Abstract
Background
Interferon-γ–release assays (IGRAs) are alternatives to the tuberculin skin test (TST). A recent meta-analysis showed that IGRAs have high specificity, even among populations that have received bacille Calmette–Guérin (BCG) vaccination. Sensitivity was suboptimal for TST and IGRAs.
Purpose
To incorporate newly reported evidence from 20 studies into an updated meta-analysis on the sensitivity and specificity of IGRAs.
Data Sources
PubMed was searched through 31 March 2008, and citations of all original articles, guidelines, and reviews for studies published in English were reviewed.
Study Selection
Studies that evaluated QuantiFERON-TB Gold, QuantiFERON-TB Gold In-Tube (both from Cellestis, Victoria, Australia), and T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom) or its precommercial ELISpot version, when data on the commercial version were lacking. For assessing sensitivity, the study sample had to have microbiologically confirmed active tuberculosis. For assessing specificity, the sample had to comprise healthy, low-risk individuals without known exposure to tuberculosis. Studies with fewer than 10 participants and those that included only immunocompromised participants were excluded.
Data Extraction
One reviewer abstracted data on participant characteristics, test characteristics, and test performance from 38 studies; these data were double-checked by a second reviewer. The original investigators were contacted for additional information when necessary.
Data Synthesis
A fixed-effects meta-analysis with correction for overdispersion was done to pool data within prespecified subgroups. The pooled sensitivity was 78% (95% CI, 73% to 82%) for QuantiFERON-TB Gold, 70% (CI, 63% to 78%) for QuantiFERON-TB Gold In-Tube, and 90% (CI, 86% to 93%) for T-SPOT.TB. The pooled specificity for both QuantiFERON tests was 99% among non–BCG-vaccinated participants (CI, 98% to 100%) and 96% (CI, 94% to 98%) among BCG-vaccinated participants. The pooled specificity of T-SPOT.TB (including its precommercial ELISpot version) was 93% (CI, 86% to 100%). Tuberculin skin test results were heterogeneous, but specificity in non–BCG-vaccinated participants was consistently high (97% [CI, 95% to 99%]).
Limitations
Most studies were small and had limitations, including no gold standard for diagnosing latent tuberculosis and variable TST methods and cutoff values. Data on the specificity of the commercial T-SPOT.TB assay were limited.
Conclusion
The IGRAs, especially QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube, have excellent specificity that is unaffected by BCG vaccination. Tuberculin skin test specificity is high in non–BCG-vaccinated populations but low and variable in BCG-vaccinated populations. Sensitivity of IGRAs and TST is not consistent across tests and populations, but T-SPOT.TB appears to be more sensitive than both QuantiFERON tests and TST.
The tuberculin skin test (TST) was formerly the only test for detecting latent tuberculosis infection; however, interferon-γ–release assays (IGRAs) have emerged as attractive alternatives. Two IGRAs, QuantiFERON-TB Gold (Cellestis, Carnegie, Australia) and T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom), are now commercially available, and their use is expanding. Although IGRAs are intended for diagnosing latent tuberculosis infection, active tuberculosis is used as a surrogate standard to estimate accuracy in the absence of a gold standard for latent tuberculosis infection.
In a recent meta-analysis (1), Menzies and colleagues showed that IGRAs have high specificity, especially in populations who have received bacille Calmette-Guérin (BCG) vaccination. However, the sensitivity of both TST and IGRAs was suboptimal, and none of these tests could distinguish between latent tuberculosis and active disease. Since the publication of this meta-analysis, the evidence base for IGRAs has rapidly grown with publication of several guidelines and statements (2–6). We present an updated meta-analysis that will provide helpful information for clinicians and for agencies developing updated guidelines.
Methods
Study Selection and Eligibility
Using the same search strategy as that published elsewhere (1), we searched PubMed for new studies through 31 March 2008 that reported data on the sensitivity and specificity of commercial IGRAs. We reviewed citations of all original articles, guidelines, and reviews for studies published in English.
The inclusion criteria for this update were narrower than for the original meta-analysis, which included research, in-house, or commercial versions of QuantiFERON or enzyme-linked immunospot (ELISpot) tests that used early-secreted antigenic target 6, with or without culture filtrate protein 10 and with or without TB7.7 antigens. For the update, we restricted the studies to QuantiFERON-TB Gold (also known as QFT-2G), QuantiFERON-TB Gold In-Tube (also known as QFT-3G) (both from Cellestis, Victoria, Australia), and T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom) or its pre-commercial ELISpot version, when data on the commercial version were lacking. Unlike the original meta-analysis, we excluded studies with fewer than 10 participants, studies that included only immunocompromised populations, and studies that used only early-secreted antigenic target 6.
For studies assessing sensitivity, the study sample had to comprise participants with microbiologically confirmed active tuberculosis, but not include only immunocompromised participants. For studies assessing specificity, the sample had to comprise healthy, low-risk individuals without known exposure to tuberculosis who were from countries with a low tuberculosis incidence rate.
Two independent reviewers performed searches and selected articles meeting the inclusion criteria. One reviewer abstracted data on participant characteristics and test characteristics and performance, and a second reviewer double-checked these data. When necessary, we contacted the original investigators for additional information.
Data Synthesis
For each study, we calculated sensitivity or specificity and 95% CIs and summarized the results in forest plots. To pool estimates across the studies, we did a fixed-effects meta-analysis with correction for overdispersion to account for between-study variability by using MetaDiSc software, version 1.4 (Hospital Ramón y Cajal, Madrid, Spain; www.hrc.es/investigacion/metadisc_en.htm). We evaluated heterogeneity by using the chi-square and I2 tests. Because we found heterogeneity, we performed subgroup analyses; we analyzed each commercial test (and test version, in the case of QuantiFERON) separately and evaluated BCG-vaccinated and nonvaccinated groups separately for specificity studies. When data on the sensitivity and specificity of concurrently done TSTs were reported, we extracted and summarized the data in tables and forest plots.
Role of the Funding Source
The Canadian Institutes of Health Research and the Fonds de la recherche en santé du Québec had no role in the design, conduct, and analysis of the study or the decision to submit the manuscript for publication.
