SUMMARY
SETTING
Urine antigen testing is an attractive strategy for the diagnosis of active tuberculosis (TB), but accuracy data are scarce.
OBJECTIVE
To prospectively evaluate the diagnostic performance of commercial urinary lipoarabinomannan (LAM) antigen testing for active TB among pulmonary and extra-pulmonary TB suspects.
DESIGN
Prospective blinded evaluation of 200 adult TB suspects at a tertiary referral hospital in India. Reference standards included culture and clinical diagnosis.
RESULTS
Patients were 61% male (mean age 40.4 years): 8.5% were human immunodeficiency virus (HIV) infected and 47 of 200 (23.5%) were culture-positive for TB. Compared to positivity on either Löwenstein-Jensen (LJ) or BACTEC cultures, LAM sensitivity was 17.8% (95%CI 8.5–32.6), while specificity was 87.7% (95%CI 81.3–92.3). Compared to positivity on both LJ and BACTEC, LAM sensitivity was 5.8% (95%CI 12.5–44.9), with a specificity of 88.8% (95%CI 82.7–92.9). Compared to the clinical diagnosis, LAM sensitivity was 20.0% (95%CI 1.1–70.1), with a specificity of 83.3% (95%CI 50.9–97.0). HIV and smear status did not influence test accuracy.
CONCLUSION
In its current form, LAM is insensitive for the diagnosis of active TB, although its specificity is adequate.
Keywords: tuberculosis, lipoarabinomannan, diagnostic accuracy, urine
The deficiencies inherent in the current tools used to diagnose active tuberculosis (TB) have been well described,1,2 and contribute to the low global case detection rate of only 62%, reflecting a failure to achieve the 2005 goal of 70% set by the World Health Assembly.3 The development and evaluation of new diagnostic tools is a priority for the Global Plan to Stop TB.4 Increasing numbers of cases of active TB are infected with the human immunodeficiency virus (HIV), which contributes to the failure of conventional smear-based diagnosis.5
A new diagnostic capable of replacing sputum smear could save 400 000 lives annually.6 Ideal new assays would involve rapid point-of-care diagnosis using non-invasive clinical specimens that are affordable and easy to perform,7 would detect both pulmonary and extra-pulmonary disease, and would not be influenced by the level of immunosuppression of the host.
As part of the global effort to refine diagnostic tools for TB, non-conventional specimens, including urine, breath condensate and blood are being explored. Urine is simple and feasible to collect, process and store in TB-endemic settings.
Antibody detection using current assays does not offer adequate diagnostic accuracy,8–10 but antigen detection assays may correlate with bacterial burden and may not be as influenced by HIV infection. Detection of mycobacterial lipoarabinomannan (LAM), a heat-stable glycolipid, was originally described using serum,11,12 but this test was limited by immune complex formation.13 Subsequent investigations determined that LAM was expressed in urine,14 and a case-control study demonstrated promising diagnostic performance15 that was later supported by a study in Tanzania.16 Based on these early data, a prototype urinary LAM detection test was produced by Chemogen (South Portland, ME, USA). A commercial version of this test is now marketed as Clearview® TB enzyme-linked immunosorbent assay (ELISA; Inverness Medical Innovations Inc, Scarborough, ME, USA).
LAM is specific to mycobacteria,17 is released by metabolically active bacteria,18 and is filtered by the kidney.19 LAM excreted by patients with active TB may be present in urine in variable amounts, between 0.5 and several hundred ng/ml.20 The influence of urine concentration and anti-tuberculosis treatment on urine LAM excretion is not known.
Given the paucity of data on LAM in high-burden countries, we performed a prospective blinded evaluation of the diagnostic performance of urine LAM ELISA (MTB ELISA Urinary Antigen Test Kit, Chemogen, USA, version 7, 28 February 2007) for the diagnosis of active TB among adult TB suspects in South India.
