Abstract
Epidemiological data have demonstrated an association between artificial sweetener use and weight gain. Evidence of a causal relationship linking artificial sweetener use to weight gain and other metabolic health effects is limited. However, recent animal studies provide intriguing information that supports an active metabolic role of artificial sweeteners. This systematic review examines the current literature on artificial sweetener consumption in children and its health effects. Eighteen studies were identified. Data from large, epidemiologic studies support the existence of an association between artificially-sweetened beverage consumption and weight gain in children. Randomized controlled trials in children are very limited, and do not clearly demonstrate either beneficial or adverse metabolic effects of artificial sweeteners. Presently, there is no strong clinical evidence for causality regarding artificial sweetener use and metabolic health effects, but it is important to examine possible contributions of these common food additives to the global rise in pediatric obesity and diabetes.
Keywords: Acesulfame-K, artificial sweetener, aspartame, neotame, obesity, saccharine, sucralose, weight gain
Introduction
Artificial sweeteners and the obesity epidemic
As a means to help curtail the obesity epidemic, small dietary changes to prevent weight gain in children and adolescents have been encouraged (1). Artificial sweeteners have gained attention as dietary tools (2) that provide sweet taste without the extra energy derived from foods and drinks containing caloric sugars (3–7), and thus may assist in weight-loss plan adherence (8). A key question is whether replacement of sugar-sweetened products with those containing artificial sweeteners is truly beneficial.
Since their FDA approval, artificial sweeteners and their benefits on metabolic health have been questioned (9,10). An association between artificial sweetener intake and weight gain was first observed in epidemiological studies with adults. Several largescale studies, including the National Health and Nutrition Examination Survey (NHANES) and the San Antonio Heart Study, have shown a positive association between artificial sweetener use and increases in weight and/or BMI (11–14). Other large, prospective cohort studies in adults have shown associations between artificial sweetener intake and incidence of the metabolic syndrome and its components, including waist circumference, blood pressure, and fasting blood glucose (15–18). Some studies in adults have demonstrated links between artificial sweetener consumption and insulin resistance, incidence of type 2 diabetes, and poor glucose control in patients with pre-existing diabetes (19,20), while others have found no association with diabetes incidence or glycemia control (21,22).
Beneficial effects of artificial sweeteners have been shown in adults, as well. For example, the Nurses Health Study II found decreased weight gain among adults who consumed artificially-sweetened beverages (23). More importantly, randomized controlled studies in adults have shown mildly beneficial results of artificial sweetener use, including decreased weight regain after dieting (24), and weight-stability or minimal short-term weight loss compared with caloric-sweetener supplementation (25,26). In response to these conflicting data, several reviews were conducted (27,28). The consensus in the early 1990s was that artificial sweeteners had not been found to increase weight in humans (29,30).
Mechanism of action of artificial sweeteners
Multiple behavioral mechanisms have been proposed to account for the epidemiologic association between artificial sweetener use and weight gain. It has been suggested that the dissociation of the sensation of sweet taste from caloric intake may promote appetite, leading to greater food consumption and weight gain. In addition, increased consumption of added caloric sweeteners has been associated with lower diet quality in children (31), perhaps by altering taste preferences toward sweetened foods in place of more healthful foods, such as fruits and vegetables; this mechanism could apply to artificial sweeteners as well.
New data from both humans and animal models have provided convincing evidence that artificial sweeteners play an active role in the gastrointestinal tract, thus providing a mechanistic explanation for observed metabolic effects. Sweet-taste receptors, including the taste receptor T1R family and α-gustducin, respond not only to caloric sugars, such as sucrose and glucose, but also to artificial sweeteners, including sucralose (Splenda™) and acesulfame-K (32,33). In both humans and animals, these receptors have been shown to be present not only in lingual taste buds, but also in glucagon-like peptide-1 (GLP-1) secreting L cells of the gut mucosa (34–36), where they serve as critical mediators of GLP-1 secretion (36). Mace et al. showed in rat studies that stimulation of intestinal taste receptors with sucralose led to more rapid absorption of sugars from the intestine into the bloodstream (32). We have demonstrated in young healthy volunteers that consumption of diet soda before an oral glucose challenge potentiates GLP-1 secretion, thus potentially altering both gastric emptying and insulin secretion (62). Translating these results into the clinical realm, consumption of an artificial sweetener in conjunction with a sugarcontaining food or drink could lead to more rapid sugar absorption, as well as increased GLP-1 and insulin secretion, potentially affecting weight, appetite, and glycemia.