Results
A total of 38 articles (7–44) met our inclusion criteria, 20 of which (comprising 1879 participants) were new articles not included in our previous meta-analysis. Of these, 15 included QuantiFERON-TB Gold or QuantiFERON-TB Gold In-Tube and 9 included T-SPOT.TB.
Eight articles in the original meta-analysis (1) were excluded from this update because they included noncommercial assays (45–48), had fewer than 10 participants (49, 50), included only immunocompromised populations (51), or used only early-secreted antigenic target 6 as the antigen (45).
Appendix Tables 1 through 4 (available at www.annals.org) provide details on the included studies. All studies were cross-sectional. Of the 38 studies, 21 (55%) had some sort of industry involvement or support, such as sponsorship, donation of test kits, participation in advisory boards, involvement of test developers, or ownership of patents.
Sensitivity of Interferon-γ–Release Assays
We identified 22 studies of QuantiFERON tests, comprising 1369 participants (Appendix Table 1), and 13 studies of T-SPOT.TB, comprising 726 participants (Appendix Table 2). Active tuberculosis was confirmed by culture in most cases, and most studies included participants without HIV infection. Three of the QuantiFERON studies were from countries with a high rate of tuberculosis incidence, whereas none of the T-SPOT.TB studies was from a high-incidence country. Almost all of the sensitivity studies included only adults.
Appendix Table 1.
Sensitivity of QuantiFERON-TB Gold (QFT) among Patients with Active Tuberculosis (TB)
| Study, Year (Reference) |
Industry Supported?* |
Sample | Setting | Patients with Active (Culture-Confirmed) TB, n (%) | HIV-Positive Patients, % |
QFT Result, n (%) | Sensitivity of TST | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Indeterminate† | Cutoff Value | Patients, n/n (%) | ||||||
| Mori et al., 2004 (7) | Yes | Predominantly adults |
Japan | 119 (100) | 0 | 105 (88) | 13 (11) | 1 (1) | 5 mm | 50/76 (66) |
| Ferrara et al., 2005 (8) | No | Predominantly adults |
Italy | 11 (45) | 0 | 6 (55) | 3 (27) | 2 (18) | Risk stratified | 3/9 (33) |
| Ravn et al., 2005 (9) | Yes | Predominantly adults |
Denmark | 48 (56) | 6 | 41 (85) | 7 (15) | 0 (0) | NR | NR |
| Kang et al., 2005 (10) | Yes | Adults | South Korea | 58 (100) | 0 | 44 (76) | 10 (17) | 4 (7) | 10 mm | 42/54 (78) |
| Lee et al., 2006 (11) | Yes | Predominantly adults |
South Korea | 87 (63) | 0 | 61 (70) | 18 (21) | 8 (9) | 10 mm | 58/87 (67) |
| Ferrara et al., 2006 (12) | Yes | Predominantly adults |
Italy | 24 (NR) | NR | 17 (71) | 6 (25) | 1 (4) | Risk stratified | 14/20 (70) |
| Goletti et al., 2006 (13) | Yes | Predominantly adults |
Italy | 23 (100) | 0 | 19 (83) | 4 (17) | 0 (0) | NR | NR |
| Dewan et al., 2007 (14) | No | Adults | United States | 45 (82) | 7 | 25 (56) | 17 (38) | 3 (7) | 5 mm | 21/24 (88) |
| Kobashi et al., 2006 (15) | No | Predominantly adults |
Japan | 50 (100) | 0 | 43 (86) | 2 (4) | 5 (10) | NR | 32/50 (64) |
| Mazurek et al., 2007 (16) | Yes | Adults | United States | 96 (72) | 11 | 62 (65) | 24 (25) | 10 (10) | 5 mm | 74/96 (77) |
| Kang et al., 2007 (17) | Yes | Adults | South Korea | 67 (100) | 0 | 58 (87) | 7 (10) | 2 (3) | 10 mm | 45/67 (67) |
| Bua et al., 2007 (18) | NR | Adults | Italy | 30 (NR) | 7 | 23 (77) | 2 (7) | 5 (17) | NR | 24/30 (80) |
| Soysal et al., 2008 (19) | NR | Predominantly adults |
Turkey | 100 (100) | 0 | 77 (77) | 22 (22) | 1 (1) | 5 mm | 80/99 (81) |
| Tsiouris et al., 2006 (20) | Yes | Adults | South Africa | 154 (100) | 17 | 100 (65) | 31 (20) | 23 (15) | Risk stratified | 131/146 (90) |
| Pai et al., 2007 (21) | No | Adults | India | 60 (97) | 5 | 44 (73) | 16 (27) | 0 (0) | NR | NR |
| Adetifa et al., 2007 (22) | Yes | Adults | The Gambia | 75 (100) | 9 | 48 (64) | 27 (36) | 0 (0) | NR | NR |
| Domínguez et al., 2008 (23) | Yes | Adults and children‡ |
Spain | 42 (NR) | 0 | 33 (79) | 9 (21) | 0 (0) | 5 mm | 40/42 (95) |
| Palazzo et al., 2008 (24) | NR | Predominantly adults |
Italy | 17 (100) | 0 | 14 (82) | 3 (18) | 0 (0) | NR | 9/12 (75) |
| Detjen et al., 2007 (25) | No | Children | Germany | 28 (100) | 0 | 26 (93) | 2 (7) | 0 (0) | 10 mm | 28/28 (100) |
| Kobashi et al., 2008 (26) | NR | Adults (including elderly) |
Japan | 130 (100) | 0 | 110 (85) | 6 (5) | 14 (10) | 5 mm | 78/130 (60) |
| Nishimura et al., 2008 (27) | NR | Adults | Japan | 77 (80) | 0 | 59 (77) | 18 (23) | 0 (0) | NR | NR |
| Kobashi et al., 2008 (28) | NR | Adults | Japan | 28 (100) | 0 | 22 (79) | 2 (7) | 4 (14) | 5 mm | 16/28 (57) |
NR = not reported; TST = tuberculin skin test.
“Industry support” refers to any industry involvement or support (e.g., sponsorship, donation of test kits, participation in advisory boards, and involvement of test developers or ownership of patents).
Indeterminate results were not excluded in calculating sensitivity estimates.
Children formed 27% of the sample.
