Abstract
During the secretory phase of the menstrual cycle, a Natural Killer (NK) cell subset expressing CD56bright, appears in the decidualizing uterus, and remains until onset of menses. If pregnancy occurs, decidual (d)NK cells increase to become the predominant uterine lymphocytes of early pregnancy. To elucidate mechanisms of CD56bright cell recruitment to the uterus, an in vitro adhesion assay was used to assess the effect of the menstrual cycle, as well as cycle-associated hormones on adhesive properties of human lymphocytes. Adhesion of human peripheral blood lymphocytes to pregnant mouse lymph nodes and Peyer’s Patches tissue sections was constant throughout the cycle. When uterine tissue was used as the substrate, adhesive CD56+ cells were found only in decidua basalis. Adhesion increased at the luteinizing hormone surge. Adhesion was mediated through both L-selectin and α4 integrin-dependent mechanisms. Furthermore, we observed increased adhesive function in CD56+ cells from male donors which had been cultured with estradiol or LH as compared to cell aliquots cultured without additives. Lymphocytes adherent to mouse uterine tissue were predominantly CD56bright, suggesting that peripheral NK cells may be actively recruited to the uterus in an important, brief endocrine-regulated fashion at the time of ovulation to establish the dNK population of early pregnancy.
Keywords: Cell Trafficking, NK Cells, Luteinizing Hormone, Adhesion Molecules, Uterus
Introduction
Uterine immune cell populations undergo remarkable changes during the course of each ovulatory cycle. Leucocytes constitute 5% of uterine stromal cells in the proliferative phase of the cycle, but increase to 25% of total cells in the late secretory phase, primarily as a result of a large influx of natural killer (NK) cells (1;2). This migration is concurrent with decidualization, a process whereby endometrial fibroblasts transform, under the influences of estrogen and progesterone (P4), into decidual cells (3;4). In decidualized tissue, 70% of lymphocytes are CD56bright CD16null, a phenotype which constitutes less than 1% of circulating blood lymphocytes and about 10% of total peripheral NK cells, which are predominantly CD56dim, CD16+ (5). CD56bright cells are detectable in the uterus 2–3 days after the luteinizing hormone (LH) surge until LH+12 days, then undergo apoptosis prior to the onset of menses. Loss of decidual NK (dNK) cells has been attributed to declining progesterone levels (3). In the event of pregnancy, dNK cells increase rapidly and distribute broadly throughout decidua. As the fetus develops, dNK cells are found in close proximity to fetally-derived extravillous trophoblast invading the decidua (3). This suggests dNK cells have roles in implantation and establishment of the placenta.
Murine uterine (u)NK cells are derived from circulating precursors. Intravenous infusions of cell suspensions derived from any lymphoid organ of wild-type mice re-constitutes uNK cells in impregnated, alymphoid mice, with maximal reconstitution from splenocytes of pregnant donors (6). Lymphocyte homing depends on a multi-step cascade of interactions with vascular endothelial cells and occurs in response to conditions such as fever, inflammation, wounding or infection. This process is well-described in secondary lymphoid tissues (7;8). Initial rolling and tethering of circulating lymphocytes is mediated by transient binding of L-selectin and α4β7 integrin homing receptors expressed on lymphocytes to endothelial ligands (PNAd and MadCAM-1, respectively) on specialized high endothelial venules (HEV). Extravasation is mediated by chemokine signals from the site of injury or inflammation. The binding of chemokines to ligands expressed on lymphocyte surfaces stimulates G-protein dependent activation of LFA-1 which results in firm adhesion to its endothelial ligand ICAM-1. The final step is transendothelial migration (9).
Evidence that L-selectin and α4 integrin may orchestrate lymphocyte trafficking to decidualized mouse uterus is accumulating. In previous studies, we demonstrated dynamic, pregnancy-associated endothelial surface changes in murine lymphoid and decidualized uterine tissues but not in other tissues. These changes were recognized by L-selectin and/or α4 integrin receptors on human lymphocytes (10). When the mouse tissue donor was ovariectomized and treated with either 17β-estradiol (E2) or P4, gains in adhesion were particularly pronounced on decidual substrate. Complementary gains in adhesive capacity were found in the lymphocytes from these pregnant or hormone-treated mice as compared to virgin or sham-treated ovariectomized controls. This suggests independent but co-ordinated hormonal regulation of adhesion pathways in decidual endothelium and lymphocytes(6;10). At gestation day (gd)8 of murine pregnancy, Kruse et al. (11;12) identified α4 integrin+, LFA-1+ NK cells in decidua basalis (DB), close to VCAM-1 expressing endothelium. VCAM-1, an alternate ligand for α4 integrin, is up-regulated by pro-inflammatory cytokines such as TNFα and IL-1, in concert with transcription factors such as the IFN-related factor (IRF)-1 in vascular endothelium (13;14) and IRF-2 in muscle (15). Human CD56+ dNK cells express α4 integrin and LFA-1, while the α4 integrin ligand, VCAM-1 is expressed on decidual blood vessels in the human uterus (16–18). Both E2 and P4 are reported to stabilize expression of VCAM-1, ICAM-1 and E-selectin in primary vascular endothelial cell cultures (19;20). E2 is also reported to directly stimulate VCAM-1 expression by endothelial cells in culture (21). While these effects would enhance extravasation of lymphocytes, they are insufficient to induce specific homing of pre-dNK cells to the uterus since lymphocytes require specific chemotactic signals to activate the homing receptors expressed constitutively on their surfaces.
The present study was undertaken to define the effects of the normal hormonal fluctuations during the menstrual cycle on functional interactions between human lymphocytes and endothelium. Since our previous experiments used human lymphocytes purchased in quantity and the current study required serial donations from specific donors, we optimized the adhesion assay for use with smaller lymphocyte samples. Using this optimized protocol, we found that the functional ability of human blood lymphocytes and the CD56bright subset in particular, to interact with uterine endothelium was dynamically altered at the LH surge or in association with high physiological levels of E2 and LH.