In light of this new information regarding the metabolic activity of artificial sweeteners, we have conducted a systematic review of the effects of artificial sweeteners on food intake, weight, and metabolic health in children.
Commonly used artificial sweeteners
Currently, the FDA has approved five artificial sweeteners for consumption: acesulfame-K, aspartame, neotame, saccharin, and sucralose (Table I). In addition, the FDA has determined that a new sugar substitute, stevia, is a dietary supplement “generally recognized as safe” (GRAS). Artificial sweeteners are known by several names, which include: low-calorie sweeteners, high intensity sweeteners, non-sucrose sweeteners, intense sweeteners, non-nutritive sweeteners, sugar substitutes, and sugar-free sweeteners (37). For the purposes of this review, we will use the term “artificial sweeteners.”
Table I.
FDA-approved artificial sweeteners.
| Chemical Name | Trade Names | Sweetness (compared with sucrose) | Acceptable Daily Intake (ADI) | Structure |
|---|---|---|---|---|
| Saccharin (C7H5NO3S) | Sweet N' Low | 300× | 5 mg/kg | 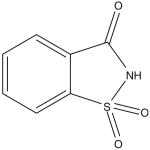 |
| Aspartame (C14H18N2O5) | NutraSweet, Equal | 160–220× | 50 mg/kg | 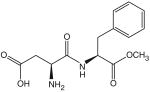 |
| Acesulfame-Potassium (C4H4KNO4S) | Sunett, Sweet & Safe, Sweet One | 200× | 15 mg/kg | 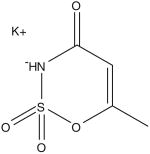 |
| Sucralose (C12H19Cl3O8) | Splenda | 600× | 5 mg/kg | 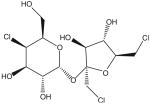 |
| Neotame (C2OH3ON2O5) | Made by NutraSweet | 7 000–13 000× | 0.10 mg/kg | 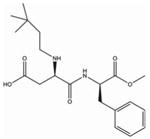 |
Artificial sweetener intake in children
Beverages have been identified as a major source of artificial sweeteners in the diet (38,39), hence estimates of artificial sweetener consumption are typically based on artificially-sweetened drinks or sodas. Nationally representative surveys from the 1990s estimated that artificially-sweetened sodas accounted for approximately 4–18% of total carbonated beverage intake in children (40,41). Artificially-sweetened soft drink consumption appears to be increasing in children, both with age and over time (40–43).
Methods
Search strategy
Eligible studies were identified by searching the following electronic databases: PubMed, Web of Science, and EMBASE. The following terms were used: artificial sweeteners, sweetening agent, sweetener, sugar substitute, nonnutritive sweetener, intense sweetener, sucralose, aspartame, saccharin, sugar free, diabetes, weight, obesity, obese, metabolic syndrome, cholesterol, and blood pressure. The year of publication was not restricted. Additional studies were identified by manual search of reference lists of key original and review articles.
Study selection
For inclusion, studies were required to: (a) be published in peer reviewed journals in the English language, (b) include pediatric subjects age 0 to 18; (c) specifically address artificial sweetener consumption in association with metabolic health effects, such as food intake, weight change, diabetes, and metabolic syndrome components; and (d) provide original data. Articles published solely in abstract form were omitted.
Data extraction
Two authors (RJB and MAdB) independently identified potentially relevant articles based on titles and abstracts. A full text record was retrieved when articles met eligibility criteria or when reviewers disagreed. A third reviewer (KIR) with no involvement in the initial search process resolved disagreements.
Data extracted from eligible articles included author, date and duration of study, study design, characteristics of the participants (sample size, age, gender), outcome measures and results.
Results
The initial literature search produced 116 potentially eligible articles. Of these articles, 111 were excluded as they did not meet requirements. An additional 13 articles were identified based on a reference list search of review articles. Our search yielded a total of 18 human studies, summarized in Table II.
