Abstract
Objective
We performed a longitudinal study using BALB/c mice expressing a T cell receptor (TCR) recognizing the arthritogenic ATEGRVRVNSAYQDK peptide of human cartilage proteoglycan (PG) to determine whether this genetic preponderance is sufficient for the development of arthritis.
Methods
PG-specific TCR transgenic (PG-TCR-Tg) mice were inspected weekly for peripheral arthritis until 12 months of age. The histology of peripheral joints was examined, and T cell responses, T cell activation markers, serum cytokines and autoantibodies were measured. Apoptosis and signaling studies were performed in vitro on T cells from aged PG-TCR-Tg mice.
Results
Spontaneous arthritis developed as early as 5–6 months of age, and the incidence increased to 40–50% by 12 months of age. Progressive inflammation began in the interphalangeal joints with cartilage and bone erosions and later expanded to the proximal joints of the front and hind paws. Spontaneous arthritis was associated with a high proportion of activated CD4+ T cells, enhanced interferon-gamma (IFNγ) and Interleukin (IL)-17 production and elevated levels of serum autoantibodies. IL-4-deficient PG-TCR-Tg mice developed arthritis earlier with higher incidence. Antigen-specific activation-induced cell death was diminished in CD4+ T cells of spontaneously arthritic PG-TCR-Tg mice in vitro, especially in those lacking IL-4.
Conclusion
The presence of CD4+ T cells expressing a TCR specific for an arthritogenic PG epitope is sufficient to trigger spontaneous autoimmune inflammation in the joints of BALB/c mice. IL-4 appears to be a negative regulator of this disease through attenuation of activation-induced cell death.
Introduction
Extracellular matrix components of avascular hyaline cartilage contain “tissue-restricted” antigens, such as those encrypted in a tertiary complex (e.g., the G1 domain of proteoglycan [PG] aggrecan) or hidden in the triple helix of type II collagen (CII). Although some of these cartilage macromolecules are involved in central tolerance (1), they are considered potential autoantigens in rheumatoid arthritis (RA) (2–5). Epitope-mapping studies in PG-induced arthritis (PGIA) have identified a dominant arthritogenic epitope (5/4E8) ATEGRVRVNSAYQDK (core peptide is underlined) within the G1 domain (6–8). Importantly, this peptide sequence, partially or completely, is incorporated in peptides that have been shown to stimulate T cells from RA patients (9–11). It is of special interest that a synthetic peptide containing the citrullinated 5/4E8 epitope (citrullinated at the TCR-binding arginine (12), bold in sequence shown above) induced positive T cell responses of approximately 60% of human patients with RA (11). The T cell hybridoma specific for this 5/4E8 peptide was used to generate T cell receptor transgenic (henceforth “PG-TCR-Tg”) mice (13). When compared to wild-type (wt) BALB/c mice, PG-TCR-Tg mice on the BALB/c background developed exacerbated arthritis upon PG immunization (14). Because T cell autoreactivity plays a central role in the etiology and pathological mechanisms of RA and corresponding mouse models (15–17), (auto)antigen-specific TCR signaling is of special interest. Therefore, the PG-TCR-Tg BALB/c mouse is a useful tool for studying T cell activation by self-peptides as well as the link between autoreactivity and arthritis development.
Depending on the threshold of stimulation, TCR signaling might result in either activation (proliferation and differentiation) or apoptosis (18), both of which are regulated by costimulatory molecules and cytokine receptor signaling pathways (19–21). TCR signal-induced apoptosis, also called activation-induced cell death (AICD), is a key mechanism of deleting activated T cell clones to downregulate superfluous immune responses (22). Thus, defective AICD may underlie the sustained T cell activation that is usually associated with autoimmune diseases (23). IL-4 is an anti-inflammatory cytokine that controls several target genes through the activation of STAT6. Deficiency in either IL-4 or STAT-6 in the BALB/c background has been shown to increase the severity of PGIA (24). The regulatory function of IL-4 in AICD has also been demonstrated (25). Therefore, autoepitope-specific (PG-TCR-Tg) CD4+ T cells, in combination with IL-4 deficiency, especially in an arthritis-prone genetic background (BALB/c), may mediate an accelerated autoimmune response.
Spontaneous arthritis has been reported in a number of genetically modified/altered mice. For example, the K/B×N mouse was generated by intercrossing KRN TCR-Tg mice, specific for bovine pancreas ribonuclease, with the NOD strain (26). In the context of NOD MHC-II (H2g7), the KRN transgenic TCR recognizes an epitope in glucose-6-phosphate isomerase, which is the actual autoantigen in the K/B×N spontaneous arthritis model (27,28). SKG mice develop arthritis due to a spontaneous mutation in the SH2 domain of Zap70 (17). Altered thymic selection in these SKG mice leads to the survival of otherwise negatively selected T cell clones, which then spontaneously differentiate into Th17 cells in the periphery and attack the joints. IL-1R antagonist protein (IRAP) knock-out mice, on the other hand, develop spontaneous arthritis due to increased production of pro-inflammatory cytokines (IL-1β, IL-6, IL-17 and TNFα) and autoantibodies because a negative regulator of IL-1 signaling is absent (29,30). Importantly, spontaneous arthritis develops in SKG and IRAP-deficient mice only on the BALB/c genetic background (17,29,30).
Here we report that PG-TCR-Tg mice on the BALB/c background develop spontaneous arthritis at an advanced age. Inflammation of the interphalangeal joints is observed in association with cartilage and bone erosions after 6 months of age. Inflammation expands slowly but steadily, involving the metacarpal/metatarsal and then the wrist/ankle joints. The morphological alterations are associated with increasing activation of PG-TCR-Tg CD4+ T cells and production of increasing amounts of anti-PG autoantibodies. The lack of the anti-inflammatory cytokine IL-4 results in a further increase in the severity of inflammation and an earlier disease onset. Based on these observations, we conclude that the dominant presence of an arthritogenic epitope-specific TCR is sufficient to trigger and maintain spontaneous autoimmune inflammation in the joints of aging mice in an appropriate (BALB/c) genetic background.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) or Fischer Scientific (Chicago, IL), unless indicated otherwise. Mouse recombinant cytokines and enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN) or BD Biosciences (San Diego, CA). Phosphate buffered saline (PBS; pH 7.4) was used for washing and short-term storage of cells until use. Cell-surface labeling with monoclonal antibodies (mAb) was carried out in flow cytometry wash buffer (PBS containing 0.1% NaN3 and 0.1 % bovine serum albumin). Cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) under standard tissue culture conditions.