Appendix Table 2.
Sensitivity of T-SPOT.TB among Patients with Active Tuberculosis (TB)
| Study, Year (Reference) |
Industry Supported?* |
Sample | Setting | Patients with Active (Culture-Confirmed) TB, n (%) | HIV-Positive Patients, % | T-SPOT.TB Result, n (%) | Sensitivity of TST | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Indeterminate† | Cutoff Value | Patients, n/n (%) | ||||||
| Meier et al., 2005 (29) | Yes | Adults | Germany | 73 (86) | 2 | 70 (97) | 2 (3) | 1 (1) | NR | 40/45 (89) |
| Lee et al., 2006 (11) | Yes | Predominantly adults |
South Korea | 87 (63) | 0 | 83 (95) | 4 (5) | 0 (0) | 10 mm | 58/87 (67) |
| Goletti et al., 2006 (13) | Yes | Predominantly adults |
Italy | 23 (100) | 0 | 21 (91) | 2 (9) | 0 (0) | NR | NR |
| Ferrara et al., 2006 (12) | No | Predominantly adults |
Italy | 24 (NR) | NR | 20 (83) | 4 (17) | 0 (0) | Risk stratified | 14/20 (70) |
| Jafari et al., 2006 (30) | Yes | Predominantly adults |
Germany | 12 (67) | NR | 12 (100) | 0 (0) | 0 (0) | 10 mm | 10/12 (83) |
| Domínguez et al., 2008 (23) | Yes | Adults and children‡ |
Spain | 42 (NR) | 0 | 36 (86) | 3 (7) | 3 (7) | 5 mm | 40/42 (95) |
| Kang et al., 2007 (17) | Yes | Adults | South Korea | 64 (100) | 0 | 59 (92) | 5 (8) | 0 (0) | 10 mm | 45/67 (67) |
| Wang et al., 2007 (31) | NR | Predominantly adults |
Taiwan | 39 (95) | 7 | 34 (87) | 5 (13) | 0 (0) | NR | NR |
| Janssens et al., 2007 (32) | No | Adults | Switzerland | 58 (100) | 0 | 57 (98) | 1 (2) | 0 (0) | NR | NR |
| Detjen et al., 2007 (25) | No | Children | Germany | 28 (100) | 0 | 26 (93) | 2 (7) | 0 (0) | 10 mm | 28/28 (100) |
| Ozekinci et al., 2007 (33) | No | Adults | Turkey | 28 (NR) | NR | 26 (93) | 2 (7) | 0 (0) | 10 mm and 15 mm | 23/28 (82) |
| Soysal et al., 2008 (19) | NR | Predominantly adults |
Turkey | 100 (100) | 0 | 80 (80) | 16 (16) | 4 (4) | 5 mm | 80/99 (81) |
| Dosanjh et al., 2008 (34) | Yes | Adults | United Kingdom | 148 (100) | 5 | 128 (86) | 20 (14) | 0 (0) | 10 mm | 98/119 (82) |
NR = not reported; TST = tuberculin skin test.
“Industry support” refers to any industry involvement or support (e.g., sponsorship, donation of test kits, participation in advisory boards, and involvement of test developers or ownership of patents).
Indeterminate results were not excluded in calculating sensitivity estimates.
Children formed 27% of the sample.
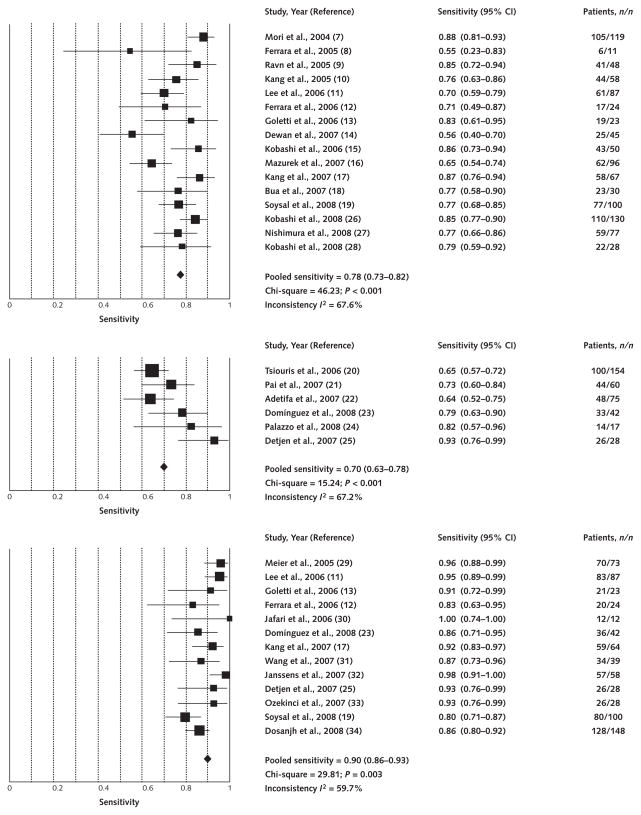
Figure 1 shows the forest plot and pooled sensitivity estimates. The pooled sensitivity of all 22 QuantiFERON studies was 76% (95% CI, 72% to 80%) (plot not shown). Pooled sensitivity was 78% (CI, 73% to 82%) for QuantiFERON-TB Gold, 70% (CI, 63% to 78%) for QuantiFERON-TB Gold In-Tube, and 90% (CI, 86% to 93%) for T-SPOT.TB (Figure 1). Although we found no obvious differences in sensitivity between QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube, the non-overlapping CIs suggest that the pooled sensitivity of T-SPOT.TB was higher than that of either QuantiFERON-TB test.
Figure 1. Forest plot of studies estimating sensitivity of interferon-γ–release assays in patients with active tuberculosis as a surrogate for latent tuberculous infection.
Point estimates for sensitivity and 95% CIs are shown along with pooled estimates. Top. QuantiFERON-TB Gold (16 studies). Middle. QuantiFERON-TB Gold In-Tube (6 studies). Bottom. T-SPOT.TB (13 studies).