Table II.
Review of pediatric studies on the effects of artificial sweeteners on weight gain and glucose metabolism.
| Study | Subjects | Age | Duration | Result |
|---|---|---|---|---|
| Acute effects on food intake | ||||
| Anderson et al. (48) | 20 healthy children | 9–10 years | – | 6% compensation* in ad lib lunch intake 90 min after aspartame vs. sucrose-sweetened preload. |
| Bellissimo et al. (45) | 14 boys | 9–14 years | – | 94% compensation in ad lib lunch intake 30 min after sucralose vs. glucose-sweetened preload. |
| Bellissimo et al. (44) | 14 boys | 9–14 years | – | 112% compensation in ad lib lunch intake 30 min after sucralose vs. glucose-sweetened Kool-Aid preload (only 66% compensation if watching TV during lunch). |
| Birch et al. (49) | 18 children | 3–5 years | – | 90% compensation in ad lib snack intake 20 min after aspartame vs. maltodextrin-sweetened pudding. When subsequently given intermediate caloric density pudding, children who previously had aspartame-sweetened pudding ate 50 kcal more ad lib snack than those previously given maltodextrin-sweetened pudding. |
| Birch et al. (46) | 22 children | 2.5–5 years | – | Children showed 109% compensation in ad lib snack intake 20 min after aspartame vs. maltodextrin-sweetened pudding, while adults showed 0% compensation. |
| 26 adults | 25–35 years | |||
| Birch et al. (47) | 24 children | 2–5 years | – | 60%, 1% and 11% compensation in ad lib snack intake 0, 30 and 60 min after aspartame vs. sucrose-sweetened preload. Children reduced ad lib snack intake 30 min after aspartame-sweetened preload (compared with water), but not after 0 or 60 minutes. |
| Interventional studies: randomized controlled trials | ||||
| Knopp et al. (56) | 55 children and young adults | 10–21 years | 13 wk | No significant differences in weight loss between 2.7 g/day encapsulated aspartame vs. placebo |
| Ebbeling et al. (57) | 103 children (56♀ 47♂) | 13–18 years | 25 wk | No significant difference in BMI between those in intervention (replacing SSBs with ASBs) vs. control group except among heaviest subjects |
| Williams et al. (3) | 32 overweight girls | 13.2±1.4 years | 12 wk | No significant difference in BMI between those permitted sugar-sweetened soda vs. those only artificially-sweetened soda |
| Observational studies: cross-sectional studies | ||||
| Forshee et al. (52) | 3 311 children (1 624♀ 1 687♂); USDA CSFII 1994–6, 1998 | 6–19 years | – | BMI positively associated with ASB consumption |
| Giammattei et al. (53) | 385 children (199♀ 186♂) | 11–13 years | – | Higher BMI z-score in those consuming ≥3 servings per day of SSBs and ASBs |
| O'Connor et al. (54) | 1 160 children (581♀ 579♂); NHANES 1999–2002 | 2–5 years | – | No association between ASB consumption and BMI |
| Observational studies: prospective cohort studies | ||||
| Ludwig et al. (55) | 548 children (263♀ 285♂); Planet Health Project | 11.7±0.8 years | 2 yr | Obesity positively associated with SSB intake but negatively associated with ASB intake |
| Berkey et al. (50) | 11 654 children (6 636♀ 5 067♂); Growing Up Today study | 9–14 years r | 3 yr | ASB intake associated with weight gain in boys, but not in girls |
| Blum et al. (43) | 166 (92♀ 74♂) | 9.3±1 years | 2 yr | Increased ASB intake associated with BMI z-score at end of study |
| Striegel-Moore et al. (41) | 2 371 girls | 9–10 years | 10 yr | Diet soda intake significantly associated with total daily energy intake |
| Johnson et al. (51) | 1 203 children | 5–7 years | 9 yr | ASB consumption associated with baseline BMI and fat mass at age 9 |
| Kral et al. (42) | 177 children | 3–6 years | 3 yr | No association between ASB consumption and obesity risk status |
Compensation after a preload is defined as the difference in subsequent ad libitum caloric intake between two conditions, divided by the calories in the preload.