Mice and clinical assessment of arthritis
All animal procedures were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Rush University Medical Center, Chicago. IL-4−/− mice (The Jackson Laboratory, Bar Harbor, ME) and PG-TCR-Tg mice, in which more than 95% of the CD4+ T cells express PG (5/4E8 epitope)-specific Vβ4/Vα1.1 TCR (13), both on the BALB/c background, were intercrossed. Previously, both IL-4−/− and PG-TCR-Tg mice showed exacerbated arthritis upon PG-immunization (14,24). All experiments were performed with homozygous female PG-TCR-Tg/IL-4+/+ and PG-TCR-Tg/IL-4−/− mice. Mice were monitored for the clinical signs of arthritis once a week from 1 month of age until the end of the experiment. Redness and swelling of the toes were considered the first sign of inflammation. Animals were sacrificed at different time points to assess disease progression by histology and finally at 12 months of age (end of experiments).
T cell separation, activation of transgenic T cells with peptide presenting irradiated A20 cells and detection of TCR signaling
T cells from the spleens of PG-TCR-Tg mice were purified using the EasySep magnetic T cell enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada). T cells were seeded on irradiated A20 antigen presenting cells (ATCC, Rockville, MD), which can present the 5/4E8 peptide (31). A20 cells (1×105 cells/well) were plated in 48-well plates and pre-cultured with or without synthetic 5/4E8 peptide (50 μg/ml) for 12 hours and then washed with serum-free DMEM.
For the signaling studies, 3×105 purified T cells were spun onto the A20 cell layer by short centrifugation (900×g, 5 min), and then T cells were harvested after 1, 2, 3 and 5 hours of co-culture. Phosphorylation of Zap70 and ERK1/2 was detected by phospho-flow technique according to the manufacturers' instructions (32). Cells were labeled with anti-CD4-PerCP-Cy5.5 and phospho-specific antibodies, either phycoerythrine (PE)-conjugated anti-mouse Zap70 (clone 17A/P-Zap70) recognizing the pY319 or PE-conjugated mouse anti-ERK1/2 (clone 20A) recognizing the pT203/pY205 in ERK1 and the pT183/pY185 in ERK2 (both purchased from BD Biosciences).
Apoptosis detection using Annexin V/propidium-iodide (PI) staining
Annexin V/PI staining was used to distinguish between early and late apoptotic cells (33). Labeling was done according to the manufacturer's instructions, and cells were immediately analyzed by flow cytometry. Live cells are negative for both Annexin V and PI, early apoptotic cells are Annexin V+PI−, and late apoptotic cells are Annexin V+PI+.
Monoclonal antibodies (mAbs), fluorescent cell surface labeling and flow cytometry
Fluorochrome-labeled rat anti-mouse antibodies specific for TCR Vβ4, CD3, CD4, CD5, CD8, CD19, CD23, CD25, CD43, CD44, CD62L, CD69, B220, IgD and IgM, as well as AnnexinV-FITC and PI, were purchased from BD Biosciences. We used a multicolor labeling technique for simultaneous detection of cell surface molecules on cells harvested from the spleens of mice as described (34). Samples were measured using a FACS Canto II flow cytometer, and data was analyzed by FACS DIVA software (BD Flow Cytometry Systems, San Jose, CA). Cell populations were defined by surface marker expression as described (34). Specific cell populations were expressed as percent of total cells unless otherwise stated. We used fluorescence histogram plots to compare mean fluorescence intensities (MFIs) of different samples and to calculate the proportions of positively stained cells.
Measurement of PG-specific antibodies and T cell responses
Serum samples and spleen cells were harvested from mice at the end of the experiment. Mouse PG-specific IgG1 or IgG2a (auto)antibodies in serum were measured using ELISA as described (35). Antigen-specific T cell responses were measured in quadruplicate samples of spleen cells (3×105 cells/well in 200 μl in 96-well plates) cultured in the absence or presence of 25 μg 5/4E8 peptide/ml. T cell proliferation was assessed using [3H]thymidine incorporation on day three of culture (14,36). Spontaneous and antigen-specific production of IL-4, IL-6, IL-17, TNFα and IFNγ was measured in spleen cell culture supernatants (1.8×106 cells/well in 600 μl in 48-well-plates) harvested on day 4 using capture ELISAs, and the results were expressed as nanogram (ng) cytokine secreted by 1×106 cells (14).
Statistical analysis
Descriptive statistics were used to determine group means and standard error of the mean (mean ± SEM) unless otherwise stated. The difference between two groups was tested for statistical significance using Student's t-test, and the difference among three or more groups was tested using analysis of variance (ANOVA). A p≤0.05 value was considered to be statistically significant.
RESULTS
Spontaneous arthritis develops in PG-specific PG-TCR-Tg mice at an advanced age
The 5/4E8 sequence-specific PG-TCR-Tg mouse on the BALB/c background was developed to study the mechanisms of PGIA and the role of antigen-specific TCR signaling and AICD in the clinical phenotype of arthritis (13,14). During the backcross process onto the BALB/c background, we occasionally found mild interphalangeal joint swelling in aging naïve PG-TCR-Tg mice. This observation compelled us to perform a longitudinal study of homozygous PG-TCR-Tg mice to monitor for spontaneous development of arthritis.
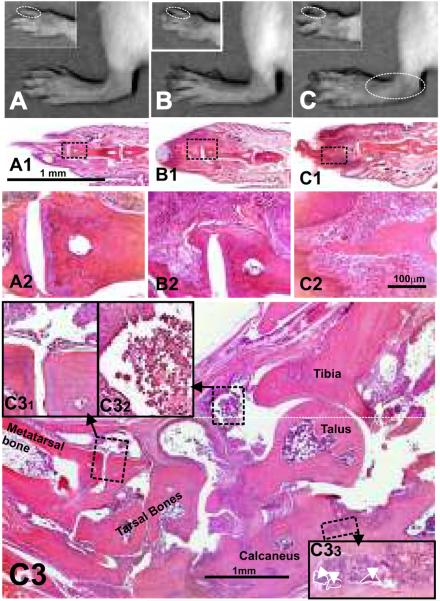
PG-TCR-Tg mice developed inflammation in the interphalangeal joints beginning at five to six months of age (Fig. 1A). The incidence increased gradually from 10–20 % at 6 months of ago to ~40 % at 12 months of age (Fig. 1A). Inflammation typically started in the distal interphalangeal joints, first in the hind paws, and a couple weeks later in the front paws as well (Figs 2A–2C). Additional (proximal) interphalangeal joints became inflamed, followed by metacarpo/metatarsophalangeal, carpo-metacarpo and tarso-metatarso joints as the mice aged (Fig. 2C3). Although stiffness of affected fingers was characteristic, cartilaginous or bony ankylosis did not occur, but cartilage and bone were eroded especially in tarsal/carpal joints at advanced age. Repeated inflammatory episodes in joints led to the thickening of fingers, nails were lost, and finally a “drumstick-finger” deformation developed (Fig. 2B). Ultimately, inflammation expanded to the proximal joints (wrist and ankle) in older animals, which was more pronounced in animals with the earliest onset of arthritis. Although approximately half of the PG-TCR-Tg mice were clinically healthy at the age of 12 months, histology frequently revealed proliferation of synovial lining cells and mild cartilage and bone erosions, even in symptom-free animals after 5–6 months of age. Although not all asymptomatic animals were examined histologically, by 12 months of age, and especially by 15 months of age, all animals tested were positive for synovial inflammation (most recent information, data not shown). Therefore, we expect that with sufficient time all PG-TCR-Tg mice would develop arthritis, although their life expectancy is approximately 25–30% shorter than their wild-type littermates. Such changes were never seen in age-matched non-transgenic BALB/c mice (results not shown).
