Abstract
The primary cilium organizes numerous signal transduction cascades and an understanding of signaling receptors trafficking to cilia is now emerging. A defining feature of cilia is the periciliary diffusion barrier that separates the ciliary and plasma membranes despite the topological continuity between these two membranes. Although lateral transport through this barrier may take place, polarized exocytosis to the base of the cilium has been the prevailing model for delivering membrane proteins to cilia. Key players for this polarized exocytosis model include the GTPases Rab8 and Rab11, the exocyst and possibly the intraflagellar tranport machinery. Sorting membrane proteins to cilia critically relies on the recognition of ciliary targeting signals by sorting machines such as the BBSome coat complex or the GTPase Arf4. Finally, signaling at the cilium entails the bidirectional movement of proteins between cytoplasm and cilia and ubiquitination may promote exit from cilia.
Keywords: CILIA, FLAGELLA, SIGNALING
DIFFUSION BARRIERTHE CENTRAL PROBLEM: SEPARATION BETWEEN THE SIGNALING COMPARTMENT AND THE SITE OF PROTEIN SYNTHESIS
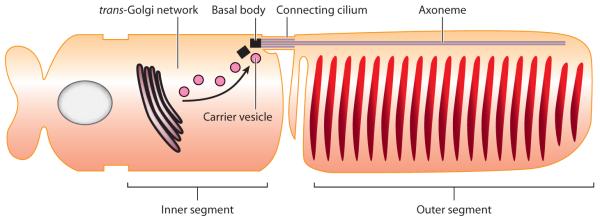
Cilia and flagella are some of the most ancient eukaryotic structures and thus define the cenancestral eukaryote in the same way as nuclei and mitochondria do (Cavalier-Smith 2002). Although the structural organization of cilia and flagella appears fairly invariant, consisting of a core of nine microtubule doublets ensheathed within the ciliary membrane, the functions of cilia and flagella are remarkably diverse. Flagella and motile cilia (these terms can be used interchangeably) bend in a sine-wave fashion to generate fluid movement (if the cell is immobilized) or cell propulsion (if the cell is free). Conversely, a single nonmotile cilium---also called the primary cilium or monocilium---is present on most cells in the body, and primary cilia actively participate in signaling pathways such as phototransduction, olfactant sensing, Hedgehog (Hh) signaling, and planar cell polarity ( PCP). Together with the concentration of signaling receptors in the membrane of cilia, these fascinating aspects have led to the view of cilia as “signaling antennas” that extend a few micrometers away from the cell body to present signaling receptors to the outside world (Singla & Reiter 2006). The antenna paradigm applies particularly well to the photoreceptor cell of the retina, in which a primary cilium (also called an outer segment) acts as a collecting antenna for photons by concentrating more than 109 molecules of the photosensory G protein-coupled receptor (GPCR) rhodopsin in 103 stacked disks (Besharse et al. 1977; Figure 1). This enormous concentration of rhodopsin in photoreceptor outer segments and the histological organization of the retina allow the human eye to accomplish the astounding feat of detecting single photons in perfectly dark conditions (Rieke & Baylor 1998).
Figure 1.
The photoreceptor cell, an exemplar of polarized trafficking to cilia. This schematic diagram of a photoreceptor cell illustrates the key steps in rhodopsin trafficking from the Golgi complex to the outer segment.
Flagella: equivalent to motile cilia. Describes longer (>10 μm) structures present in small number (one or two per cell)
Cilia: hair-like projections at the cell surface consisting of a core of nine microtubule doublets ensheathed within a membrane; an be motile or nonmotile
Hedgehog (Hh): named after a Drosophila mutant, it is a morphogen required for neural tube and limb patterning
Planar cell polarity (PCP): the polarization of cells within the plane of an epithelium, e.g. cell spikes on the fly wing consistently point to the posterior end
However, this concentration of rhodopsin (as well as other signaling molecules such as the trimeric G protein transducin, the cGMP gated channel, and the phosphodiesterase PDE6) in the cilium poses a tremendous problem for the photoreceptor because no biosynthetic machinery is present in cilia. Therefore, all membrane and soluble proteins of the cilium need to be transported from the cell body (i.e., the inner segment). This separation between the site of protein synthesis (the cell body) and the site of protein function (the cilium) constitutes a central problem of ciliary biology and is particularly salient in photoreceptors because of the constant shedding of older disks at the distal end of their cilia. Classic pulse chase studies have estimated that, in frog photoreceptors, 8% of the disks are turned over every day (Besharse et al. 1977). Given that each disk contains 106 molecules of rhodopsin, nearly 1,000 rhodopsin molecules are transported through the connecting cilium every second. For comparison, the plasma cell, a professional immunoglobulin (IgG)-producing cell, secretes 1,400 IgG molecules per second. The trafficking feat accomplished by photoreceptors is even more remarkable when one considers that all of the rhodopsin molecules must pass through the connecting cilium, whose diameter is only 0.3 μm. The net mass flow of rhodopsin through the connecting cilium thus amounts to 40 MDa per second per cilium, in vast excess of the estimated protein mass flow through the nuclear pore complex (7 MDa s−1) (Ribbeck & Görlich 2001). This extreme example of rhodopsin trafficking in photoreceptors begs the question of how membrane proteins are transported to the primary cilium. Yet, compared with other membrane trafficking routes, our understanding of trafficking to the cilium remains in its infancy.
FUNCTIONAL SEPARATION BETWEEN CILIARY AND PLASMA MEMBRANES
At first glance, the transport of membrane proteins to cilia need not follow the general paradigm of carrier vesicles targeted to and fusing with an acceptor organelle. Indeed, the ciliary and plasma membranes are topologically continuous, and simple lateral diffusion of lipids and membrane proteins could provide a straightforward means for membrane proteins to enter the cilium. Although this model has some merits, there is substantive evidence for the presence of a membrane diffusion barrier at the base of the cilium that would prevent the lateral diffusion of membrane proteins between plasma and cilia membranes. Such membrane diffusion barriers have been described in a variety of cells; the best-characterized examples lie between the apical and basolateral membranes of polarized epithelial cells and at the base of the axon in mature neurons (Caudron & Barral 2009). In epithelial cells and in neurons, the existence of a diffusion barrier was rigorously demonstrated by showing that a freely diffusible lipid is unable to exchange between apical and basolateral membranes (van Meer et al. 1986, Nakada et al. 2003). In the following subsections, we examine the evidence for a membrane diffusion barrier at the base of the cilium.
Concentration of Signaling Receptors at the Ciliary Membrane
Cilia were originally implicated in signaling pathways such as Hh or PCP and in diseases such as polycystic kidney disease (PKD) based on the phenotypes of mouse mutants unable to assemble cilia (Huangfu et al. 2003, Pazour et al. 2000). However, the importance of cilia in signaling pathways only began to make sense when it was discovered that signaling factors localize to cilia (Nauli et al. 2003, Pazour et al. 2002, Yoder et al. 2002). In particular, each element of the Hh transduction cascade localizes to cilia at one point or another during Hh signaling (Chen et al. 2009, Corbit et al. 2005, Haycraft et al. 2005, Kim et al. 2009, Rohatgi et al. 2007). In unstimulated cells, the Hh receptor Patched is detected in cilia and represses signaling, whereas the 7-transmembrane Hh transducer Smoothened (Smo) is absent from cilia. Upon Hh pathway engagement, the situation is diametrically opposite: Patched disappears from cilia, Smo accumulates in cilia and the Gli2 and Gli3 transcription factors become enriched at the tip of the cilium where they presumably become activated. However, whether the movement of Smo into cilia is absolutely required for signaling or relates to a mere epiphenomenon remains an important yet unanswered question. This concentration of transmembrane signaling proteins in the ciliary membrane is also apparent in platelet-derived growth factor αα (PDGFαα) signaling by fibroblasts (Schneider et al. 2005), in mechanosensing by the Ca2+ channels polycystin1 and 2 (Nauli et al. 2003), in gustatory sensing by tracheal epithelial cells (Shah et al. 2009), and in olfactory sensation by olfactory receptor neurons (Mayer et al. 2008). In the latter case, it was shown that membrane proteins of the olfactory transduction cascade such as adenylate cyclase III and the cyclic nucleotide gated channel are concentrated 50-fold in ciliary extracts compared with total cell extracts (Mayer et al. 2008).
Although it is clear that several membrane proteins are concentrated in the membrane of the cilium, this mere fact cannot be taken to indicate the presence of a diffusion barrier. Indeed, it has been demonstrated in several cases that membrane proteins are anchored in the cilium through interactions with the intraflagellar transport (IFT) machinery (Qin et al. 2005). Even in cases in which interactions with the IFT machinery are not obvious, it is easily conceivable that ciliary enrichment could be the result of continuous active transport to cilia and slow outward diffusion into the plasma membrane.
Intraflagellar transport (IFT): the process by which proteins are transported up and down inside the ciliary shaft; the IFT-A and IFT-B complexes mediate IFT
Functionally Distinct Pools of Agglutinins at the Plasma and Flagellar Membranes
The system most often cited for providing evidence of a membrane diffusion barrier at the base of the cilium is based on the study of gamete adhesion in the unicellular green algae Chlamydomonas reinhardii. In the earliest fertilization event, gametes of opposite mating type adhere (“agglutinate”) to one another via giant (>1 MDa) glycoproteins termed agglutinin that are present on the naked surfaces of their flagella (the cell body of Chlamydomonas is covered by an impermeable cell wall). Surprisingly, in a naive gamete, only 10% of the agglutinins localize to the flagella; the remaining 90% of agglutinins are present in the plasma membrane (Hunnicutt et al. 1990). The fact that Chlamydomonas gametes never adhere through their cell body (even after it its removed) indicates that the surface-exposed cell body agglutinins are inactive and thus functionally different from the adhesion-competent agglutinins on the flagellum. This led to the initial conclusion that “a functional barrier prevents utilization of preexisting cell body agglutinins by the flagella” (Hunnicutt et al. 1990) and was later interpreted to suggest the existence of a membrane diffusion barrier that separates the ciliary membrane from the plasma membrane (Pazour & Bloodgood 2008). However, cell body agglutinins could be prevented from diffusing into the flagellar membrane by anchoring through interaction with the cortical cytoskeleton. In support of this anchoring model, fused gametes do not detectably exchange agglutinins over a 30 min period despite having fused their plasma membranes together (Musgrave et al. 1986).