BMI: Body mass index; SSB: sugar-sweetened beverage; ASB: artificially-sweetened beverage; USDA CSFII: United States Department of Agriculture Continuing Survey of Food Intake by Individuals; NHANES, National Health and Nutrition Examination Survey.
Effects of artificial sweeteners on food intake in children
Several studies have looked at the effect of caloric versus artificial sweetener preloads on subsequent ad libitum food intake in children. In two experiments, boys aged 9–14 with a wide range of body mass indices (BMIs) exhibited complete caloric compensation in ad libitum lunch intake 30 minutes following a sucralose versus sugar-sweetened drink, meaning that they reduced their intake at lunch by the number of calories contained in the preload (44,45). In another study, 2.5 to 5-year-old children showed complete caloric compensation 20 minutes after a low-calorie, aspartame-sweetened pudding preload versus high-calorie, maltodextrin-sweetened pudding (46). Interestingly, adults participating in the same experiment showed no caloric compensation, meaning they ate the same number of calories at lunch regardless of which preload they received.
The timing of artificial sweetener consumption with respect to meals may also affect food intake. Children who consumed sugar-sweetened beverages immediately prior to a test meal ate less than those who consumed artificially-sweetened beverages before the meal (47). However, no compensation in meal intake was observed when sugar- versus artificially-sweetened beverages were given 30, 60, or 90 minutes prior to the test meal (47,48). In addition, children reduced ad libitum lunch intake 30 minutes after an aspartame-sweetened preload (compared with water), but not after 0 or 60 minutes (47).
These studies, of course, do not describe the effect of chronic consumption of artificial sweeteners on food intake. Birch et al. explored this idea by giving 3 to 5-year-old children an ad libitum snack 20 minafter either low-calorie (aspartame-sweetened) or high-calorie (maltodextrin-sweetened) pudding during multiple trials (49). With each trial, children consistently showed complete caloric compensation for the preload. However, when subsequently given an intermediate caloric density pudding of the same flavor they had received previously, children who had eaten low-calorie pudding beforehand ate significantly more snack than those previously given high-calorie pudding, by approximately 50 kcal.
Observational studies of artificial sweeteners and weight gain in children
The majority of pediatric epidemiologic studies have found a positive correlation between weight gain and artificially-sweetened beverage intake. Blum et al. examined beverage consumption and BMI Z-scores in 164 elementary school-aged children (43). This longitudinal study found that increased diet soda consumption was positively correlated with follow-up BMI Z-score after two years. Comparable results were found by Berkey et al., who examined the relationship between BMI and diet soda consumption in over 10 000 children (aged 9 to 14 years) of Nurses' Health Study II participants over the course of one year (50). Artificially-sweetened beverage intake was significantly correlated with weight gain in boys, but not in girls, during the study period. A long-term prospective study of 1 203 children in England found that artificially-sweetened beverage consumption at ages 5 and 7 was correlated both with baseline BMI and fat mass at age 9 (51). Another longitudinal study of 2 371 girls (aged 9 and 10) participating in the National Heart, Lung and Blood Institute Growth and Health Study showed that diet soda consumption was significantly associated with higher daily caloric intake, but not with BMI (41). A much smaller study of 177 children aged 3 to 6 years showed no association between diet soda consumption and risk of obesity (42).
Several cross-sectional studies in children have added to the association between artificially-sweetened beverage use and adverse health effects. Forshee et al. analyzed data from the US Department of Agriculture's Continuing Survey of Food Intake by Individuals from 1994–96 and 1998. This nationally representative sample of US children between 6 and 19 years of age found that BMI was positively correlated with diet soda consumption (52). These results were consistent with Giammattei et al.'s findings in 385 sixth and seventh graders, which showed that both diet and sugar-sweetened soda intake were positively correlated with BMI z-score and percent body fat (53). However, a study of 2 to 5-year-old children using NHANES data did not show an association between artificially-sweetened beverage consumption and BMI in this age group (54).
