Abstract
To aid the development of symptomatic and disease modifying therapies in Parkinson's disease (PD), there is a strong need to identify non-invasive measures of basal ganglia function that are sensitive to disease severity. This study examines the relation between blood oxygenation level dependent (BOLD) activation in every nucleus of the basal ganglia and symptom-specific disease severity in early stage, de novo PD. BOLD activation measured at 3 Tesla was compared between 20 early stage de novo PD patients and 20 controls during an established precision grip force task. In addition to the basal ganglia nuclei, activation in specific thalamic and cortical regions was examined. There were three novel findings. First, there were significant negative correlations between total motor Unified Parkinson's Disease Rating Scale (UPDRS) and BOLD activation in bilateral caudate, bilateral putamen, contralateral external segment of the globus pallidus, bilateral subthalamic nucleus, contralateral substantia nigra, and thalamus. Second, bradykinesia was the symptom that most consistently predicted BOLD activation in the basal ganglia and thalamus. Also, BOLD activation in the contralateral internal globus pallidus was related to tremor. Third, the reduced cortical activity in primary motor cortex and supplementary motor area in de novo PD did not relate to motor symptoms. These findings demonstrate that BOLD activity in nuclei of the basal ganglia relates most consistently to bradykinesia. The findings demonstrate that functional magnetic resonance imaging has strong potential to serve as a non-invasive marker for the state of basal ganglia function in de novo PD.
Keywords: fMRI, Parkinson's disease, Basal Ganglia, BOLD, disease severity
Introduction
Objective biomarkers of Parkinson's disease (PD) are pivotal to therapeutic development to confirm diagnosis (trait), and track disease progression (state). Based on research advances in the 1990's, new technologies for in vivo brain imaging are now available. In the case of PD, both positron emission tomography (PET) and single photon emission computed tomography (SPECT) have been developed as biomarkers of striatal function, and these techniques meet many of the criteria for a viable biomarker.1 However, these techniques rely on radioactive tracers which often have short half lives, remain expensive, and have limited availability.2 In recent work using diffusion tensor imaging (DTI) in the substantia nigra (SN), it was shown that hand-drawn regions of interest in the ventral and lateral SN differentiated individual patients with PD from healthy individuals on a patient-by-patient basis.3 However, DTI in the ventrolateral SN did not correlate with the severity of PD.
Another technique that has the potential to serve as a non-invasive state biomarker of the basal ganglia in PD is functional magnetic resonance imaging (fMRI). During resting state fMRI, it was found that the only nucleus of the basal ganglia (BG) that correlated with the severity of PD in the off state was the putamen.4 However, since PD is classically a motor disorder, it is possible that fMRI during a motor task is required to detect a relationship between activation in other BG nuclei and the severity of PD. In a recent study using fMRI, we provided the first in-vivo evidence that every nucleus of the BG is hypoactive in untreated (de novo) patients with early stage PD during a 2-second grip force task which required switching force on and off.5 It remains unclear however if fMRI during a motor task can be used as a state measure relating specific symptoms to activity in the BG, thalamus, and cortex in early stage, de novo PD using a cross-sectional design. As such, the current study tests the hypothesis that fMRI in specific nuclei of the BG relates to the severity of PD during a robust 2-s visually-guided grip force task. Based upon previously identified factor loadings from the motor examination of the Unified Parkinson's Disease Rating Scale (UPDRS),6 the current study also determines which motor symptoms (bradykinesia, tremor, rigidity, and axial function/balance/gait) relate most closely to the fMRI signal in every nucleus of the BG.
Methods
Subjects
This research was a prospective case-controlled study that included 20 patients with PD and 20 controls. Patients were included if they had never been treated with antiparkinsonian medications, and had a Mini Mental State Examination greater than 26. Antiparkinsonian medication was defined to include any drug designed to alter symptoms of PD or posited to slow the progression of PD. All patients were diagnosed with PD by one of eight movement disorder Neurologists, and the diagnosis was confirmed by the other seven using the PD Society Brain Bank diagnostic criteria.7, 8 Table 1 shows the characteristics of each patient. Healthy control subjects were matched for age, sex, and handedness to each patient with PD. The age of the PD group (mean=57.9 years) was not different from the control group (mean=58.3 years) (t=-0.12, df=38, p=0.90). The control participants had no history of neuropsychiatric or neurological disease. On the day of scanning the control participants were also evaluated using questions 20, 21, 23, 24, 27, 28, and 29 from the UPDRS. All control subjects scored a 0 on these items. All subjects gave written informed consent consistent with the Declaration of Helsinki, which was approved by the Institutional Review Boards at Rush University Medical Center and the University of Illinois at Chicago.
