Scholar]
- Birch EE, O’Connor AR. Preterm birth and visual development. Seminars in Neonatology. 2001;6:487–497. doi: 10.1053/siny.2001.0077. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager D, Leffler J, Weakley D. Early treatment of congenital unilateral cataract minimizes unequal competition. Investigative Ophthalmology & Visual Science. 1998;39:1560–1566. [PubMed] [Google Scholar]
- Bjerkedal T, Kristensen P, Skjeret GA, Brevik JI. A follow up of persons who received basic and/or supplemental benefits in childhood. Tidsskrift for Den Norske Laegeforening. 2006;126:436–439. [PubMed] [Google Scholar]
- Blakemore C, Vital-Durand F. Visual deprivation prevents the postnatal maturation of spatial resolution and contrast sensitivity for neurones of the monkey’s striate cortex. The Journal Physiology. 1983;345:40P. [Google Scholar]
- Blanchard R. Fraternal birth order and the maternal immune hypothesis of male homosexuality. Hormones and Behavior. 2001;40:105–114. doi: 10.1006/hbeh.2001.1681. [DOI] [PubMed] [Google Scholar]
- Blanchard R. Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. Journal of Theoretical Biology. 2004;230:173–187. doi: 10.1016/j.jtbi.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Blanchard R. Review and theory of handedness, birth order, and homosexuality in men. Laterality. 2008;13:51–70. doi: 10.1080/13576500701710432. [DOI] [PubMed] [Google Scholar]
- Blanchard R, Bogaert AF. Homosexuality in men and number of older brothers. American Journal of Psychiatry. 1996;153:27–31. doi: 10.1176/ajp.153.1.27. [DOI] [PubMed] [Google Scholar]
- Blanchard R, Klassen P. H-Y antigen and homosexuality in men. Journal of Theoretical Biology. 1997;185:373–378. doi: 10.1006/jtbi.1996.0315. [DOI] [PubMed] [Google Scholar]
- Bogaert AF. Biological versus nonbiological older brothers and men’s sexual orientation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10771–10774. doi: 10.1073/pnas.0511152103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, van Beijsterveld TCEM, Beem AL, Hoekstra RA, Polderman TJC, Bartels M. Intelligence and birth order in boys and girls. Intelligence. 2008;36:630–634. [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and non-human primates. Annual Review of Neuroscience. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Bartlett MS, Dobkins KR. Image statistics of American Sign Language: Comparison with faces and natural scenes. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2006;23:2085–2096. doi: 10.1364/josaa.23.002085. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Dobkins KR. Chromatic (red/green) and luminance contrast sensitivity in fullterm and “late” preterm infants: Effects of early visual experience on magnocellular and parvocellular pathway processing [Abstract] Journal of Vision. 2008;8(17):46. 46a, http://journalofvision.org/8/17/46/, doi:10.1167/8.17.46.
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Research. 1999;39:257–269. doi: 10.1016/s0042-6989(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: Motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41:1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 1981;21:467–476. [PubMed] [Google Scholar]
- Brenner E, Cornelissen F, Nuboer W. Striking absence of long-lasting effects of early color deprivation on monkey vision. Developmental Psychobiology. 1990;23:441–448. doi: 10.1002/dev.420230506. [DOI] [PubMed] [Google Scholar]
- Brody GH. Siblings’ direct and indirect contributions to child development. Current Directions in Psychological Science. 2004;13:124–126. [Google Scholar]
- Brown AM, Lindsey DT, McSweeney EM, Walters MM. Infant luminance and chromatic contrast sensitivity: Optokinetic nystagmus data on 3-month-olds. Vision Research. 1995;35:3145–3160. doi: 10.1016/0042-6989(95)00017-t. [DOI] [PubMed] [Google Scholar]
- Camp BW, Jamieson-Darr K, Hansen R, Schmidt B. Growth parameters and attention to faces at 4 to 6 months of age. Journal of Developmental and Behavioral Pediatrics. 1990;11:229–233. [PubMed] [Google Scholar]