To date, only one observational study has shown an inverse association between artificial sweetener use and weight gain. In this study of 548 ethnically diverse school children (mean age 11.7 years) in Massachusetts, Ludwig et al. found that increased diet soda consumption over a 19-month time period was associated with decreased incidence of obesity, whereas the odds ratio of becoming obese increased 1.6 fold for each sugar-sweetened drink consumed (55).
Interventional studies of artificial sweeteners and weight gain in children
Three small interventional studies that manipulated artificial sweetener intake have been conducted in children, and have failed to show metabolic effects. Shortly after the approval of aspartame by the FDA, its effects during active weight reduction and its role in glucoregulatory hormone changes were studied in 55 overweight children and young adults, aged 10 to 21, during a 13-week 1 000 kcal/day diet (56). There were no differences in weight loss for subjects receiving 2.7 g/day of encapsulated aspartame versus placebo.
A randomized, controlled pilot study of 103 adolescents, aged 13 to 18 years, examined the effect of replacing sugar-sweetened drinks with artificially-sweetened beverages or water during a 25-week period (57). Changes in BMI for intervention versus control (no replacement of sugar-sweetened drinks) were not significant for the entire group, although an exploratory post-hoc analysis showed that the intervention made the greatest difference in the heaviest subjects, whose BMIs declined by 0.63 ± 0.23 kg/m2, compared with a 0.12 ± 0.26 kg/m2 gain in the control group. However, the authors did not separately report consumption of water versus artificially-sweetened beverages during the intervention, and thus the effect of artificial sweeteners in this study could not be isolated.
In the third randomized, controlled trial, girls aged 11 to 15 years consumed a 1500 kcal/day diet for 12 weeks. In one group, sugar-sweetened soda was permitted as a snack, while in the other group, only diet sodas were permitted. There were no differences between groups for BMI change, and reported intake of either sugar-sweetened or artificially-sweetened soda did not affect BMI change (3).
Artificial sweeteners and the metabolic syndrome
Components of the metabolic syndrome have been assessed in two pediatric studies. The previously discussed study of encapsulated aspartame versus placebo in young people found no differences in blood pressure, glucose, or lipid profiles between groups (56). Similarly, in the study in which teenage girls were permitted either sugar-sweetened or artificially-sweetened soda as a snack during weight loss, there were no differences between groups in blood pressure, waist circumference, or lipid profile (3).
Discussion
Recent epidemiological, clinical and laboratory findings question whether recommendations for the use of artificial sweeteners are indeed appropriate. A careful review of this literature by health professionals including physicians, epidemiologists, and dietitians is necessary to help consumers make well-informed decisions about their health. In this review, we have examined the existing evidence supporting or refuting a link between artificial sweetener use and weight change and other metabolic effects in children.
Epidemiologic studies of artificial sweetener use in children have generally shown a positive association between artificial sweetener intake (most commonly as diet soda) and weight gain. In interpreting such studies, it is critical to consider the conditions required to support causality in such studies, including the strength of the association, consistency in findings, temporality, biological gradient, plausibility, coherence between epidemiological and laboratory findings, and strength of the dose-response relationship (58). Based on these criteria, causality is far from established with regard to artificial sweetener use and weight gain in children. It is particularly difficult to establish causality between artificial sweetener consumption, weight gain, and metabolic abnormalities, as artificial sweetener intake is likely to be an indicator for other variables. For example, the decision to consume artificial sweeteners is often made by individuals who are concerned about their weight in an effort to reduce their caloric intake. In the case of children, this decision is frequently made by parents who are concerned about their own weight and consequently the weight of their offspring, thus further confounding the choice to use artificial sweeteners with genetic and behavioral variables.
Studies of artificial sweeteners and food intake demonstrate that caloric compensation is more complete in children than in adults, in whom food intake is substantially influenced by social cues and learned behaviors. However, even in children the degree of caloric compensation depends on the timing of the preload relative to the ad libitum meal, as well as the age of the child and other experimental circumstances. Although not all studies agree, the general trend is that artificial sweeteners may reduce total caloric intake when consumed between meals, but when consumed with meals, children may compensate for low-calorie snacks or drinks by increasing meal-associated calories. One study (49) supported the hypothesis that training children to associate sweet taste with low caloric density may result in overeating. Such studies, while not realistically mimicking actual human behavior, may provide insight into underlying mechanisms.