Figure 1.
Timeline of the development of spontaneous arthritis in the 5/4E8 peptide (ATEGRVRVNSAYQDK)-specific PG-TCR-Tg (PG-TCR-Tg) mice. Swelling and mild redness of the distal interphalangeal joints were considered the first signs of inflammation. Values are calculated as percent of arthritic animals per total number of PG-TCR-Tg mice in each group followed up to 12 months of age. (A) Spontaneous arthritis in 28 female 5/4E8 peptide-specific PG-TCR-Tg BALB/c mice. (B) Spontaneous arthritis in 20 female IL-4-deficient PG-TCR-Tg (PG-TCR-Tg/IL-4−/−) BALB/c mice.
Figure 2.
Macroscopic and histology images of front and hind paws of (A) wild type (healthy 9-month-old BALB/c), (B) spontaneously arthritic PG-TCR-Tg/IL-4+/+ and (C) PG-TCR-Tg/IL-4−/− mice. Corresponding sections (at low and high magnifications) of fingers underneath the macroscopic images are depicted in panels A1–A2, B1–B2 and C1–C2. Thickening of the distal interphalangeal joints and phalanges and loss of nails were the earliest macroscopic abnormalities, which were followed by progression of inflammation to the proximal interphalangeal, metatarsophalangeal and tarso-matatarsal or carpo-metacarpal joints. Panel C3 is a low magnification montage picture of a hind paw (ankle area) from a 1-year-old PG-TCR-Tg/IL-4−/− BALB/c mouse (approximately 6 months after arthritis onset). Extensive cartilage and bone erosions of affected joints are the prominent histopathological abnormalities demonstrated in high-magnification inserts (C31 and C33). Dominantly mononuclear cells infiltrate (C32) the synovium and the joint cavities. Contours of multinuclear osteoclasts are indicated by white broken lines and arrowheads (C33). Representative hematoxylin-stained sections are shown at 4× (A1, B1, C1 and C3), 10× (A2, B2 and C2) and 40× (C31, C32 and C33) original magnification.
Immunological characterization of mice with spontaneous arthritis
To gain insight into the potential mechanisms involved in the spontaneous development of arthritis in PG-TCR-Tg mice, groups of mice were sacrificed at different ages. Sera and spleens were harvested, and T and B cell responses and serum cytokines were assayed. Because spontaneous arthritis was usually observed in mice older than six months and immunosenescence appears to play a role in PGIA (37), we compared the T and B cell responses of old (12 months of age, arthritic or yet nonarthritic) and young (1.5 months of age) PG-TCR-Tg mice (Tables 1 and 2). These ~1.5-month-old mice were chosen as “young” controls because BALB/c mice at this age have been found to be resistant to PGIA (37). The age-related expansion of CD69high or CD25high (activated) or CD44high (activated/memory) transgenic CD4+ T cells, with a marked decrease of the CD62Lhigh (naïve) population (Table 1), appeared to create an optimal milieu for the development of autoimmunity.
Table 1.
Cellular composition of the spleen in PG-TCR-Tg/IL-4+/+ or PG-TCR-Tg/IL-4−/− spontaneously arthritic and healthy control mice as assessed by flow cytometry.
| PG-TCR-Tg/IL-4+/+ |
PG-TCR-Tg/IL-4−/− |
|||||
|---|---|---|---|---|---|---|
| Arthritic | Non-arthritic |
Arthritic | Non-arthritic |
|||
| Age (months) | 12 | 12 | 1.5 | 12 | 12 | 1.5 |
| Number of animals | 6 | 3 | 4 | 19 | 3 | 4 |
| T cell (Vβ4+CD3+)#^ | 23.1±1.2 | 27.2±1.4 | 28.7±1.5 | 19.0±0.9 | 17.8±1.6 | 25.1±0.3 |
| CD8+T cell§ | 2.8±0.1 | 2.7±0.4 | 0.5±0.01 | 5.2±0.4* | 2.8±0.5 | 0.4±0.1 |
| CD4+T cell§ | 96.1±0.1 | 95.2±1.0 | 98.7±0.1 | 92.0±0.8 | 93.6±1.8 | 98.8±0.1 |
| CD69highCD4+T cell¶ | 3.2±0.1 | 4.0 ± 0.5 | 1.0 ± 0.1 | 9.3 ± 1.2* | 2.7±0.6 | 1.1±0.1 |
| CD25highCD4+T cell¶ | 7.4±1.5 | 6.8 ± 1.6 | 3.4 ± 0.2 | 10.2±0.9 | 6.8 ± 1.9 | 3.4 ± 0.2 |
| CD62LhighCD4+T cell¶ | 61.3±1.5 | 50.3 ± 2.0 | 90.6 ± 1.0 | 50.1±1.7 | 57.5 ± 5.5 | 91.5 ± 0.1 |
| CD44highCD4+T cell¶ | 15.9±1.5 | 19.5 ± 2.2 | 5.5 ± 0.3 | 31.4 ± 1.4* | 12.4±1.0 | 5.8±0.3 |
| B cell (B220+)# | 37.3±0.8 | 36.3±1.0 | 39.2±0.5 | 34.1±1.1 | 33.3±4.7 | 41.2±0.9 |
| B1 & MZ & TB cells# | 5.5 ± 0.2 † | 8.1±0.3 | 6.6±0.4 | 4.5 ± 0.3† | 8.2±0.6 | 8.6±0.4 |
Percent of total cells.