Table 1.
Patient Characteristics
| UPDRS Part III |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Gender | Handedness | Hand Tested | HY Stage | Total | Bradykinesia | Tremor | Rigidity | Axial function/Balance/Gait |
| PD 1 | 47 | F | R | R | I | 10 | 6 | 1 | 2 | 1 |
| PD 2 | 72 | M | R | L | II | 31 | 13 | 7 | 7 | 4 |
| PD 3 | 66 | F | R | L | II | 20 | 8 | 4 | 6 | 2 |
| PD 4 | 55 | F | R | L | I | 12 | 6 | 5 | 1 | 0 |
| PD 5 | 57 | M | L | R | II | 25 | 12 | 1 | 7 | 5 |
| PD 6 | 60 | M | R | R | I | 12 | 4 | 1 | 5 | 2 |
| PD 7 | 69 | M | R | L | II | 18 | 8 | 1 | 4 | 5 |
| PD 8 | 45 | F | R | L | II | 18 | 8 | 1 | 5 | 4 |
| PD 9 | 57 | M | R | L | II | 18 | 8 | 2 | 4 | 4 |
| PD 10 | 36 | M | R | L | I | 4 | 1 | 1 | 2 | 0 |
| PD 11 | 55 | M | L | R | II | 31 | 11 | 3 | 8 | 9 |
| PD 12 | 60 | F | R | R | II | 11 | 3 | 2 | 3 | 3 |
| PD 13 | 58 | F | R | R | II | 16 | 6 | 4 | 4 | 2 |
| PD 14 | 64 | M | R | L | II | 25 | 9 | 3 | 7 | 6 |
| PD 15 | 60 | F | R | R | I | 5 | 2 | 1 | 1 | 1 |
| PD 16 | 70 | F | R | L | II | 9 | 2 | 3 | 1 | 3 |
| PD 17 | 55 | F | R | R | II | 13 | 6 | 1 | 3 | 3 |
| PD 18 | 50 | M | R | R | II | 10 | 6 | 1 | 2 | 1 |
| PD 19 | 66 | M | R | L | II | 24 | 10 | 3 | 6 | 5 |
| PD 20 | 56 | F | R | R | II | 12 | 3 | 2 | 6 | 1 |
HY = Hoehn and Yahr; UPDRS = Unified Parkinson's Disease Rating Scale; F= female; M= male; R = right; L =left.
Force Data Acquisition
Figure 1A shows that subjects produced force against a custom fiber optic force transducer (Aither Engineering). PD patients used their most affected limb. Since control subjects were matched for handedness, each control subject used the same hand as the matched patient. The Si425 Fiber Optic Interrogator digitized the force data at 125 Hz and customized software written in LabView collected the force data and converted it to Newtons. Force output was presented to the subject using a visual display inside the MRI scanner (Figure 1B).9
Figure 1.
A, grip force transducer and fiber optic device used to collect force data. B, visual display seen during the scan for the grip force task at rest and during force production. The arrow shows movement direction of the white force cursor but was not part of the visual display. C, actual force traces of the motor task performed by one control subject (top) and one PD patient (bottom).
MRI Data Acquisition
Magnetic resonance images were collected using a quadrature volume head coil inside a 3 Tesla MR Scanner (GE Healthcare 3T94 Excite 2.0). The subject's head was stabilized using padding. The functional images were obtained using a T2*-sensitive, single shot, gradient-echo echo-planar pulse sequence (echo-time 25ms; time to repeat (TR) 2500ms; flip angle 90°; field of view 200mm2; imaging matrix 64×64; 42 axial slices at 3mm thickness; 0mm gap between slices). T1 anatomical scans were obtained using a T1-weighted fast spoiled gradient echo pulse sequence (echo-time 1.98ms; repeat-time 9ms; flip angle 25°; field of view 240mm2; imaging matrix 256×256; 120 axial slices at 1.5mm thickness; 0mm gap between slices).