The strongest evidence for causation between artificial sweetener use and either adverse or beneficial health effects comes from randomized controlled trials. The few small, randomized controlled trials conducted in children did not find an association between artificial sweetener consumption and weight change. However, these studies were not specifically designed to look for effects of artificial sweeteners on weight change, and were presumably underpowered to find such effects. Currently, several trials are in progress to study the effects of artificially-sweetened carbonated soft drinks on body weight and other metabolic parameters in both children and adults (59,60), and studies of mechanisms underlying metabolic effects of artificial sweeteners are ongoing as well (61). These studies, and other similar investigations, will be critical for advancing understanding of the role of artificial sweeteners in metabolic health.
At the current time, the jury remains out regarding a possible role of increased artificial sweetener use in the obesity and diabetes epidemics, whether adverse, beneficial or neutral. In particular, very little data exist regarding the role of artificial sweeteners in glucose metabolism in children. Our growing understanding of the active metabolic role played by such chemicals in animal models should spur further research into the effects of these common food additives in humans.
Acknowledgments
This work was supported by the intramural research program of the National Institute of Diabetes, Digestive and Kidney Diseases.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Rodearmel SJ, Wyatt HR, Stroebele N, et al. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the Move family study. Pediatrics. 2007;120(4):e869–79. doi: 10.1542/peds.2006-2927. [DOI] [PubMed] [Google Scholar]
- 2.Benton D. Can artificial sweeteners help control body weight and prevent obesity? Nutr Res Rev. 2005;18(1):63–76. doi: 10.1079/NRR200494. [DOI] [PubMed] [Google Scholar]
- 3.Williams CL, Strobino BA, Brotanek J. Weight control among obese adolescents: A pilot study. Int J Food Sci Nutr. 2007;58(3):217–30. doi: 10.1080/09637480701198083. [DOI] [PubMed] [Google Scholar]
- 4.Bellisle F, Altenburg de Assis MA, Fieux B, et al. Use of ‘light’ foods and drinks in French adults: biological, anthropometric and nutritional correlates. J Hum Nutr Diet. 2001;14(3):191–206. doi: 10.1046/j.1365-277x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Husoy T, Mangschou B, Fotland TO, et al. Reducing added sugar intake in Norway by replacing sugar sweetened beverages with beverages containing intense sweeteners - A risk benefit assessment. Food Chem Toxicol. 2008;46(9):3099–105. doi: 10.1016/j.fct.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100(1):43–51. doi: 10.1016/S0002-8223(00)00018-3. quiz 49–50. [DOI] [PubMed] [Google Scholar]
- 7.Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61(6):691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- 8.Morris D, Cuneo P, Stuart M, et al. High-intensity sweetener, energy and nutrient intakes of overweight women and men participating in a weight-loss program. Nutr Res. 1993;13:123–32. [Google Scholar]
- 9.Friedhoff R, Simon JA, Friedhoff AJ. Sucrose solution vs no-calorie sweetener vs water in weight gain. J Am Diet Assoc. 1971;59(5):485–6. [PubMed] [Google Scholar]
- 10.Blundell JE, Hill AJ. Paradoxical effects of an intense sweetener (aspartame) on appetite. Lancet. 1986;1(8489):1092–3. doi: 10.1016/s0140-6736(86)91352-8. [DOI] [PubMed] [Google Scholar]
- 11.Fowler SP, Williams K, Resendez RG, et al. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16(8):1894–900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 12.Stellman SD, Garfinkel L. Artificial sweetener use and one-year weight change among women. Prev Med. 1986;15(2):195–202. doi: 10.1016/0091-7435(86)90089-7. [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA, Willett WC, Stampfer MJ, et al. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51(6):1100–5. doi: 10.1093/ajcn/51.6.1100. [DOI] [PubMed] [Google Scholar]
- 14.Duffey KJ, Popkin BM. Adults with healthier dietary patterns have healthier beverage patterns. J Nutr. 2006;136(11):2901–7. doi: 10.1093/jn/136.11.2901. [DOI] [PubMed] [Google Scholar]