METHODS
Setting
Our study was conducted at the Christian Medical College (CMC), a 2200-bed tertiary referral centre in Vellore, a town in Tamil Nadu State in South India. The study protocol was approved by the institutional review boards at both CMC Vellore and McGill University, Montreal, Canada. Vellore District has an annual TB case detection rate of 144 per 100 000 population.21
Participants
Between October 2007 and July 2008, 200 patients with suspected pulmonary or extra-pulmonary TB consented to participate and were recruited by a trained research officer as part of a larger diagnostics study. Sample size estimate was approximated using a nomogram22 based on test sensitivity of 50%, with 50% of TB suspects meeting the final clinical diagnosis of TB, a 7% confidence interval (CI) and a significance of 0.05.
Inclusion criteria were as follows: 1) age ≥18 years; 2) assessed by a physician who feels the diagnosis of TB is possible; 3) willing to consent to study protocol including an 8-week follow-up visit, an HIV test and specimen submission as outlined; 4) being symptomatic:
Pulmonary TB (at least two of the following symptoms): a) cough for ≥2 weeks, b) weight loss of at least 10% of healthy body weight, c) symptoms of fever for ≥2 weeks or one measured temperature above 38.5°C.
Extra-pulmonary TB: a) one focal symptom lasting ≥3 weeks AND b) weight loss of at least 10% of healthy body weight AND/OR c) symptoms of fever for ≥2 weeks or one measured temperature above 38.5°C.
Exclusion criteria were as follows: 1) received any anti-tuberculosis treatment within the last 6 months; 2) extra-pulmonary specimen submitted in formalin.
Pulmonary TB suspects submitted one spot and one morning sputum sample for TB culture, and extra-pulmonary TB suspects submitted one sterile fluid or tissue sample for TB culture. All patients also submitted one fresh urine specimen (≥5 ml) in a sterile container at the first study visit, prior to TB treatment.
Laboratory methods
The LAM assay was performed according to the manufacturer’s package insert.20
Urine processing
Urine was refrigerated immediately after collection and processed the next day. Urine collections occurring on weekend days were processed on Monday. No stabilising agent or buffer was added to the urine. Two ml of urine was placed in a 1.5 ml microcentrifuge tube and heated at 95°C for 30 min. After cooling to room temperature, it was centrifuged at 10 000 rpm for 15 min. Supernatant was removed into a second tube and stored at −20°C for subsequent batch testing.
ELISA
Manufacturer’s strips of antibody-coated wells were used. Positive and negative controls provided with the kit were processed in duplicate in appropriate wells: 100 μl of processed urine was added in duplicate to appropriate wells, and the plates were sealed using film provided in the kit. The plates were incubated at 24°C in an air-conditioned room for 1 h, then decanted into a collection basin, and tapped firmly over a paper towel. Wells were manually washed four times with 300 μl of wash buffer and decanted into a collection basin. LAM-specific horseradish peroxidase-conjugated polyclonal antibody (100 μl) was added and sealed plates were incubated at 24°C for 1 h, then washed four more times. Tetramethylbenzidine single component chromogenic substrate (100 μl) was added and plates were incubated at 24°C for 15 min. The reaction was stopped with 100 μl of 1M H2SO4 (in-house) with gentle shaking, to achieve a yellow colour. Optical density (OD) was read immediately at 450 nm.
As recommended by the manufacturer, the sample was reported as positive if OD was at least 0.1 above the signal of the negative control. If both replicates of patient samples failed to agree or were not both within 15% of the mean OD value, the test was repeated. If the negative control was not between 0.15 and 0.25 absorbance units, or if the positive control was not between 0.3 and 0.5 absorbance units above background, the test was repeated.
The test was performed in a large clinical microbiology laboratory at an advanced tertiary hospital by one individual operator who was supervised by a senior technician with 20 years of experience in serology. The department routinely performs 15 auto-immune serology tests and six microbiology serology tests at high volume.
Smears
Direct smears were prepared from sputum, tissue or fluid. Auramine staining was used, and slides were examined by one technician using the 25× objective, viewing at least 30 fields.