Materials and Methods
Human Subjects and Blood Sampling
Healthy male volunteers of legal age donated either 15 mL thrice over several weeks (cohort A (n=4)) or up to 50 mL of blood once (cohort D (n=4)). Non-pregnant female subjects of legal and reproductive age (23–45), having regular menstrual cycles between 26 and 33 days in length, using no form of hormonal birth control for at least 1 year and in good health were recruited to donate serial blood samples. Based on the experiment for which their blood was used, they were divided into 2 cohorts, B (n=7) and C (n=12). All subjects were informed about the risks of participation in this study and provided an informed written consent (Ethics Committee, Office of Research, University of Guelph). About half of the female donors were multiparous (n=3, cohort B and n=6, cohort C). The others had never attempted to become pregnant. Female donors provided either 25 mL (cohort B) or 20 mL (cohort C) of venous blood in sterile blood collection tubes containing the anticoagulant acid citrate dextrose (ACD), which was immediately layered onto an equal volume of warmed (room temperature) Histopaque 1.077 (Sigma, Mississauga, ON) and centrifuged (400×g; 30 min; RT). The lymphocytes at the Histopaque/plasma interface were collected, washed thrice, counted and adjusted to 2.5×107 cells/mL in 37°C RPMI (Sigma) with no additives.
Mice and tissue dissections
C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME) aged 7–8 wk were used for timed matings with the morning of the copulation plug designated gd0. Non pregnant (NP) controls were virgin females who had never been paired with males. All procedures were performed under approved animal utilization protocols (Animal Care Committee, University of Guelph). Virgin mice and mice at gd 6–8 were euthanized and the following tissues were collected; a pool of 10–12 lymph nodes (LN) from subcutaneous and intermuscular sites, a pool of 10–12 Peyer’s Patches (PP) and uteri. Implantation sites from pregnant uteri were trimmed and cut in the median sagittal plane and placed cut face down into biopsy cryomolds (Fisher Scientific, Mississauga, ON). All tissues were embedded in OCT (Miles Laboratories, Fisher Scientific), flash frozen in liquid nitrogen-cooled isopentane and stored at −20°C. Tissues were used within 2 weeks of collection.
Antibodies
For assays involving LN or uterine tissue substrates, cell aliquots were incubated (30 min, RT) with function blocking mAb specific for human L-selectin (CD62L, BD Pharmingen, Mississauga, ON). When PP was used as the tissue substrate, lymphocyte aliquots were pre-treated (30 min, RT) with a function-blocking antibody to alpha 4 integrin (HP2/1, Coulter Immunology, Hialeah, FL, 10 μg/mL)(23). After treatment, cells were washed in RPMI and readjusted to 5×107 cells/mL. In some experiments, aliquots of 5×106 lymphocytes were pre-labeled with CD56-PE (Immunotech, Beckman Coulter, Mississauga, ON) to detect NK cells at 1:100 for 20 min RT, then washed with RPMI and re-suspended in 100 μL. Preliminary assessment of labeled and unlabeled cells in bright field microscopy indicated that no change in adhesion occurred due to labeling (data not shown). In some experiments, endothelial cells in frozen mouse tissues were labeled with 100 μl of 1:100 dilution of Alexa488 conjugated isolectin from griffonia simplicifolia (Molecular Probes, Burlington, ON)(22).
To characterize populations in the starting cell suspension prior to application onto frozen sections, flow cytometric analysis was conducted using the following panel of mouse anti-human antibodies (Caltag Laboratories); CD4 R-PE-8 FITC (1:100), CD19 FITC (1:10), CD33-4D3 R-PE (1:10), CD34 FITC (1:10) and isotype controls; mouse IgG2a FITC/R-PE (1:100), mouse IgG1 FITC (1:10), mouse IgG1 R-PE (1:10). To detect NK cells, CD56-PE (Immunotech, Beckman-Coulter) (1:100) was used. All antibody dilutions were optimized in preliminary studies (data not shown).
Flow Cytometry
Cells suspended in PBS were labeled for 30 min RT, then washed twice with PBS containing 2% BSA and 0.01% Na azide. Analyses of cell data were performed on a FACScan using CellQuest™ software (Becton-Dickenson, San Jose, CA) immediately after the last wash, without the use of fixative. The lymphocyte gate was defined by forward and side scatter properties (data not shown).
Assay of cell adhesion under shear to frozen tissue sections
Twelve micron sections of frozen mouse tissue on cover slips were placed on rubber stoppers on a table, rotating at 80 rpm in a cold chamber, and immediately overlain with 100 μL of either untreated human lymphocytes or lymphocytes treated with antibody to L-selectin, α4 integrin and/or pre-labeled with CD56-PE. Rotation was increased to 112 rpm and continued for 30 min at 4°C. After incubation, slides were gently rinsed in PBS, then fixed 30 min in 3% glutaraldehyde at 4°C or, if fluorescent labeling was done, in 2% paraformaldehyde for 20 min at 4°C, then rinsed in PBS for 10 min, then in ddH2O and mounted with AquaPolymount (Polysciences, Inc, Washington, PA ). The sections were assessed for lymphocyte adhesion by microscopy. Two hundred HEV were counted per blind encoded slide of LN or PP or 50 high power fields (HPF, 400×) in uterine tissues by 2 independent researchers.
Optimization of experimental procedures
Preliminary experiments were performed to determine the lowest number of cells necessary to detect significant differences in lymphocyte adhesion between untreated and pre-labeled cells. Replicate experiments used 107, 5×106, 2.5×106, and 106 cells per 100 μL of test suspension from four different male donors (cohort A). These cells were applied to sections of LN from both virgin and pregnant animals in a standard adhesion assay. One aliquot of cells was pre-treated with anti-CD62L to block L-selectin mediated adhesion.
To assess the effects of anticoagulants on adhesive properties of isolated PBL, 10 mL of blood were collected per subject from cohort A into each of 4 different anticoagulants. These were ACD, EDTA, Sodium Citrate and Heparin. Lymphocytes were isolated and used simultaneously as described above.
Because fever range temperature promotes adhesion of PBL (23), the effect of blood storage temperature on the adhesive properties of PBL was assessed. Whole blood samples (collected in ACD) from each member of cohort A were exposed to the following conditions; 1) 4h at 20°C, 2) 4 h at 37°C, 3) 4h at 4°C or 4) 3.5h at 4°C, then warmed back to 37°C for 30 min. Lymphocytes were isolated as described above. Control lymphocytes came from freshly drawn blood taken from the same donor 4 h after the initial 40 mL donation.
Effect of the menstrual cycle on adhesion of human PBL
Seven female volunteers (cohort B) were recruited to donate 25 mL of blood thrice weekly through an entire menstrual cycle (12 samples collected per subject). Subjects maintained basal body temperature charts to monitor their cycles. White blood cells were isolated and 2.5 × 106 cells, either untreated or pre-treated with anti CD62L or anti HP2/1, were applied to frozen tissue sections of LN and PP from virgin and pregnant (d8) mice. The women started their donations together, on various cycle days. At completion of the experiment, they submitted their temperatures charts. All cycles were normalized by arbitrarily setting the day of temperature rise as cycle day 14. Results were correlated with the phase of the cycle as indicated by temperature shift (indicating that ovulation had occurred) and confirmed by analysis of individual serum hormone levels (moderate estrogen and low progesterone levels indicate proliferative phase and high levels of progesterone and estrogen indicate the secretory phase).