- 15.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–61. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 16.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 17.Winkelmayer WC, Stampfer MJ, Willett WC, et al. Habitual caffeine intake and the risk of hypertension in women. JAMA. 2005;294(18):2330–5. doi: 10.1001/jama.294.18.2330. [DOI] [PubMed] [Google Scholar]
- 18.Nettleton JA, Lutsey PL, Wang Y, et al. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32(4):688–94. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenzie T, Brooks B, O'Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006;16(9):688–91. doi: 10.1016/j.annepidem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 20.McNaughton SA, Mishra GD, Brunner EJ. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II study. Diabetes Care. 2008;31(7):1343–8. doi: 10.2337/dc07-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer JR, Boggs DA, Krishnan S, et al. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–92. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grotz VL, Henry RR, McGill JB, et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103(12):1607–12. doi: 10.1016/j.jada.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 24.Blackburn GL, Kanders BS, Lavin PT, et al. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65(2):409–18. doi: 10.1093/ajcn/65.2.409. [DOI] [PubMed] [Google Scholar]
- 25.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51(6):963–9. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 26.Raben A, Vasilaras TH, Moller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 27.Drewnowski A. Intense sweeteners and the control of appetite. Nutr Rev. 1995;53(1):1–7. doi: 10.1111/j.1753-4887.1995.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn GL. Sweeteners and weight control. World Rev Nutr Diet. 1999;85:77–87. doi: 10.1159/000059707. [DOI] [PubMed] [Google Scholar]
- 29.Rolls BJ. Effects of intense sweeteners on hunger, food intake, and body weight: a review. Am J Clin Nutr. 1991;53(4):872–8. doi: 10.1093/ajcn/53.4.872. [DOI] [PubMed] [Google Scholar]
- 30.Renwick AG. Intense sweeteners, food intake, and the weight of a body of evidence. Physiol Behav. 1994;55(1):139–43. doi: 10.1016/0031-9384(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 31.Libuda L, Alexy U, Buyken AE, et al. Consumption of sugar-sweetened beverages and its association with nutrient intakes and diet quality in German children and adolescents. Br J Nutr. 2009;101(10):1549–57. doi: 10.1017/S0007114508094671. [DOI] [PubMed] [Google Scholar]
- 32.Mace OJ, Affleck J, Patel N, et al. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(Pt 1):379–92. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson G, Hoon MA, Chandrashekar J, et al. Mammalian sweet taste receptors. Cell. 2001;106(3):381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 34.Dyer J, Salmon KS, Zibrik L, et al. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33(Pt 1):302–5. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 35.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104(38):15075–80. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagonlike peptide-1. Proc Natl Acad Sci USA. 2007;104(38):15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy VB, Sigman-Grant M. Position of the American Dietetic Association: use of nutritive and nonnutritive sweeteners. J Am Diet Assoc. 2004;104(2):255–75. doi: 10.1016/j.jada.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Morgan KJ, Stults VJ, Zabik ME. Amount and dietary sources of caffeine and saccharin intake by individuals ages 5 to 18 years. Regul Toxicol Pharmacol. 1982;2(4):296–307. doi: 10.1016/0273-2300(82)90003-4. [DOI] [PubMed] [Google Scholar]
- 39.Ilback NG, Alzin M, Jahrl S, et al. Estimated intake of the artificial sweeteners acesulfame-K, aspartame, cyclamate and saccharin in a group of Swedish diabetics. Food Addit Contam. 2003;20(2):99–114. doi: 10.1080/0265203021000042896. [DOI] [PubMed] [Google Scholar]
- 40.French SA, Lin BH, Guthrie JF. National trends in soft drink consumption among children and adolescents age 6 to 17 years: prevalence, amounts, and sources, 1977/1978 to 1994/1998. J Am Diet Assoc. 2003;103(10):1326–31. doi: 10.1016/s0002-8223(03)01076-9. [DOI] [PubMed] [Google Scholar]