Seven studies (Appendix Table 5) reported head-to-head comparisons of T-SPOT.TB and QuantiFERON sensitivity. In 6 of those studies, T-SPOT.TB had a higher sensitivity than QuantiFERON-TB Gold, with difference ranging from 3% to 25%. One study reported identical sensitivity estimates for both assays. Studies that used QuantiFERON-TB Gold showed lower sensitivity relative to T-SPOT.TB than did studies that used QuantiFERON-TB Gold In-Tube.
Appendix Table 5.
Head-to-Head Comparisons of Sensitivity of QuantiFERON-TB (QFT) Gold versus T-SPOT.TB among Patients with Active Tuberculosis (TB)
| Study, Year (Reference) |
Industry Supported?* |
Sample | Setting | Patients with Active (Culture-Confirmed) TB, n (%) | Sensitivity† | Difference between T-SPOT.TB and QFT Gold Sensitivity, percentage points | |
|---|---|---|---|---|---|---|---|
| Patients with Positive QFT Gold Result, n (%) | Patients with Positive T-SPOT.TB Result, n (%) | ||||||
| Lee et al., 2006 (11) | Yes | Predominantly adults | South Korea | 87 (63) | 61 (70)‡ | 83 (95) | 25 |
| Ferrara et al., 2006 (12) | Yes | Predominantly adults | Italy | 24 (NR) | 17 (71)‡ | 20 (83) | 12 |
| Goletti et al., 2006 (13) | Yes | Predominantly adults | Italy | 23 (100) | 19 (83)‡ | 21 (91) | 8 |
| Kang et al., 2007 (17) | Yes | Adults | South Korea | 67 (100) | 58 (87)‡ | 59 (92) | 5 |
| Soysal et al., 2008 (19) | NR | Predominantly adults | Turkey | 100 (100) | 77 (77)‡ | 80 (80) | 3 |
| Domínguez et al., 2008 (23) | Yes | Adults and children§ | Spain | 42 (NR) | 33 (79)|| | 36 (86) | 7 |
| Detjen et al., 2007 (25) | No | Children | Germany | 28 (100) | 26 (93)|| | 26 (93) | 0 |
NR = not reported.
“Industry support” refers to any industry involvement or support (e.g., sponsorship, donation of test kits, participation in advisory boards, and involvement of test developers or ownership of patents).
Indeterminate results were not excluded in calculating sensitivity estimates.
QFT Gold.
Children formed 27% of the sample.
QFT Gold In-Tube.
Specificity of Interferon-γ–Release Assays
We identified 16 studies of QuantiFERON tests, 8 of BCG-vaccinated and 8 of non–BCG-vaccinated samples, comprising 1624 participants (Appendix Table 3). None of the specificity studies was from a country with a high rate of tuberculosis incidence. Almost all of the specificity studies included only adults. The BCG vaccination policies in the study countries varied; for example, Japan and South Korea have a policy of repeated BCG vaccinations, whereas the United States and the Netherlands do not recommend BCG vaccination.
Appendix Table 3.
Specificity of QuantiFERON-TB Gold (QFT) in Bacille Calmette–Guérin (BCG)–Vaccinated and Non–BCG-Vaccinated Patients with an Expected Low Prevalence of Tuberculous Infection*
| Study, Year (Reference) |
Industry Supported?† |
Sample | Setting | Not BCG Vaccinated or Predominantly Nonvaccinated | BCG Vaccinated or Predominantly Vaccinated | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | QFT | TST | Patients, n | QFT | TST | ||||||||
| False- Positive Result, n |
Specificity, % | False- Positive Result, n |
Specificity, % | False- Positive Result, n |
Specificity, % | False- Positive Result, n |
Specificity, % | ||||||
| Brock et al., 2001 (35) | Yes | Predominantly adults |
Denmark | 15 | 0 | 100 | 0 | 100 | 19 | 2 | 89 | 9 | 53 |
| Mori et al., 2004 (7) | Yes | Predominantly adults |
Japan | – | – | – | – | – | 213 | 4 | 98.1 | 73/113 | 35 |
| Ravn et al., 2005 (9) | Yes | Predominantly adults |
Denmark | – | – | – | – | – | 39 | 1 | 97 | NR | NR |
| Brock et al., 2004 (36) | Yes | Adults and children |
Denmark | 40 | 2 | 95 | 3 | 93 | 32 | 2 | 94 | NR | NR |
| Kang et al., 2005 (10)‡ | Yes | Adults only | South Korea | – | – | – | – | – | 99 | 4 | 96 | 50 | 49§ |
| Taggart et al., 2006 (37) | NR | Adults only | United States | 81 | 0 | 100 | 3 | 96 | – | – | – | – | – |
| Lee et al., 2006 (11)‡ | Yes | Predominantly adults |
South Korea | – | – | – | – | – | 131 | 11 | 91.6 | 28 | 78.6§ |
| Kobashi et al., 2006 (15) | No | Predominantly adults |
Japan | – | – | – | – | – | 50 | 3 | 94 | 18 | 64 |
| Palazzo et al., 2008 (24) | NR | Predominantly adults | Italy | 13 | 0 | 100 | – | – | – | – | – | – | – |
| Bua et al., 2007 (18) | NR | Adults only | Italy | 16 | 0 | 100 | – | – | – | – | – | – | – |
| Mazurek et al., 2007 (38) | Yes | Adults only | United States | 544 | 1 | 99.8 | 9 | 98.4§ | – | – | – | – | – |
| Franken et al., 2007 (39) | NR | Adults only | The Netherlands | 171 | 5 | 97.1 | 9/145 | 93.8§ | – | – | – | – | – |
| Soborg et al., 2007 (40) | Yes | Adults only | The Netherlands | – | – | – | – | – | 139 | 2 | 98.6 | 47 | 66.2|| |
| Detjen et al., 2007 (25) | No | Children only | Germany | 22 | 0 | 100 | 0 | 100§ | – | – | – | – | – |
NR = not reported; TST = tuberculin skin test.
Participants were healthy volunteers from the general population, students, or recruits with no history of exposure to tuberculosis.
“Industry support” refers to any industry involvement or support (e.g., sponsorship, donation of test kits, participation in advisory boards, and involvement of test developers or ownership of patents).
Because South Korea is an intermediate-incidence country, some of the low-risk participants may have been latently infected.