Percent of CD3+T cells.
Percennt of CD4+T cells.
All values are expressed percent (mean ± SEM). Significantly higher (*) or lower (†) values (p<0.05) in arthritic mice compared to age-matched (old) non-arthritic groups are boldfaced. Significant (p<0.05) age-related changes in cellular composition are indicated with underlined italics.
Table 2.
Immune parameters and biomarkers of the PG-TCR-Tg/IL-4+/+ and PG-TCR-Tg/IL-4−/− BALB/c mice at 6 weeks and 12 months of age
| PG-TCR-Tg/IL-4+/+ |
PG-TCR-Tg/IL-4−/− |
|||||
|---|---|---|---|---|---|---|
| Arthritic | Non-arthritic |
Arthritic | Non-arthritic |
|||
| Age (months) | 12 | 12 | 1.5 | 12 | 12 | 1.5 |
| Number of animals | 6 | 3 | 4 | 19 | 3 | 4 |
| Proliferation (Δcpm, ×104) | 8.5±0.4 | 7.5±0.3 | 7.1±0.1 | 7.2±0.4 | 6.3±1.2 | 7.5±0.7 |
| In vitro 5/4E8-peptide-induced spleen cell cytokine production (ng/106cells) | ||||||
| IL-4 | ND* | ND* | ND | ND | ND | ND |
| IL-6 | 0.22±0.09 | 0.10±0.01 | 0.70±0.03 | 0.19±0.02 | 0.10±0.03 | 0.66±0.04 |
| IL-17 | 0.51±0.38 | 0.40±0.10 | 1.01±0.14 | 0.89±0.12 | 0.63±0.24 | 1.19±0.12 |
| IFNγ | 6.76 ± 2.71 * | 4.59±0.27 | ND | 6.16 ± 0.46 * | 4.94±0.53 | 0.89±0.63 |
| TNFα | 0.15±0.04 | 0.15±0.03 | 0.39±0.08 | 0.13±0.01 | 0.14±0.02 | 0.47±0.04 |
| Serum cytokines (pg/ml) | ||||||
| IL-1β | 3.3±8.9 | ND | ND | 6.3±1.7 | 2.3±1.4 | ND |
| IL-4 | ND | ND | ND | ND | ND | ND |
| IL-6 | ND | ND | ND | 29.7±2.1 | ND | ND |
| IL-17 | ND | ND | ND | 78.2±7.1 | ND | ND |
| IFNγ | ND | ND | ND | ND | ND | ND |
| TNFα | ND | ND | ND | ND | ND | ND |
| Serum autoantibodies (μg/ml) | ||||||
| IgG1 | ND | ND | ND | ND | ND | ND |
| IgG2a | 29.5±16.2 | ND | ND | 33.6±13.8 | ND | ND |
Age, number of animals and incidence of arthritis in each group, in vitro T cell responses in spleen cell cultures (proliferation and cytokine production) and serum markers (cytokines and mouse PG-specific autoantibodies) are summarized. Values are mean ± SEM. Significantly (p<0.05) higher values are boldfaced and labeled with asterisk (*) in old PG-TCR-Tg arthritic compared to non-arthritic young mice. Serum biomarkers (cytokines and autoantibody) were measured in arthritic old (either IL-4-deficient or IL-4-positive) PG-TCR-Tg mice. ND: not detectable.
Note: Although sera were negative for IL-4, PG-TCR-Tg T cells isolated from old (6–7 months) mice secreted significant amounts of IL-4 in vitro in response to human PG or recombinant human G1 (antigen) stimulation.
There was no significant difference in 5/4E8 epitope peptide-induced proliferation of spleen cells in young and old (arthritic or still symptom-free) mice (Table 2). This was more or less the same when a number of in vitro stimulation-induced cytokines were measured in supernatants of spleen cell cultures; with the exception of peptide stimulation-induced IFNγ production, which was significantly higher in aged (12-month-old) than in young mice (Table 2). Thus IFNγ, a Th1 pro-inflammatory cytokine, may play a role in the development of spontaneous arthritis, similar to that reported in PGIA (38).
Higher incidence of spontaneous arthritis and higher frequency of autoreactive CD4+ T cells in PG-TCR-Tg/IL-4−/− than in PG-TCR-Tg/IL-4+/+ BALB/c mice
Earlier studies from our laboratory showed that IL-4 regulates arthritis severity in a STAT-6 dependent manner (24). Therefore, we intercrossed PG-TCR-Tg with IL-4 knockout (IL-4−/−) mice (both in BALB/c background) to determine whether IL-4 has a regulatory role in spontaneous arthritis. As shown in Figure 1, earlier onset of spontaneous arthritis was observed in IL-4-deficient PG-TCR-Tg mice as compared with IL-4 sufficient PG-TCR-Tg mice (henceforth PG-TCR-Tg/IL-4+/+). Interphalangeal joint inflammation developed in ~10% of IL-4-deficient PG-TCR-Tg (PG-TCR-Tg/IL-4−/−) mice at 4 months of age, which increased gradually to ~60% by 12 months of age (Fig. 1B). This difference in onset time and incidence indicated that IL-4 was indeed involved in the regulation of spontaneous arthritis. However, the macroscopic abnormalities (Figs 2B–2C) and histopathology were similar in PG-TCR-Tg/IL-4+/+ (Fig. 2B) and PG-TCR-Tg/IL-4−/− mice (Fig. 2C and Fig. 2C3, only the PG-TCR-Tg/IL-4−/− ankle joint is shown).
Spleen cells harvested from arthritic PG-TCR-Tg (either IL-4-deficient or IL-4 sufficient) mice produced high concentrations of IFNγ upon 5/4E8 peptide stimulation in vitro (Table 2). Pro-inflammatory IL-1β, IL-6, and IL-17 were found in the sera of arthritic PG-TCR-Tg/IL-4−/− mice, but, except IL-1β in old non-arthritic mice, these cytokines were not detected in the non-arthritic old or young control groups (Table 2). The discrepancy between the serum and in vitro produced cytokines reflects the difference between the cytokine levels measured in the circulation versus a more selective group (mainly T lymphocytes in the spleen in response to antigen stimulation) of cells examined in in vitro tests. Similar to the PG-TCR-Tg/IL-4+/+ BALB/c mice, anti-mouse PG (only IgG2 isotype) autoantibodies were detected in the sera of arthritic PG-TCR-Tg/IL-4−/− mice (Table 2).