Experimental Design
Before scanning, each subject participated in a 1-hour training session outside the scanner. Each subject's maximum voluntary contraction (MVC) was calculated using a separate force transducer (Jamar Hydraulic Pinch Gauge) before entering the MR environment. The MVC was calculated as the peak force amplitude.
During the fMRI rest blocks, subjects fixated on a stationary red target and stationary white cursor but did not produce force. There were five rest blocks and four task blocks. During task blocks, subjects completed 2s pulse-hold contractions using a pinch grip followed by 1s of rest (Figure 1C). The target represented 15% of the individual subject's MVC and was displayed on the screen as a horizontal bar (Figure 1B). A force cursor was displayed on the screen as a white bar that moved vertically related to the force produced by the subject. Each force pulse began as the target bar turned green and remained green for 2s. The force pulse ended when the target bar turned red for 1s, indicating rest. This sequence was repeated 10 times per task block.
Data Analysis
The supplemental material describes the force data analysis and results. The following describes the voxel-wise fMRI analyses. AFNI, the public domain software (http://afni.nimh.nih.gov/afni/), was used to analyze the fMRI data. Before analysis, the fMRI data were transposed for those subjects that used their left hand so that the left and right hemispheres in all datasets were contralateral and ipsilateral to the tested hand, respectively. Head motion was less than 1mm in the x, y, and z directions for all subjects.
We previously found that the fMRI signal was hypoactive in the BG, thalamus, and motor cortex when comparing 14 patients with PD to 14 control subjects.5 As such, the first analysis was to confirm that we replicate these previous findings when 6 additional subjects are added to each group. A voxel-wise analysis was performed on the whole brain fMRI data in order to identify group differences in BOLD activation. Motion-corrected individual datasets were normalized by dividing the instantaneous signal in each voxel at each point in the time series by the mean signal in that voxel across each scan. After this, a Gaussian filter was applied to the resultant datasets (full-width half-maximum at 3mm). Then, the time series data were regressed to a simulated hemodynamic response function for the task sequence (3Ddeconvolve, AFNI). Before group analysis, each subject's anatomical and functional datasets were transformed to Talairach space using AFNI.
The data were analyzed using a mixed-effect two-way ANOVA with the group (control, PD) as a fixed factor and the subject as a random factor. This yielded the estimated difference in group means (control-PD) for task minus rest for the 2-second task. These data were corrected for Type I error using a Monte Carlo Simulation model (AFNI, Alphasim). The datasets were thresholded to remove all voxels with t<3 with an activation cluster minimum of 205μL (p<0.05, corrected).
Disease Severity Analysis
To examine the relation between percent signal change in each region of interest (ROI) and disease severity, correlation analyses were performed using Pearson's correlation coefficient from which the coefficient of determination (r2) was computed. All BG, thalamic, and cortical ROIs were drawn a priori from templates in standardized space based on the Basal Ganglia Human Area Template,10 Human Motor Area Template,11 and previous work on PD.5 The supplemental material describes the details for percent signal change quantification for the ROI analysis. The average percent signal change for each ROI across subjects was correlated with the total score from the motor section (Part III) of the UPDRS. The UPDRS ratings were performed by a Neurologist who specializes in movement disorders, and the Neurologist was blinded to the imaging data. The total score from the motor section of the UPDRS was broken down into previously identified factor loading subscales for bradykinesia (items 23-26), rigidity (item 22), tremor (items 20-21), and axial function/balance/gait (items 18,19, 27-31).6 The current study differed from the study of Stebbins and colleagues6 in that we combined resting and postural tremor into one subscale. The scores used in the disease severity subscale analyses are shown in Table 1. Following correlation analysis, forward stepwise multiple regression analysis was performed. The dependent variable was percent signal change in each ROI and the predictor variables were the four UPDRS subscales of bradykinesia, rigidity, tremor, and axial function/balance/gait. Separate multiple regression was performed for each ROI.