- Campbell FW, Kulikowski JJ, Levinson J. The effect of orientation on the visual resolution of gratings. The Journal of Physiology. 1966;187:427–436. doi: 10.1113/jphysiol.1966.sp008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JA. The relationship between intelligence and birth weight in twins. Neurology. 1965;15:341–347. doi: 10.1212/wnl.15.4.341. [DOI] [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. American Journal of Sociology. 1995;100:1261–1293. [Google Scholar]
- Coppola DM, Purves HR, McCoy AN, Purves D. The distribution of oriented contours in the real world. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4002–4006. doi: 10.1073/pnas.95.7.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: Permissive or instructive? Current Opinion in Neurobiology. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Crawford ML, Harwerth RS, Smith EL, 3rd, Shen F, Carter-Dawson L. Glaucoma in primates: Cytochrome oxidase reactivity in parvo- and magnocellular pathways. Investigative Ophthalmology & Visual Science. 2000;41:1791–1802. [PubMed] [Google Scholar]
- Crognale MA. Development, maturation, and aging of chromatic visual pathways: VEP results. Journal of Vision. 2002;2(6):2. doi: 10.1167/2.6.2. 438–450, http://journalofvision.org/2/6/2/, doi:10.1167/2.6.2. [DOI] [PubMed]
- Cynader M, Cmerneko G. Abolition of direction selectivity in the visual cortex of the cat. Science. 1976;193:504–505. doi: 10.1126/science.941025. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Mareschal I, Bex PJ. An oblique effect for local motion: Psychophysics and natural movie statistics. Journal of Vision. 2005;5(10):9. doi: 10.1167/5.10.9. 878–887, http://journalofvision.org/5/10/9/, doi:10.1167/5.10.9. [DOI] [PubMed]
- Dalby JT. Environmental effects of prenatal development. Journal of Pediatric Psychology. 1978;3:105–109. [Google Scholar]
- Dannemiller JL. Variations in birth weight within the normal range are related to visual orienting in infancy for boys but not for girls. Infant Behavior & Development. 2004;27:204–215. [Google Scholar]
- Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes of magnocellular and parvocellular visual function in early- and late-onset strabismic amblyopia. Investigative Ophthalmology & Visual Science. 2006;47:4836–4841. doi: 10.1167/iovs.06-0382. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Morgan HC, Polson MC, Mead WR, Hull EM. Psychophysical studies of monkey vision—I. Macaque luminosity and color vision tests. Vision Research. 1974;14:53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Delgado H, Martorell R, Brineman E, Klein RE. Nutrition and length of gestation. Nutrition Research. 1982;2:117–126. [Google Scholar]
- Demirci H, Gezer A, Sezen F, Ovali T, Demiralp T, Isoglu-Alkoc U. Evaluation of the functions of the parvocellular and magnocellular pathways in strabismic amblyopia. Journal of Pediatric Ophthalmology and Strabismus. 2002;39:215–221. doi: 10.3928/0191-3913-20020701-09. [DOI] [PubMed] [Google Scholar]
- Dobkins KR. Moving colors in the lime light. Neuron. 2000;25:15–18. doi: 10.1016/s0896-6273(00)80867-3. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM, Kelly JP. Development of psychophysically-derived detection contours in L- and M- cone contrast space. Vision Research. 2001;41:1791–1807. doi: 10.1016/s0042-6989(01)00070-0. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: Evidence for precocious magnocellular development? Vision Research. 1999;39:3223–3239. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Gunther KL, Peterzell DH. What covariance mechanisms underlie green/red equiluminance, chromatic contrast sensitivity and luminance contrast sensitivity? Vision Research. 2000;40:613–628. doi: 10.1016/s0042-6989(99)00211-4. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Lia B, Teller DY. Infant color vision: Temporal contrast sensitivity functions (tCSFs) for chromatic (red/green) stimuli in 3-month-olds. Vision Research. 1997;37:2699–2716. doi: 10.1016/s0042-6989(97)81180-7. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Teller DY. Infant contrast detectors are selective for direction of motion. Vision Research. 1996a;36:281–294. doi: 10.1016/0042-6989(95)00094-g. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Teller DY. Infant motion: detection (M:D) ratios for chromatic-defined and luminance-defined moving stimuli. Vision Research. 1996b;36:3293–3310. doi: 10.1016/0042-6989(96)00069-7. [DOI] [PubMed] [Google Scholar]