Role of LH surge in determining level of adhesion
Data from the menstrual cycle study indicated that the peri-ovulatory period was a time of change in lymphocyte-endothelial interaction. Therefore, cohort C women were recruited to donate blood on day 8, day of LH surge and day 20 of their cycles. In addition to basal body temperature charts, cohort C used a commercial LH detection kit (Clearplan, Novartis, Mississauga, ON) from day 11 of their cycle until the LH surge. Two cell aliquots were prepared from each sample for use in adhesion assays; one not labeled and one pre-treated with anti-CD62L. Similarly, 2 aliquots of cells were prepared from a second group of 6 subjects; both aliquots were labeled with anti-CD56-PE, and one was also pre-treated with anti-CD62L. Serum from each of these samples was assayed by ELISA for E2 (DRG Instruments, Biophase Diagnostic Laboratories, Mississauga, ON), LH and P4 concentrations(Biocheck, Inc. Burlingame, CA).
Role of ovarian steroids in stimulating adhesive ability in CD56bright cells
To determine the effect of ovarian steroids on adhesive capability of CD56bright cells and to avoid endogenous female hormone effects on NK cell adhesiveness, male donors from cohort D were recruited to donate up to 50 mL venous blood. Freshly isolated lymphocytes were adjusted to 7×106 cells per mL of serum-free RPMI. One mL of cell suspension was placed in individual wells of a 12 well plate containing 1 mL of serum-free RPMI containing either no additives, E2 (Sigma), P4 (Sigma) or LH (Dr AF Parlow, National Hormone and Peptide Program, Torrence, CA), such that the final concentrations were E2; 100, 200, 400, 800 pg/mL, P4 1, 4, 8, 16 ng/mL, and LH 10, 50, 100, 200 ng/mL. After 4 hours of culture at 37°C, 5% CO2, cells were aspirated, washed with serum-free 37°C RPMI and labeled with anti-CD56-PE as above. Cell numbers were adjusted to 5×107 cells per mL and 100 μL of labeled cells were used immediately in an adhesion assay.
Statistical Analyses
Results depict the mean ± SEM. To analyze experimental effects of cell number, blocking, temperature or anticoagulant on lymphocyte adhesion, either a one- way or two-way ANOVA was used, as indicated in figure legends. Further comparison of means was conducted using Bonferroni’s or Tukey’s procedure to adjust for multiple comparisons. We used Bonferroni when a fixed number of comparisons were made, and Tukey’s procedure to adjust for all pair-wise comparisons. Since some data points were missing in the timecourse study, due to loss of tissue substrate during processing, the mixed linear model (a generalization of repeated measures ANOVA) was used to analyze the timecourse studies to test for differences over time and between conditions (untreated cells vs function-blocking antibody) as well as for associations between adhesion and hormone concentrations. A posthoc paired t-test was used to determine whether mean adhesion in the peri-ovulatory period was different from the rest of the cycle. Analyses were performed using SigmaStat and SAS. All analyses passed the Kolmogorov-Smirnoff test for normality (with Lillefor’s correction) as performed by SigmaStat.
Results
Adhesion Substrate
Since frozen murine tissues were used to assess adhesion of human lymphocytes as a model of trafficking, it was important to establish the adhesive partner cell in the tissue. Figure 1 illustrates adhesion of fluorescently labeled CD56+ cells and endothelial cells. It is apparent that the NK cells selectively adhere to vascular endothelium specific to the decidua basalis, as adhesion was non-existent in non-decidualized areas of the same tissue.
Figure 1.
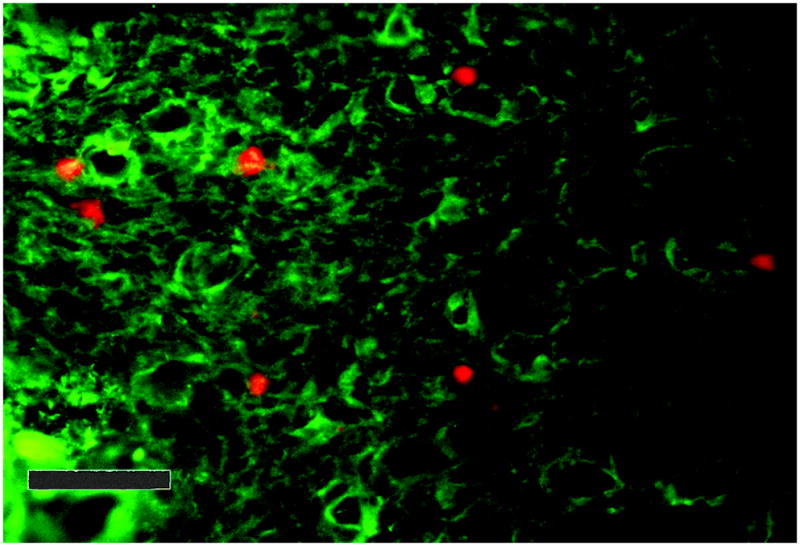
Photomicrograph of results of Stamper-Woodruff adhesion assay. Human lymphocytes (5×106 in 100 μL) were pre-labeled with anti-CD56-PE, then applied to decidua from a C57Bl/6J mouse at day 7 of gestation under rotation, to mimic shear forces in blood vessels. Murine endothelium was labeled with Alexa 488 conjugated isolectin (bright green). Non-adherent cells were rinsed off, the tissues were fixed and mounted. The number of CD56+ cells (bright red cells) per high power field (HPF) were counted. (400X). Size bar shows 50μm.