Löwenstein-Jensen culture
Clinical specimens were decontaminated with 4% NaOH with NALC.23,24 Five drops of decontaminated specimen were inoculated onto Löwenstein-Jensen (LJ) slants (in-house) using a disposable pipette and incubated on a slant in the dark at 37°C for 6 weeks. Slants were examined weekly, and contaminated slants were discarded and re-inoculated with redecontaminated specimen. Positive growth was confirmed with Ziehl-Neelsen (ZN) smear. Every new batch of LJ media was controlled using American Type Culture Collection (ATCC) H37Ra.
BACTEC 460 culture
BACTEC 12B vials (Becton Dickinson, Gurgaon, Haryana, India) containing 0.1 ml of polymyxin B, amphotericin B, nalidixic acid, trimethoprim, azlocillin (PANTA; Becton Dickinson) were inoculated with 0.5 ml of decontaminated specimen after cleaning the rubber septum with 70% ethanol and flushing the bottle with CO2. Vials were incubated at 37°C for 6 weeks, and tested for growth every day in the first 2 weeks, followed by alternate day testing for 4 weeks. Each bottle was cleaned with 70% ethanol before testing. Positive bottles were smeared when the growth index reached 50. Contaminated vials were discarded and re-inoculated with frozen decontaminate. ρ-nitro-α-acetylamino-β-hydroxy-propiophenone (NAP) test was used for identification.25
Two uninoculated 12B bottles were tested at the end of each run, at the last position, to serve as negative controls. Positive controls (ATCC H37Ra) were inoculated with every batch, and machine performance was tested monthly with performance standards. NAP test was controlled at baseline using ATCC H37Ra and ATCC Mycobacterium gordonae.
Blinding
Urine specimens were labelled with a four-digit random number by the laboratory investigator. The technician was not aware of the identity of each specimen. A table connecting random numbers with study numbers was kept by the laboratory investigator in a locked file.
Analysis
Two hundred pulmonary and extra-pulmonary TB suspects were recruited as part of a diagnostic evaluation project, in which the sample size had been calculated to evaluate the performance of several new diagnostic tests for TB. Data were entered by two separate operators, and manually rechecked after entry. Data analysis was performed using SPSS 15.0 (LEAD Technologies Inc, Charlotte, NC, USA). Performance was reported as sensitivity and specificity (with 95% CIs) of LAM as compared to three reference standards: 1) positivity in either solid (LJ) or liquid (BACTEC 460) culture; 2) positivity in both solid and liquid culture; 3) final clinical diagnosis based on culture, smear, histology and response to empirical treatment at 8 weeks (presence of two or more pre-defined clinical and laboratory response criteria (modified from Wilson26). See Table 1 for response to treatment criteria.
Table 1.
Response to empirical treatment criteria applied at 8-week visit (modified from Wilson26)
| Response criteria | Definition of response (compared to baseline) |
|---|---|
| Smear conversion | Change from positive to negative |
| Body weight | Increase of 5% |
| Haemoglobin | Increase of at least 1.0 g/dl |
| C-reactive protein | Decrease of at least 70% |
| Karnofsky performance index | Increase of at least 20 points (or 10 points if baseline was ≥ 80) |
| Symptom count ratio (number of symptoms improved or resolved, divided by number of symptoms at baseline. Symptoms from predefined list of acceptable symptoms) | Improvement or resolution of at least 50% of symptoms |
RESULTS
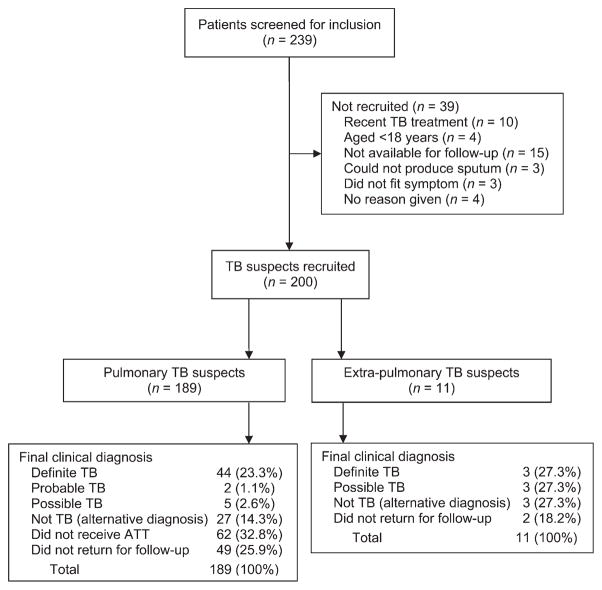
Figure 1 shows the recruitment process and clinical diagnosis and Table 2 provides a description of the study participants. Patients were mostly male (122/200, 61%), out-patients (184/200, 92.0%) with a mean age of 40.4 years (standard deviation [SD] 16.2) and a mean body mass index of 19.7 (SD 4.10). Seventeen of the 200 (8.5%) were HIV-infected and 47/200 (23.5%) were culture-positive for TB. No adverse events were observed during the study.