The Glycosylphosphatidylinositol-Fluorescent Protein Black Hole: Evidence for a Diffusion Barrier at the Base of the Cilium
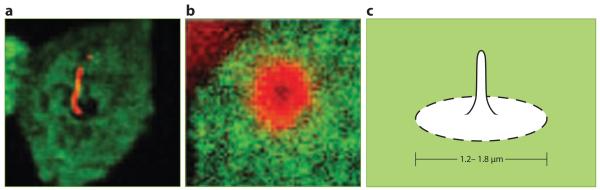
A more convincing demonstration for the existence of a diffusion barrier made use of a fluorescent protein (FP) genetically encoded to become lipid-anchored and incorporated into the outer leaflet. This glycosylphosphatidylinositol-FP (GPI-FP) is specifically targeted to the apical plasma membrane in polarized epithelial cells such as the kidney tubule cell line Malin-Darby Canine Kidney (MDCK). However, GPI-FP fails to diffuse from the apical plasma membrane into the ciliary membrane, which is seen as a black hole in the GPI-FP channel (Vieira et al. 2006) (Figure 2a). Quite surprisingly, the diameter of the structure that excludes GPI-FP is 1.2--1.8 μm, much greater than the 0.3 μm diameter of the cilium (Figure 2c). Furthermore, the lectin Galectin-3 is localized in a doughnut-shaped ring that fits precisely inside the excluded zone of GPI-FP (Figure 2b). Thus, the diffusion barrier that separates the ciliary and plasma membranes may isolate a much greater area than what is traditionally defined as the ciliary membrane. One argument against the use of GPI as a freely diffusable lipid is that GPI is itself incorporated into 50-nm size microdomains of the plasma membrane called lipid rafts (Simons & Toomre 2000). However, other lipid raft markers are either equally distributed in the ciliary membrane and the plasma membrane (e.g. Forssman glycolipid and the ganglioside GM1) or highly concentrated in the ciliary membrane itself (GM3) (Vieira et al. 2006, Janich & Corbeil 2007). Secondly, the GPI-FP used in the above study has been shown to diffuse as rapidly as a nonraft protein within the plasma membrane, which indicates that GPI-FP must rapidly partition into and out of rafts (Kenworthy et al. 2004). Therefore, the exclusion of GPI-FP from the ciliary membrane can be taken as evidence that a lipid diffusion barrier encircles the base of the cilium.
Figure 2.
The diffusion barrier that separates ciliary and plasma membranes. (a) Glycosylphosphatidylinositol-fluorescent protein (GPI-FP, green), a GPI-anchored FP, is excluded from a zone surrounding the base of the primary cilium (acetylated tubulin, red). (b) Galectin-3 (red), a carbohydrate-binding protein, concentrates at the base of the cilium within the zone of exclusion of GPI-FP (green). (c) Schematic representation of the periciliary diffusion barrier. Images in (a) and (b) reproduced from Vieira et al. (2006), copyright © 2006, National Academy of Sciences, U.S.A.
IS THE CILIUM A CELLULAR ORGANELLE?
Even though cilia do not adhere to the strictest definition of organelles as “membrane-enclosed compartments” (Alberts et al. 2002), the existence of a membrane diffusion barrier that encloses the cilium away from the plasma membrane makes cilia de facto organelles. As such, it will be interesting to test whether specialized lipids are enriched in the ciliary membrane in the same way as the phosphoinositide PI(4)P marks the Golgi complex, PI(3)P the endosomes, and PI(4,5)P2 the plasma membrane (Di Paolo & De Camilli 2006). Some efforts made at defining the ciliary lipidome in the 1980s showed that Paramecium cilia contain four times more sphingolipids than the cell bodies (Kaneshiro 1987) and hinted at cholesterol enrichment in ciliary membranes (Chailley & Boisvieux-Ulrich 1985, Montesano 1979, Souto-Padrón & de Souza 1983). Several sphingolipids have now been localized to trypanosome flagella (galactocerebroside and GM1) and to mammalian primary cilia (GM1 and GM3) and some sterols localize to trypanosome flagella (Janich & Corbeil 2007, Tyler et al. 2009). In addition, the inositol 5-phosphatase INPP5E has been localized to cilia, and mutations in INPP5E in humans and mice produce phenotypes characteristic of ciliary dysfunction (kidney cysts, polydactyly, neural tube closure defects) (Bielas et al. 2009, Jacoby et al. 2009). Cilia may therefore be enriched in the products of INPP5E, i.e., PI(4)P and PI(3,4)P2. More generally, the application of modern lipidomics tools is likely to uncover specialized lipids of the ciliary membrane and may cast light on why the cilium has been selected as a locale by so many signaling pathways. For example, the fatty acid omega-3 docosahexaenoic acid (DHA) accounts for more than 40% of all acyl chains in the retinal outer segment disks (Aveldaño & Bazán 1983). The preponderance of multikinked chains renders the disk membrane hyperfluid and gives rhodopsin the highest known diffusion coefficient of any membrane protein at 0.4 μm2 s−1 (Poo & Cone 1974), a value 100 times higher than that of other typical plasma membrane proteins (Jacobson 1983). In turn, the membrane hyperfluidity and high mobility of rhodopsin enable ultrafast protein-protein interactions, and membrane hyperfluidity may therefore play a central role in the extreme sensitivity of phototransduction (Niu et al. 2004).
Omega-3 docosahexaenoic acid (DHA): a polyunsaturated 22-carbon long chain fatty acid with six double bonds that is most abundant in photoreceptors
Given that the membrane of the cilium does not appreciably intermix with the plasma membrane, one wonders if a similar diffusion barrier exists for soluble molecules. The diffusion of soluble molecules between the ciliary lumen and the cytoplasm has only been examined in photoreceptor cells, and the 27 kDa green fluorescent protein (GFP, a barrel 4.2 nm long by 2.4 nm diameter) can equilibrate between frog photoreceptors’ inner and outer segments within ~3 min, very close to the theoretical value of 3.5 min assuming free diffusion through the connecting cilium (Nair et al. 2005, Calvert et al. 2006, Calvert et al. 2010). Remarkably, it is now thought that the vectorial transport of arrestin, the rhodopsin desensitizer, and transducins Gα and Gβγ between the inner and outer segments are driven solely by diffusion through the connecting cilium and by retention at binding sites provided by rhodopsin in the outer segment (Calvert et al. 2006). Although one may argue that the absence of a diffusion barrier for solutes is a unique feature of the ultraspecialized photoreceptor cilium, it should be noted that the ultrastructure of the connecting cilium and its associated base do not deviate in any manner from the run-of-the-mill cilium. Thus, even though the ciliary membrane is a privileged compartment, the ciliary lumen is likely to have a composition that closely resembles the cytoplasm, at least in terms of ions, small molecules, and small proteins. Furthermore, whereas GFP diffuses freely though the connecting cilium, we know little of the fate of larger proteins. The periciliary base may behave similar to the nuclear pore complex, which excludes proteins larger than 60 kDa, slows the diffusion of proteins 30--60 kDa in size, and allows in anything smaller than 30 kDa. The use of inert GFP fusions of increasing sizes in photoreceptors should provide an answer to this important question. An indication that large proteins do not enter the ciliary lumen freely is that the transport into flagella of the radial spoke complex and the outer dynein arm, two very large molecular assemblies, requires the activity of the IFT complex (Qin et al. 2004, Hou et al. 2007).
ULTRASTRUCTURAL ORGANIZATION OF THE PERICILIARY BASE
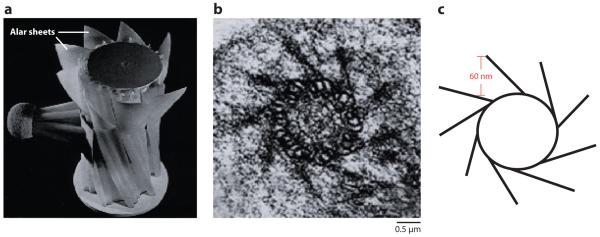
The above-noted parallel between the nuclear pore and the periciliary base has led to the notion of a ciliary pore complex (Satir & Christensen 2007) that establishes a diffusion barrier for membrane proteins and possibly some large soluble proteins and thus functions as a gate for the entry of proteins into cilia. This ciliary pore complex is comprised of the basal body, its accessory structures, the most proximal portion of the ciliary axoneme, and a poorly defined membrane zone that encircles the cilium. The basal body itself consists of nine triplet microtubules, designated A, B, and C with respect to increasing distance from the center of the basal body. Whereas the A and B tubules extend to form the axoneme, the C tubules terminate before the axoneme at a region known as the transition zone. The transition zone also contains the transition fibers, which anchor the C microtubules of the basal body to the most proximal region of the ciliary membrane (Figure 3b). These transition fibers are therefore the obvious candidate to preclude the movement of proteins and vesicles from the cytoplasm to the ciliary lumen and are thought to constitute the ciliary gate itself. Remarkably, the most accurate reconstructions of the transition fibers suggest them to be rather unfiber-like; instead they appear as thin trapezoidal “wings” appropriately termed alar sheets (Anderson 1972) (Figure 3a). The basal edge of each of the nine alar sheets is anchored along the longitudinal axis of each C tubule (Figure 3b), and the opposite edge contacts the plasma membrane. Thus, the alar sheets form a nine-bladed propeller-like structure jammed at the entrance of the ciliary lumen, and the space between two consecutive alar sheets accommodates particles smaller than 60 nm in diameter (Anderson 1972; Figure 3c).
Figure 3.
Ultrastructure of the periciliary base. (a) A brick-and-mortar basal body reconstruction (Anderson 1972). The so-called transition fibers appear as wing-like structures (alar sheets) that cover most of the space at the base of the cilium. (b) Transverse electron microscopic section through the basal body transition zone (Anderson 1972). (c) Tracing of the basal body microtubule barrel and alar sheets from (b). The bottleneck shown in red can only accommodate particles 60 nm and smaller.
Alar sheet: also called transition fibers, these structural elements connect the basal body to the ciliary membrane and restrict access to the ciliary lumen
TRAFFICKING OF MEMBRANE PROTEINS TO THE CILIUM: HYPOTHESES
Given that the cilium has all the properties of an organelle, it must contend with a central problem of any organelle: how to get specific proteins delivered to its membrane. Typical interorganelle trafficking entails packaging of membrane proteins into a carrier vesicle, transport of the vesicle to the acceptor organelle, and fusion of the vesicle with the acceptor membrane. However, given the specific geometry of the periciliary base, the models for trafficking to cilia are likely to be more complex.