Reliability of assay
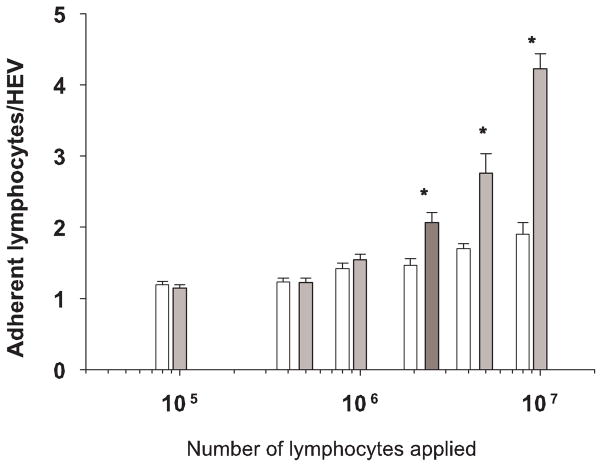
A limited number of lymphocytes can be purified from 25 mL of blood (approximately 1–10×107). Therefore, a dose response study was conducted to determine the lowest number of cells that could be used per tissue section while still retaining statistical differences between untreated cells and cells treated with function-blocking antibody to L-selectin. Results are shown in Figure 2. A two-way ANOVA showed a significant interaction between the presence of blocking antibody and number of lymphocytes applied. We subsequently tested for significant differences between the blocked and unblocked conditions at each of the 6 lymphocyte concentrations. Using Bonferroni’s procedure to adjust for multiple comparisons, a p-value of less than 0.00833 on an individual test is required to show statistical significance, in order to maintain an overall Type-I error rate of 0.05. The minimum number of applied lymphocytes with which a significant increase in adhesion between untreated cells and cells pre-treated with antibody to L-selectin could be detected was 2.5×106 cells/tissue section (p=0.0033). Differences between normal and blocked cells at concentrations of 5×106 and 107 cells were likewise significant (p<0.0001). Thus 2.5×106 cells were used in each adhesion assay throughout the study permitting more experimental conditions using a single blood sample.
Figure 2.
Adhesion outcomes (# adherent lymphocytes) after application of varying numbers of cells from the same donors to constant tissue (LN from a pregnant mouse) within single experiments. Solid bars depict the mean number of adherent cells ± se when 105 to 107 lymphocytes were applied to frozen sections of LN. Each solid bar is paired with an open bar which represents the mean number of adherent lymphocytes pre-treated with a function-blocking antibody to L-selectin. The minimum number of applied lymphocytes required to detect a significant difference from control cells was determined to be 2.5 × 106 (dark bar). * denotes a significant difference in adhesion between untreated and treated cells applied in equal number.
The effect of the anticoagulant on the adhesive properties of the isolated lymphocytes was investigated with 4 anticoagulants for each of 3 subjects (data not shown). While lymphocytes isolated from blood containing ACD, the anticoagulant used in leukopacks employed in our previous studies, appeared superior in adhesion to lymphocytes isolated from Na Citrate, EDTA or heparin, the difference was not significant (one-way ANOVA). ACD was selected as the anticoagulant for these experiments.
The results of the temperature assay were analyzed by one-way ANOVA which found significant differences between the various treatments (p=0.01). Bonferroni’s procedure, which requires p<0.0125 to maintain an overall significance level of 0.05 was used to determine which treatments differed from fresh lymphocytes (data not shown). Holding whole blood at 37°C results in adhesive ability significantly greater than the freshly isolated lymphocytes (p=0.0048). Storage at room temperature has no effect on adhesion (p=0.8106), while chilling blood reduces the effectiveness of the lymphocytes isolated from it to recognize and bind to ligands expressed on mouse endothelium, although the difference is not significant (p=0.6720). Chilled blood, which is then re-warmed to 37°C demonstrates a rebound in adhesion, but with a Bonferroni adjustment, this is not significantly different from fresh lymphocytes (p=0.0311). Thus, cells were kept warm following their collection.
Effect of the menstrual cycle on functional adhesive properties of lymphocytes
Lymphocytes from cohort B women appeared to increase in adhesiveness on LN or PP from virgin mice or pregnant mice at days 10–12 of the cycle (data not shown). However, when data from this peri-ovulatory period (d10-12 of the cycle, day of temperature shift which occurs post-ovulation was set as day 14) were pooled and compared to the rest of the cycle, human lymphocytes collected at the peri-ovulatory phase were not found to be more adherent to LN (p=0.0529, t-test) than those from the proliferative and luteal phases of the menstrual cycle. Treatment of the lymphocytes with function blocking antibodies to L-selectin or α4 integrin resulted in a significant reduction in adhesion (p<0.0005, mixed linear model F-test, data not shown) which was constant over the menstrual cycle, regardless of the tissue used.
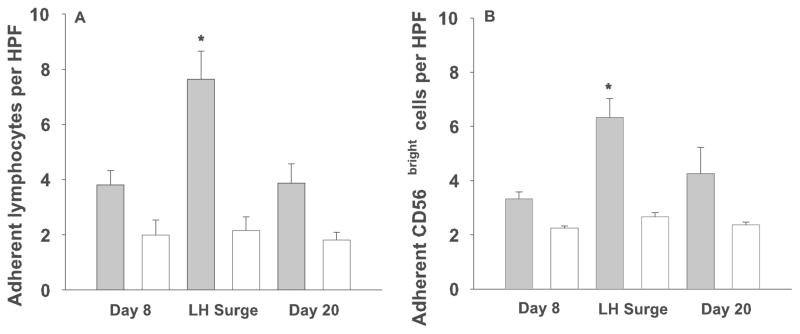
This experiment indicated the peri-ovulatory period was of potential interest, so LH detection was incorporated into our protocol to focus on the effect of the LH surge on the modification of lymphocyte-endothelial interactions. Lymphocytes from women in cohort C, collected at cycle day 8, day of LH surge and cycle day 20 were applied either untreated, blocked, labeled with anti-CD56PE or labeled and blocked, to LN and uteri from pregnant mice only. Mean adhesion of blood lymphocytes and CD56bright cells to LN peaked at peri-ovulation, but there was no change in mean adhesion (data not shown). When uterine tissue was used as the substrate, a significant increase in both lymphocyte (Figure 3A) and CD56bright cell (Figure 3B) adhesion was observed on the day of LH surge as compared to proliferative (d8) and mid-secretory (d20) lymphocytes (p=0.0004 and p=0.0092, mixed linear model, F-test). Blocking with anti-L-selectin resulted in a significant decline in adhesion at all timepoints (p=0.0004, mixed linear model, F-test), but did not completely eliminate adhesion of CD56+ cells, indicating that other adhesion molecules are involved in mediating lymphocyte-endothelial adherence. The LH-associated gain in adhesion to uterus was L-selectin-dependent because there was no change in adhesion in the blocked samples at the LH surge compared with other timepoints.
Figure 3.
Adhesion of lymphocytes from 3 timepoints in the menstrual cycle to LN and uterus from pregnant B6 mice.