Cutoff value ≥10 mm.
Cutoff value ≥12 mm.
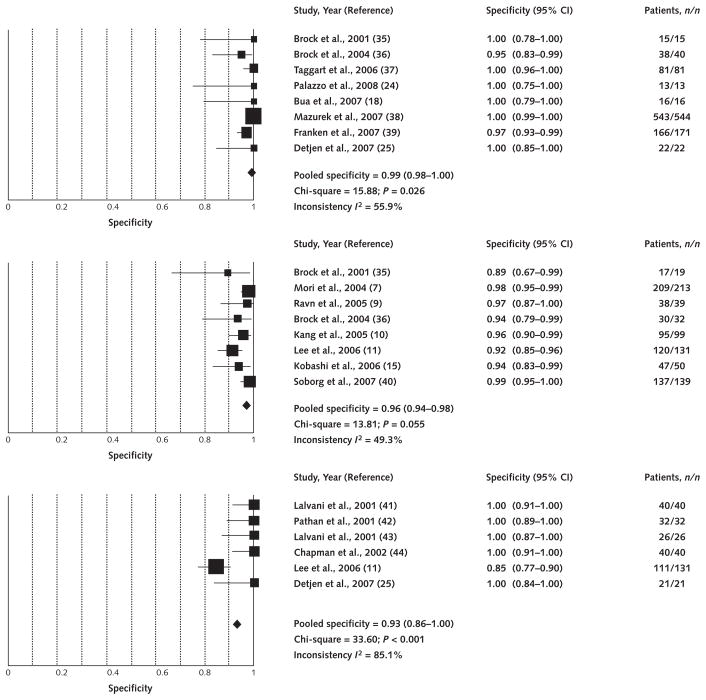
We identified 2 studies that used the commercial T-SPOT.TB assay and 4 studies that used the precommercial ELISpot version, with a combined total of 290 participants. Appendix Table 4 summarizes these 6 studies. Figure 2 presents the forest plot and pooled estimates. The pooled specificity was 98% (CI, 96% to 99%) for all QuantiFERON studies, 99% (CI, 98% to 100%) for QuantiFERON among non–BCG-vaccinated populations, and 96% (CI, 94% to 98%) for QuantiFERON among BCG-vaccinated populations (Figure 2). The pooled specificity of T-SPOT.TB/ELISpot was 93% (CI, 86% to 100%) (Figure 2). All but 1 T-SPOT.TB study included BCG-vaccinated participants. When only the 2 commercial T-SPOT.TB studies were pooled, the specificity was 87% (CI, 80% to 92%).
Appendix Table 4.
Specificity of Enzyme-Linked Immunospot (ELISpot) and T-SPOT.TB in Bacille Calmette–Guérin (BCG)–Vaccinated and Non–BCG-Vaccinated Participants with an Expected Low Prevalence of Tuberculous Infection*
| Study, Year (Reference) |
Industry Supported?† |
Sample | Setting | Test (Antigens) | Not BCG-Vaccinated or Predominantly Nonvaccinated | BCG-Vaccinated or Predominantly Vaccinated | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | ELISpot/T-SPOT.TB | TST | No | ELISpot/T-SPOT.TB | TST | |||||||||
| False- Positive Result, n |
Specificity, % | False- Positive Result, n |
Specificity, % | False- Positive Result, n |
Specificity, % | False- Positive Result, n |
Specificity, % | |||||||
| Lalvani et al., 2001 (41) | Yes | Adults | United Kingdom |
ELISpot (ESAT-6) | – | – | – | – | – | 40 | 0 | 100 | NR | NR |
| Pathan et al., 2001 (42) | Yes | Adults | United Kingdom |
ELISpot (ESAT-6) | – | – | – | – | – | 32 | 0 | 100 | NR | NR |
| Lalvani et al., 2001 (43) | Yes | Adults | United Kingdom |
ELISpot (ESAT-6) | – | – | – | – | – | 26 | 0 | 100 | NR | NR |
| Chapman et al., 2002 (44) | Yes | Adults | United Kingdom |
ELISpot (ESAT-6/CFP-10) | – | – | – | – | – | 40 | 0 | 100 | NR | NR |
| Lee et al., 2006 (11)‡ | Yes | Adolescents | South Korea | T-SPOT.TB (ESAT-6/CFP-10) | – | – | – | – | – | 131 | 20 | 84.7 | NR | 78.6|| |
| Detjen et al., 2007 (25) | No | Children only |
Germany | T-SPOT.TB (ESAT-6/CFP-10) | 21 | 0 | 100 | 0 | 100|| | – | – | – | – | – |
CFP-10 = culture filtrate protein 10; ESAT-6 = early-secreted antigenic target 6; NR = not reported; TST = tuberculin skin test.
Participants were healthy volunteers from the general population, students, or recruits with no history of exposure to tuberculosis.
“Industry support” refers to any industry involvement or support (e.g., sponsorship, donation of test kits, participation in advisory boards, and involvement of test developers or ownership of patents).
Because South Korea is an intermediate-incidence country, some of the low-risk participants may have been latently infected.
Cutoff value ≥10 mm.
Figure 2. Forest plot of studies estimating specificity of interferon-γ–release assays in populations at very low risk for latent tuberculous infection.
Point estimates for specificity and 95% CIs are shown along with pooled estimates. Top. QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube (braille Calmette–Guérin [BCG] nonvaccinated; 8 studies). Middle. QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube (BCG vaccinated; 8 studies). Bottom. T-SPOT.TB (predominantly BCG vaccinated; 6 studies).