Vesicular Targeting to the Inside of the Cilium
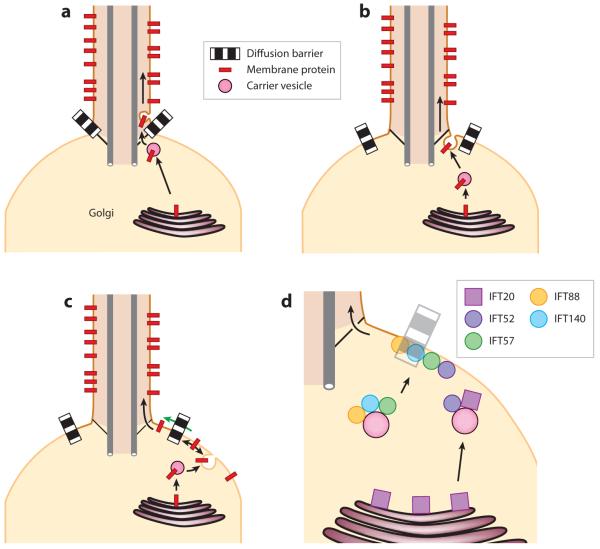
One of the simplest models for trafficking of membrane proteins to the cilium posits that vesicles emerging from the Golgi complex are transported to the base of the cilium, enter the ciliary lumen, and then fuse with the ciliary membrane (Figure 4a). However, given the structural constraints posed by the alar sheets, the possibility of vesicular traffic through the ciliary pore is essentially ruled out because the size of the smallest transport vesicles exceeds 60 nm. Intriguingly, there exist clear examples of intraciliary vesicles in electron micrographs of olfactory cilia (Reese 1965), and every olfactory cilium has small vesicles in its proximal segment. Furthermore, small vesicles have been repeatedly observed in the primary cilia of chondrocytes (Poole et al. 1985). There are three possible explanations for the existence of intraciliary vesicles. First, these vesicles could be artifacts of the chemical fixation methods used. The conspicuous absence of such vesicles from the Chlamydomonas flagellum despite 40 years of intense study certainly casts doubt about their universal existence. Repeating the ultrastructural studies on olfactory cilia and chondrocyte cilia using high pressure freezing and freeze substitution, a method devoid of bubbling artifacts, would unquestionably ascertain the existence of intraciliary vesicles. Second, if such intraciliary vesicles do exist, it is possible that endocytosis and exocysosis take place at ciliary membrane. Indeed, an example of intraciliary endocytosis is provided by the formation of disks inside the outer segment of photoreceptor. In this case, intraciliary vesicles would be self-contained within cilia and would not provide a means of exchange between the endomembrane system and the ciliary membrane. Third, the alar sheets could deform to accommodate vesicles larger than 60 nm. Although this remains a distant possibility, one would expect such a dramatic structural remodeling to be a rate-limiting step in the transport of vesicles from cytoplasm to cilia and thus to be readily captured in electron micrographs of the periciliary base. Because no such vesicle distending the alar sheet has ever been observed, it is safe to assume that the former two possibilities are the most likely. Therefore, it would appear highly unlikely that the passage of vesicles through the ciliary gate could solve the problem of membrane proteins targeting to the ciliary membrane.
Figure 4.
Three models for the trafficking of membrane proteins to the cilium. All models attempt to solve the problem of crossing the lipid diffusion barrier. The first two models are variations on the same theme, as both rely on polarized trafficking of post-Golgi vesicles to the base of the cilium. (a) Post-Golgi vesicles could deliver membrane proteins to the cilium by fusing with the ciliary membrane inside the ciliary shaft. (b) An extension of the previous model builds on the observation that the periciliary diffusion barrier is likely positioned at least 0.5 μm away from the base of the ciliary shaft. This 0.5-μm zone between the cilium proper and the diffusion barrier can still be considered ciliary membrane and may provide the site for vesicle docking and fusion. (c) In the last model, vesicles are delivered isotropically to the plasma membrane, and membrane proteins somehow cross the diffusion barrier (green arrow) to reach the ciliary membrane. (d) A speculative model for the sequential sorting and delivery of cargoes to cilia by intraflagellar transport (IFT) proteins. IFT20 is the only IFT protein present on the Golgi complex, and IFT20 associates with post-Golgi vesicles together with IFT52 while IFT88, -57, and -140 are present on a distinct vesicle population. En route to the base of the cilium, IFT20 is shed from the IFT complexes, and additional IFT subunits are incorporated into the cilia-destined IFT train.
Vesicular Targeting to a Privileged Domain at the Base of the Cilium
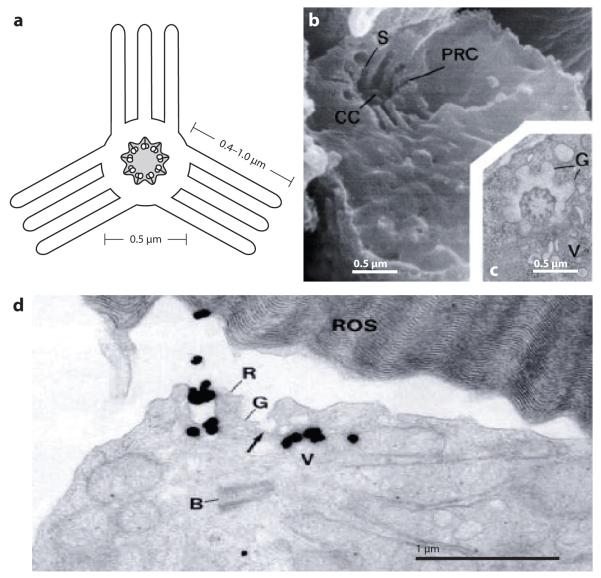
The most widely accepted model for trafficking of membrane proteins to cilia relies on the precise exocytosis of post-Golgi vesicles at the periciliary base (Rosenbaum & Witman 2002, Pazour & Bloodgood 2008; Figure 4b). Vesicular targeting to the bud neck formed between mother and daughter cells in Saccharomyces cerevisiae provides precedent for such a model (Drubin & Nelson 1996). Remarkably, polarized trafficking to cilia and to the yeast bud neck both employ the GTPase Rab8/sec4, its exchange factor Rabin8/sec2, and the exocyst complex (Goud et al. 1988, Walch-Solimena et al. 1997,Moritz et al. 2001, Nachury et al. 2007, Zuo et al. 2009). In both cases, the tubulin and actin cytoskeletons are clearly polarized toward the target, with actin cables and parallel microtubules oriented toward the bud neck (Kilmartin & Adams 1984, Adams & Pringle 1984) and microtubules anchored at the basal body (Sorokin 1962). In the case of the cilium, the targeted exocytosis model was initially proposed based on ultrastructural studies of mastigoneme transport in Chlamydomonas (Bouck 1971). It received considerable support from pulse-chase studies in photoreceptor cells (Papermaster et al. 1985), in which 135 min after the application of a pulse of radioactive amino acids to frog retina, most of the radioactivity has been incorporated into rhodopsin and is found in vesicles close to or fusing with the base of the cilium (Figure 5d). Further immunocytochemical studies demonstrated that the vesicles fusing with the periciliary base do indeed contain rhodopsin (Papermaster et al. 1985). Interestingly, the periciliary base of frog photoreceptors shows a characteristic structure named the periciliary ridge complex (PRC) (Peters et al. 1983) (Figure 5a--c). The PRC consists of nine symmetrically arrayed ridges and grooves that extend laterally ~0.4--1 μm along the rod inner segment plasma membrane. Most remarkably, vesicle fusion occurs exclusively at the grooves of the PRC (Figure 5d), indicating that the PRC provides docking sites for rhodopsin carrier vesicles (Papermaster et al. 1985). Whereas the PRC has only been imaged in frog photoreceptor, it is likely that such a structure exists in all cilia but remains inconspicuous unless hypertrophied to meet the enormous trafficking demands seen in photoreceptors. The similar diameters of the PRC (Figure 5a) and of the GPI-FP black hole (Figure 2c) are certainly suggestive of a periciliary complex establishing the diffusion barrier and providing docking sites for polarized exocytic transport.
Figure 5.
The periciliary ridge complex (PRC) of photoreceptors. (a) Schematic cross-section of the PRC adapted from Peters et al. (1983). Note the connecting cilium (CC) at the center. (b) Scanning electron micrograph of the PRC as seen from the inner segment (Peters et al. 1983). The PRC is organized into grooves and ridges. Steps (S) are found between the deep and shallow parts inside the periciliary groove. The CC can be seen at the center of the PRC. (c) Transverse section of the PRC (Peters et al. 1983). V, vesicle. Note the central location of the CC. (d) Thin-section electron micrograph of the periciliary region 2.25 hours following radioactive amino acid labeling (Papermaster et al. 1985). Most of the label is incorporated into rhodopsin that is found in vesicles fusing in the grooves (G) of the PRC. B is the daughter centriole of the basal body complex.
Exocyst: an octameric rod-like tethering complex required for vesicle docking and fusion in polarized exocytic trafficking to the basolateral membrane and the yeast bud tip
Lateral Transport from the Plasma Membrane
The last model states that ciliary membrane proteins are first incorporated in the plasma membrane and then enter the cilium after crossing the membrane diffusion barrier and being retained in the cilium (Figure 4c). The first indication of the diffusion and retention mechanism came from Bill Snell’s study of agglutinin activation (Hunnicutt et al. 1990). As noted above, agglutinins are distributed in two pools on the cell surface: 90% in an inactive pool at the plasma membrane and 10% in an adhesion-competent pool at the flagellar membrane. Since the initial adhesion event activates a signal transduction cascade within flagella that leads to the inactivation of paired agglutinins, Chlamydomonas gametes must continuously replenish agglutinins in their flagella. Surprisingly, preexisting plasma membrane agglutinins rather than neosynthesis refill the flagellar agglutinin pool. Although the presence of flagellar agglutinin precursors on the plasma membrane suggested the possibility that the transport process merely involved crossing the diffusion barrier, it did not exclude the hypothesis that plasma membrane agglutinins became internalized and that polarized trafficking of endosomal vesicles would ensure the delivery of agglutinins to the base of the cilium.
Recently, an elegant study concluded that lateral transport is responsible for directing Smo to cilia. It had been previously shown that, following Hh pathway activation, Smo appears at the ciliary membrane but the origin of ciliary Smo had remained unknown. In unstimulated cells, Smo is found at the plasma membrane and in the endomembrane system (Milenkovic et al. 2009). Surprisingly, pulse labeling studies show that at t1h post-Hh stimulation, most of the ciliary Smo originates from a pool of Smo that resided at the plasma membrane at t0. Similar to agglutinins, Smo turns over rapidly in stimulated cells and at t4h post-Hh stimulation, ciliary Smo is largely contributed by endomembrane-localized Smo at t0 (Wang et al. 2009). This lag phase in the trafficking of Smo from endomembrane compartments to cilia can be interpreted in two ways. First, Smo may always pass through the plasma membrane before entering the cilium. Second, the cell may utilize the plasma membrane pool of Smo for the initial rapid response to Hh and may then rely on directed exocytosis of Smo-containing vesicles to replenish the cilium with Smo. Since dynamin is not required for the movement of Smo to cilia, the first scenario would predict that Smo traffics from the plasma membrane to the cilium by lateral transport and independently from endocytosis (Milenkovic et al. 2009). However, because the effects of dynamin inhibition were assessed at t4h post-Hh stimulation, the second interpretation would leave open the possibility that polarized exocytosis of Smo-carrier vesicles delivered Smo to cilia while the movement of Smo from the plasma membrane to the cilium was blocked by dynamin inhibition. Hence a definitive proof for the transport of Smo into cilia by lateral diffusion from the plasma membrane may require following single molecules of Smo after Hh stimulation.