Solid bars represent the mean number of adherent lymphocytes ± se (A) or CD56+ cells (B) per HPF in decidua from gd 6–8 B6 mice. Open bars show adhesion of lymphocytes pre-treated with a function-blocking antibody to L-selectin (n=6) which was significantly less than the untreated cells (p=0.0004, mixed linear model, F-test). * denotes significant increase in adhesion of untreated cells at LH as compared to untreated cells at cycle day 8 and 20 as determined by a mixed linear model, p=0.0004 (A) and p=0.0092 (B).
Cytometric analysis of PBL
The histogram of Figure 4 summarizes mean percentages (± se) of CD4, CD8, CD19, CD56dim and CD56bright cells within the lymphocyte gate during the monitored cycles of cohort C donors. There was considerable variation in lymphocyte subset percentages between subjects, and inconsistent variations from subject to subject over the course of the cycle, with some subjects showing an increase in the luteal phase, some a decrease, and others no change. Thus we found no significant changes in lymphocyte populations over the course of a menstrual cycle.
Figure 4.
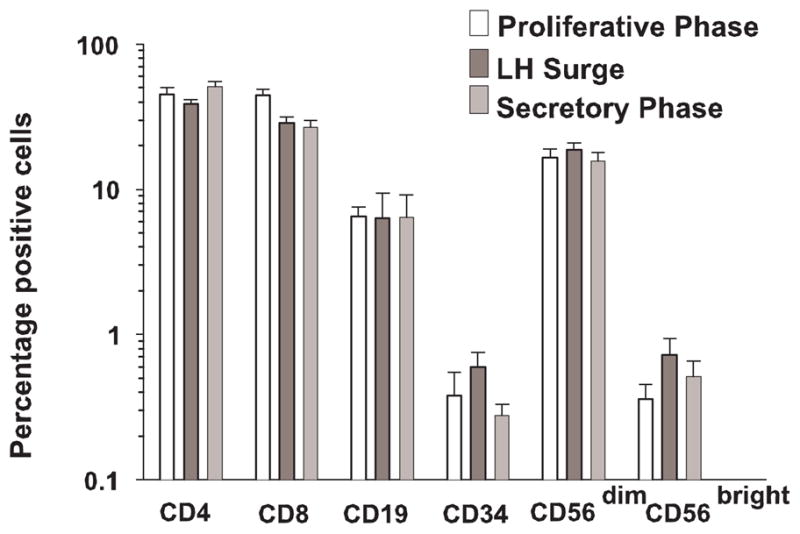
The relative percentages of lymphocyte subsets over the course of a menstrual cycle. As analyzed by flow cytometry, the numbers of CD4, CD8, CD19, CD33 and CD56dim cells remained stable. Each bar represents the mean of 3–6 samples.
Hormonal profile of subjects
Serum concentrations of E2, LH and P4 for cohort C donors are summarized as mean ± se in Table I. The ELISA kits provided expected reference values for phases of the menstrual cycle as shown below each of the means of the measured values. All subjects were within the normal range of hormone values for each phase of the cycle with the exception of two subjects who had high pre-ovulatory levels of P4 and one subject had consistently low levels of P4.
Table I.
Serum hormone concentrations in cycling blood donors (n=6)
| 17 β Estradiol pg/ml | Luteinizing Hormone mIU/ml | Progesterone ng/ml | |
|---|---|---|---|
| Cycle day 8 (Normal range) | 83.26 ± 32.59 (30 – 120) | 9.56 ± 1.98 (≤15) | 1.38 ± 1.77 (0.15 – 0.70) |
| Surge of LH (Normal range) | 152.39 ± 58.50 (130 – 370) | 39.51 ± 23.55 (20 – 120) | 1.50 ± 1.25 (1 – 2) |
| Cycle day 20 (Normal range) | 88.46 ± 20.24 (70 – 250) | 7.39 ± 2.42 (≤12) | 10.51 ± 4.39 (2– 25) |
To assess whether the hormone concentrations were correlated with adhesion to LN and uterus, a statistical mixed linear model was applied. Estrogen was significantly associated with adhesion to uterus (p=0.0149), but not LN. No association between progesterone and adhesion in either uterus or LN was found. Finally, LH was significantly associated with adhesion in uterus (p=0.0002) and in LN (p=0.0186).
Effect of reproductive hormones on adhesion of CD56+ cells
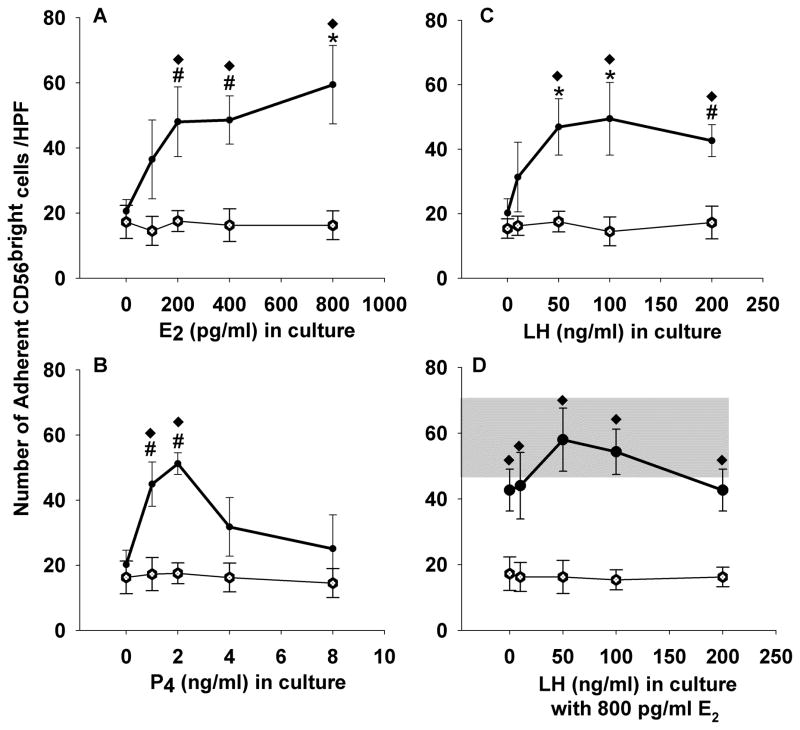
To address directly the effects of cycle hormones on lymphocyte adhesive function, NK cells from 4 male donors of cohort D were cultured in RPMI media with no additives, RPMI with E2, RPMI with P4 or RPMI with LH. Figure 5 summarizes these experiments. Adhesion of CD56 expressing cells increased following culture compared to adhesion of fresh cells. Cells cultured with hormones, especially E2 and LH, but also low levels of P4 demonstrated greater adherence to vascular endothelium than did cells cultured without hormone additives (Figure 5). Furthermore, flow cytometric analysis of cells before and after culture showed a consistent and dramatic increase in CD56 expression in all samples, regardless of additives to culture media (not shown).