Figure 1.
Study recruitment process and final clinical diagnosis. TB = tuberculosis; ATT = anti-tuberculosis treatment.
Table 2.
Demographics of study participants, N = 200
| n | % | |
|---|---|---|
| Total TB suspects included (pulmonary and extra-pulmonary) | 200 | 100 |
| Male | 122 | 61.1 |
| Age, years, mean (SD) | 40.4 (16.2) | |
| BMI, mean (SD) (n = 189) | 19.7 (4.1) | |
| Pulmonary TB suspected | 189 | 94.5 |
| Out-patient | 184 | 92.0 |
| Living in Tamil Nadu State | 184 | 92.0 |
| Monthly family income <US$115 | 180 | 90.0 |
| Experiencing cough | 193 | 96.5 |
| Experiencing chest pain | 106 | 53.0 |
| Experiencing fever | 172 | 86.0 |
| HIV-infected | 17 | 8.5 |
| Given treatment for TB after recruitment (n = 139) | 39 | 28.1 |
TB = tuberculosis; SD = standard deviation; BMI = body mass index; HIV = human immunodeficiency virus.
The LAM assay provided evaluable results in all 200 participants, with no indeterminate or invalid results. The LAM assay had very poor sensitivity and adequate specificity for active TB diagnosis, as determined by positivity on LJ and BACTEC culture. When positivity on either LJ or BACTEC was considered (Table 3), LAM sensitivity was 17.8% (95%CI 8.5–32.6), with a specificity of 87.7% (95%CI 81.3–92.3), providing a positive predictive value (PPV) of 29.6% (95%CI 14.5–50.3) and a negative predictive value (NPV) of 78.6% (95% CI 71.6–84.3). When positivity on both LJ and BACTEC was considered (data not shown), sensitivity was 5.8% (95%CI 12.5–44.9), with a specificity of 88.8% (95%CI 82.7–92.9), providing a PPV of 29.6% (95%CI 14.5–50.3) and an NPV of 86.7% (95%CI 80.5–91.2).
Table 3.
Diagnostic accuracy of LAM as compared to L J or BACTEC culture*
| Any L J or BACTEC culture-positive |
Total | ||
|---|---|---|---|
| Negative | Positive | ||
| LAM interpretation | |||
| Negative | 136 | 37 | 173 |
| Positive | 19 | 8 | 27 |
| Total | 155 | 45 | 200 |
Sensitivity 17.8% (95%CI 8.5–32.6); specificity 87.7% (95%CI 81.3–92.3); PPV 29.6% (95%CI 14.5–50.3); NPV 78.6% (95%CI 71.6–84.3).
LAM = lipoarabinomannan; LJ = Löwenstein Jensen; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value.
Final clinical diagnosis was determined for all patients. Patients were grouped into two groups: definite (culture-positive), probable (smear-positive with response to treatment) and possible TB (response to treatment), as compared to all others, including patients who had firm alternative diagnoses, those who did not receive empirical treatment, and those who did not return for follow up. When compared to this binary definition of clinical diagnosis, LAM sensitivity was 20.0% (95%CI 1.05–70.1), with a specificity of 83.3% (95%CI 50.9–97.0), providing a PPV of 33.3% (95%CI 1.77–87.5) and an NPV of 71.4% (95%CI 42.0–90.4; Table 4).