The lateral transport model poses many exciting questions. At first glance, diffusion between the whole of the plasma membrane and the periciliary base, a target area that constitutes less than a hundredth of the total plasma membrane area, seems like an extremely slow and inefficient process. However, modeling the problem as a 2D diffusion to capture by an annular absorber (the periciliary diffusion barrier, 1.5-μm diameter) centered within an impermeable boundary (the apical surface of an epithelial cell treated as a 15-μm diameter circle) shows that the mean time to capture of a given membrane protein (of D = 4 × 10−−9 cm2 s−−1) by the periciliary base is less than 2 min (Berg & Purcell 1977). Clearly, lateral diffusion need not be a slow or inefficient mechanism. Secondly, how generally used is the hypothetical Golgi complex → plasma membrane → cilia route? Is it limited to signaling components such as Smo or the agglutinins that need to be stockpiled in a readily accessible location for the cell to rapidly respond to signaling cues? Or is it the prevalent trafficking route for ciliary membrane proteins? In the latter scenario, the targeted trafficking of rhodopsin would be interpreted as an adaptation to the extreme demands of rhodopsin turnover. Indeed, the photoreceptor’s trafficking machinery is so utterly dedicated to moving rhodopsin from its site of synthesis to the outer segment that any membrane protein that is not retained in the ER or targeted to the synapse is taken along for a ride on the rhodopsin freight train to the outer segment (Baker et al. 2008). Still, the utilization of polarized trafficking factors such as Rab8 and the exocyst for ciliogenesis in cultured mammalian cells such as telomerase-immortalized retinal pigmented epithelial (RPE) cells and MDCK cells (Nachury et al. 2007, Yoshimura et al. 2007, Zuo et al. 2009) strongly suggests that targeted exocytosis is not simply an exception. It is thus likely that both lateral transport from the plasma membrane and targeted exocytosis coexist within the cell.
Lastly, one wonders how membrane proteins could cross a seemingly impassable lipid diffusion barrier. Here, it is worth examining the known mechanisms for establishing a diffusion barrier in other settings.
HOW TO MAKE AND CROSS A MEMBRANE DIFFUSION BARRIER
Biophysics of Membrane Diffusion Barriers
The most rigorous work on diffusion barriers comes from single lipid tracking experiments performed by the Kusumi lab in cultured neurons. Historically, one of the most puzzling observations in membrane biophysics was that lipid diffusion in the plasma membrane of cells is 10 to 100 times slower than in artificial liposomes (Lee et al. 1993). It was found that this discrepancy is the result of a fence anchored in place by an actin-based membrane skeleton (Fujiwara et al. 2002). Lipids rapidly diffuse within each 230-nm nanocell delineated by the picket fence, but the hop from one nanocell to the next is only observed occasionally, thereby effectively restraining diffusion across large distances. Although hypothetical at first, nanocells formed by the actin-based membrane skeleton were directly observed by ultrastructural imaging of the cytoplasmic side of the plasma membrane (Morone et al. 2006). In the case of the axonal diffusion barrier, the local accumulation of immobilized membrane proteins such as Ankyrin-G and the sodium channel at the axon/cell body junction was found to coincide with the appearance of the diffusion barrier at the base of the axon (Nakada et al. 2003). According to Nakada et al. (2003), “these immobilized transmembrane proteins effectively function as rows of pickets against free diffusion of molecules in the membrane (through steric hindrance) and increase packing near the protein (in terms of the free-volume theory) or increase hydrodynamic-like friction (in terms of the hydrodynamic theory).” Indeed, Monte-Carlo simulations showed that covering more than 25% of the edges of the nanocells with immobilized 1 nm diameter pickets is sufficient to slow lipid diffusion by nearly three orders of magnitude to values below the detection limit (3.5 × 10−12 cm2 s−1) of the single-lipid tracking assay. With such a low diffusion coefficient, it would take nearly an hour for a lipid to cross a 2-μm zone, thus establishing a de facto diffusion barrier. In the axonal diffusion barrier, the immobilization of membrane proteins requires the actin cytoskeleton (Nakada et al. 2003). In the case of the diffusion barrier at the yeast bud neck separating mother and daughter membranes, the septin cytoskeleton establishes the diffusion barrier, thus indicating that the cytoskeletal element immobilizing the pickets may vary in different systems (Takizawa et al. 2000). Candidates for the immobilizing elements of the periciliary diffusion barrier include the Usher syndrome transmembrane proteins SANS, whirlin, USH2b, and VLGR1b (Maerker et al. 2008), which are localized to the periciliary ridge complex of photoreceptors; the centrosomal component Cep164, which is localized to a doughnut-shaped zone at the ciliary base (Graser et al. 2007); and Ankyrin-G, which is required for the movement of the cGMP gated channel to the photoreceptor outer segment and establishes the axonal diffusion barrier (Kizhatil et al. 2009). Alternatively, the very sharp membrane curvature at the base of the cilium may be sufficient to slow down the diffusion of lipids and membrane proteins.
Crossing the Periciliary Diffusion Barrier
Given what we know about the nature of membrane diffusion barriers, there are at least two conceivable mechanisms for how to cross the periciliary diffusion barrier. First, proteins could permeate the picket fence by specifically interacting with the fence elements themselves (i.e., the transmembrane domains of the immobilized proteins), thereby locally loosening the fence before hopping from one fence barrier to another. This scenario would be analogous to the nuclear transport factors known as importins and exportins that cross the nuclear envelope by permeating the hydrogel channel of the nuclear pore through multiple interactions with the cross-linking elements of the hydrogel matrix (Frey & Görlich 2007). This hypothesis invokes the existence of transport factors that act as diffusion facilitators. In this scenario, entry into the cilium need not require energy consumption. Conversely, membrane proteins could actively cross the diffusion barrier by being physically pulled through the barrier. The primary candidates for an active lateral transport machinery would be the IFT A and B complexes, which move proteins up and down inside cilia through the motor activities of Kinesin II and Dynein 1b. The IFT complexes are the components of flat coats that resemble curvatureless COPI, COPII, and clathrin coats at the ultrastructural level (Rosenbaum & Witman 2002). Coincidentally, IFT-complex polypeptides are largely composed of α-solenoids and β-propeller domains that predominate in COPI, COPII, and clathrin cage components (Avidor-Reiss et al. 2004, Jékely & Arendt 2006). The absence of curvature from the IFT train is not incompatible with the IFT coat hypothesis: Clathrin forms flat plaques that cluster membrane proteins for endocystosis when the clathrin triskelion is organized in hexagons rather than the canonical mix of hexagons and pentagons found in clathrin-coated pits (Traub 2009, Heuser 1980). Furthermore, IFT polypeptides are highly enriched at the membrane-proximal end of alar sheets, and it is therefore thought that a rate-limiting step in the assembly of IFT train (formerly known as IFT rafts) occurs right at the entry point into cilia (Deane et al. 2001). Canonical coat complexes interact directly with the cytoplasmic tails of transmembrane cargoes and utilize coat polymerization to simultaneously cluster cargoes and deform membranes to bud a carrier vesicle (Springer et al. 1999). An appealing scenario would therefore have the IFT polypeptides polymerize into a flat coat on the outer periphery of the periciliary diffusion barrier to cluster membrane proteins destined to enter the cilium (Figure 4d). These IFT clusters would provide a focal point to recruit molecular motors that could drag the IFT clusters through the picket fence of the diffusion barrier like an icebreaker advancing though ice-covered water.
It is also conceivable that directed exocytosis delivers ciliary transmembrane cargoes to the location where IFT trains assemble, thus coupling vesicle trafficking to entry into cilia and IFT. In particular, the IFT-B component IFT20 localizes to the trans-Golgi network (TGN) and to post-Golgi vesicles moving to and fusing with the base of the cilium (Follit et al. 2006). Furthermore, a highly refined immunoelectron microscopy analysis of IFT polypeptide localization in photoreceptors found IFT52 together with IFT20 on vesicles near the PRC and IFT57, -88 and -140 on a different vesicle population near the base of the cilium (Figure 4d; Sedmak & Wolfrum 2010). In one interpretation, Rosenbaum has proposed that IFT20 stays associated with the vesicular surface (presumably interacting with the transmembrane cargoes) from the initial budding event out of the TGN through the fusion of vesicles with the periciliary base until lateral transport into the cilium proper (Baldari & Rosenbaum 2010). Consistent with this interpretation, the Golgin GMAP210 anchors IFT20 to the Golgi complex, and GMAP210 is required for the efficient transport of the polycystin PKD2 to the ciliary membrane (Follit et al. 2006, Follit et al. 2008). An appealing aspect of IFT train assembly at the periphery of the periciliary base is that it would unite the two demonstrated modes of entry into cilia, lateral diffusion and polarized exocytosis. However, much work remains to be done to determine whether the IFT complexes assemble a coat that becomes progressively remodeled from the TGN to the base of the cilium. Because IFT polypeptides are required for ciliogenesis, only the development of in vitro trafficking assays coupled with biochemical reconstruction will allow scientists to dissect the coupling between polarized exocytosis and IFT train formation.
TRAFFICKING OF MEMBRANE PROTEINS TO THE CILIUM: MOLECULES
Cis-acting Elements for Targeting to the Primary Cilium
An accurate molecular understanding of trafficking to the cilium requires the identification of sequence elements that direct the sorting of membrane proteins in cis and of the machinery that recognizes those signals in trans. Ciliary targeting signals (CTSs) are both necessary and sufficient for sending a membrane protein to the cilium (Table 1), and the first CTS to be defined was in rhodospin. Mutations clustered within the last five amino acids of rhodospin (QVSPA) lead to one of the most severe forms of autosomal dominant retinal degeneration (also called retinitis pigmentosum or RP), and deletion of the QVSPA motif impairs trafficking to the outer segment in transgenic animals (Deretic et al. 1998, Li et al. 1996, Sung et al. 1994). Because grafting the last eight amino acids of rhodopsin onto a membrane-anchored GFP promotes efficient targeting to the outer segment, this octapeptide encodes the CTS of rhodopsin (Tam et al. 2000). Given that single amino acid substitutions found in RP patients primarily affect the second and fourth positions of the QVSPA motif, VxP (where x is any amino acid) likely constitutes the core element of the rhodopsin CTS (Deretic et al. 1998). Interestingly, the motif RVxP is present in the CTS of PKD2 (Geng et al. 2006; Table 1), and an RVxP motif in the cyclic nucleotide gated channel subunit CNG1B is required for ciliary localization of CNG1B but fails to direct a plasma membrane protein to cilia (Jenkins et al. 2006). Another motif common to several CTSs is the AQ box (AxS/AxQ) found in the third intracellular loop (i3) of GPCRs that localize to cilia such as somatostatin receptor 3 (SSTR3), serotonin receptor 6 (5HT6) and melanocortin concentrating hormone receptor 1 (MCHR1) (Berbari et al. 2008a). Intriguingly, substituting the A and Q residues in the AQ box does not abolish the CTS activity of SSTR3i3 when SSTR3i3 is grafted onto the single pass membrane protein CD8α (Jin et al. 2010), whereas an identical mutation prevents ciliary targeting of an SSTR5 chimera in which SSTR3i3 replaces SSTR5i3 (Berbari et al. 2008a). Given the known sensitivity of GPCRs to alterations of their intracellular loops, the latter finding may result from improper folding of the SSTR5/SSTR3i3 chimera and consequent ER retention.