Results
fMRI Results
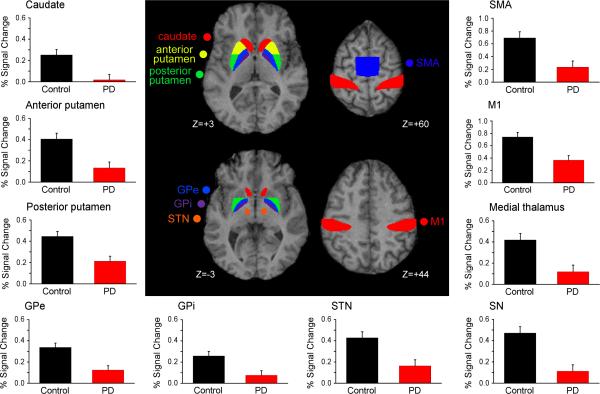
The voxel-wise analysis indicated that PD patients had less activity in all nuclei of the BG, cortex, and thalamus (Table 2). No other group differences were identified in other regions. Figure 2 shows mean percent signal change (PSC) in each BG ROI, medial thalamus, and cortical ROI for the control and PD groups. The central panel of Figure 2 shows the BG and cortical ROIs. Compared with control subjects, patients with PD had reduced PSC in all contralateral and ipsilateral nuclei of the BG (Figure 2, Table 2). Patients with PD also had reduced PSC in the medial and lateral thalamus, M1, and SMA.
Table 2.
Control>PD
| ROI | Center of Mass (X,Y,Z) | Group (df=1,38) |
|---|---|---|
| Basal Ganglia | ||
| C Caudate | (-11.2,9.0,11.2) | F=10.45, p<0.01 |
| I Caudate | (12.2,9.0,11.3) | F=7.91, p<0.01 |
| C A Putamen | (-22.3,7.0,6.8) | F=12.07, p<0.01 |
| I A Putamen | (22.3,7.0,6.8) | F=11.16, p<0.01 |
| C P Putamen | (-26.7,-5.4,3.8) | F=12.67, p<0.01 |
| I P Putamen | (26.7,-5.4,3.8) | F=10.48, p<0.01 |
| C GPe | (-19.8,-4.0,3.9) | F=14.10, p<0.001 |
| I GPe | (19.8,-4.0,3.9) | F=11.37, p=0.01 |
| C GPi | (-16.0,-4.4,1.6) | F=9.16, p<0.01 |
| I GPi | (16.0,-4.4,1.6) | F=8.17, p<0.01 |
| C STN | (-10.7,-13.7,-3.4) | F=10.91, p<0.01 |
| I STN | (10.1,-13.3,-3.4) | F=9.10, p<0.01 |
| C SN | (-13.9,-19.8,-5.1) | F=18.27, p<0.001 |
| I SN | (13.6,-19.0,-5.6) | F=6.05, p<0.05 |
| Thalamus | ||
| C Medial | (-19.2,-23.1,13.4) | F=11.66, p<0.01 |
| C Lateral | (-23.0,-24.6,13.5) | F=9.55, p<0.01 |
| Cortex | ||
| C M1 | (-37.0,-22.2,44.4) | F=12.81, p<0.001 |
| SMA | (6.8, 19.1, 58.5) | F= 11.34, p<0.01 |
Bold type indicates a significant result p<.05. Methods for region of interest analysis are described in the supplemental section. ROI = region of interest; Center of Mass = the X,Y,Z coordinates in Talairach space of the center of mass of each ROI; df = degrees of freedom; C = contralateral; I = ipsilateral; GPe = external segment of the globus pallidus; GPi = internal segment of the globus pallidus; STN = subthalamic nucleus; SN = substantia nigra; M1 = primary motor cortex; SMA = supplementary motor area.
Figure 2.
The center diagram shows the basal ganglia and cortical ROIs used. Surrounding plots show percent signal change in the contralateral basal ganglia, medial thalamus, and cortical ROIs for control subjects (black bars) and PD patients (red bars). Error bars indicate standard error for the group mean.