Impaired antigen-specific AICD may promote the development of spontaneous arthritis in PG-TCR-Tg BALB/c mice
TCR-induced strong signals lead to activation of T cells followed by AICD. Perturbed AICD is assumed to underlie autoimmune processes through accumulation of activated (and potentially self-reactive) T cells (23). Because aged PG-TCR-Tg mice developed arthritis spontaneously and arthritis was associated with the accumulation of activated self-reactive T cells (Tables 1 and 2), we next decided to characterize the antigen (5/4E8 epitope)-specific TCR signal-induced apoptosis in PG-TCR-Tg mice. Mice with IL-4- and/or STAT-6-deficiency demonstrated a regulatory role for IL-4 in AICD (25); therefore, the use of PG-TCR-Tg/IL-4−/− mice appeared to be appropriate to study the regulatory role of IL-4 on TCR signaling and apoptosis. Because only subtle differences were found between IL-4-deficient and IL-4-sufficient PG-TCR-Tg mice in the onset and incidence of spontaneous arthritis (Fig. 1) but there were more pronounced differences in the percentage of activated CD4+ T cells (Table 1), we hypothesized that IL-4 was involved in the regulation of AICD. Therefore, we compared the 5/4E8 peptide-induced apoptosis of T cells from PG-TCR-Tg/IL-4+/+ and PG-TCR-Tg/IL-4−/− mice (Fig. 3). Approximately 60–70 % of the CD4+ PG-TCR-Tg cells (either IL-4-deficient or IL-4-sufficient) were Annexin V+ after 2 days when cultured in the presence of 5/4E8 synthetic peptide presented by A20 cells (Fig. 3A). The percentage of early apoptotic cells was still above 50 % in PG-TCR-Tg/IL-4+/+ T cell cultures on day 3, whereas it was reduced to 30–40 % in IL-4-deficient PG-TCR-Tg T cell cultures (Figs. 3B and 3C). At both time points, there were significantly more live cells (especially evident on day 3) and less late apoptotic cells in the absence of IL-4 (Fig. 3).
Figure 3.
Antigen-specific activation-induced apoptosis in PG-TCR-Tg CD4+ T cells. In vitro 5/4E8 peptide stimulation-induced apoptosis of purified CD4+ T cells of spleens (from IL-4-deficient and IL-4-sufficient PG-TCR-Tg BALB/c mice) were compared. (A) Percent of live (Annexin V−PI−), early apoptotic (Annexin V+PI−) and late apoptotic (Annexin V+PI+) cells. Bars represent mean ± SEM values calculated from the data of three mice in each group on days two and three. Significant (p<0.05) differences are indicated by asterisks. Representative flow cytometric contour plots show the distribution of (B) PG-TCR-Tg /IL-4+/+ and (C) PG-TCR-Tg/IL-4−/− T cells according to their Annexin V and PI staining after three days culture in the presence of TCR-specific 5/4E8 peptide presented by semi-confluent irradiated A20 cells. Numbers in the quadrants of contour plots show the percent of total cells.
To determine whether the IL-4-dependent differences in apoptosis could be explained by alteration of the TCR signaling threshold, we performed intracellular staining with phospho-specific antibodies against Zap70 and ERK1/2, two key members of the TCR signaling cascade (39) (Fig. 4). The phosphorylation of Zap70 and ERK1/2 in spleen CD4+ T cells reached a peak at 2 hours of in vitro stimulation with 5/4E8 peptide-coated A20 cells (Fig. 4). In the absence of IL-4, the amplitude of Zap70 phosphorylation was considerably lower (Fig. 4A and 4C), while the ERK1/2 phosphorylation was only subtly lower in the IL-4-deficient than in IL-4-sufficient PG-TCRTg mice (Fig. 4B and 4D).
Figure 4.
Phosphorylation changes upon TCR stimulation in PG-TCR-Tg T cells. Flow cytometric comparison of the in vitro 5/4E8 peptide-induced signaling was performed using purified T cells (more than 95% CD4+Vβ4+) harvested from spleens of old (≥9 months) PG-TCR-Tg/IL-4+/+ or PG-TCR-Tg/IL-4−/− mice. (A and B) Diagrams show values of mean fluorescence intensities (MFI) from four representative samples, measured in the FL2 channel after labeling the cells (A) with anti-phospho-Zap70-PE or (B) anti-phospho-ERK1/2-PE antibodies. (C) Representative FL-2 histogram plots show the Zap70 phosphorylation measured at different time points in the CD4+ T cells of PG-TCR-Tg /IL-4+/+ and PG-TCR-Tg/IL-4−/− mice. (D) Representative FL-2 histogram plots show the ERK1/2 phosphorylation measured at the indicated time points in the CD4+ T cells of PG-TCR-Tg/IL-4+/+ and PG-TCR-Tg/IL-4−/− mice. Numbers inside the panels indicate the MFI value, representing the phosphorylation status of Zap70 or ERK1/2. Time points are indicated between the histogram plots of panels C and D. The vertical black line in the histogram plots (C, D) is set at the MFI value of the control sample.
DISCUSSION
Alterations in T cell activation and apoptosis have been shown to contribute to the development of autoimmune diseases (23). PG-TCR-Tg mice, which possess CD4+ T cells specific for the 5/4E8 peptide sequence, a dominant arthritogenic epitope in the G1 domain of the PG-aggrecan molecule (14), are a useful tool for studying the potential role of antigen-specific AICD in PGIA. PG-TCR-Tg mice deficient in IL-4 confirmed that IL-4 contributes to the regulation of AICD in PGIA (25). Nonetheless, both IL-4+/+ and IL-4−/− groups of PG-TCR-Tg mice developed spontaneous arthritis at an advanced age (beginning at 4–6 months of age), with the earliest signs of inflammation being localized to the interphalangeal joints.