Figure 5.
Adhesion of cultured lymphocytes of male donors (n=4) to uterine endothelium. Peripheral blood lymphocytes were cultured with concentrations of 17β estradiol varying from 0 to 800 pg/mL (A), progesterone varying from 0 to 8 ng/mL (B), luteinizing hormone varying from 0 to 200 ng/mL (C) or luteinizing hormone varying from 0 to 200 ng/mL with 800 pg/ml 17β estradiol (D) for 4 h prior to assay for adhesion to frozen sections of mouse uterus. Gray bar in panel D shows effect of 800 pg/mL alone on adhesion (mean ± sem), without added LH. Data points represent mean values ± se. Open circles denote mean number of adherent CD56+ cells pre-treated with anti-L-selectin, closed circles show mean adhesion of untreated NK cells.* increased adhesion relative to control sample with no added hormones, p<0.005 (one way ANOVA), # increased adhesion relative to control sample with no added hormones, p<0.05 (one way ANOVA), ◆ differs from samples pre-treated with antibody to L-selectin p<0.008 (one way ANOVA).
There was an approximately linear relationship between adhesion and E2 concentration (p=0.0071, mixed linear model)(Figure 5A). For every pg/mL increase in E2, there is an accompanying increase of 0.038 adherent cells/HPF. There was also an increase in adhesion between progesterone levels of 0 and 2 ng/mL (p=0.0022, mixed linear model)(Figure 5B). Specifically, adhesion is estimated to increase by 15.8 cells/HPF for every ng increase in P4. At P4 levels greater than 2 ng/mL, there is a significant decreasing relationship between adhesion and P4 (p=0.0403, mixed linear model). In particular, adhesion is estimated to decrease by 3.6 cells/HPF for every ng increase in P4. There was an increasing relationship of LH with adhesion (p=0.0055) from 0 to100 ng/mL (Figure 5C). LH concentrations higher than 100 ng/mL did not induce further increases in adhesion, rather, adhesion plateaued. Finally, there was no additive effect of combining various dosages of LH with 800 pg/ml 17β estradiol (Figure 5D).
Discussion
The highly proliferative and abundant population of CD56bright, CD16dim NK cells which inhabits the human uterus within days after the LH surge is thought to arise from uterine recruitment of circulating blood and in situ proliferation (2;24). Here, we describe significant increases in adhesion of CD56bright blood lymphocytes taken at the LH surge of fertile females, to L-selectin ligands in decidualized mouse uterus but not to ligands in either LN or PP, indicating a tissue specific effect. Furthermore, lymphocytes from male donors exhibited enhanced adhesive capacity after incubation with E2, low pre-ovulatory levels of P4 or LH as compared to cell aliquots cultured without additives.
Blood CD56bright cells constitute less than 1% of peripheral blood lymphocytes and differ in function from the CD56dim subset by producing greater amounts of IFN-γ, TNF-β, GM-CSF, IL-10 and IL-13 when stimulated by IL-12, 15, 18 or PMA (25), and by exhibiting reduced natural cytotoxicity in the unstimulated state (26;27). Uterine NK cell populations differ from peripheral blood subsets in several species (2;4;28), particularly in terms of gene transcription (29). In primates, dNK cells express the phenotype and share the cytokine and comparatively non-lytic characteristics of peripheral CD56bright, CD16null cells (28;30;31). It is attractive to postulate that a subset of blood CD56bright cells homes to the uterus and undergoes terminal differentiation in response to uterine stromal factors such as IL-15 (5;32;33). We speculate that major differences between blood and dNK cells can be attributed to these uterine events, as circulating NK cells are immature.
Migration of leukocytes to inflammatory sites is induced by chemokines, secreted by cells at the site, which bind to seven-transmembrane G-protein couples receptors (34). Hormonal effects on expression of chemokines and their receptors have been described; E2 induces increased production of inflammatory chemokines in dendritic cells and monocytes (35). Administration of exogenous estrogens during fertility treatment results in increased numbers of dNK cells and in glandular IL-8/CXCL8 production (36). Progesterone induces MIG/CXCL9, while E2 inhibits its production (37). Investigations of menstrual cycle effects on production of chemokines or their ligands reveal up-regulation of endometrial transcripts for CXCR1, CCR5, CCR2B and, to a lesser extent, CXCR4 in the luteal phase (38) and increased expression of CXCL10 (39) and CXCL9 but not CXCL11 or CXCL12 (40) in decidua. Circulating CD56bright cells express high levels of CXCR3, the ligand for CXCL9, 10 and 11 and moderate levels of CXCR4, the ligand for CXCL12, and demonstrate migration in response to these signals (41). Thus, this subset is well-equipped to respond to cyclic signals from dendritic and stromal cells in the uterus.
Here, we examined the effect of the menstrual cycle on the functional adhesiveness of peripheral blood lymphocytes, the source of dNK cell progenitors suggested from mouse models. Mouse, rather than human uterine tissue was used as the substrate to eliminate differences in stage of cycle in the uterus, and because the assay requires use of relatively fresh substrate tissue (less than 2 weeks of storage at −20°C). We found significant enhancement of adhesive capacity of lymphocytes only at LH surge and only when decidua was used as substrate. When only CD56bright cells were enumerated, it was apparent that they constituted the majority of adherent cells and thus could be preferentially recruited. Since antibody-blocked lymphocytes showed no alteration in adhesion, any changes in adhesive capacity were mediated by activation of L-selectin, presumably by circulating factors.
Direct response of CD56bright cells to menstrual cycle hormones is debatable; neither rat nor human lymphocytes express α forms of the receptor for estrogen (ER) or progesterone (PR) (42–45) but mouse NK cells do (46–48). It is reported that dNK cells express ERβ1, but these are non-circulating, terminally differentiated cells (49). Our findings of a positive relationship between estrogen and adhesion to decidua, a stronger association between LH levels and adhesion, and a biphasic effect of P4 are suggestive of complex hormonal influences in trafficking potential. It appears that rising E2 prior to the LH surge create a trafficking window enabling CD56bright cell egress from circulation. After ovulation has occurred, predominantly high P4 levels close the window and CD56bright cells cease trafficking. A critical role for E2 on NK cell trafficking to the uterus is unlikely since a study of mice genetically devoid of ERα or ERβ display no alteration in recruitment of uNK cells to the pregnant uterus (50). It has been reported that lymphocytes of pregnant women, as well as of women in the luteal phase of the menstrual cycle express the LH receptor, but at lower levels than found in ovarian tissue (51). Therefore, it is quite plausible that peripheral blood lymphocytes are directly activated during the LH surge.