Disease Severity
Figure 3 shows there were significant r2 values for the relation between total motor UPDRS score and PSC in the bilateral caudate, bilateral anterior and posterior putamen, contralateral GPe, bilateral STN, contralateral SN, and thalamus. The relation between disease severity and percent signal change was always negative (ie. negative correlation coefficient). Within the BG, all contralateral regions except STN showed higher r2 values for the relation between total motor UPDRS and PSC than ipsilateral ROIs. The r2 values for M1 and SMA did not approach significance. It is important to note that the significant r2 values in the BG and thalamus were achieved in spite of the fact that this group of patients had relatively mild disease severity.
Figure 3.
Percent variance accounted for in disease severity from percent signal change in each basal ganglia, medial thalamus, and cortical ROI. r2 values for the total motor UPDRS score for contralateral ROIs (black bars) and ipsilateral ROIs (red bars) are shown.
Correlation among the UPDRS subscales was first examined. The bradykinesia subscale correlated significantly with both rigidity (r = 0.74) and axial function/balance/gait (r = 0.70), and rigidity correlated significantly with axial function/balance/gait (r = 0.71). There were no significant correlations between tremor and the other subscales. These correlation values are within an acceptable range so as to not produce multicollinearity in multiple regression.12 Multiple regression analysis revealed that bradykinesia contributed significantly to the BOLD signal in all contralateral basal ganglia nuclei except SN, and to the BOLD signal in all ipsilateral basal ganglia nuclei except ipsilateral GPi and SN (Table 3). Bradykinesia also contributed significantly to the BOLD signal in the thalamus. Rigidity and axial function did not contribute significantly to the BOLD signal in any ROI. Tremor significantly predicted the BOLD signal in contralateral GPi. It is important to note that the beta coefficients were positive for bradykinesia and negative for tremor (Table 3). No UPDRS subscale contributed significantly to the BOLD signal in SMA or M1. Since the bradykinesia subscale had a larger potential range of scores (range 0-32) than the other subscales (range 0-28 for tremor, 0-20 for rigidity, and 0-28 for axial function/balance/gait) we determined if the results for bradykinesia and the BOLD signal were simply due to a range effect. We computed a reduced range bradykinesia score, which included scores from bradykinesia items 23-25 with a range of scores from 0-24. Overall, the pattern of results did not change suggesting that the range of scores was not the driving factor.
Table 3.
Multiple regression results
| Beta coefficient |
|||||||
|---|---|---|---|---|---|---|---|
| ROI | Bradykinesia | Rigidity | Tremor | Axial function | Adjusted R2 | F | p |
| C Caudate | -1.00 | 0.35 | 0.22 | --- | .46 | 6.34 | .005 |
| I Caudate | -0.92 | 0.31 | 0.21 | --- | .35 | 4.34 | .020 |
| C Ant Putamen | -0.67 | --- | --- | --- | .42 | 14.47 | .001 |
| I Ant Putamen | -0.49 | .20 | 5.71 | .028 | |||
| C Post Putamen | -0.67 | --- | --- | --- | .42 | 14.82 | .001 |
| I Post Putamen | -0.54 | .25 | 7.35 | .014 | |||
| C GPe | -0.60 | --- | --- | --- | .32 | 10.12 | .005 |
| I GPe | -0.48 | .19 | 5.34 | .033 | |||
| C GPi | -0.59 | --- | 0.54 | -0.16 | .44 | 5.95 | .006 |
| I GPi | -0.47 | 0.25 | .10 | 2.02 | .162 | ||
| C STN | -1.10 | 0.47 | 0.25 | --- | .50 | 7.11 | .003 |
| I STN | -0.98 | 0.33 | 0.34 | -0.04 | .41 | 4.24 | .017 |
| C SN | -0.31 | -0.32 | --- | --- | .27 | 4.45 | .028 |
| I SN | --- | -0.70 | --- | 0.47 | .16 | 2.76 | .092 |
| M Thalamus | -0.79 | 0.27 | --- | .46 | 9.26 | .002 | |
| L Thalamus | -0.86 | 0.50 | 0.33 | --- | .20 | 2.58 | .090 |
| SMA | -0.67 | 0.36 | 0.48 | --- | .16 | 2.19 | .129 |
| M1 | -0.29 | --- | --- | --- | .03 | 1.63 | .218 |
Bold type for each beta coefficient indicates a significant regression coefficient at p < .05; Bold type for the p value of the regression model indicates a significant regression model at p< .05; --- indicates an item was not kept in the regression model.