Table 1. Ciliary targeting signals. Short sequence elements that direct an unrelated protein to the cilium are listed. It is currently unkown whether the CTSs of PKHD1, SSTR3 and 5HT6 are the sole CTSs within those proteins. TM segments are underlined and key amino acids are bolded.
| Protein | Organism | Cellular function |
Topology | Lipidation? | Major CTS determinants | Reference |
|---|---|---|---|---|---|---|
| Fibrocystin (PKHD1) |
Mouse | Unknown | 1 TM; N- out; C-in |
Di- palmitoylation |
End of TM segment + cytoplasmic tail (193 last a.a.); ClvCCWFKKSKTRKIKP E |
(Follit et al. 2010 |
| Cystin | Mouse | Unknown | Peripheral | Myristoylation | 28-tAsEGGta-35 | (Tao et al. 2009) |
| Polycystin-2 (PKD2) |
Mouse | Cation channel |
6 TM; N- in; C-in |
N.D. | N-terminal cytoplasmic tail; NH2-mvnssRVqPqqpgda |
(Geng et al. 2006) |
| Rhodospin | Frog, mouse |
GPCR; photon receptor |
7 TM; N- out; C-in |
Di- palmitoylation |
C-terminal cytoplasmic tail; sssqVsPa-COOH |
(Tam et al. 2000) |
| SSTR3 | Mouse | GPCR; somatostatin receptor |
7 TM; N- out; C-in |
N.D. | Cytoplasmic loop #3, apsCq + apaCq |
(Berbari et al. 2008a, Jin et al. 2010) |
| 5HT6 | Mouse | GPCR; serotonin receptor |
7 TM; N- out; C-in |
N.D. | Cytoplasmic loop #3 | (Berbari et al. 2008a) |
| ISO1 (isoform 1 of the glucose transporter) |
Leishmania | Glucose transporter |
12 TM; N- in; C-in |
N.D. | N-terminal cytoplasmic tail; bipartite [84--100] + [110-- 118] |
(Nasser & Landfear 2004) |
| Calflagin/FCaB P (Flagellar Ca2+ binding protin) |
Trypanoso me |
Unknown; Ca2+ binding |
Peripheral | Myristoylation + palmitoylation |
N-terminal cytoplasmic tail; [1--24] |
(Godsel & Engman 1999) |
GPCR, G protein-coupled receptor; TM, transmembrane; N.D., not determined.
Unexpectedly, most known CTSs have been either predicted (fibrocystin, rhodopsin) or demonstrated (cystin, calflagin) to become incorporated into lipid rafts through myristoylation and/or palmitoylation. Furthermore, mutations in these CTSs that prevent lipidation abolish ciliary targeting (Follit et al. 2010, Godsel & Engman 1999, Tam et al. 2000, Tao et al. 2009), and depletion of the calflagin palmitoyltransferase has the same effect (Emmer et al. 2009). Because incorporation into lipid rafts directs apical targeting in epithelial cells (Simons & Toomre 2000), it is tempting to postulate that targeting to cilia generally relies on partitioning into specific lipid microdomains and on the recruitment of specific sorting complexes.
Arf4 and ASAP1: Sorting Rhodopsin out of the Trans-Golgi Network
Elegant work combining in vitro assays and mouse genetics established that the CTS of rhodospin is required for sorting rhodospin out of the TGN and into carrier vesicles (Deretic et al. 1998, Li et al. 1996). A logical inference is that the decision to direct membrane proteins to the cilium is made during exit from the Golgi complex rather than at the recycling endosome, as is the case for basolateral versus apical targeting in polarized epithelial cells (Ang et al. 2004). To identify the factors that mediate rhodopsin sorting out of the Golgi complex, Deretic and colleagues performed affinity chromatography against the CTS of rhodospin and recovered the small GTPase Arf4 (Deretic et al. 2005). The rhodopsin CTS/Arf4 interaction was functionally characterized using a cell-free assay measuring rhodopsin packaging into carrier vesicles from Golgi membranes, and addition of antibodies against Arf4 or against the CTS of rhodopsin strongly inhibits packaging (Deretic et al. 1996, Mazelova et al. 2009a).
Arf4 is closely related to Arf1, Arf2, Arf3, and Arf5 (each 81% to 96% identical to another), and these five Arfs are equally capable of recruiting COPI coats or the clathrin coat adaptor AP-1 to Golgi membranes (Liang & Kornfeld 1997). In cultured mammalian cells, Arf1 and Arf4 function redundantly in COPI recruitment to the Golgi complex, and Arf4 functions singularly in COPI-mediated trafficking from the ER-Golgi intermediate compartment (ERGIC) to the cis-Golgi complex (Volpicelli-Daley et al. 2005). Interestingly, brefeldin A (a chemical inhibitor of the Golgi complex--localized ArfGEF GBF1) displaces Arf1 and Arf3 from all endomembranes but fails to remove Arf4 and Arf5 from the ERGIC (Chun et al. 2008). However, the singular role of Arf4 in trafficking out of the ERGIC is difficult to reconcile with its function in sorting rhodopsin into carrier vesicles at the TGN. This latter function of Arf4 has been proposed to be mediated by a “module” consisting of the Arf GTPase activating protein (GAP) ASAP1, the GTPase Rab11, and the Rab11 effector FIP3, which are all required for the packaging of rhodopsin into carrier vesicles from isolated Golgi membranes in vitro. Moreover, expression of the Arf4[I46D] mutant deficient in ASAP1-induced GTP hydrolysis disrupts rhodopsin post-Golgi trafficking and causes retinal degeneration in transgenic animals (Mazelova et al. 2009a). An interesting possibility is that ASAP1 is part of a coat complex for sorting membrane proteins destined for the cilium out of the TGN. The composition of the putative ASAP1 complex, the demonstration that the ASAP1 complex forms a coat, and the general utilization of the ASAP1 complex for trafficking to cilia are major issues that now need to be addressed experimentally. Alternatively, AP-1 could be the relevant coat for Arf4-mediated exit of rhodopsin from the Golgi complex because AP-1 is a known effector of Arf4 and because the transport of the olfactory receptor ODR-4 to the sensory cilium of worm neurons requires the μ1 subunit of the AP-1 clathrin adaptor (Dwyer et al. 2001).
ASAP1: an ArfGAP containing SH3, ANK repeat, PH and BAR domains with roles at focal adhesions and at the recycling endosome
FIP3: a member of the family of Rab11-interacting proteins required to maintain the integrity of the recycling endosome compartment
Rab8 and the Exocyst: The Polarized Exocytosis Masters
One of the first factors to be implicated in vesicular transport to the primary cilium was the small GTPase Rab8. Rab GTPases mediate the transport, docking, and fusion of carrier vesicles with an acceptor compartment, and Rab8 associates with post-Golgi vesicles (Deretic et al. 1995, Huber et al. 1993,) while the yeast homologue of Rab8, Sec2, functions in vesicular trafficking to the bud neck. Strikingly, inhibition of Rab8 function in frog photoreceptors results in the massive accumulation of rhodopsin carrier vesicles near the base of the connecting cilium (Moritz et al. 2001). Furthermore, Rab8 activation is required for cilium formation in RPE cells, and overexpression of GTP-locked Rab8 leads to a dramatic elongation of primary cilia (Nachury et al. 2007, Yoshimura et al. 2007). Collectively, these data show that Rab8GTP mediates the docking and fusion of vesicles with the base of the cilium and pose the questions of how Rab8 activity is coordinated to vesicular trafficking and how Rab8GTP exerts its docking function.
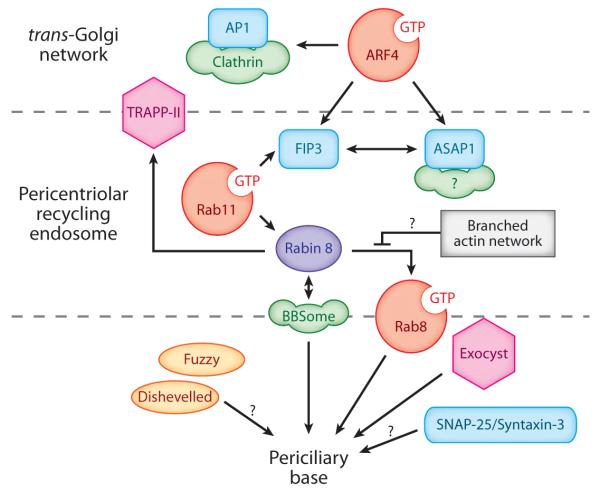
Upstream of Rab8 lies the guanosyl nucleotide exchange factor (GEF), Rabin8 (Hattula et al. 2002). Rabin8 localizes to vesiculotubular recycling endosomes near the base of the cilium (the pericentriolar recycling endosome, PRE) and the recycling endosome marker Rab11GTP directly potentiates the Rab8GEF activity of Rabin8 and targets Rabin8 to the PRE (Knödler et al. 2010, Westlake et al. 2009). Moreover, Smo and fibrocystin accumulate in the PRE in nonciliated cells, and Smo is transported through the PRE before reaching newly assembled cilia (Follit et al. 2010, Kim et al. 2010). Thus, the PRE may constitute a way station for trafficking to cilia in ciliated cells and/or a reservoir for cilia-destined proteins before cilia are assembled. A role for the PRE in ciliogenesis is further evidenced by the requirement for known PRE players such as Rab11, ASAP1, the tubulating machine Epsin15 homology domain protein 1 (EHD1) and the tethering complex trafficking transport protein particle II (TRAPPII) in cilium assembly. Most revealing, ASAP1 localizes to the PRE and is required for clustering the PRE around centrioles but is dispensable for transferin recycling by the PRE (Inoue et al. 2008). Thus, the specific requirement for ASAP1 in cilium assembly (Kim et al. 2010) highlights the necessity for close proximity between the PRE and the periciliary base. Strikingly, depletion of Arp3, an actin nucleation and branching factor, or mild treatement with the actin poison cytochalasin D stabilize Smo enrichment at the PRE, facilitate cilium assembly, and increase cilium length. Conversely, depletion of the actin severing factors gelsolin or advillin inhibits ciliogenesis (Kim et al. 2010). Given that cytochalasin D treatment has been shown to direct Rab8 to Rab11-positive vesiculotubular structures without affecting Rabin8 localization and because Rab8 localization to tubular structures correlates with GTP loading onto Rab8 (Hattula et al. 2006, Westlake et al. 2009), one may infer a molecular cascade for trafficking to the cilium that is negatively regulated by branched actin networks at the level of Rab8 activation (Figure 6). The picture is much less clear downstream of Rab8: Ciliogenic factors such as Cenexin 2, Cep290, and rabaptin5 have been identified as binding partners of Rab8, but the functional significance of these interactions remains unclear (Kim et al. 2008, Omori et al. 2008, Tsang et al. 2008, Yoshimura et al. 2007).