The enrichment of CD56bright NK cells in the uterine environment is well documented (1;2;52). However, the means by which this enrichment occurs is still not understood. Decidua contains no HEV, the usual site of lymphocyte trafficking to lymphoid tissues. Due to frequent histological observations of mouse NK cells transiting decidual arteries, we speculate that these arteries, which thin and elongate as decidualization progresses, are the site of NK cell invasion.
Menstrual cycle variation has also been described in human blood NK cell lytic function(53–55) and in number of NK cells per millilitre of blood (55). A significant increase in the number of CD56+ cells at the peri-ovulatory period reported for 39 women between the ages of 20 and 29 was postulated to be due to LH and not to E2, P4 or testosterone (55). We observed larger differences in NK cell populations between individuals in our study group than within individual. While there appeared to be an increase in the percentage of CD56bright subset (relative to the total lymphocyte population) at LH surge in our cohort B of 7 patients between the ages of 25 and 38, the difference was not significant. We did not enumerate the total NK cells/mL blood.
While it is attractive to postulate that adhesion is representative of NK cell recruitment to the decidualizing uterus, this is difficult to prove in women. Loss of this NK cell population with each menstrual cycle indicates that renewal occurs. It is generally accepted, and these data support the hypothesis, that lymphocyte recruitment is from the peripheral blood. It would appear from our work that either a threshold level of E2 or a surge of LH activates adhesion molecules on the dNK precursor cell surface, enabling a single wave of NK cells or precursor cells to leave the circulation and enter the uterus. This is consistent with a model in which NK cells localize to the DB at LH +3, proliferate until approximately LH+12, then start to disappear, as previously described (1;2). Furthermore, modulation of NK cell lytic activity by LH and βhCG at physiological levels has been reported, suggesting a direct interaction between the NK cell and the polypeptide hormones (54). Complete understanding of the migration and role of dNK cells is expected to have broad clinical impact in women’s reproductive health areas such as infertility, gestational complications (i.e. recurrent spontaneous abortion, preeclampsia), and pathologies such as endometriosis.
Acknowledgments
Supported by Awards from Natural Sciences and Engineering Council, Canada, Ontario Ministry of Agriculture, Food and Rural Affairs. MvdH is supported by the Ontario Women’s Health Postdoctoral Scholar Award.
We thank our subjects for their co-operation throughout this study and for their generosity of time and spirit. Dr AF Parlow of the National Hormone and Peptide Program (Torrence, California) kindly provided LH for culture experiments. We are grateful to Dr. Sharon Evans, Buffalo, NY for helpful discussions.
Abbreviations used
- uNK
uterine Natural Killer cell
- DB
decidua basalis
- dNK
decidual NK cell
- HEV
high endothelial venule
- gd
gestation day
- LH
luteinizing hormone
- E2
17-β estradiol
- P4
progesterone
- LN
lymph node
- PP
Peyer’s Patches
References
- 1.King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4(5):480–485. doi: 10.1093/humupd/4.5.480. [DOI] [PubMed] [Google Scholar]
- 2.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6(6):791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 3.King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6(1):28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JS, Petroff MG, Burnett TG. Uterine leukocytes: key players in pregnancy. Semin Cell Dev Biol. 2000;11(2):127–137. doi: 10.1006/scdb.2000.0158. [DOI] [PubMed] [Google Scholar]
- 5.Loke YW, King A. Immunological aspects of human implantation. J Reprod Fertil Suppl. 2000;55:83–90. [PubMed] [Google Scholar]
- 6.Chantakru S, Miller C, Roach LE, et al. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168(1):22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 7.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. 827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 8.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. 209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 9.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 10.Chantakru S, Wang WC, van den Heuvel M, et al. Coordinate Regulation of Lymphocyte-Endothelial Interactions by Pregnancy-Associated Hormones. J Immunol. 2003;171(8):1132–1145. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29(4):1116–1126. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol Reprod. 2002;66(2):333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- 13.Lechleitner S, Gille J, Johnson DR, Petzelbauer P. Interferon enhances tumor necrosis factor-induced vascular cell adhesion molecule 1 (CD106) expression in human endothelial cells by an interferon-related factor 1-dependent pathway. J Exp Med. 1998;187(12):2023–2030. doi: 10.1084/jem.187.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochi H, Masuda J, Gimbrone MA. Hyperosmotic stimuli inhibit VCAM-1 expression in cultured endothelial cells via effects on interferon regulatory factor-1 expression and activity. Eur J Immunol. 2002;32(7):1821–1831. doi: 10.1002/1521-4141(200207)32:7<1821::AID-IMMU1821>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Jesse TL, LaChance R, Iademarco MF, Dean DC. Interferon regulatory factor-2 is a transcriptional activator in muscle where It regulates expression of vascular cell adhesion molecule-1. J Cell Biol. 1998;140(5):1265–1276. doi: 10.1083/jcb.140.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes MK, Wapner RL, Jackson LG, Smith JB. Phenotypic analysis of adhesion molecules in first-trimester decidual tissue from chorion villus samples. Am J Reprod Immunol. 1997;38(6):423–430. doi: 10.1111/j.1600-0897.1997.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruck P, Marzusch K, Kaiserling E, et al. Distribution of cell adhesion molecules in decidua of early human pregnancy. An immunohistochemical study. Lab Invest. 1994;71(1):94–101. [PubMed] [Google Scholar]
- 18.Burrows TD, King A, Loke YW. The role of integrins in adhesion of decidual NK cells to extracellular matrix and decidual stromal cells. Cell Immunol. 1995;166(1):53–61. doi: 10.1006/cimm.1995.0007. [DOI] [PubMed] [Google Scholar]
- 19.Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest. 1994;93(1):17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz KE, Wakefield D. Modulation of endothelial cell expression of ICAM-1, E-selectin, and VCAM-1 by beta-estradiol, progesterone, and dexamethasone. Cell Immunol. 1996;167(1):79–85. doi: 10.1006/cimm.1996.0010. [DOI] [PubMed] [Google Scholar]