In PG-TCR-Tg mice almost all T cells recognize the dominant arthritogenic epitope GRVRVNSAY (14). Cross reactivity with the homologous mouse sequence GQVRVNSIY has been confirmed (6,13). It is of special importance that T cell responses to the human 5/4E8 epitope in its native (10) or citrullinated form (11) were frequently detected in RA patients. PG-TCR-Tg BALB/c mice were shown to be highly susceptible to PGIA, with very early onset and high severity of the disease (14). However, no study has determined whether the presence of antigen-specific TCR transgenic T cells is sufficient to induce arthritis without injection of exogenous antigen, either in our model or in type II collagen-specific TCR-Tg mice (40). In the present work, we confirmed that, indeed, arthritis develops in PG-TCR-Tg mice spontaneously at an advanced age, and disease develops even earlier in the absence of IL-4. The clinical symptoms and the early histopathological abnormalities, however, are markedly different from what we observed in “classical” PGIA or what was described in CIA (4,5). In PG-TCR-Tg mice, the disease begins with mild lesions of the fingers, which gradually (over months) lead to more severe deformities, loss of nails and thickening of the toes, but the whole paw is only affected in a minority of animals. On the other hand, PGIA usually starts 10–15 days after the 2nd intraperitoneal PG injection with adjuvant, and the complete clinical picture (redness and swelling of entire paws, and early joint deformities and ankylosis) develops rapidly after the third immunization (36).
The dominant proximal joint (finger) involvement, the late onset and the histopathology of affected small joints found here are similar to those described in a previous study in HLA-DR4 mice with spontaneous arthritis (35). Replacement of the I-Ad (class-II MHC in BALB/c) molecule with human HLA-DR4 on a BALB/c background led to the development of spontaneous arthritis, which resembled psoriatic arthritis (35). The association of HLA-DR4 with RA was described a long time ago (41), and this MHC molecule can likely initiate activation of T cells through presentation of potentially arthritogenic peptide fragments, leading to autoimmunity in susceptible individuals (7). The HLA-DR4 molecule has been shown to present 20 peptide fragments (epitopes) of the human cartilage PG (aggrecan) molecule, including the 5/4E8 epitope (7).
Based on the similarity of spontaneous arthritis described here in PG-TCR-Tg mice and earlier in HLA-DR4 Tg mice (35), we hypothesized that a common immunological mechanism operates in both cases. In addition to the same BALB/c genetic background, age seems to be an additional factor. Age-related cartilage degeneration could be a common triggering event. Over time, small amounts of arthritogenic cartilage components (including PG fragments) are released from the joints, leading to activation of T and B cells. In an appropriate genetic background, this stimulation could lead to a breach of tolerance, resulting in an autoimmune response that culminates in arthritis development. While 100 % incidence was observed in HLA-DR4 Tg mice (35), in PG-TCR-Tg mice only 40–60 % of the mice developed spontaneous arthritis, depending on the presence or absence of IL-4. In HLA-DR4 Tg mice, the DR4 molecule can present a broad spectrum of epitopes to a diverse T cell repertoire, which could lead to a “polyclonal” activation of T cells (7). In PG-TCR-Tg mice, on the other hand, the majority of T cells are specific for 5/4E8, the dominant arthritogenic peptide epitope; therefore, only one of the potentially arthritogenic epitopes, presented by the native I-Ad, is recognized, which leads to a limited “monoclonal” activation of T cells, resulting in lower disease incidence in these mice. Another possible explanation for the lower incidence of spontaneous arthritis in PG-TCR-Tg mice could be that the 5/4E8 homologous sequence in the mouse PG molecule has lower affinity for the TCR of the Tg mice than the human peptide (8). In either case, arthritogenic T cells may be continuously activated by PG fragments released from cartilage catabolism in aging animals, gradually paving the way to autoimmune joint inflammation.
Studying T cell apoptosis is of special interest in elucidating the pathogenesis of autoimmune arthritis (23,42). According to our present results, T cell apoptosis might play a role in the pathogenesis of the spontaneous arthritis in PG-TCR-Tg mice. IL-4 is an anti-inflammatory cytokine that contributes to the regulation of PGIA (24). Here we found slightly higher incidence of the spontaneous disease and a more pronounced accumulation of activated T cells in PG-TCR-Tg mice deficient in IL-4. The lack of IL-4 resulted in decreased in vitro TCR signaling and impaired apoptosis in CD4+ T cells. This is consistent with results from a previous study showing that IL-4 potentiates T cell apoptosis (25). We propose that in PG-TCR-Tg mice, repeated endogenous antigen exposure leads to T cell activation, which, in the absence of IL-4, is followed by reduced apoptosis. Thus accumulation of activated T cells in the absence of IL-4 may ultimately lead to higher disease incidence.
The threshold of TCR signaling is one of the key regulators, or critical “checkpoints”, of AICD. Zap70 and ERK1/2 have been shown to be involved in regulating T cell apoptosis (43,44). Here, decreased phosphorylation of Zap70 (at Y319) and ERK1/2 (at T203/Y205 in ERK1 and T183/Y185 in ERK2) was detected upon TCR stimulation in IL-4-deficient PG-TCR-Tg CD4+ T cells when compared to PG-TCR-Tg/IL-4+/+ CD4+ T cells. A point mutation study has shown that loss of the Y319 activator phosphorylation site in Zap70 abrogates PLCγ and LAT phosphorylation, while SLP-76 and ERK signaling remains unaffected (45). The fact that TCR-induced Y319 phosphorylation of Zap70 in PG-TCR-Tg CD4+ T cells is affected by the absence of IL-4 raised the possibility of cross-talk between the TCR and IL-4 signaling pathways. Modification of IL-4 signaling by the Ras-MAPK pathway after TCR engagement has been reported (46). However, this is the first report where attenuation of TCR signaling by IL-4 deficiency is shown in spontaneous self-reactive transgenic CD4+ T cells. IL-4-induced phosphorylation of p56 Lck and p59 Fyn was shown in CD3-activated killer cells (47). Decreased phosphorylation of Zap70 in the absence of IL-4, described here, might thus be attributed to lower activity of Lck and Fyn, which are important upstream regulators of Zap70 (39). The ERK pathway is hyper-responsive in T cells from RA patients, which is not limited to activated T effector cells but involves all naïve and central memory CD4+ and CD8+ T cells (48). This observation is of interest because an earlier study (49) proposed that pERK levels, which are central to TCR threshold tuning, make the decision between responding to exogenous high-affinity antigens (such as human PG in our case) while maintaining low response or tolerance to low-affinity self peptides (such as mouse PG here). Activation of the ERK pathway in RA patients or in PG-TCR-Tg BALB/c mice could shift this delicate balance. Higher responsiveness of the ERK pathway is found not only in patients with established RA but also in SKG mice before they develop arthritis, which may be a critical step toward the breach of tolerance by allowing for expansion and differentiation of autoreactive T cells (49).
In conclusion, aging PG-TCR-Tg mice develop spontaneous arthritis, which could be triggered by sustained low-threshold T cell activation by self-cartilage components coupled with impaired AICD. In addition, IL-4 was confirmed to be a regulator of antigen-specific AICD through Zap70 and ERK1/2, two key signaling components of TCR activation. PG-TCR-Tg mice provide useful tools for studying antigen (PG)-specific signaling pathways and the role of the threshold of T cell activation and T cell apoptosis in the pathogenesis of arthritis.