Figure 6.
A speculative molecular pathway for membrane traffic to the primary cilium. Green peanut shapes are coats, red circles are GTPases, pink hexagons are tethering complexes, and Rabin8 (purple) is an extended coiled coil.
Trafficking transport protein particle II (TRAPPII): a decameric tethering complex that mediates trafficking events within the Golgi complex and from the early endosome to the late Golgi complex
Epsin15 homology domain protein 1 (EHD1): a resident recycling endosome ATPase that tubulates membranes and is required for endosome-to-plasma membrane transport
Finally, the exocyst, a tethering complex with roles in polarized exocytosis to the yeast bud neck and to the basolateral membrane of epithelial cells, localizes to cilia and may play a role in trafficking to cilia (Rogers et al. 2004, Mazelova et al. 2009b, Zuo et al. 2009). Tethering complexes generally facilitate the docking and fusion of vesicles with an acceptor compartment in concert with soluble N-ethylmaleimide sensitive factor receptors (SNAREs) and Rabs. SNAREs are helical membrane proteins present on vesicles and target organelles that mediate fusion upon pairing of four SNARE helices (named Qabc and R) and act as zip codes for addressing specific vesicles to their destination (Jahn & Scheller 2006). In photoreceptors, the Qa-SNARE syntaxin3 is present at the base of the connecting cilium, and the fatty acid DHA potentiates the interaction between syntaxin3 and the Qbc-SNARE SNAP-25 (Mazelova et al. 2009b). Given the abundance of DHA in photoreceptor membranes, it is appealing to speculate that DHA may control fusion of vesicles with the periciliary base, and functional evidence for a role of syntaxin3 and SNAP-25 in rhodopsin transport to the outer segment is eagerly awaited. Finally, the identity of the vesicle-bound R-SNARE that completes the helical bundle with SNAP-25/syntaxin3 may provide insight into the origin of vesicles that fuse with the periciliary base.
The BBSome: A Coat Complex for Ciliary Transport
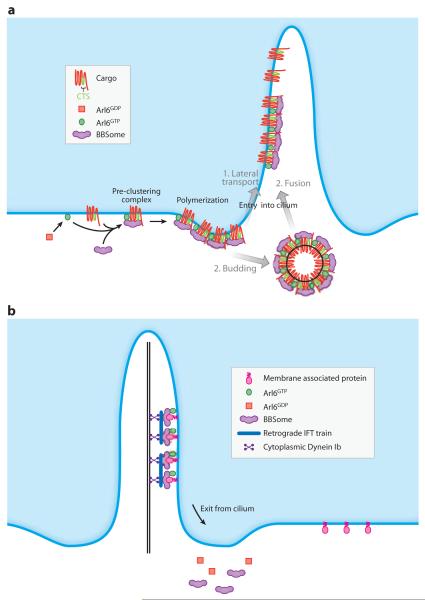
Another player in ciliary trafficking is the BBSome, a complex of seven highly conserved Bardet-Biedl Syndrome (BBS, see sidebar on A Brief History of the Ciliopathies) proteins and one novel protein (Loktev et al. 2008, Nachury et al. 2007). The BBSome contains coat-like structural elements common to COPI, COPII, and clathrin coats and is the major effector of the Arf-like GTPase Arl6/BBS3, another highly conserved BBS protein. Strikingly, Arl6GTP-mediated recruitment of the BBSome to synthetic liposomes produces distinct patches of polymerized coat apposed onto the lipid bilayer (Jin et al. 2010). In RPE cells, Arl6 and the BBSome localize to cilia in an interdependent manner, and Arl6 and the BBSome are required for targeting of the GPCR somatostatin receptor 3 (SSTR3) to hippocampal cilia (Berbari et al. 2008a, Jin et al. 2010). Consistent with the sorting function of coats, the BBSome directly recognizes the CTS of SSTR3 (SSTR3i3) and, when grafted onto the plasma membrane protein CD8α, SSTR3i3 directs the resulting chimera to cilia in a BBSome- and Arl6-dependent manner. Furthermore, a mutation in SSTR3i3 that disrupts BBSome binding prevents targeting of the CD8α-SSTR3i3 chimera to cilia. Thus, SSTR3 fullfills all the criteria of a bona fide BBSome cargo. Finally, in the absence of the BBSome or Arl6, the CD8α-SSTR3i3 chimera accumulates in the plasma membrane, thereby suggesting that the BBSome mediates trafficking from the plasma membrane to the ciliary membrane (Figure 7a; Jin et al. 2010). A major open question now concerns the mechanism by which the BBSome mediates trafficking into cilia: Does the BBSome resemble canonical coats and bud cargo-containing vesicles that fuse with the periciliary base? Or is the BBSome a more simple sorting machine that assembles a planar coat and clusters cargoes into a patch that can enter the cilium laterally from the plasma membrane?
Figure 7.
Two models for the function of the BBSome, a complex of seven highly conserved Bardet-Biedl Syndrome (BBS) proteins and one novel protein. (a) The BBSome functions as a coat complex to target membrane proteins to cilia. Upon GTP binding, Arl6 associates with membranes and recruits the BBSome. Preclustering complexes are then formed through the direct recognition of ciliary targeting signals (CTSs) by the BBSome. These BBSome/CTS/Arl6GTP complexes polymerize to form the BBSome coat and target membrane proteins to cilia. It is currently unclear whether the BBSome assembles a planar coat that mediates active lateral transport through the diffusion barrier (route 1) or a canonical coat that buds out vesicles that then fuse with the ciliary membrane (route 2). (b) The BBSome functions as an adaptor for retrograde IFT to export proteins from cilia. Note that models (a) and (b) need not be mutually exclusive.
The central role of the BBSome in trafficking membrane proteins to from cilia provides a logical framework to account for the etiology of BBS. Although the BBSome is not generally required for the assembly of the cilium, its trafficking function implies that specific signaling receptors and transmembrane proteins fail to reach the cilia of BBS patients, thereby resulting in organ-specific signaling abnormalities and pathological symptoms. In particular, Val Sheffield’s lab recently discovered that the leptin signaling cascade is disrupted downstream of the leptin receptor in bbs2−/− and bbs4−/− mice (Rahmouni et al. 2008, Seo et al. 2009). Given that the cytoplasmic tail of the leptin receptor interacts with BBSome subunit BBS1, it is tempting to speculate that the BBSome may traffic the leptin receptor to cilia (Seo et al. 2009). However, attempts to localize the leptin receptor protein in situ have been hampered by the lack of highly specific immunological reagents, and it remains to be seen if leptin signaling takes place inside cilia.
Removal of Membrane Proteins from Cilia: Emerging Concepts
Whereas most of this chapter---and most of what we currently know---centers around trafficking to the cilium, the removal of proteins from cilia is likely to be of great biological significance. When cells resorb their cilia before cell cycle entry in vertebrate cells or before conjugation in Chlamydomonas, membrane and soluble proteins need to exit the cilia. Furthermore, in Hh signaling, once stimulation is terminated, Smo disappears from cilia. The mechanisms that underlie membrane protein removal from cilia and the regulation of this trafficking step remain largely unexplored.
Surprisingly, the BBSome may carry out bidirectional trafficking into and out of the cilium. Recent work from the Witman lab showed that the Chlamydomonas BBSome colocalizes with IFT trains in flagella and is required to remove specific signaling components from flagella (Lechtreck et al. 2009). Four proteins abnormally accumulate in bbs flagella: a truncated hemoglobin (THB1), a type c phospholipase D (PLDc), a serine/threonine protein kinase (STPK), and a protein of unknown function. While none of these proteins contains a transmembrane domain, the latter three contain putative myristoylation sites. Remarkably, THB1 also accumulates in the flagella of a retrograde IFT mutant, suggesting that THB1 is exported from cilia by the BBSome and the retrograde IFT machinery. Because the BBSome undergoes IFT motility in all organisms examined (Lechtreck et al. 2009, Blacque et al. 2004, Nachury et al. 2007), the BBSome may function as an adaptor for the retrograde IFT-A complex (Figure 7b). Given that not all IFT particles carry BBSomes and Chlamydomonas polycystin 2 accumulates in IFT mutant flagella but not in BBSome mutant flagella (Huang et al. 2007, Lechtreck et al. 2009), it is likely that the BBSome is only one of many adaptors for the retrograde IFT machinery. Conversely, because PLDc does not accumulate in retrograde IFT mutant flagella, the BBSome may remove proteins from flagella independently of the IFT machinery.
Another advance on the ciliary export front is the discovery that ubiquitination of ciliary proteins may constitute a mark for removal from cilia. Ubiquitination of plasma membrane proteins such as the epidermal growth factor receptor is well known to trigger their endocytosis and facilitate their movement through the endocytic route toward the multivesicular body and finally the lysosome, thus ultimately leading to degradation. Interestingly, clathrin adaptors and the multivesicular body sorting machinery [the endosomal sorting complexes required for transport (ESCRT complexes)] all recognize the ubiquitin (Ub) moiety on Ub-conjugated cargoes (Huang et al. 2009, Hurley & Emr 2006, Tanaka et al. 2008). During flagellar resorption in Chlamydomonas, the level of ubiquitinated proteins in flagella increases dramatically, particularly in mutants deficient in retrograde IFT, and PKD2 and α-tubulin are highly ubiquitinated (Huang et al. 2009). Moreover, in C. elegans, a PKD2-Ubiquitin fusion is absent from cilia, whereas PKD2 normally remains in cilia at steady-state. Interestingly, the ESCRT-0 protein Hrs/STAM is required for removing Ub-PKD2 from cilia: In Hrs/STAM mutants, Ub-PKD2 accumulates at the base of cilia (Hu et al. 2007). Thus, Ub may be used as a tag for transport to the base of the cilium and also for further trafficking toward the degradative endocytic route. In this context, it will be particularly interesting to test whether ubiquitination plays a role in signaling pathways that utilize the cilium. In particular, the steady-state localization of any protein at the primary cilium results from the difference between the rate of ciliary entry and the rate of ciliary exit. Whereas it would seem that regulating the rate of entry is the most parsimonious solution, the finding that Smo continuously shuttles into and out of the cilium in unstimulated cells (Ocbina & Anderson 2008, Kim et al. 2009) leaves open the possibility that either entry or exit rates could be the target for regulation upon pathway engagement.