- 21.Winkler M, Kemp B, Hauptmann S, Rath W. Parturition: steroids, prostaglandin E2, and expression of adhesion molecules by endothelial cells. Obstet Gynecol. 1997;89(3):398–402. doi: 10.1016/S0029-7844(96)00500-5. [DOI] [PubMed] [Google Scholar]
- 22.Sahagun G, Moore SA, Fabry Z, Schelper RL, Hart MN. Purification of murine endothelial cell cultures by flow cytometry using fluorescein-labeled griffonia simplicifolia agglutinin. Am J Pathol. 1989;134(6):1227–1232. [PMC free article] [PubMed] [Google Scholar]
- 23.Frey M, Packianathan NB, Fehniger TA, et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161(1):400–408. [PubMed] [Google Scholar]
- 24.King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol. 1991;1(3):169–190. doi: 10.1155/1991/83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 26.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143(10):3183–3191. [PubMed] [Google Scholar]
- 27.Ellis TM, Fisher RI. Functional heterogeneity of Leu 19″bright″+ and Leu 19″dim″+ lymphokine-activated killer cells. J Immunol. 1989;142(8):2949–2954. [PubMed] [Google Scholar]
- 28.Slukvin II, Watkins DI, Golos TG. Phenotypic and functional characterization of rhesus monkey decidual lymphocytes: rhesus decidual large granular lymphocytes express CD56 and have cytolytic activity. J Reprod Immunol. 2001;50(1):57–79. doi: 10.1016/s0165-0378(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 29.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198(8):1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jokhi PP, King A, Loke YW. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine. 1997;9(2):126–137. doi: 10.1006/cyto.1996.0146. [DOI] [PubMed] [Google Scholar]
- 31.Saito S, Nishikawa K, Morii T, et al. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol. 1993;5(5):559–563. doi: 10.1093/intimm/5.5.559. [DOI] [PubMed] [Google Scholar]
- 32.Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2003;63(3):683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62(4):959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 34.Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65(1):6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- 35.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17{beta}-Estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104(5):1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 36.DeLoia JA, Stewart-Akers AM, Brekosky J, Kubik CJ. Effects of exogenous estrogen on uterine leukocyte recruitment. Fertil Steril. 2002;77(3):548–554. doi: 10.1016/s0015-0282(01)03062-x. [DOI] [PubMed] [Google Scholar]
- 37.Daikoku N, Kitaya K, Nakayama T, Fushiki S, Honjo H. Expression of macrophage inflammatory protein-3beta in human endometrium throughout the menstrual cycle. Fertil Steril. 2004;81 (Suppl 1):876–881. doi: 10.1016/j.fertnstert.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9(4):189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 39.Kai K, Nasu K, Nakamura S, Fukuda J, Nishida M, Miyakawa I. Expression of interferon-gamma-inducible protein-10 in human endometrial stromal cells. Mol Hum Reprod. 2002;8(2):176–180. doi: 10.1093/molehr/8.2.176. [DOI] [PubMed] [Google Scholar]
- 40.Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab. 2004;89(5):2470–2476. doi: 10.1210/jc.2003-031293. [DOI] [PubMed] [Google Scholar]
- 41.Campbell JJ, Qin S, Unutmaz D, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166(11):6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JA, Bulmer JN, Murdoch AP. Endometrial leucocytes: expression of steroid hormone receptors. J Clin Pathol. 1998;51(2):121–126. doi: 10.1136/jcp.51.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansour I, Reznikoff-Etievant MF, Netter A. No evidence for the expression of the progesterone receptor on peripheral blood lymphocytes during pregnancy. Hum Reprod. 1994;9(8):1546–1549. doi: 10.1093/oxfordjournals.humrep.a138746. [DOI] [PubMed] [Google Scholar]
- 44.Ogle TF, Dai D, George P, Mahesh VB. Stromal cell progesterone and estrogen receptors during proliferation and regression of the decidua basalis in the pregnant rat. Biol Reprod. 1997;57(3):495–506. doi: 10.1095/biolreprod57.3.495. [DOI] [PubMed] [Google Scholar]
- 45.King A, Gardner L, Loke YW. Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod. 1996;11(5):1079–1082. doi: 10.1093/oxfordjournals.humrep.a019300. [DOI] [PubMed] [Google Scholar]
- 46.Curran EM, Berghaus LJ, Vernetti NJ, Saporita AJ, Lubahn DB, Estes DM. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001;214(1):12–20. doi: 10.1006/cimm.2002.1886. [DOI] [PubMed] [Google Scholar]
- 47.Sakazaki H, Ueno H, Nakamuro K. Estrogen receptor alpha in mouse splenic lymphocytes: possible involvement in immunity. Toxicol Lett. 2002;133(2–3):221–229. doi: 10.1016/s0378-4274(02)00203-5. [DOI] [PubMed] [Google Scholar]
- 48.van den Heuvel M, McBey BA, Hahnel AC, Croy BA. An analysis of the uterine lymphocyte-derived hybridoma cell line GWM 1-2 for expression of receptors for estrogen, progesterone and interleukin 2. J Reprod Immunol. 1996;31(1–2):37–50. doi: 10.1016/0165-0378(96)00966-7. [DOI] [PubMed] [Google Scholar]
- 49.Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88(1):440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- 50.Borzychowski AM, Chantakru S, Paffaro VA, et al. Functional analysis of murine uterine natural killer cells genetically devoid of oestrogen receptors. Placenta. 2003;24(4):403–411. doi: 10.1053/plac.2002.0924. [DOI] [PubMed] [Google Scholar]
- 51.Lin J, Lojun S, Lei ZM, Wu WX, Peiner SC, Rao CV. Lymphocytes from pregnant women express human chorionic gonadotropin/luteinizing hormone receptor gene. Mol Cell Endocrinol. 1995;111(1):R13–R17. doi: 10.1016/0303-7207(95)03565-o. [DOI] [PubMed] [Google Scholar]
- 52.Flynn L, Byrne B, Carton J, Kelehan P, O’Herlihy C, O’Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol. 2000;43(4):209–217. doi: 10.1111/j.8755-8920.2000.430405.x. [DOI] [PubMed] [Google Scholar]
- 53.Sulke AN, Jones DB, Wood PJ. Variation in natural killer activity in peripheral blood during the menstrual cycle. Br Med J (Clin Res Ed ) 1985;290(6472):884–886. doi: 10.1136/bmj.290.6472.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sulke AN, Jones DB, Wood PJ. Hormonal modulation of human natural killer cell activity in vitro. J Reprod Immunol. 1985;7(2):105–110. doi: 10.1016/0165-0378(85)90064-6. [DOI] [PubMed] [Google Scholar]
- 55.Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81(2):254–262. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]