Sensitivity and Specificity of Tuberculin Skin Test
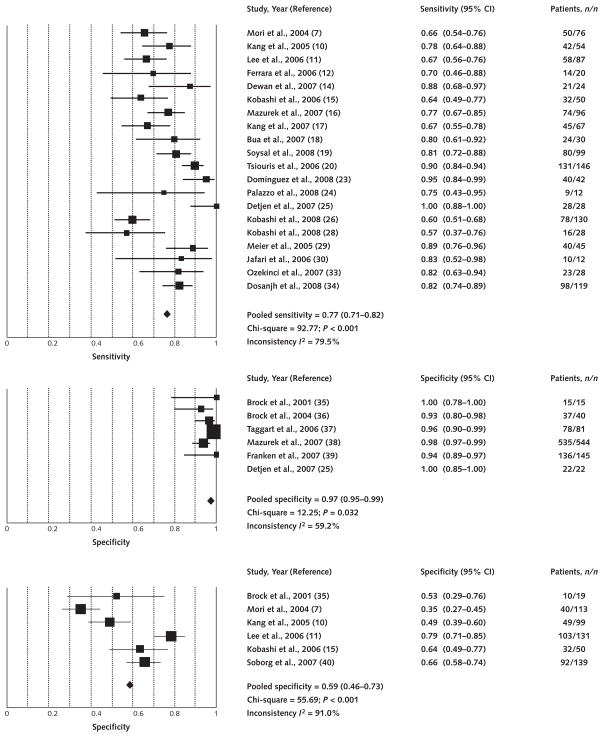
Figure 3 shows the forest plot of TST sensitivity and specificity estimates. Sensitivity estimates (20 studies with 1193 participants) were heterogeneous, with a pooled estimate of 77% (CI, 71% to 82%). Specificity in non–BCG-vaccinated populations (6 studies with 847 participants) was consistently high, with a pooled estimate of 97% (CI, 95% to 99%). Specificity in BCG-vaccinated populations (6 studies with 551 participants) was low and highly heterogeneous.
Figure 3. Forest plot of studies estimating sensitivity and specificity of the tuberculin skin test.
Point estimates for sensitivity and specificity and 95% CIs are shown along with pooled estimates. Top. Sensitivity (20 studies). Middle. Specificity in non–bacille Calmette–Guérin-vaccinated populations (6 studies). Bottom. Specificity in bacille Calmette–Guérin-vaccinated populations (6 studies).
Discussion
This updated meta-analysis includes the results of 20 new studies and synthesizes a substantial body of new IGRA literature. Our results confirm that IGRAs have excellent specificity that is unaffected by BCG vaccination. In particular, we found that both QuantiFERON tests have excellent specificity on the basis of a large number of consistent studies. In contrast, data on the specificity of the commercial T-SPOT.TB assay are limited. Further research is needed to better define the specificity of the T-SPOT.TB assay and to assess the trade-offs between sensitivity and specificity.
Our results suggest that TST specificity is high in non–BCG-vaccinated populations but low and highly variable in BCG-vaccinated populations. Overall, the high specificity of IGRAs, especially QuantiFERON, might prove to be useful in BCG-vaccinated individuals, particularly in settings where TST specificity is compromised by BCG vaccination after infancy or by multiple BCG vaccinations (52). Specificity estimates for IGRAs were highly consistent across studies, which may be because almost all specificity studies were conducted in settings with a low rate of tuberculosis incidence and test methods and cutoff values are better standardized than for the TST.
The sensitivity of IGRAs and the TST was not consistent across the tests and samples. This may have been because of the spectrum (case-mix) and severity of tuberculosis cases included in various studies, the varying background rates of tuberculosis and HIV across countries, or the inherent differences among the various test formats. For example, 3 studies of QuantiFERON-TB Gold InTube in countries with a high rate of tuberculosis incidence showed lower sensitivity than studies in countries with a low rate of incidence. Persons with tuberculosis in high-incidence countries often have advanced disease and are likely to be infected with HIV or malnourished. Anergy due to advanced disease, malnutrition, and HIV-associated immune suppression may lower the sensitivity of IGRAs.
The pooled T-SPOT.TB sensitivity was higher than that of the QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube assays. This finding should be carefully interpreted, however, because it is not based on direct head-to-head comparison studies. Seven studies (5 that used QuantiFERON-TB Gold and 2 that used QuantiFERON-TB Gold In-Tube) that did provide head-to-head comparisons showed higher sensitivity for T-SPOT.TB, although the difference ranged from 0% to 25% (median, 7%). Tuberculin skin test sensitivity results are hard to interpret because of the heterogeneity; however, the pooled estimate of 77% suggests that TST is probably as sensitive as QuantiFERON but less sensitive than T-SPOT.TB.
The higher sensitivity of T-SPOT.TB may be clinically useful in evaluating high-risk populations with immunosuppressive conditions. However, the diagnosis of active tuberculosis rests on microbiological detection of Mycobacterium tuberculosis. Immune-based tests, such as IGRAs and TST, do not directly detect M. tuberculosis; they merely indicate a cellular immune response to recent or remote sensitization with M. tuberculosis. In settings with high tuberculosis incidence, in which latent infection is widespread, a positive IGRA result may not necessarily indicate active tuberculosis (53, 54). Furthermore, a negative IGRA result would not conclusively rule out active disease in an individual suspected to have tuberculosis (53, 54); this also applies to the TST.
Our meta-analysis has limitations. Most studies were small and had limitations, including no gold standard for diagnosing latent tuberculosis infection and variable TST methods, cutoff values, and results. Our meta-analysis of TST accuracy did not include all the available literature on TST; we included only TST studies that also included a comparison of IGRAs. Thus, several older studies on TST were not eligible for inclusion.
Although sensitivity and specificity are useful and easily measured test characteristics, they have limitations (55). Given the lack of a gold standard, sensitivity and specificity for active tuberculosis may not translate to accuracy for latent tuberculosis (which cannot be directly estimated). Also, studies that reported sensitivity did not always report specificity and vice versa. Thus, the trade-offs between these test characteristics are not easy to interpret. Furthermore, the sensitivity and specificity of single tests do not provide information on their incremental or added value.
Despite the substantial body of literature on IGRAs, several questions remain unanswered (56), including the prognostic ability of these tests to accurately identify individuals with latent infection who are at the highest risk for progressing to active tuberculosis and therefore most likely to benefit from preventive therapy (57–59). The IGRAs appear to have dynamic characteristics that increase the likelihood of conversions and reversions over time (60). Data on high-risk populations, such as children and immunocompromised persons, are limited. Ongoing studies should resolve these issues within the next few years and inform evidence-based guidelines on how to implement IGRAs in clinical practice.
Acknowledgments
Grant Support: By the Canadian Institutes of Health Research (grant MOP-81362). Dr. Pai is a recipient of a New Investigator Career Award from the Canadian Institutes of Health Research. Dr. Menzies is a recipient of a career award from the Fonds de la recherche en santé du Québec.