ESCRT: a set of four “endosomal sorting complexes required for transport” to the multivesicular body where membrane proteins and lipids are poised for degradation Ubiquitin (Ub): a 76-amino acid polypeptide that becomes conjugated to acceptor proteins to mark them for degradation, endocytosis, or regulation
Roles of the Intraflagellar Transport Machinery Outside Cilia
Until quite recently, the role of IFT polypeptides had been equated with cilium assembly. One report suggested that IFT88 might be involved in cell cycle control independently of its role in ciliogenesis based on experiments in HeLa cells, which the authors assumed were not ciliated (Robert et al. 2007). However, we have recently confirmed that HeLa cells are mostly ciliated (H. Jin and M.V. Nachury, unpublished observations; Alieva & Vorobjev 2004), and depletion of IFT88 in HeLa cells accelerates cell cycle progression owing to aberrations in ciliary signaling pathways. However, this cilia-centric view of the IFT complexes is now bound to change thanks to two recent reports (Finetti et al. 2009, Sedmak & Wolfrum 2010).
The first report addresses the role of IFT proteins in trafficking of the T cell receptor (TCR) to the immune synapse of T cells, which are not ciliated. T cells undergoing activation adhere to antigen-presenting cells through a close membrane juxtaposition called the immune synapse. Remarkably, the TCRs (on the T cell side) and the cognate major histocompatibility complexes-antigen complexes (on the antigen-presenting cell side) are clustered at the center of the immune synapse. Because the T cell centrosome migrates to the immune synapse very early during the activation process, it had been suggested that the centrosome polarizes the microtubule cytoskeleton to direct trafficking of the TCR to the immune synapse (Kupfer et al. 1987, Martín-Cófreces et al. 2008). Surprisingly, all tested IFT polypeptides are expressed in T cells, and polarized TCR trafficking to the immune synapse is severely impaired when T cells are treated with IFT20 small hairpin RNA (Finetti et al. 2009). Lastly, upon T cell activation by the antigen-presenting cell, the T cell receptor associates with the IFT-B complex subunits IFT20, IFT57, and IFT88, thereby suggesting assembly of an IFT coat on TCR carrier vesicles or on TCR clusters at the synapse. Given the availability of IFT20 and IFT88 conditional mouse mutants, future experiments will need to rigorously test whether different IFT-B complex polypeptides are required for T cell activation in vivo.
The second report found IFT20, -52 and -57 as well as the IFT motor KIF17 localized to the postsynaptic compartment of the synapses connecting photoreceptor cells to the dendritic processes of secondary retinal neurons. Because these secondary neurons are not ciliated and because IFT20, -52 and -57 associate with the surface of vesicles in the dendritic processes of secondary neurons, a novel IFT complex may mediate the exocytic insertion of postsynaptic components at the synapse. A role for IFT at the signal-receiving end (the dendrite) of a nonciliated neuron would elegantly expand upon the well-characterized function of IFT at the signal-receiving antenna (the cilium) of ciliated cells and may indicate a general utilization of IFT at signal-receiving structures (cilia, dendrites, T cell synapse). Nonetheless, the functional relevance of IFT to the organization of the postsynaptic compartment of secondary retinal neurons remains to be tested.
Fuzzy and Dishevelled: Planar Polarity Moonlighting at the Basal Body
The final category of molecular players for ciliary trafficking came from un unexpected direction. Genetic analysis of PCP in Drosophila uncovered 6 core PCP proteins functioning upstream of many effector PCP proteins. Unexpectedly, mice knockouts for the PCP effector Fuzzy display very mild PCP phenotypes but severe Hh signaling defects and stunted primary cilia (Gray et al. 2009, Heydeck et al. 2009). Furthermore, exocytosis is severely disrupted in the mucus-secreting cells of Fuzzy knockdown frogs, with mucus granules found apposed to the apical membrane seemingly unable to fuse. Thus, Fuzzy participates in the terminal step of mucus granule exocytosis and may play a similar role in exocytosis at the periciliary base (Gray et al. 2009). In another departure from the Drosophila model, the core PCP protein Dishevelled localizes to the basal body of ciliated epithelial cells in frog embryos and is required for activation of the actin remodeling GTPase Rho, the ensuing apical docking of basal bodies, and the capture of exocyst-positive vesicles by basal bodies (Park et al. 2008). Thus, similar to how some of the Hh signaling elements are utilized for ciliogenesis and/or Hh signal transduction depending on the evolutionary branch examined (Wilson et al. 2009, Rink et al. 2009), PCP proteins may function in polarized exocytosis and/or PCP signaling in different organisms. These deeply rooted linkages between PCP or Hh signaling and cilium assembly suggest that cilia may have initially coevolved with their signaling obligations---such as clustering receptors on the cell surface---before acquiring motile characteristics (Jékely & Arendt 2006).
SUMMARY POINTS
-
■
The cilium, even though it is not fully membrane-enclosed, has the properties of a cellular organelle: Its membrane is segregated away from the plasma membrane by a diffusion barrier and contains a distinct set of receptors and possibly lipids.
-
■
The transport of small soluble proteins to the cilium may proceed by diffusion and retention rather than active transport. A protein as large as GFP can freely diffuse through the connecting cilium of photoreceptors.
-
■
Ultrastructural studies coupled with pulse-chase labeling of rhodopsin in photoreceptor cells have offered strong support to the polarized exocytosis model in which post-Golgi vesicles dock and fuse with the periciliary base.
-
■
A set of proteins has been proposed to pave the way for the transport of membrane proteins from the Golgi complex to cilia. These include the GTPases Arf4, Rab11, and Rab8; the tethering complexes TRAPPII and exocyst; the Rab11 effector FIP3; the ArfGAP ASAP1; the tubulating machine EHD1; and the Rab8GEF Rabin8. This cast of actors suggests a role for recycling endosomes in vesicular trafficking to cilia.
-
■Two protein complexes that recognize CTSs and sort cargoes to the cilium have recently been identified:
-
○Arf4 recognizes the rhodopsin CTS, and together with the putative coat complex component ASAP1, functions in rhodospin sorting to cilia at the TGN.
-
○The BBSome is the major effector of the GTPase Arl6 and functions as a coat complex that sorts the GPCR SSTR3 to cilia. The BBSome coat may also remove proteins from cilia.
-
○
-
■
A novel route for transport of membrane proteins into cilia has been proposed for the Hh signaling factor Smo. It has been proposed that Smo may be transported laterally from the plasma membrane into the ciliary membrane, presumably by diffusion, crossing of the periciliary diffusion barrier, and retention in cilia.
-
■
The IFT complexes function mainly in lateral transport within cilia, but IFT20 may function at the Golgi complex in the transport of PKD2 and in the polarization of recycling endosomes for the transport of the TCR to the immune synapse.
ACKNOWLEDGMENTS
We thank Susan White, Phil Beachy, and Joel Rosenbaum for comments on the manuscript, Dusanka Deretic for the photoreceptor scheme used in Figure 1, and Cynthia Jensen for stimulating discsussions. Research in the Nachury lab is supported by grants from the American Heart Association (AHA-0930365N), the March of Dimes (5-FY09-112), the AACR/Pancreatic Cancer Action Network (09-20-25-NACH), a Sloan Research Fellowship (BR-5014), a Klingenstein Fellowship, and NIH/NIGMS (1R01GM089933-01).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
FUTURE ISSUES
Where is the sorting decision made for proteins targeted to the cilium versus the plasma membrane? Are ciliary membrane proteins packaged into vesicles distinct from those of plasma membrane resident proteins at the level of the TGN, or is there an intermediate step (plasma membrane, recycling endosomes) in sorting?
What is the molecular machinery that recognizes ciliary targeting sequences and sorts proteins to the cilium? How is this machinery regulated? This and the previous question are likely to shed light on the mechanisms that regulate the dynamic trafficking of signaling proteins (e.g., Smo) to the primary cilium.
What is the molecular nature of the periciliary diffusion barrier? It will be important to determine how many membrane proteins reach the cilium by crossing this barrier versus utilizing the polarized exocytosis route.
How are proteins removed from cilia? Is there a specific machinery involved in endocytosis of ciliary proteins? Is this step regulated by ubiquitination?
LITERATURE CITED
- Adams AE, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 1984;98:934–45. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walters P. Molecular Biology of the Cell. 4th ed. Garland Sci.; New York, NY: 2002. p. 1268. [Google Scholar]
- Alieva IB, Vorobjev IA. Vertebrate primary cilia: a sensory part of centrosomal complex in tissue cells, but a “sleeping beauty” in cultured cells? Cell Biol. Int. 2004;28:139–50. doi: 10.1016/j.cellbi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Anderson RG. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J. Cell Biol. 1972;54:246–65. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A very careful reconstruction of the base of the cilium shows that the so-called transition fibers are actually large sheets covering the entrance to the ciliary lumen. Ang AL, Taguchi T, Francis S, Fölsch H, Murrells LJ, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–43. doi: 10.1083/jcb.200408165.
- Aveldaño MI, Bazán NG. Molecular species of phosphatidylcholine, -ethanolamine, -serine, and -inositol in microsomal and photoreceptor membranes of bovine retina. J. Lipid Res. 1983;24:620–27. [PubMed] [Google Scholar]
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, et al. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–39. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: It’s not just for cilia anymore. Curr. Opin. Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Haeri M, Yoo P, Gospe SM, Skiba NP, et al. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J. Cell Biol. 2008;183:485–98. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N, Johnson A, Lewis J, Askwith C, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell. 2008a;19:1540–47. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N, Lewis J, Bishop G, Askwith C, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA. 2008b;105:4242–46. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC, Purcell EM. Physics of chemoreception. Biophys. J. 1977;20:193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J. Cell Biol. 1977;75:507–27. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas S, Silhavy J, Brancati F, Kisseleva M, Al-Gazali L, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009;41:1032–36. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck GB. The structure, origin, isolation, and composition of the tubular mastigonemes of the Ochromonas flagellum. J. Cell Biol. 1971;50:362–84. doi: 10.1083/jcb.50.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Schiesser WE, Pugh EN. Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J. Gen. Physiol. 2010;135:173–96. doi: 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The first comprehensive study of diffusion through the connecting cilium finds no diffusion barrier for soluble GFP. Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–68. doi: 10.1016/j.tcb.2006.09.001.
- Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- Chailley B, Boisvieux-Ulrich E. Detection of plasma membrane cholesterol by filipin during microvillogenesis and ciliogenesis in quail oviduct. J. Histochem. Cytochem. 1985;33:1–10. doi: 10.1177/33.1.3965567. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Wilson CW, Li Y-J, Law KKL, Lu C-S, et al. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–28. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Shapovalova Z, Dejgaard SY, Presley JF, Melançon P. Characterization of class I and II ADP-ribosylation factors (Arfs) in live cells: GDP-bound class II Arfs associate with the ER-Golgi intermediate compartment independently of GBF1. Mol. Biol. Cell. 2008;19:3488–500. doi: 10.1091/mbc.E08-04-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001;11:1586–90. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J. Cell Sci. 1995;108(Pt. 1):215–24. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Puleo-Scheppke B, Trippe C. Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J Bio.l Chem. 1996;271:2279–86. doi: 10.1074/jbc.271.4.2279. [DOI] [PubMed] [Google Scholar]
- Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc. Natl. Acad. Sci. USA. 1998;95:10620–25. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, et al. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc. Natl. Acad. Sci. USA. 2005;102:3301–6. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–57. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–44. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–87. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci. 2009;122:867–74. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009;11:1–10. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows a requirement for IFT20 in TCR trafficking to the immune synapse. This suggests that IFT complexes may function outside of ciliogenesis. Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–93. doi: 10.1038/nrm2278.
- Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, et al. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008;4:e1000315. doi: 10.1371/journal.pgen.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 2006;17:3781–92. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Görlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 2007;130:512–23. doi: 10.1016/j.cell.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 2002;157:1071–81. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 2006;119:1383–95. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18:2057–65. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–68. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Graser S, Stierhof Y-D, Lavoie SB, Gassner OS, Lamla S, et al. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 2007;179:321–30. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Abitua P, Wlodarczyk B, Szabo-Rogers H, Blanchard O, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat. Cell Biol. 2009;11:1225–32. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Arffman A, Peränen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol. Biol. Cell. 2002;13:3268–80. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpää K, Laakkonen P, Peränen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 2006;119:4866–77. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J. Cell Biol. 1980;84:560–83. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydeck W, Zeng H, Liu A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev. Dyn. 2009;238:3035–42. doi: 10.1002/dvdy.22130. [DOI] [PubMed] [Google Scholar]
- Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, et al. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007;176:653–65. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wittekind SG, Barr MM. STAM and Hrs down-regulate ciliary TRP receptors. Mol. Biol. Cell. 2007;18:3277–89. doi: 10.1091/mbc.E07-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Diener DR, Rosenbaum JL. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J. Cell Biol. 2009;186:601–13. doi: 10.1083/jcb.200903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, et al. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J. Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J. Cell Biol. 1990;111:1605–16. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The first study to suggest that transport to the ciliary membrane may occur by lateral diffusion from the plasma membrane. Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 2006;35:277–98. doi: 10.1146/annurev.biophys.35.040405.102126.
- Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol. Biol. Cell. 2008;19:4224–37. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. Lateral diffusion in membranes. Cell Motil. 1983;3:367–73. doi: 10.1002/cm.970030504. [DOI] [PubMed] [Google Scholar]
- Jacoby M, Cox J, Gayral S, Hampshire D, Ayub M, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009;41:1027–31. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs---engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–87. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, et al. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 2006;16:1211–16. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Jékely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–98. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, et al. The conserved Bardet-Biedl Syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010 doi: 10.1016/j.cell.2010.05.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro ES. Lipids of Paramecium. J. Lipid Res. 1987;28:1241–58. [PubMed] [Google Scholar]
- Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, et al. Dynamics of putative raft-associated proteins at the cell surface. J. Cell Biol. 2004;165:735–46. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AE. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol. 1984;98:922–33. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc. Natl. Acad. Sci. USA. 2009;106:21666–71. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Krishnaswami S, Gleeson J. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 2008;17:3796–805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen S, Suyama E, Ono K, et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010 doi: 10.1038/nature08895. In press. doi:10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Baker SA, Arshavsky VY, Bennett V. Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science. 2009;323:1614–17. doi: 10.1126/science.1169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knödler A, Feng S, Zhang J, Zhang X, Das A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. USA. 2010 doi: 10.1073/pnas.1002401107. Epub ahead of print. doi:10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J. Exp. Med. 1987;165:1565–80. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 2009;187:1117–32. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM, Zhang F, Ishihara A, McNeil CL, Jacobson KA. Unconfined lateral diffusion and an estimate of pericellular matrix viscosity revealed by measuring the mobility of gold-tagged lipids. J. Cell Biol. 1993;120:25–35. doi: 10.1083/jcb.120.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc. Natl. Acad. Sci. USA. 1996;93:14176–81. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JO, Kornfeld S. Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J. Biol. Chem. 1997;272:4141–48. doi: 10.1074/jbc.272.7.4141. [DOI] [PubMed] [Google Scholar]
- Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev. Cell. 2008;15:854–65. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Maerker T, van Wijk E, Overlack N, Kersten FF, McGee J, et al. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum. Mol. Genet. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- Martín-Cófreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordón-Alonso M, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell Biol. 2008;182:951–62. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Ungerer N, Klimmeck D, Warnken U, Schnölzer M, et al. Proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem. Senses. 2008;33:145–62. doi: 10.1093/chemse/bjm073. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009a;28:183–92. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Ransom N, Astuto-Gribble L, Wilson M, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J. Cell Sci. 2009b;122:2003–13. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 2009:1–40. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carefully examines all hypotheses for how Smoothened reaches the ciliary membrane and finds the lateral transport model to be most likely. Montesano R. Inhomogeneous distribution of filipin-sterol complexes in the ciliary membrane of rat tracheal epithelium. Am. J. Anat. 1979;156:139–45. doi: 10.1002/aja.1001560115.
- Moritz OL, Tam BM, Hurd LL, Peränen J, Deretic D, et al. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol. Biol. Cell. 2001;12:2341–51. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This definitive study demonstrates that Rab8 functions in the docking and/or fusion of rhodopsin carrier vesicles with the base of the cilium. Morone N, Fujiwara T, Murase K, Kasai RS, Ike H, et al. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J. Cell Biol. 2006;174:851–62. doi: 10.1083/jcb.200606007.
- Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, et al. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–33. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- Musgrave A, Wildt P, Etten I, Pijst H, Scholma C, et al. Evidence for a functional membrane barrier in the transition zone between the flagellum and cell body of Chlamydomonas eugametos gametes. Planta. 1986;167:544–53. doi: 10.1007/BF00391231. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–13. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Discovery of the BBSome and first suggestion that Bardet-Biedl syndrome proteins may function in membrane trafficking to the cilium. Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, et al. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–67. doi: 10.1016/j.neuron.2005.03.023.
- Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 2003;5:626–32. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- Nasser MIA, Landfear SM. Sequences required for the flagellar targeting of an integral membrane protein. Mol. Biochem. Parasitol. 2004;135:89–100. doi: 10.1016/j.molbiopara.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Lim SY, Wen ZM, Kim HY, et al. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J. Biol. Chem. 2004;279:31098–104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- Ocbina PJR, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn. 2008;237:2030–38. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, et al. elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 2008;10:437–44. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest. Ophthalmol. Vis. Sci. 1985;26:1386–404. [PubMed] [Google Scholar]
- Classic pulse-chase labeling experiments lead to the visualization of vesicular fusion events at the base of the cilium. Park T, Mitchell B, Abitua P, Kintner C, Wallingford J. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 2008;40:871–79. doi: 10.1038/ng.104.
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr. Top. Dev. Biol. 2008;85:115–49. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000;151:709–18. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr. Biol. 2002;12:R378–80. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Peters KR, Palade GE, Schneider BG, Papermaster DS. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J. Cell Biol. 1983;96:265–76. doi: 10.1083/jcb.96.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Description of a structure at the base of the cilium that provides a site for rhodopsin carrier vesicle docking and fusion. Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247:438–41. doi: 10.1038/247438a0.
- Poole CA, Flint MH, Beaumont BW. Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 1985;5:175–93. doi: 10.1002/cm.970050302. [DOI] [PubMed] [Google Scholar]
- Qin H, Burnette DT, Bae Y-K, Forscher P, Barr MM, et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 2005;15:1695–99. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 2004;164:255–66. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J. Clin. Invest. 2008;118:1458–67. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T. Olfactory cilia in the frog. J. Cell Biol. 1965;25:209–30. doi: 10.1083/jcb.25.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K, Görlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 2001;20:1320–30. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Single-photon detection by rod cells of the retina. Rev. Mod. Phys. 1998;70:1027–36. [Google Scholar]
- Rink JC, Gurley KA, Elliott SA, Sánchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–10. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Margall-Ducos G, Guidotti J-E, Brégerie O, Celati C, et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 2007;120:628–37. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, et al. The exocyst localizes to the primary cilium in MDCK cells. Biochem. Biophys. Res. Commun. 2004;319:138–43. doi: 10.1016/j.bbrc.2004.04.165. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–76. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, et al. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 2005;15:1861–66. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J. Cell Biol. 2010 doi: 10.1083/jcb.200911095. In press. DOI: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Guo D-F, Bugge K, Morgan DA, Rahmouni K, et al. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 2009;18:1323–31. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–34. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 1962;15:363–77. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Padrón T, de Souza W. Freeze-fracture localization of filipin-cholesterol complexes in the plasma membrane of Trypanosoma cruzi. J. Parasitol. 1983;69:129–37. [PubMed] [Google Scholar]
- Springer S, Spang A, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–48. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J. Neurosci. 1994;14:5818–33. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–44. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J. Cell Biol. 2000;151:1369–80. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Kyuuma M, Sugamura K. Endosomal sorting complex required for transport proteins in cancer pathogenesis, vesicular transport, and non-endosomal functions. Cancer Sci. 2008;99:1293–303. doi: 10.1111/j.1349-7006.2008.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao B, Bu S, Yang Z, Siroky B, Kappes JC, et al. Cystin localizes to primary cilia via membrane microdomains and a targeting motif. J. Am. Soc. Nephrol. 2009;20:2570–80. doi: 10.1681/ASN.2009020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. The nonmotile ciliopathies. Genet. Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- Traub LM. Clathrin couture: fashioning distinctive membrane coats at the cell surface. PLoS Biol. 2009;7:e1000192. doi: 10.1371/journal.pbio.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Bossard C, Khanna H, Peränen J, Swaroop A, et al. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell. 2008;15:187–97. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, et al. Flagellar membrane localization via association with lipid rafts. J. Cell Sci. 2009;122:859–66. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986;322:639–41. doi: 10.1038/322639a0. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WLC, et al. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA. 2006;103:18556–61. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Li Y, Zhang C-J, Kahn RA. Isoform-selective effects of the depletion of ADP-ribosylation factors 1--5 on membrane traffic. Mol. Biol. Cell. 2005;16:4495–508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 1997;137:1495–509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou Z, Walsh C, McMahon A. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc. Natl. Acad. Sci. USA. 2009;106:2623–28. doi: 10.1073/pnas.0812110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake CJ, Wright KJ, Nachury MV, Baye LM, Rahajeng J, et al. Building the primary cilium membrane: regulation of GEF trafficking and activity and a Rab11-Rab8 cascade; American Society for Cell Biology Annual Meeting; 2009. Abstr. 34. [Google Scholar]
- Wilson C, Nguyen C, Chen M, Yang J, Gacayan R, et al. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature. 2009;459:98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 2002;13:2508–16. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Yoshimura S-I, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 2007;178:363–69. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell. 2009;20:2522–29. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]