Table 4.
Diagnostic accuracy of LAM as compared to clinical diagnosis*
| Final diagnosis |
Total | ||
|---|---|---|---|
| Not TB or not treated or did not return | Definite or probable or possible TB | ||
| LAM interpretation | |||
| Negative | 126 | 47 | 173 |
| Positive | 17 | 10 | 27 |
| Total | 143 | 57 | 200 |
Sensitivity 17.5% (95%CI 9.2–30.4); specificity 88.1% (95%CI 81.4–92.7); PPV 37.0% (95%CI 20.1–57.5); NPV 72.8% (95%CI 65.5–79.2).
LAM = lipoarabinomannan; TB = tuberculosis; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value.
The proportion of LAM positives was not different between those patients who did not return for follow-up and those patients classified as ‘not TB or not treated’ (results not shown, χ2 P = 0.186).
When data from HIV-infected individuals were analysed separately, LAM performance was not different (Table 5; sensitivity 20.0%, 95%CI 1.05–70.1; specificity 83.3%, 95%CI 50.9–97.0; PPV 33.3%, 95%CI 1.77–87.5; NPV 71.4%, 95%CI 42.0–90.4, as compared to positivity on LJ or BACTEC), although accuracy estimates were imprecise because of the smaller numbers of participants.
Table 5.
Diagnostic accuracy of LAM as compared to LJ or BACTEC among HIV-infected individuals*
| Any L J or BACTEC culture-positive |
Total | ||
|---|---|---|---|
| Negative | Positive | ||
| LAM interpretation | |||
| Negative | 10 | 4 | 14 |
| Positive | 2 | 1 | 3 |
| Total | 12 | 5 | 17 |
Sensitivity 20.0% (95%CI 1.1–70.1); specificity: 83.3% (95%CI 50.9–97.0); PPV 33.3% (95%CI 1.8–87.5); NPV 71.4% (95%CI 42.0–90.4).
LAM = lipoarabinomannan; L J = Löwenstein Jensen; CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value.
Among 45 patients who were smear-positive, LAM was slightly more sensitive than the overall population (sensitivity 23.0%, 95%CI 9.8–44.0; specificity 84.2%, 95%CI 59.5–95.8; PPV 66.6%, 95%CI 30.9–90.0; NPV 44.4%, 95%CI 28.3–61.7). Among 155 patients who were smear-negative, performance was similar to the overall population (sensitivity 25.0%, 95%CI 4.45–64.0; specificity 89.1%, 95%CI 82.6–93.4; PPV 11.1%, 95%CI 1.94–3.60; NPV 95.6%, 95%CI 90.2–98.2; data not shown).
LAM results were analysed using a receiver operating characteristic (ROC) curve to assess the global performance (Figure 2). The area under the curve was 0.508 (95%CI 0.407–0.610), suggesting poor overall diagnostic performance as compared to positivity on LJ or BACTEC. This would suggest that there is no optimal LAM cut-off value where sensitivity and specificity are optimised.
Figure 2.