Acknowledgements
The authors thank Beata Tryniszewska, B.S., for assistance with animal breeding and Dr. T. Kobezda for collecting human cartilage. We appreciate our earlier co-authors (Dr. Suzanne E. Berlo, Dr. Chris P. Broeren (who passed away in 2003) and Prof. Willem van Eden (The Netherlands), who were technically involved in, or intellectually contributed to, the development of transgenic mice.
Dr.Glant's work was supported the NIH (AR040310) and J.O. Galante endowment chair
Dr. Glant and Dr. Mikecz was supported The Grainger Foundation, Forest Park, IL
List of abbreviations
- 5/4E8 peptide
ATEGRVRVNSAYQDK
- AICD
activation-induced cell death
- AP-1
activator protein 1
- APC
allo-phycocyanin
- CII
collagen type II
- CIA
collagen-induced arthritis
- DMEM
Dulbecco's modified minimal essential medium
- ELISA
enzyme-linked immunosorbent assay
- ERK 1/2
extracellular signal regulated kinase 1/2
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HLA
human leukocyte antigen
- IFNγ
interferon-gamma
- IL-1
IL-4, interleukin-1, -4
- IL-4R
IL-4 receptor
- IRAP
IL-1R antagonist protein
- LAT
linker for activated T cells
- Lck
leukocyte-specific protein tyrosine kinase
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MFI
mean fluorescence intensity
- MHC
major histocompatibility antigen
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PBS
phosphate-buffered saline
- NOD
non-obese diabetic mice
- PE
phycoerythrine
- PG
proteoglycan
- PGIA
PG-induced arthritis
- PI
propidiumiodide
- PLCγ
phospholipaseCgamma
- RA
rheumatoid arthritis
- Ras
Harvey rat sarcoma virus oncogene
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
- Tg
transgenic
- TNFα
TNF-alpha
- wt
wild-type
- Sos
son of sevenless homolog
- Zap70
zeta-chain associated 70 kDa kinase.
Footnotes
Competing interests The authors declare that they have no competing interests.
REFERENCES
- (1).Campbell IK, Kinkel SA, Drake SF, van Nieuwenhuijze A, Hubert FX, Tarlinton DM, et al. Autoimmune regulator controls T cell help for pathogenetic autoantibody production in collagen-induced arthritis. Arthritis Rheum. 2009;60:1683–93. doi: 10.1002/art.24501. [DOI] [PubMed] [Google Scholar]
- (2).Trentham DE, Dynesius RA, Rocklin RE, David JR. Cellular sensitivity to collagen in rheumatoid arthritis. N Engl J Med. 1978;299:327–32. doi: 10.1056/NEJM197808172990703. [DOI] [PubMed] [Google Scholar]
- (3).Glant T, Csongor J, Szücs T. Immunopathologic role of proteoglycan antigens in rheumatoid joint diseases. Scand J Immunol. 1980;11:247–52. doi: 10.1111/j.1365-3083.1980.tb00232.x. [DOI] [PubMed] [Google Scholar]
- (4).Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunization against heterologous type II collagen induces arthritis in mice. Nature. 1980;282:666–8. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- (5).Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- (6).Glant TT, Buzas EI, Finnegan A, Negroiu G, Cs-Szabó G, Mikecz K. Critical role of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812–9. [PubMed] [Google Scholar]
- (7).Szanto S, Bárdos T, Szabo Z, David CS, Buzás E, Mikecz K, et al. Induction of arthritis in HLA-DR4-humanized and HLA-DQ8-humanized mice by human cartilage proteoglycan aggrecan but only in the presence of an appropriate (non-MHC) genetic background. Arthritis Rheum. 2004;50:1984–95. doi: 10.1002/art.20285. [DOI] [PubMed] [Google Scholar]
- (8).Buzas E, Vegvari A, Murad YM, Finnegan A, Mikecz K, Glant TT. T-cell recognition of differentially tolerated epitopes of cartilage proteoglycan aggrecan in arthritis. Cell Immunol. 2005;235:98–108. doi: 10.1016/j.cellimm.2004.08.006. [DOI] [PubMed] [Google Scholar]
- (9).Guerassimov A, Zhang YP, Banerjee S, Cartman A, Leroux JY, Rosenberg LC, et al. Cellular immunity to the G1 domain of cartilage proteoglycan aggrecan is enhanced in patients with rheumatoid arthritis but only after removal of keratan sulfate. Arthritis Rheum. 1998;41:1019–25. doi: 10.1002/1529-0131(199806)41:6<1019::AID-ART8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- (10).de Jong H, Berlo SE, Hombrink P, Otten HG, van Eden W, Lafeber FP, et al. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T cell responses in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2009;69:255–62. doi: 10.1136/ard.2008.103978. [DOI] [PubMed] [Google Scholar]
- (11).von Delwig A, Locke J, Robinson JH, Ng WF. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 2009;62:143–9. doi: 10.1002/art.25064. [DOI] [PubMed] [Google Scholar]
- (12).Buzas EI, Hanyecz A, Murad Y, Hudecz F, Rajnavolgyi E, Mikecz K, et al. Differential recognition of altered peptide ligands distinguishes two functionally discordant (arthritogenic and non-arthritogenic) autoreactive T cell hybridoma clones. J Immunol. 2003;171:3025–33. doi: 10.4049/jimmunol.171.6.3025. [DOI] [PubMed] [Google Scholar]
- (13).Berlo SE, Van Kooten PJ, ten Brink CB, Hauet-Broere F, Oosterwegel MA, Glant TT, et al. Naive transgenic T cells expressing cartilage proteoglycan-specific TCR induce arthritis upon in vivo activation. J Autoimmun. 2005;25:172–80. doi: 10.1016/j.jaut.2005.09.017. [DOI] [PubMed] [Google Scholar]
- (14).Berlo SE, Guichelaar T, ten Brink CB, Van Kooten PJ, Hauet-Broere F, Ludanyi K, et al. Increased arthritis susceptibility in cartilage proteoglycan-specific T cell receptor-transgenic mice. Arthritis Rheum. 2006;54:2423–33. doi: 10.1002/art.22013. [DOI] [PubMed] [Google Scholar]
- (15).Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10(Suppl 1):S1. doi: 10.1186/ar2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–18. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- (17).Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–60. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- (18).She J, Matsui K, Terhorst C, Ju ST. Activation-induced apoptosis of mature T cells is dependent upon the level of surface TCR but not on the presence of the CD3 zeta ITAM. Int Immunol. 1998;10:1733–40. doi: 10.1093/intimm/10.11.1733. [DOI] [PubMed] [Google Scholar]