- 41.Striegel-Moore RH, Thompson D, Affenito SG, et al. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2006;148(2):183–7. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Kral TV, Stunkard AJ, Berkowitz RI, et al. Beverage consumption patterns of children born at different risk of obesity. Obesity (Silver Spring) 2008;16(8):1802–8. doi: 10.1038/oby.2008.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blum JW, Jacobsen DJ, Donnelly JE. Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr. 2005;24(2):93–8. doi: 10.1080/07315724.2005.10719449. [DOI] [PubMed] [Google Scholar]
- 44.Bellissimo N, Pencharz PB, Thomas SG, et al. Effect of television viewing at mealtime on food intake after a glucose preload in boys. Pediatr Res. 2007;61(6):745–9. doi: 10.1203/pdr.0b013e3180536591. [DOI] [PubMed] [Google Scholar]
- 45.Bellissimo N, Thomas SG, Goode RC, et al. Effect of short-duration physical activity and ventilation threshold on subjective appetite and short-term energy intake in boys. Appetite. 2007;49(3):644–51. doi: 10.1016/j.appet.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Birch LL, Deysher M. Caloric compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite. 1986;7(4):323–31. doi: 10.1016/s0195-6663(86)80001-0. [DOI] [PubMed] [Google Scholar]
- 47.Birch LL, McPhee L, Sullivan S. Children's food intake following drinks sweetened with sucrose or aspartame: time course effects. Physiol Behav. 1989;45(2):387–95. doi: 10.1016/0031-9384(89)90145-5. [DOI] [PubMed] [Google Scholar]
- 48.Anderson GH, Saravis S, Schacher R, et al. Aspartame: effect on lunch-time food intake, appetite and hedonic response in children. Appetite. 1989;13(2):93–103. doi: 10.1016/0195-6663(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 49.Birch LL, Deysher M. Conditioned and Unconditioned Caloric Compensation: Evidence for Self-Regulation of Food Intake in Young Children. Learn Motiv. 1985;16:341–55. [Google Scholar]
- 50.Berkey CS, Rockett HR, Field AE, et al. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12(5):778–88. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 51.Johnson L, Mander AP, Jones LR, et al. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition. 2007;23(7–8):557–63. doi: 10.1016/j.nut.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Forshee RA, Storey ML. Total beverage consumption and beverage choices among children and adolescents. Int J Food Sci Nutr. 2003;54(4):297–307. doi: 10.1080/09637480120092143. [DOI] [PubMed] [Google Scholar]
- 53.Giammattei J, Blix G, Marshak HH, et al. Television watching and soft drink consumption: associations with obesity in 11- to 13-year-old schoolchildren. Arch Pediatr Adolesc Med. 2003;157(9):882–6. doi: 10.1001/archpedi.157.9.882. [DOI] [PubMed] [Google Scholar]
- 54.O'Connor TM, Yang SJ, Nicklas TA. Beverage intake among preschool children and its effect on weight status. Pediatrics. 2006;118(4):e1010–8. doi: 10.1542/peds.2005-2348. [DOI] [PubMed] [Google Scholar]
- 55.Ludwig DS. Relation between consumption of sugar sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 56.Knopp RH, Brandt K, Arky RA. Effects of aspartame in young persons during weight reduction. J Toxicol Environ Health. 1976;2(2):417–28. doi: 10.1080/15287397609529443. [DOI] [PubMed] [Google Scholar]
- 57.Ebbeling CB, Feldman HA, Osganian SK, et al. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117(3):673–80. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- 58.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richselsen B, Nielsen M. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Effect of carbonated soft drinks on the body weight. [cited 2008 Nov 20] Available from http://clinicaltrials.gov/ct2/show/locn/NCT00777647 NLM Identifier: NCT00777647. [Google Scholar]
- 60.Antonelli T. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Reducing sugar-sweetened beverage consumption in overweight adolescents (BASH) [cited 2008 Nov 20] Available from http://clinicaltrials.gov/ct2/show/locn/NCT00381160 NLM Identifier: NCT00381160. [Google Scholar]
- 61.DiSabatino A. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Zero calorie drink products. [cited 2008 Nov 20] Available from http://clinicaltrials.gov/ct2/show/locn/NCT00525694 NLM Identifier: NCT00525694. [Google Scholar]
- 62.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1. Diab care. 2009;32(12):2184–6. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]