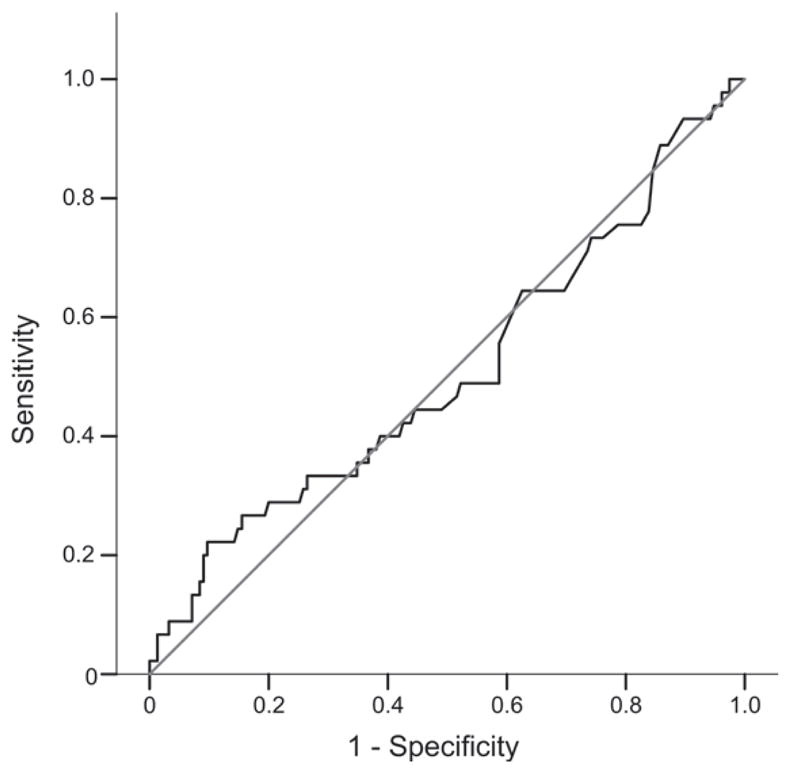
Receiver operating characteristic (ROC) curve of LAM diagnostic performance with positivity on LJ or BACTEC as the reference standard. LAM = lipoarabinomannan; LJ = Löwenstein-Jensen.
DISCUSSION
Although early data on urinary LAM were positive, our data suggest that urinary LAM antigen detection does not have adequate sensitivity to be used for the detection of active TB. However, urine LAM does have a reasonably high NPV, suggesting that a negative result could be used as evidence against active TB. Our study did not demonstrate a significant impact of HIV status on urine LAM performance, but it lacked power to assess this subgroup.
A prospective evaluation of the same commercial detection system for LAM in urine was performed in Tanzania among 231 pulmonary TB suspects (69% HIV-positive) and 103 healthy volunteers;16 132 (57.1% of total suspects) were sputum culture-positive, and were compared to healthy volunteers. Sensitivity was 80.3%, with 99.0% specificity. HIV infection had no effect on sensitivity. Patients with strongly positive sputum smears had higher LAM concentrations.
A second clinical evaluation was undertaken in 2005 (unpublished data from Chemogen).20 Among 600 TB suspects (53% HIV-positive), 78 were AFB smear-positive (13.0%); of these, 53 were LAM-positive (69.0% sensitivity compared to AFB smear), while 226 healthy volunteers gave two false-positive LAM results (specificity 99.1%).
These evaluations have used healthy volunteers instead of culture-negative TB suspects to define specificity, a weakness in study design, as healthy volunteers are not the group the test is designed to be used on. This may have biased the results towards a higher specificity.
A recent abstract demonstrated low sensitivity of LAM (28–44%), but higher sensitivity among HIV-infected patients than HIV-negatives.27 These evaluations have been performed in high HIV prevalence areas, which are unique to Africa. The manufacturer clearly recommends that the test be used only among HIV-infected patients.20 It is conceivable that HIV-infected patients may excrete greater amounts of mycobacterial antigens in urine.
Our study is the first to evaluate LAM technology in a TB-endemic country with low HIV prevalence. We evaluated the Chemogen version of the LAM assay, and not the more recent ClearView kit, which was not available at the time of our study. Our data show poor diagnostic performance. Urine-based TB diagnosis is attractive, and further work is needed to improve the LAM assay. The use of larger volumes of urine, alternative methods of extraction that avoid high temperatures, and stabilising agents that can be added to urine at the time of collection, may improve sensitivity. It is also important to evaluate other urine antigens that may have diagnostic accuracy.
Acknowledgments
The study was funded by the McGill India Strategic Research Initiative (MISRI), and in part by the Canadian Institutes of Health Research (CIHR grant MOP-89918). MP is supported by a CIHR New Investigator Award.
