Abstract
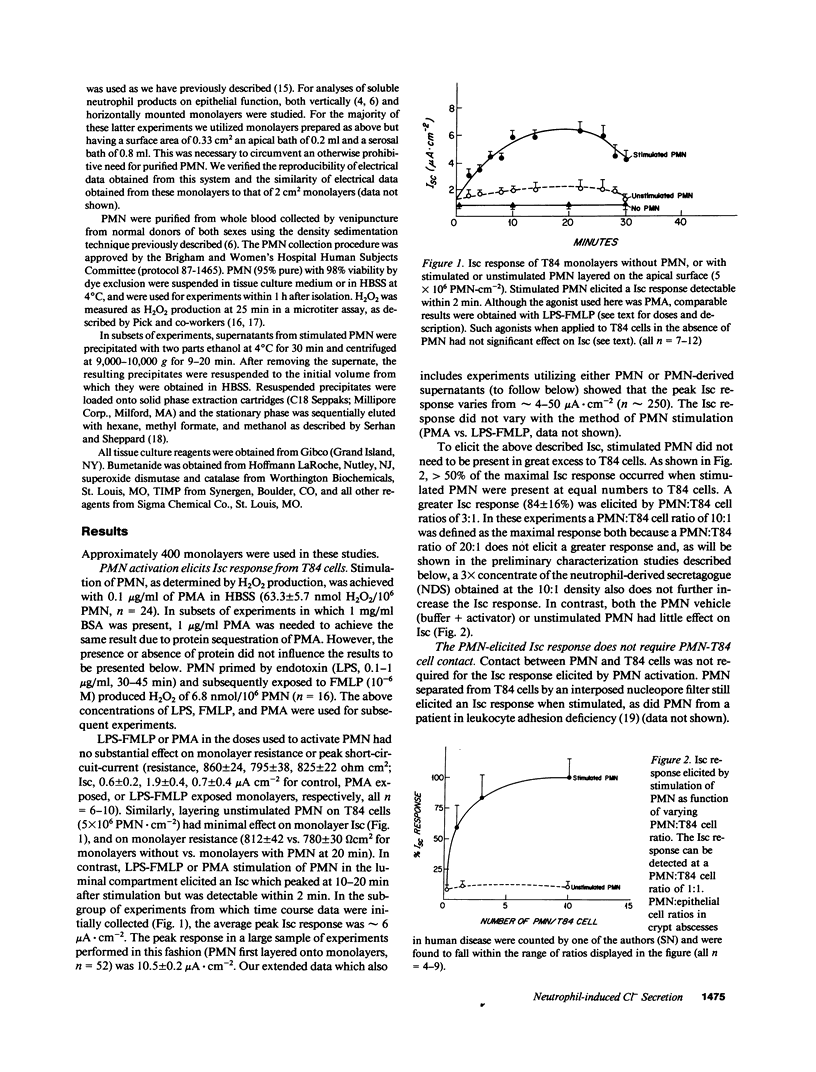
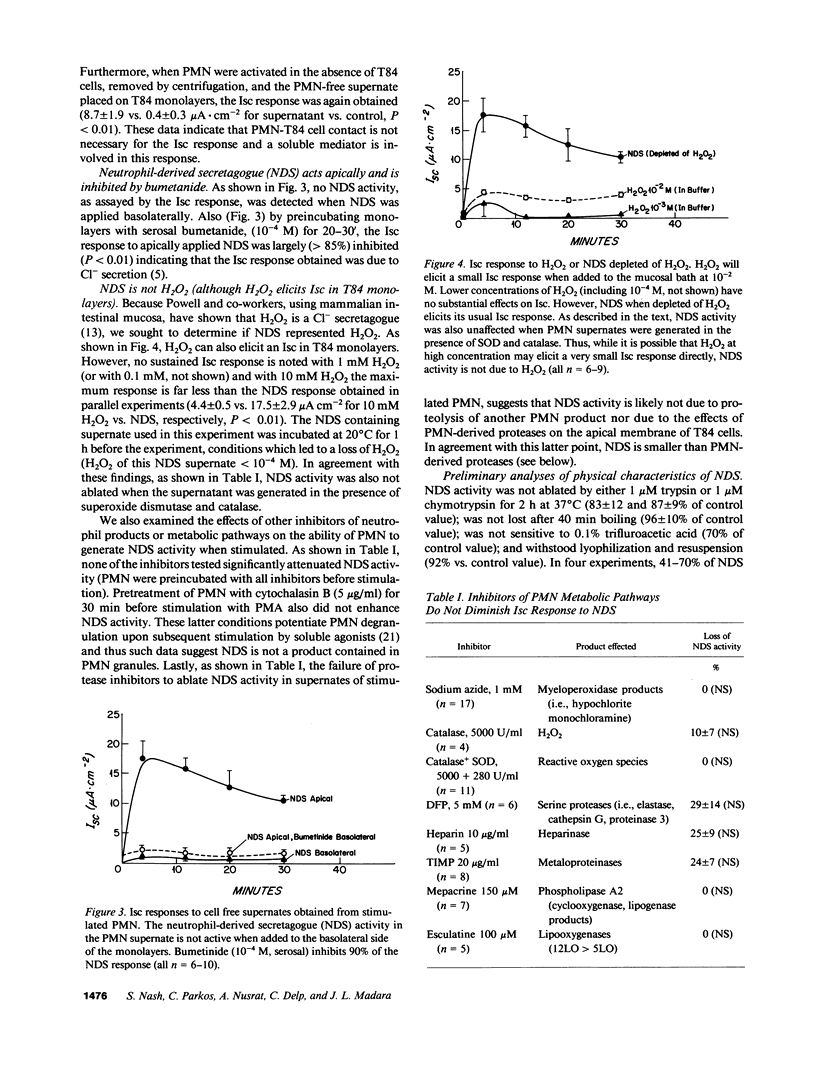
In order to model crypt abscesses, a histological finding which correlates with disease activity in intestinal inflammation, human polymorphonuclear leukocytes (PMN) were layered onto monolayers of the human intestinal epithelial cell line T84, a crypt-like epithelium which is capable of Cl- secretion. Such PMN-epithelial interaction had no substantial effect on monolayer integrity or function. However, when PMN were stimulated by conditions including those present naturally in the human colonic lumen, monolayers responded with a bumetanide-sensitive short circuit current (Isc) indicative of Cl- secretion, the basis of secretory diarrhea. This Isc response was induced by a neutrophil-derived secretagogue (NDS), which was only active when applied to the luminal surface of monolayers and did not require PMN-epithelial contact. NDS activity is resistant to boiling, acid, and trypsin and passes a 500 nominal mol wt cutoff filter. NDS activity is not secondary to the respiratory burst products O2- or H2O2 and does not appear to be a myeloperoxidase product. We speculate NDS elicited Cl- secretion may contribute to the secretory diarrhea seen in patients with intestinal inflammation and crypt abscesses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett K. E., Huott P. A., Shah S. S., Dharmsathaphorn K., Wasserman S. I. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol. 1989 Jan;256(1 Pt 1):C197–C203. doi: 10.1152/ajpcell.1989.256.1.C197. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Mellor D. M., Myers D. B., Selden A. C., Keshavarzian A., Broom M. F., Hobson C. H. Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand J Gastroenterol. 1988 Jan;23(1):121–128. doi: 10.3109/00365528809093861. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Madara J. L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huott P. A., Liu W., McRoberts J. A., Giannella R. A., Dharmsathaphorn K. Mechanism of action of Escherichia coli heat stable enterotoxin in a human colonic cell line. J Clin Invest. 1988 Aug;82(2):514–523. doi: 10.1172/JCI113626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayalcin S. S., Sturbaum C. W., Wachsman J. T., Cha J. H., Powell D. W. Hydrogen peroxide stimulates rat colonic prostaglandin production and alters electrolyte transport. J Clin Invest. 1990 Jul;86(1):60–68. doi: 10.1172/JCI114715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. B., Nostrant T. T., Appelman H. D. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis). Am J Surg Pathol. 1982 Sep;6(6):523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol. 1985 Dec;101(6):2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987 Oct;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. The selective and superoxide-independent disruption of intestinal epithelial tight junctions during leukocyte transmigration. Lab Invest. 1988 Oct;59(4):531–537. [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Sheppard K. A. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990 Mar;85(3):772–780. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer S. J., ten Cate J. W., Tytgat G. N. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988 Mar;94(3):825–831. doi: 10.1016/0016-5085(88)90261-2. [DOI] [PubMed] [Google Scholar]