Discussion
There were three novel findings in this study. First, there were significant negative correlations between total motor UPDRS score and fMRI BOLD activation in bilateral caudate, bilateral anterior and posterior putamen, contralateral GPe, bilateral STN, contralateral SN, and thalamus in early stage de novo PD. Second, using multiple regression analysis to identify which UPDRS factors significantly predicted the BOLD signal in each ROI, it was found that bradykinesia significantly predicted the BOLD signal in all basal ganglia nuclei except ipsilateral GPi and bilateral SN. Also, bradykinesia significantly predicted the BOLD signal in the thalamus. In contralateral GPi, tremor significantly predicted the BOLD signal with a negative beta coefficient. Third, while BOLD activation in M1 and SMA was reduced in PD, none of the UPDRS subscales significantly predicted the BOLD signal in these cortical areas. These novel findings suggest that fMRI has the potential to serve as a non-invasive state marker that relates symptom-specific disease severity with BG function in de novo PD.
Previous studies have used other imaging modalities such as SPECT and PET to examine brain function and disease severity in early stage PD. For example, SPECT performed on thirty six de novo PD patients showed a significant negative correlation between contralateral putaminal binding potential and increased UPDRS score (r2= 0.18).13 PET using 18F-fluorodeoxyglucose has been used to examine regional glucose utilization and spatial covariance patterns using network analysis and their relationship to disease progression.14 At baseline testing, PD patients who were within two years of initial diagnosis showed no difference from controls in glucose metabolism in the STN and GPi. When scanning was repeated 48 months later, PD patients showed significantly increased metabolism in the STN and GPi compared to controls. Changes in the PD-related motor metabolic covariance pattern (a measure of abnormal network activity in PD) correlated with increased motor UPDRS scores (r2 = 0.38). Other studies which have used either SPECT or PET to examine the degree of correlation between overall striatal or putaminal binding potential and UPDRS motor scores have shown r2 values ranging from 0.14 to 0.38.15, 16, 17, 18 However, the study sample in each of these studies included a mixture of de novo and treated patients. The results from the present study which examined only de novo patients with PD are within this range of r2 values and, in the case of the anterior putamen, slightly better. Therefore, when using a robust behavioral task, BOLD activation in specific nuclei of the BG examined with fMRI can be significantly correlated with total motor UPDRS even in early stage de novo PD.
Bradykinesia, tremor, rigidity, and axial functional/balance/gait are symptoms that impair the normal daily activities of patients with PD, and are recognized as factors in the UPDRS.6 The clinical presentation of these symptoms in each patient can be different in terms of their distribution and progression. This, taken together with evidence that each symptom can respond differently to therapeutic intervention, makes it seem likely that the pathophysiology underlying each symptom may be different.19 A previous neuroimaging study of patients more advanced in the disease process who were already taking anti-parkinsonian medication examined the metabolic substrate of bradykinesia and tremor.20 Using PET and 18F-fluoro-2-deoxyglucose in seventeen patients, they found that the severity of bradykinesia was related to higher cerebral glucose metabolic rate in the putamen and globus pallidus. In contrast, resting tremor was related to lower cerebral glucose metabolic rate in the putamen and cerebellar vermis. Within the putamen there was a large overlap of active voxels in the putamen bilaterally that were both negatively correlated with tremor scores and positively correlated with bradykinesia scores. The current findings are consistent with these findings for bradykinesia since bradykinesia significantly predicted the BOLD signal with a positive beta coefficient in both bilateral putamen and bilateral GPe and contralateral GPi. In addition, the current study found that bradykinesia subscales significantly predicted BOLD activation in bilateral caudate and bilateral STN. The only other UPDRS subscale that significantly predicted the BOLD signal in any BG nucleus was tremor. Both bradykinesia and tremor significantly predicted the BOLD signal in contralateral GPi, and the regression coefficient was positive for bradykinesia whereas it was negative for tremor. Taken together, these data provide support for the general hypothesis that major clinical features of PD, particularly bradykinesia and tremor, are related to distinct neuronal systems.19