References
- 1.Pai M, O’Brien R. New diagnostics for latent and active tuberculosis: state of the art and future prospects. Semin Respir Crit Care Med. 2008;29:560–568. doi: 10.1055/s-0028-1085707. [DOI] [PubMed] [Google Scholar]
- 2.Pai M, Ramsay A, O’Brien R. Evidence-based tuberculosis diagnosis. PLoS Med. 2008;5:e156. doi: 10.1371/journal.pmed.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO/HTM/TB/2008.393. Geneva, Switzerland: WHO; 2008. Global tuberculosis control: surveillance, planning, financing. [Google Scholar]
- 4.World Health Organization. Towards a world free of tuberculosis. Geneva, Switzerland: WHO; 2006. Global Plan to Stop TB 2006–2015. Actions for life. [Google Scholar]
- 5.Burman WJ, Jones BE. Clinical and radiographic features of HIV-related tuberculosis. Semin Respir Infect. 2003;18:263–271. doi: 10.1053/s0882-0546(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 6.Keeler E, Perkins MD, Small P, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444 (Suppl 1):S49–S57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 7.Urdea M, Penny LA, Olmsted SS, et al. Requirements for high impact diagnostics in the developing world. Nature. 2006;444 (Suppl 1):S73–S79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 8.Steingart KR, Henry M, Laal S, et al. A systematic review of commercial serological antibody detection tests for the diagnosis of extra-pulmonary tuberculosis. Thorax. 2007;62:911–918. doi: 10.1136/thx.2006.075754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steingart KR, Henry M, Laal S, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202. doi: 10.1371/journal.pmed.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Diagnostics Evaluation Series No. 2. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 11.Doskeland SO, Berdal BP. Bacterial antigen detection in body fluids: methods for rapid antigen concentration and reduction of nonspecific reactions. J Clin Microbiol. 1980;11:380–384. doi: 10.1128/jcm.11.4.380-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sada E, Brennan PJ, Herrera T, Torres M. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J Clin Microbiol. 1990;28:2587–2590. doi: 10.1128/jcm.28.12.2587-2590.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tessema TA, Bjune G, Hamasur B, et al. Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis. Scand J Infect Dis. 2002;34:97–103. doi: 10.1080/00365540110077263. [DOI] [PubMed] [Google Scholar]
- 14.Hamasur B, Bruchfeld J, Haile M, et al. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J Microbiol Methods. 2001;45:41–52. doi: 10.1016/s0167-7012(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 15.Tessema TA, Hamasur B, Bjun G, Svenson S, Bjorvatn B. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand J Infect Dis. 2001;33:279–284. doi: 10.1080/003655401300077306. [DOI] [PubMed] [Google Scholar]
- 16.Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg. 2005;99:893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 18.Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 20.Product instructions. Revision 7. Portland, ME, USA: Chemogen; 2007. MTB ELISA urinary antigen test kit. 96 well plate format. [Google Scholar]
- 21.TB India. RNTCP status report. New Delhi, India: Central TB Division; 2008. [Google Scholar]
- 22.Carley S, Dosman S, Jones SR, Harrison M. Simple nomograms to calculate sample size in diagnostic studies. Emerg Med J. 2005;22:180–181. doi: 10.1136/emj.2003.011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubica GP, Dye WE, Cohn ML, Middlebrook G. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963;87:775–779. doi: 10.1164/arrd.1963.87.5.775. [DOI] [PubMed] [Google Scholar]
- 24.Ratnam S, Stead FA, Howes M. Simplified acetylcysteine-alkali digestion-decontamination procedure for isolation of mycobacteria from clinical specimens. J Clin Microbiol. 1987;25:1428–1432. doi: 10.1128/jcm.25.8.1428-1432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqi SH, Hwangbo CC, Silcox V, et al. Rapid radiometric methods to detect and differentiate Mycobacterium tuberculosis/M. bovis from other mycobacterial species. Am Rev Respir Dis. 1984;130:634–640. doi: 10.1164/arrd.1984.130.4.634. [DOI] [PubMed] [Google Scholar]
- 26.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10:31–38. [PubMed] [Google Scholar]
- 27.Mutetwa R, Boehme C, Dimairo M, et al. Evaluation of a commercial urine lipoarabinomannan ELISA kit for diagnosis of TB in a high HIV prevalence setting. 16th Conference on Retroviruses and Opportunistic infections; February 8–11, 2009; Montreal, Canada. Session 134 Poster Abstracts. [Google Scholar]