Footnotes
Potential Financial Conflicts of Interest: Other: Dr. Pai serves as an external consultant for the Foundation for Innovative New Diagnostics, Geneva, a nonprofit agency that collaborates with several industry partners, including Cellestis, Carnegie, Australia, for the development of new diagnostics for neglected infectious diseases. No industry partner was involved in the preparation of this manuscript.
References
- 1.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Tuberculosis Committee. Interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS) Can Commun Dis Rep. 2007;33:1–18. [PubMed] [Google Scholar]
- 3.HPA Tuberculosis Programme Board. (Health Protection Agency) Health Protection Agency Position Statement on the use of Interferon Gamma Release Assay (IGRA) tests for tuberculosis (TB): Draft for Consultation. Oct, 2007. [Google Scholar]
- 4.Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC) Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–55. [PubMed] [Google Scholar]
- 5.National Tuberculosis Advisory Committee. Position statement on interferon-gamma release immunoassays in the detection of latent tuberculosis infection, October 2007. Commun Dis Intell. 2007;31:404–5. doi: 10.33321/cdi.2007.31.44. [DOI] [PubMed] [Google Scholar]
- 6.KNCV/EuroTB Workshop. Use of gamma-interferon assays in low- and medium-prevalence countries in Europe: a consensus statement of a Wolfheze Workshop organised by KNCV/EuroTB, Vilnius Sept 2006. Euro Surveill. 2007;12:E070726.2. doi: 10.2807/esw.12.30.03242-en. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005;172:631–5. doi: 10.1164/rccm.200502-196OC. [DOI] [PubMed] [Google Scholar]
- 9.Ravn P, Munk ME, Andersen AB, Lundgren B, Lundgren JD, Nielsen LN, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005;12:491–6. doi: 10.1128/CDLI.12.4.491-496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–61. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006;28:24–30. doi: 10.1183/09031936.06.00016906. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara G, Losi M, D’Amico R, Roversi P, Piro R, Meacci M, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet. 2006;367:1328–34. doi: 10.1016/S0140-6736(06)68579-6. [DOI] [PubMed] [Google Scholar]
- 13.Goletti D, Carrara S, Vincenti D, Saltini C, Rizzi EB, Schininà V, et al. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin Microbiol Infect. 2006;12:544–50. doi: 10.1111/j.1469-0691.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 14.Dewan PK, Grinsdale J, Kawamura LM. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis. 2007;44:69–73. doi: 10.1086/509928. [DOI] [PubMed] [Google Scholar]
- 15.Kobashi Y, Obase Y, Fukuda M, Yoshida K, Miyashita N, Oka M. Clinical reevaluation of the QuantiFERON TB-2G test as a diagnostic method for differentiating active tuberculosis from nontuberculous mycobacteriosis. Clin Infect Dis. 2006;43:1540–6. doi: 10.1086/509327. [DOI] [PubMed] [Google Scholar]
- 16.Mazurek GH, Weis SE, Moonan PK, Daley CL, Bernardo J, Lardizabal AA, et al. Prospective comparison of the tuberculin skin test and 2 whole-blood interferon-gamma release assays in persons with suspected tuberculosis. Clin Infect Dis. 2007;45:837–45. doi: 10.1086/521107. [DOI] [PubMed] [Google Scholar]
- 17.Kang YA, Lee HW, Hwang SS, Um SW, Han SK, Shim YS, et al. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007;132:959–65. doi: 10.1378/chest.06-2805. [DOI] [PubMed] [Google Scholar]
- 18.Bua A, Molicotti P, Delogu G, Pirina P, Mura MS, Madeddu G, et al. QuantiFERON TB Gold: a new method for latent tuberculosis infection. New Microbiol. 2007;30:477–80. [PubMed] [Google Scholar]
- 19.Soysal A, Torun T, Efe S, Gencer H, Tahaoglu K, Bakir M. Evaluation of cut-off values of interferon-gamma-based assays in the diagnosis of M. tuberculosis infection. Int J Tuberc Lung Dis. 2008;12:50–6. [PubMed] [Google Scholar]
- 20.Tsiouris SJ, Coetzee D, Toro PL, Austin J, Stein Z, El-Sadr W. Sensitivity analysis and potential uses of a novel gamma interferon release assay for diagnosis of tuberculosis. J Clin Microbiol. 2006;44:2844–50. doi: 10.1128/JCM.02411-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai M, Joshi R, Bandyopadhyay M, Narang P, Dogra S, Taksande B, et al. Sensitivity of a whole-blood interferon-gamma assay among patients with pulmonary tuberculosis and variations in T-cell responses during anti-tuberculosis treatment. Infection. 2007;35:98–103. doi: 10.1007/s15010-007-6114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adetifa IM, Lugos MD, Hammond A, Jeffries D, Donkor S, Adegbola RA, et al. Comparison of two interferon gamma release assays in the diagnosis of Mycobacterium tuberculosis infection and disease in The Gambia. BMC Infect Dis. 2007;7:122. doi: 10.1186/1471-2334-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domínguez J, Ruiz-Manzano J, De Souza-Galvão M, Latorre I, Milà C, Blanco S, et al. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin Vaccine Immunol. 2008;15:168–71. doi: 10.1128/CVI.00364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palazzo R, Spensieri F, Massari M, Fedele G, Frasca L, Carrara S, et al. Use of whole-blood samples in in-house bulk and single-cell antigen-specific gamma interferon assays for surveillance of Mycobacterium tuberculosis infections. Clin Vaccine Immunol. 2008;15:327–37. doi: 10.1128/CVI.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45:322–8. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- 26.Kobashi Y, Mouri K, Yagi S, Obase Y, Miyashita N, Okimoto N, et al. Clinical utility of the QuantiFERON TB-2G test for elderly patients with active tuberculosis. Chest. 2008 doi: 10.1378/chest.07-1995. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura T, Hasegawa N, Mori M, Takebayashi T, Harada N, Higuchi K, et al. Accuracy of an interferon-gamma release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:269–74. [PubMed] [Google Scholar]
- 28.Kobashi Y, Mouri K, Yagi S, Obase Y, Fukuda M, Miyashita N, et al. Usefulness of the QuantiFERON TB-2G test for the differential diagnosis of pulmonary tuberculosis. Intern Med. 2008;47:237–43. doi: 10.2169/internalmedicine.47.0389. [DOI] [PubMed] [Google Scholar]