- Donovan W, Taylor N, Leavitt L. Maternal sensory sensitivity and response bias in detecting change in infant facial expressions: Maternal self-efficacy and infant gender labeling. Infant Behavior & Development. 2007;30:436–452. doi: 10.1016/j.infbeh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Brent HP. Influence of monocular deprivation during infancy on the later development of spatial and temporal vision. Vision Research. 2000;40:3283–3295. doi: 10.1016/s0042-6989(00)00165-6. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Lui CH, Brent HP. Spatial and temporal vision in patients treated for bilateral congenital cataracts. Vision Research. 1999;39:3480–3489. doi: 10.1016/s0042-6989(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Current Opinion in Neurobiology. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Foster JW, Archer SJ. Birth order and intelligence: An immunological interpretation. Perceptual and Motor Skills. 1979;48:79–93. doi: 10.2466/pms.1979.48.1.79. [DOI] [PubMed] [Google Scholar]
- Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Investigative Ophthalmology & Visual Science. 1991;32:484–491. [PubMed] [Google Scholar]
- Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. British Medical Journal. 1996;312:410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb B, Andersen RA, Nakayama K, MacLeod DI, Wong A. Visual thresholds for shearing motion in monkey and man. Vision Research. 1985;25:813–820. doi: 10.1016/0042-6989(85)90189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychology Review. 1998;105:792–802. doi: 10.1037/0033-295x.105.4.792-802. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Probabilistic epigenesis. Developmental Science. 2007;10:1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Graham NVS. Visual pattern analyzers. New York: Oxford University Press; 1989. [Google Scholar]
- Gunther KL, Dobkins KR. Individual differences in chromatic (red/green) contrast sensitivity are constrained by the relative number of L- versus M-cones in the eye. Vision Research. 2002;42:1367–1378. doi: 10.1016/s0042-6989(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Gunther KL, Dobkins KR. Independence of mechanisms tuned along cardinal and non-cardinal axes of color space: Evidence from factor analysis. Vision Research. 2003;43:683–696. doi: 10.1016/s0042-6989(02)00689-2. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Held R. From visual acuity to hyperacuity: A 10-year update. Canadian Journal of Psychology. 1989;43:109–120. doi: 10.1037/h0084217. [DOI] [PubMed] [Google Scholar]
- Hammarrenger B, Lepore F, Lippe S, Labrosse M, Guillemot JP, Roy MS. Magnocellular and parvocellular developmental course in infants during the first year of life. Documenta Ophthalmologica. 2003;107:225–233. doi: 10.1023/b:doop.0000005331.66114.05. [DOI] [PubMed] [Google Scholar]
- Hanna E, Meltzoff AN. Peer imitation by toddlers in laboratory, home and day-care contexts: Implications for social learning and memory. Developmental Psychology. 1993;29:701–710. doi: 10.1037/0012-1649.29.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JE. The nutritional basis of the fetal origins of adult disease. International Journal of Epidemiology. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- Hartmann EE, Banks MS. Temporal contrast sensitivity in human infants. Vision Research. 1992;32:1163–1168. doi: 10.1016/0042-6989(92)90018-e. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Boltz RL, Crawford ML, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: Temporal modulation sensitivity. Vision Research. 1983;23:1511–1517. doi: 10.1016/0042-6989(83)90163-3. [DOI] [PubMed] [Google Scholar]
- Hawken MJ, Parker AJ. Detection and discrimination mechanisms in the striate cortex of the old-world monkey. In: Blakemore C, editor. Vision: Coding and efficiency. Cambridge: Cambridge University Press; 1990. pp. 103–116. [Google Scholar]
- Heckman GM, Engel SA. Perceptual learning of contrast detection is color selective [Abstract] Journal of Vision. 2006;6(6):160. 160a, http://journalofvision.org/6/6/160/, doi:10.1167/6.6.160.