Previous studies using F-6-Fluorodopa PET have begun to shed some light on which clinical signs best reflect the nigrostriatal lesion in PD. For example, Vingerhoets and colleagues21 used F-6-Fluorodopa PET in thirty five patients with moderately advanced PD in the off medicated state to provide an in vivo measure of nigrostriatal dopaminergic deficit. They correlated the PET results with clinical measures of function. They found that the nigrostriatal dopaminergic lesion correlated best with bradykinesia as measured by both the bradykinesia subscale of the modified Columbia score and the Purdue pegboard test. Inclusion of rigidity and postural disturbance scores in a regression model did not improve correlation with nigrostriatal lesion. This is in agreement with our findings where rigidity and axial function/balance/gait were not significant predictors in the regression model in any ROI. Given that there was some degree of correlation between the UPDRS subscales of bradykinesia, rigidity, and axial function/balance/gait, including clinical measures of rigidity and axial function/balance/gait to predict the BOLD signal may be redundant. One possible explanation is that these variables scored over a reduced range in our sample and therefore the bradykinesia results are due to a larger range effect. However, by computing a new bradykinesia variable which had a lower range, we found that he pattern of results did not change suggesting that a range effect does not explain the results of the current study. Another possibility is that the reliability of the rigidity and axial function/balance/gait scales of the UPDRS is lower than for bradykinesia.22-24
While bradykinesia may be a direct result of the nigrostriatal dopaminergic lesion that impairs cortical function via the BG-thalamocortical loop,21 tremor may have a different neural substrate. Intermittent oscillations of neurons in the motor cortex, ventrolateral thalamus, GPi, and STN have been shown to correlate temporarily with tremor, while lesioning of these regions can suppress tremor.25 In addition, resting state cerebral metabolic rate of glucose in specific voxels within the putamen has been shown to be negatively correlated with tremor.20 The results of the current study did not find that tremor significantly predicted the BOLD signal in putamen. Our ROI analysis was performed by averaging the BOLD signal across voxels, which may have affected our ability to find voxel-wise correlations between the BOLD signal and tremor in the putamen. Nevertheless, our findings for a significant regression model between tremor and the BOLD signal in GPi is consistent with measures of dopamine levels using high performance liquid chromatography in autopsy PD brains. Rajput and colleagues26 found that dopamine levels were greater in GPi of tremor-dominant PD patients compared with akinetic rigid PD patients.
An interesting observation was that bradykinesia subscales were generally better related to BOLD activation in BG nuclei (Table 3) than the relation between total motor UPDRS and BOLD activation (Figure 3). For example, the percent variance accounted for in the BOLD signal in contralateral caudate by bradykinesia was r2 = 0.46 whereas the relation between total UPDRS and BOLD signal in contralateral caudate had an r2 = 0.32. Lozza and colleagues20 found that tremor was negatively correlated with metabolic measures from PET and we found that the regression coefficient for predicting contralateral GPi BOLD activation was positive for bradykinesia whereas it was negative for tremor. These findings raise the possibility that measures of tremor may detract from the overall level of correlation between total motor UPDRS and BOLD activation. As such, neuroimaging biomarkers of the state of PD may be better suited to reflect specific symptoms of the disease, such as bradykinesia, rather than the total motor section of the UPDRS.
Supplementary Material
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (R01-NS-52318, R01-NS-58487, R01-NS-40902, R01-NS-28127). We thank the staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center, Chicago IL, and the patients for their time and commitment to this research.
Financial Disclosures of all Authors for the Past Year
Drs. Vaillancourt and Corcos have received funding from the National Institutes of Health (NIH; R01-NS-52318, R01-NS-58487, R01-NS-40902, R01-NS-28127). Dr. Comella has served as a consultant for Allergan, Merz, Ipsen, Esai, and Boehringer, and has received royalties from Kluwer publishing and Cambridge publishing. Dr. Comella has received research grants that go to her institution from Boehringer, Ipsen, Merz, and NIH.