- 29.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24:529–36. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 30.Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med. 2006;174:1048–54. doi: 10.1164/rccm.200604-465OC. [DOI] [PubMed] [Google Scholar]
- 31.Wang JY, Chou CH, Lee LN, Hsu HL, Jan IS, Hsueh PR, et al. Diagnosis of tuberculosis by an enzyme-linked immunospot assay for interferon-gamma. Emerg Infect Dis. 2007;13:553–8. doi: 10.3201/eid1304.051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssens JP, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur Respir J. 2007;30:722–8. doi: 10.1183/09031936.00028507. [DOI] [PubMed] [Google Scholar]
- 33.Ozekinci T, Ozbek E, Celik Y. Comparison of tuberculin skin test and a specific T-cell-based test, T-Spot.TB, for the diagnosis of latent tuberculosis infection. J Int Med Res. 2007;35:696–703. doi: 10.1177/147323000703500515. [DOI] [PubMed] [Google Scholar]
- 34.Dosanjh DP, Hinks TS, Innes JA, Deeks JJ, Pasvol G, Hackforth S, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med. 2008;148:325–36. doi: 10.7326/0003-4819-148-5-200803040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brock I, Munk ME, Kok-Jensen A, Andersen P. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis. 2001;5:462–7. [PubMed] [Google Scholar]
- 36.Brock I, Weldingh K, Leyten EM, Arend SM, Ravn P, Andersen P. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J Clin Microbiol. 2004;42:2379–87. doi: 10.1128/JCM.42.6.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taggart EW, Hill HR, Ruegner RG, Litwin CM. Evaluation of an in vitro assay for interferon gamma production in response to the Mycobacterium tuberculosis-synthesized peptide antigens ESAT-6 and CFP-10 and the PPD skin test. Am J Clin Pathol. 2006;125:467–73. [PubMed] [Google Scholar]
- 38.Mazurek GH, Zajdowicz MJ, Hankinson AL, Costigan DJ, Toney SR, Rothel JS, et al. Detection of Mycobacterium tuberculosis infection in United States Navy recruits using the tuberculin skin test or whole-blood interferon-gamma release assays. Clin Infect Dis. 2007;45:826–36. doi: 10.1086/521106. [DOI] [PubMed] [Google Scholar]
- 39.Franken WP, Timmermans JF, Prins C, Slootman EJ, Dreverman J, Bruins H, et al. Comparison of Mantoux and QuantiFERON TB Gold tests for diagnosis of latent tuberculosis infection in Army personnel. Clin Vaccine Immunol. 2007;14:477–80. doi: 10.1128/CVI.00463-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soborg B, Andersen AB, Larsen HK, Weldingh K, Andersen P, Kofoed K, et al. Detecting a low prevalence of latent tuberculosis among health care workers in Denmark detected by M. tuberculosis specific IFN-gamma whole-blood test. Scand J Infect Dis. 2007;39:554–9. doi: 10.1080/00365540601148483. [DOI] [PubMed] [Google Scholar]
- 41.Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 42.Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–25. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 43.Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–8. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 44.Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002;16:2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 45.Johnson PD, Stuart RL, Grayson ML, Olden D, Clancy A, Ravn P, et al. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin Diagn Lab Immunol. 1999;6:934–7. doi: 10.1128/cdli.6.6.934-937.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schölvinck E, Wilkinson KA, Whelan AO, Martineau AR, Levin M, Wilkinson RJ. Gamma interferon-based immunodiagnosis of tuberculosis: comparison between whole-blood and enzyme-linked immunospot methods. J Clin Microbiol. 2004;42:829–31. doi: 10.1128/JCM.42.2.829-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicol MP, Pienaar D, Wood K, Eley B, Wilkinson RJ, Henderson H, et al. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis. 2005;40:1301–8. doi: 10.1086/429245. [DOI] [PubMed] [Google Scholar]
- 48.Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah D, Lugos MD, et al. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006;6:66. doi: 10.1186/1471-2334-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dogra S, Narang P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM, Jr, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect. 2007;54:267–76. doi: 10.1016/j.jinf.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. 2006;61:616–20. doi: 10.1136/thx.2005.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 52.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–204. [PubMed] [Google Scholar]
- 53.Pai M, Menzies D. Interferon-gamma release assays: what is their role in the diagnosis of active tuberculosis? [Editorial] Clin Infect Dis. 2007;44:74–7. doi: 10.1086/509927. [DOI] [PubMed] [Google Scholar]
- 54.Menzies D. Using tests for latent tuberculous infection to diagnose active tuberculosis: can we eat our cake and have it too? [Editorial] Ann Intern Med. 2008;148:398–9. doi: 10.7326/0003-4819-148-5-200803040-00011. [DOI] [PubMed] [Google Scholar]
- 55.Kunst H, Khan KS. New tests for the diagnosis of latent tuberculosis infection [Letter] Ann Intern Med. 2007;147:672–3. doi: 10.7326/0003-4819-147-9-200711060-00020. author reply 673–4. [DOI] [PubMed] [Google Scholar]
- 56.Pai M, Dheda K, Cunningham J, Scano F, O’Brien R. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis. 2007;7:428–38. doi: 10.1016/S1473-3099(07)70086-5. [DOI] [PubMed] [Google Scholar]
- 57.Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole-blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008;177:1164–70. doi: 10.1164/rccm.200711-1613OC. [DOI] [PubMed] [Google Scholar]
- 58.Hill PC, Jackson-Sillah DJ, Fox A, Brookes RH, de Jong BC, Lugos MD, et al. Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS ONE. 2008;3:e1379. doi: 10.1371/journal.pone.0001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen P, Doherty TM, Pai M, Weldingh K. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol Med. 2007;13:175–82. doi: 10.1016/j.molmed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Pai M, O’Brien R. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med. 2007;4:e208. doi: 10.1371/journal.pmed.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]