- Held R, Thorn F, Gwiazda J, Bauer J. Development of binocularity and its sexual differentiation. In: Vital-Durand F, Atkinson J, Braddick OJ, editors. Infant Vision. Oxford: Oxford University Press; 1996. pp. 265–274. [Google Scholar]
- Hendrickson A, Boothe R. Morphology of the retina and dorsal lateral geniculate nucleus in dark-reared monkeys (Macaca nemestrina) Vision Research. 1976;16:517–521. doi: 10.1016/0042-6989(76)90033-x. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Reid RC. The koniocellular pathway in primate vision. Annual Review of Neuroscience. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: Evidence for a two type classification. Vision Research. 1977;17:1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Hirsch HV, Spinelli DN. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970;168:869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- Hoffman DR, Theuer RC, Castaneda YS, Wheaton DH, Bosworth RG, O’Connor AR, et al. Maturation of visual acuity is accelerated in breast-fed term infants fed baby food containing DHA-enriched egg yolk. Journal of Nutrition. 2004;134:2307–2313. doi: 10.1093/jn/134.9.2307. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. Journal of Neuroscience. 1996;16:1791–1807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. Timing of the critical period for plasticity of ocular dominance columns in macaque striate cortex. Journal of Neuroscience. 1997;17:3684–3709. doi: 10.1523/JNEUROSCI.17-10-03684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood AM, Riddell PM. Gender differences in early accommodation and vergence development. Ophthalmic & Physiological Optics. 2008;28:115–126. doi: 10.1111/j.1475-1313.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Primate color vision: A comparative perspective. Visual Neuroscience. 2008;25:619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: Evidence from the Inuit of arctic Quebec. Journal of Pediatrics. 2008;152:356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Jefferis B, Power C, Hertzman C. Birth weight, child socioeconomic environment, and cognitive development in the 1985 British birth cohort. British Medical Journal. 2002;325:305–310. doi: 10.1136/bmj.325.7359.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil MS, Cristobal G. Separating the chaff from the wheat: Possible origins of the oblique effect. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2000;17:697–710. doi: 10.1364/josaa.17.000697. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Borchert K, Teller DY. The development of chromatic and achromatic contrast sensitivity in infancy as tested with the sweep VEP. Vision Research. 1997;37:2057–2072. doi: 10.1016/s0042-6989(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Orban GA. Response properties of visual cortical neurons in cats reared in strobo-scopic illumination. Journal of Neurophysiology. 1983;49:686–704. doi: 10.1152/jn.1983.49.3.686. [DOI] [PubMed] [Google Scholar]
- Khera KS. Common fetal aberrations and their teratologic significance: A review. Fundamental and Applied Toxicology. 1981;1:13–18. doi: 10.1093/toxsci/1.1.13. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Neural limitations on visual development in primates. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. pp. 159–173. [Google Scholar]
- Leader A, Wong KH, Deitel M. Maternal nutrition in pregnancy: Part I. A review. Canadian Medical Association Journal. 1981;125:545–549. [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1990;7:2223–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- Lennie P, D’Zmura M. Mechanisms of color vision. Critical Reviews in Neurobiology. 1988;3:333–400. [PubMed] [Google Scholar]
- Levi M, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1977;16:90–95. [PubMed] [Google Scholar]
- Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J, Hemmingsen R. Prenatal stressors of human life affect fetal brain development. Developmental Medicine and Child Neurology. 1994;36:826–832. doi: 10.1111/j.1469-8749.1994.tb08192.x. [DOI] [PubMed] [Google Scholar]
- MacLeod DIA. Colour discrimination, colour constancy, and natural scene statistics (the Verriest lecture) In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and defective colour vision. London: Oxford University Press; 2003. [Google Scholar]
- Makrides M, Neumann M, Simmer K, Pater J, Gibson R. Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet. 1995;345:1463–1468. doi: 10.1016/s0140-6736(95)91035-2. [DOI] [PubMed] [Google Scholar]