Footnotes
Potential conflict of interest: None reported.
References
- 1.Brooks DJ, Frey KA, Marek KL, et al. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson's disease. Exp Neurol. 2003;184(Suppl 1):S68–79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Lang AE, Mikulis D. A new sensitive imaging biomarker for Parkinson disease? Neurology. 2009;72(16):1374–1375. doi: 10.1212/01.wnl.0000343512.36654.41. [DOI] [PubMed] [Google Scholar]
- 3.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp. 2009;30(5):1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson's disease. Human Brain Mapping. doi: 10.1002/hbm.20987. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stebbins GT, Goetz CG, Lang AE, Cubo E. Factor analysis of the motor section of the unified Parkinson's disease rating scale during the off-state. Mov Disord. 1999;14(4):585–589. doi: 10.1002/1531-8257(199907)14:4<585::aid-mds1006>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. 1992. Neurology. 2001;57(10 Suppl 3):S34–38. [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases [see comments]. Journal of Neurology Neurosurgery and Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaillancourt DE, Thulborn KR, Corcos DM. Neural Basis for the Processes that Underlie Visually-Guided and Internally-Guided Force Control in Humans. J Neurophysiol. 2003;90(5):3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- 10.Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. Neuroimage. 2008;39(3):956–965. doi: 10.1016/j.neuroimage.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31(4):1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabachinick BG, Fidell LS. Using multivariate statistics. 3rd ed. HarperCollins College Publishers; New York: 1996. [Google Scholar]
- 13.Berti V, Pupi A, Ramat S, et al. Clinical correlation of the binding potential with 123I-FP-CIT in de novo idiopathic Parkinson's disease patients. European journal of nuclear medicine and molecular imaging. 2008;35(12):2220–2226. doi: 10.1007/s00259-008-0872-4. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson's disease. Brain. 2007;130(Pt 7):1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshuis SA, Maguire RP, Leenders KL, Jonkman S, Jager PL. Comparison of FP-CIT SPECT with F-DOPA PET in patients with de novo and advanced Parkinson's disease. European journal of nuclear medicine and molecular imaging. 2006;33(2):200–209. doi: 10.1007/s00259-005-1904-y. [DOI] [PubMed] [Google Scholar]
- 16.Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson's disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15(4):692–698. doi: 10.1002/1531-8257(200007)15:4<692::aid-mds1014>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal progression of sporadic Parkinson's disease: a multi-tracer positron emission tomography study. Brain. 2009 doi: 10.1093/brain/awp209. [DOI] [PubMed] [Google Scholar]
- 18.Djaldetti R, Treves TA, Ziv I, Melamed E, Lampl Y, Lorberboym M. Use of a single [(123)I]-FP-CIT SPECT to predict the severity of clinical symptoms of Parkinson disease. Neurol Sci. 2009 doi: 10.1007/s10072-009-0100-4. [DOI] [PubMed] [Google Scholar]
- 19.Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol. 2004;14(6):715–719. doi: 10.1016/j.conb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Lozza C, Marie RM, Baron JC. The metabolic substrates of bradykinesia and tremor in uncomplicated Parkinson's disease. Neuroimage. 2002;17(2):688–699. [PubMed] [Google Scholar]
- 21.Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson's disease best reflects the nigrostriatal lesion? Ann Neurol. 1997;41(1):58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F. Unified Parkinson's Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9(1):76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 23.Richards M, Marder K, Cote L, Mayeux R. Interrater reliability of the Unified Parkinson's Disease Rating Scale motor examination. Mov Disord. 1994;9(1):89–91. doi: 10.1002/mds.870090114. [DOI] [PubMed] [Google Scholar]
- 24.Prochazka A, Bennett DJ, Stephens MJ, et al. Measurement of rigidity in Parkinson's disease. Mov Disord. 1997;12(1):24–32. doi: 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- 25.Elble RJ. Origins of tremor. Lancet. 2000;355(9210):1113–1114. doi: 10.1016/S0140-6736(00)02054-7. [DOI] [PubMed] [Google Scholar]
- 26.Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 2008;70(16 Pt 2):1403–1410. doi: 10.1212/01.wnl.0000285082.18969.3a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.