- (19).Metz DP, Farber DL, Taylor T, Bottomly K. Differential role of CTLA-4 in regulation of resting memory versus naive CD4 T cell activation. J Immunol. 1998;161:5855–61. [PubMed] [Google Scholar]
- (20).Zhang J, Bardos T, Li D, Gál I, Vermes C, Xu J, et al. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol. 2002;169:2236–40. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- (21).Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J Immunol. 2008;180:7958–68. doi: 10.4049/jimmunol.180.12.7958. [DOI] [PubMed] [Google Scholar]
- (22).Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002;14:354–9. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- (23).Gatzka M, Walsh CM. Apoptotic signal transduction and T cell tolerance. Autoimmunity. 2007;40:442–52. doi: 10.1080/08916930701464962. [DOI] [PubMed] [Google Scholar]
- (24).Finnegan A, Grusby MJ, Kaplan CD, O'Neill SK, Eibel H, Koreny T, et al. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J Immunol. 2002;169:3345–52. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- (25).Zhang J, Bardos T, Shao Q, Tschopp J, Mikecz K, Glant TT, et al. IL-4 potentiates activated T cell apoptosis via an IL-2-dependent mechanism. J Immunol. 2003;170:3495–503. doi: 10.4049/jimmunol.170.7.3495. [DOI] [PubMed] [Google Scholar]
- (26).Kouskoff V, Korganow A-S, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- (27).Korganow A-S, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- (28).Mangialaio S, Ji H, Korganow A-S, Kouskoff V, Benoist C, Mathis D. The arthritogenic T cell receptor and its ligand in a model of spontaneous arthritis. Arthritis Rheum. 1999;42:2517–23. doi: 10.1002/1529-0131(199912)42:12<2517::AID-ANR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- (29).Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist- deficient mice. J Exp Med. 2000;191:313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–90. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Brennan FR, Negroiu G, Buzas EI, Fülöp C, Mikecz K, Glant TT. Presentation of cartilage proteoglycan to a T cell hybridoma derived from a mouse with proteoglycan-induced arthritis. Clin Exp Immunol. 1995;100:104–10. doi: 10.1111/j.1365-2249.1995.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J Immunol. 2005;175:2366–73. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- (33).Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- (34).Boldizsar F, Tarjanyi O, Nemeth P, Mikecz K, Glant TT. Th1/Th17 polarization and acquisition of an arthritogenic phenotype in arthritis-susceptible BALB/c, but not in MHC-matched, arthritis-resistant DBA/2 mice. Int.Immunol. 2009;21:511–522. doi: 10.1093/intimm/dxp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bardos T, Zhang J, Mikecz K, David CS, Glant TT. Mice lacking endogenous major histocompatibility complex class II develop arthritis resembling psoriatic arthritis at an advanced age. Arthritis Rheum. 2002;46:2465–75. doi: 10.1002/art.10637. [DOI] [PubMed] [Google Scholar]
- (36).Hanyecz A, Berlo SE, Szanto S, Broeren CPM, Mikecz K, Glant TT. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–76. doi: 10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- (37).Tarjanyi O, Boldizsar F, Nemeth P, Mikecz K, Glant TT. Age-related changes in arthritis susceptibility and severity in a murine model of rheumatoid arthritis. Immunity Ageing. 2009;6:8. doi: 10.1186/1742-4933-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Mikecz K, et al. IFN-{gamma} Regulates the Requirement for IL-17 in Proteoglycan-Induced Arthritis. J Immunol. 2010;184:1552–9. doi: 10.4049/jimmunol.0902907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).van Leeuwen JE, Samelson LE. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–8. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- (40).Osman GE, Cheunsuk S, Allen SE, Chi E, Liggitt HD, Hood LE, et al. Expression of a type II collagen-specific TCR transgene accelerates the onset of arthritis in mice. Int Immunol. 1998;10:1613–22. doi: 10.1093/intimm/10.11.1613. [DOI] [PubMed] [Google Scholar]
- (41).Stastny P, Ball E, Kahn M, Olsen N, Pincus T, Gao X. HLA-DR4 and other genetic markers in rheumatoid arthritis. Br J Rheumatol. 1988;27:132–8. doi: 10.1093/rheumatology/xxvii.suppl_2.132. [DOI] [PubMed] [Google Scholar]
- (42).Zhang J, Bardos T, Mikecz K, Finnegan A, Glant TT. Impaired Fas signaling pathway is involved in defective T cell apoptosis in autoimmune arthritis. J Immunol. 2001;166:4981–6. doi: 10.4049/jimmunol.166.8.4981. [DOI] [PubMed] [Google Scholar]
- (43).van den Brink MR, Kapeller R, Pratt JC, Chang JH, Burakoff SJ. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J Biol Chem. 1999;274:11178–85. doi: 10.1074/jbc.274.16.11178. [DOI] [PubMed] [Google Scholar]
- (44).Zhong L, Wu CH, Lee WH, Liu CP. Zeta-associated protein of 70 kDa (ZAP-70), but not Syk, tyrosine kinase can mediate apoptosis of T cells through the Fas/Fas ligand, caspase-8 and caspase-3 pathways. J Immunol. 2004;172:1472–82. doi: 10.4049/jimmunol.172.3.1472. [DOI] [PubMed] [Google Scholar]
- (45).Williams BL, Irvin BJ, Sutor SL, Chini CC, Yacyshyn E, Bubeck WJ, et al. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 1999;18:1832–44. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Yamashita M, Kimura M, Kubo M, Shimizu C, Tada T, Perlmutter RM, et al. T cell antigen receptor-mediated activation of the Ras/mitogen- activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci U S A. 1999;96:1024–9. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Wang J, Hargrove ME, Ting CC. IL-2 and IL-4 mediate through two distinct kinase pathways for the activation of alphaCD3-induced activated killer cells. Cell Immunol. 1996;174:138–46. doi: 10.1006/cimm.1996.0303. [DOI] [PubMed] [Google Scholar]
- (48).Singh K, Deshpande P, Pryshchep S, Colmegna I, Liarski V, Weyand CM, et al. ERK-dependent T cell receptor threshold calibration in rheumatoid arthritis. J Immunol. 2009;183:8258–67. doi: 10.4049/jimmunol.0901784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]