- Matte TD, Bresnahan M, Begg MD, Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: Cohort study. British Medical Journal. 2001;323:310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Lewis TL. Visual outcomes after infantile cataract. In: Simons K, editor. Early Visual Development: Normal and Abnormal. New York: Oxford University Press; 1993. pp. 454–484. [Google Scholar]
- Maurer D, Lewis TL, Cavanagh P, Anstis S. A new test of luminous efficiency for babies. Investigative Ophthalmology & Visual Science. 1989;30:297–303. [PubMed] [Google Scholar]
- McCleery JP, Allman E, Carver LJ, Dobkins KR. Abnormal magnocellular pathway visual processing in infants at risk for autism. Biological Psychiatry. 2007;62:1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- McHale SM, Updegraff KA. Sibling influences on gender development in middle childhood and early adolescence: A longitudinal study. Developmental Psychology. 2001;37:115–125. [PubMed] [Google Scholar]
- Mitchell DE, Freeman RD, Westheimer G. Effect of orientation on the modulation sensitivity for interference fringes on the retina. Journal of the Optical Society of America. 1967;57:246–249. doi: 10.1364/josa.57.000246. [DOI] [PubMed] [Google Scholar]
- Moore DS, Johnson SP. Mental rotation in human infants: A sex difference. Psychol Science. 2008;19:1063–1066. doi: 10.1111/j.1467-9280.2008.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JO, Sanchez R, de Paredes B, Herrera MG. Sex related effects of nutritional supplementation during pregnancy on fetal growth. Early Human Development. 1981;5:243–251. doi: 10.1016/0378-3782(81)90032-3. [DOI] [PubMed] [Google Scholar]
- Moreland JD. Spectral sensitivity measured by motion photometry. Documenta Ohthalmologica Proceedings Series. 1982;33:61–66. [Google Scholar]
- Morrone MC, Burr DC, Fiorentini A. Development of infant contrast sensitivity to chromatic stimuli. Vision Research. 1993;33:2535–2552. doi: 10.1016/0042-6989(93)90133-h. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Van Sluyters RC. Visual neural development. Annual Review of Psychology. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Muir DW, Mitchell DE. Behavioral deficits in cats following early selected visual exposure to contours of a single orientation. Brain Research. 1975;85:459–477. doi: 10.1016/0006-8993(75)90820-3. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Paré EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, Connor SL, et al. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: A prospective, randomized controlled trial. Pediatrics. 2001;108:359–371. doi: 10.1542/peds.108.2.359. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish oil trials in pregnancy (FOTIP) team. British Journal of Obstetrics and Gynaecology. 2000;107:382–395. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, et al. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet. 1992;339:1003–1007. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- Oshima-Takane Y, Goodz E, Derevensky JL. Birth order effects on early language development: Do secondborn children learn from overheard speech. Child Development. 1996;67:621–634. [Google Scholar]
- Ounsted M, Moar VA, Scott A. Associations between size and development at four years among children who were small-for-dates and large-for-dates at birth. Early Human Development. 1984;9:259–268. doi: 10.1016/0378-3782(84)90036-7. [DOI] [PubMed] [Google Scholar]
- Palmer C, Cheng SY, Seidemann E. Linking neuronal and behavioral performance in a reaction-time visual detection task. Journal of Neuroscience. 2007;27:8122–8137. doi: 10.1523/JNEUROSCI.1940-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramei GV, Bimler DL, Mislavskaia NO. Colour perception in twins: Individual variation beyond common genetic inheritance. Clinical and Experimental Optometry. 2004;87:305–312. doi: 10.1111/j.1444-0938.2004.tb05059.x. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Schumer RA, Gizzi MS, Movshon JA. Abolition of visual cortical direction selectivity affects visual behavior in cats. Experimental Brain Research. 1985;61:214–217. doi: 10.1007/BF00235638. [DOI] [PubMed] [Google Scholar]
- Paternoster R, Brame R, Mazerolle P, Piquero A. Using the correct statistical test for the equality of regression coefficients. Criminology. 1998;36:859–866. [Google Scholar]
- Pereverzeva M, Hui-Lin Chien S, Palmer J, Teller DY. Infant photometry: Are mean adult isoluminance values a sufficient approximation to individual infant values? Vision Research. 2002;42:1639–1649. doi: 10.1016/s0042-6989(02)00089-5. [DOI] [PubMed] [Google Scholar]
- Perner J, Ruffman T, Leekham SR. Theory of mind is contagious: You catch it from your sibs. Child Development. 1994;65:1228–1238. [Google Scholar]
- Perry VH, Silveira LC, Cowey A. Pathways mediating resolution in the primate retina. Ciba Foundation Symposium. 1990;155:5–14. doi: 10.1002/9780470514023.ch2. discussion 14–21. [DOI] [PubMed] [Google Scholar]
- Peterzell DH, Chang SK, Teller DY. Spatial frequency tuned covariance channels for red-green and luminance-modulated gratings: Psychophysical data from human infants. Vision Research. 2000;40:431–444. doi: 10.1016/s0042-6989(99)00188-1. [DOI] [PubMed] [Google Scholar]
- Peterzell DH, Kaplan PS, Werner JS. Individual differences in contrast sensitivity functions: Longitudinal study of 4-, 6- and 8-month-old human infants. Vision Research. 1995;35:961–979. doi: 10.1016/0042-6989(94)00117-5. [DOI] [PubMed] [Google Scholar]
- Peterzell DH, Teller DY. Spatial frequency tuned covariance channels for red-green and luminance-modulated gratings: Psychophysical data from human adults. Vision Research. 2000;40:417–430. doi: 10.1016/s0042-6989(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Sanchez RM, Dunkelberger GR, L’Hernault NL, Baginski TA. Chronic glaucoma selectively damages large optic nerve fibers. Investigative Ophthalmology & Visual Science. 1987;28:913–920. [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rasengane TA, Allen D, Manny RE. Development of temporal contrast sensitivity in human infants. Vision Research. 1997;37:1747–1754. doi: 10.1016/s0042-6989(96)00300-8. [DOI] [PubMed] [Google Scholar]
- Rayco-Solon P, Fulford AJ, Prentice AM. Maternal preconceptional weight and gestational length. American Journal of Obstetrics and Gynecology. 2005;192:1133–1136. doi: 10.1016/j.ajog.2004.10.636. [DOI] [PubMed] [Google Scholar]
- Regal DM, Boothe R, Teller DY, Sackett GP. Visual acuity and visual responsiveness in dark-reared monkeys (Macaca nemestrina) Vision Research. 1976;16:523–530. doi: 10.1016/0042-6989(76)90034-1. [DOI] [PubMed] [Google Scholar]
- Rose SA. Relation between physical growth and information processing in infants born in India. Child Development. 1994;65:889–902. [PubMed] [Google Scholar]
- Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics. 1980;65:683–697. [PubMed] [Google Scholar]
- Scarr S. Effects of birth weight on later intelligence. Social Biology. 1969;16:249–256. doi: 10.1080/19485565.1969.9987828. [DOI] [PubMed] [Google Scholar]
- Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers’ recall of birthweight and gestational age. British Journal of Obstetrics and Gynaecology. 1987;94:731–735. doi: 10.1111/j.1471-0528.1987.tb03717.x. [DOI] [PubMed] [Google Scholar]
- Shapley R. Visual sensitivity and parallel retinocortical channels. Annual Review of Psychology. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Sladkevicius P, Saltvedt S, Almstrom H, Kublickas M, Grunewald C, Valentin L. Ultrasound dating at 12–14 weeks of gestation. A prospective cross-validation of established dating formulae in in-vitro fertilized pregnancies. Ultrasound Obstetrician Gynecology. 2005;26:504–511. doi: 10.1002/uog.1993. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J, Davis M, Yeh T. Mechanisms subserving temporal modulation sensitivity in silent-cone substitution. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1995;12:241–249. doi: 10.1364/josaa.12.000241. [DOI] [PubMed] [Google Scholar]
- Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstetrician Gynecology. 2003;101:469–479. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- Sokol S, Moskowitz A, Hansen V. Electro-physiological evidence for the oblique effect in human infants. Investigative Ophthalmology & Visual Science. 1987;28:731–735. [PubMed] [Google Scholar]
- Sokol S, Moskowitz A, Hansen V. Evoked potential and preferential looking correlates of the oblique effect in 3-month-old infants. Document Ophthalmology. 1989;71:321–328. doi: 10.1007/BF00170980. [DOI] [PubMed] [Google Scholar]
- Stavros KA, Kiorpes L. Behavioral measurement of temporal contrast sensitivity development in macaque monkeys (Macaca nemestrina) Vision Research. 2008;48:1335–1344. doi: 10.1016/j.visres.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Karraker KH. Sex stereotyping of infants: A review of gender labeling studies. Sex Roles. 1989;20:501–522. [Google Scholar]
- Stiles J. The Fundamentals of Brain Development: Integrating Nature and Nurture. Cambridge, MA: Harvard University Press; 2008. [Google Scholar]
- Sugita Y. Experience in early infancy is indispensable for color perception. Current Biology. 2004;14:1267–1271. doi: 10.1016/j.cub.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Switkes E, Mayer MJ, Sloan JA. Spatial frequency analysis of the visual environment: Anisotropy and the carpentered environment hypothesis. Vision Research. 1978;18:1393–1399. doi: 10.1016/0042-6989(78)90232-8. [DOI] [PubMed] [Google Scholar]
- Teller DY. The forced-choice preferential looking procedure: A psychophysical technique for use with human infants. Infant Behavior & Development. 1979;2:135–153. [Google Scholar]
- Teller DY, Lindsey DT. Motion nulls for white versus isochromatic gratings in infants and adults. Journal of the Optical Society of America A, Optics and Image Science. 1989;6:1945–1954. doi: 10.1364/josaa.6.001945. [DOI] [PubMed] [Google Scholar]
- Teller DY, Lindsey DT. Motion at isoluminance: Motion dead zones in three-dimensional color space. Journal of the Optical Society of America A, Optics and Image Science. 1993;10:1324–1331. doi: 10.1364/josaa.10.001324. [DOI] [PubMed] [Google Scholar]
- Teller DY, Morse R, Borton R, Regal D. Visual acuity for vertical and diagonal gratings in human infants. Vision Research. 1974;14:1433–1439. doi: 10.1016/0042-6989(74)90018-2. [DOI] [PubMed] [Google Scholar]
- Thurston C, Dobkins KR. Stimulus-specific perceptual learning for chromatic, but not luminance, contrast detection [Abstract] Journal of Vision. 2007;7(9):469. 469a, http://journalofvision.org/7/9/469/, doi:10.1167/7.9.469.
- Tunon K, Eik-Nes SH, Grottum P, Von During V, Kahn JA. Gestational age in pregnancies conceived after in vitro fertilization: A comparison between age assessed from oocyte retrieval, crown-rump length and biparietal diameter. Ultrasound in Obstetrics and Gynecology. 2000;15:41–46. doi: 10.1046/j.1469-0705.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- Tytla ME, Maurer D, Lewis TL, Brent HP. Contrast sensitivity in children treated for congenital cataract. Clinical Vision Science. 1988;2:251–264. [Google Scholar]
- van der Schaaf A, van Hateren JH. Modelling the power spectra of natural images: Statistics and information. Vision Research. 1996;36:2759–2770. doi: 10.1016/0042-6989(96)00002-8. [DOI] [PubMed] [Google Scholar]
- Watson AB. Probability summation over time. Vision Research. 1979;19:515–522. doi: 10.1016/0042-6989(79)90136-6. [DOI] [PubMed] [Google Scholar]
- Webster MA, Mollon JD. Adaptation and the color statistics of natural images. Vision Research. 1997;37:3283–3298. doi: 10.1016/s0042-6989(97)00125-9. [DOI] [PubMed] [Google Scholar]
- Weibull W. A statistical distribution function of wide applicability. Journal of Applied Mechanics. 1951;18:292–297. [Google Scholar]
- Wellen C. Effects of older siblings on the language young children hear and produce. Journal of Speech and Hearing Disorders. 1985;50:84–99. doi: 10.1044/jshd.5001.84. [DOI] [PubMed] [Google Scholar]
- Welzl H, D’Adamo P, Wolfer D, Lipp H. Mouse models of hereditary mental retardation. In: Fisch GS, Flint J, editors. Transgenic and Knockout Models of Neuropsychiatric Disorders. Totowa, NJ: Humana Press Inc; 2006. pp. 101–125. [Google Scholar]
- Wilmer JB, Backus B. Precision of depth judgment from binocular disparity is heritable [Abstract] Journal of Vision. 2007;7(9):831. 831a, http://journalofvision.org/7/9/831/, doi:10.1167/7.9.831.
- Wilmer JB, Backus B. Behavioral genetic evidence for plasticity in the oculomotor system [Abstract] Journal of Vision. 2008;8(6):633. 633a, http://journalofvision.org/8/6/633/, doi:10.1167/8.6.633.
- Woollett A. The influence of older siblings on the language young children hear and produce. Journal of Speech and Hearing Disorders. 1986;50:84–99. doi: 10.1044/jshd.5001.84. [DOI] [PubMed] [Google Scholar]
- Zele AJ, Pokorny J, Lee DY, Ireland D. Anisometropic amblyopia: Spatial contrast sensitivity deficits in inferred magnocellular and parvocellular vision. Investigative Ophthalmology & Visual Science. 2007;48:3622–3631. doi: 10.1167/iovs.06-1207. [DOI] [PubMed] [Google Scholar]