Despite the existence of effective treatments for gout, persons with gout continue to experience acute attacks, with 69% having at least one recurrent attack in a year.1 Not surprisingly, persons with gout experience diminished quality of life and work disability,2 related not only to these recurrent flares, but also the presence of painful and at times disfiguring tophi. Given that gout is a common crystal-induced inflammatory arthritis, with up to 6.1 million US adults ever having gout in their lifetime,3 this disease presents a substantial public health burden.
Management of gout consists of three strategies: 1) treatment of acute attacks; 2) lowering serum uric acid to prevent acute flares and tissue deposition of uric acid crystals (tophi); and 3) prophylaxis against flares, particularly during initiation of urate-lowering therapy, and other strategies to prevent flares. Unfortunately, management of hyperuricemia in gout is suboptimal4 and inappropriate treatment of acute attacks occurs frequently.1 The recommended options for management of acute attacks include agents that non-specifically target inflammation: NSAIDs, colchicine, corticosteroids, and possibly ACTH.5
Although NSAIDs and colchicine are accepted as effective for gout attacks and typically used as first-line agents for acute attacks,5 there are few placebo-controlled trials of these agents.6, 7 In fact, despite its widespread use, oral colchicine was only recently approved for use in acute gout by the FDA in 2009, supported by the results of a recent trial.7 While the relative efficacy of colchicine compared with NSAIDs is not known, the various NSAIDs appear to have similar benefits in acute gout. Unfortunately, NSAIDs and/or colchicine may be poorly tolerated or contraindicated in the presence of certain comorbidities. In such instances, intra-articular corticosteroids may be given for monoarticular attacks, while systemic corticosteroids or ACTH may be used for polyarticular attacks or when sites not easily amenable to intra-articular injection are involved. However, the body of evidence for intra-articular corticosteroids and ACTH is poor.8 Systemic oral corticosteroids have been studied recently in two randomized placebo-controlled trials, with one trial evaluating prednisolone 30mg daily and the other prednisolone 35mg daily, both for 5 days. Both trials demonstrated equivalence to relatively standard regimens of NSAIDs (indomethacin and naproxen, respectively).9, 10 An alternate route for systemic administration of corticosteroids, intramuscular injection, was evaluated for acute gout in two studies. A 60mg intramuscular triamcinolone dose with optional repeat administration demonstrated equivalent efficacy with its comparators, indomethacin and ACTH, respectively. However, both studies were small and of low quality.11 Most studies in acute gout have evaluated single drug regimens despite the regular use of combination therapies in clinical practice,8 suggesting that such practices merit further examination.
While several effective management options for acute gout exist, refractory flares are a problem, particularly for persons with polyarticular and tophaceous gout. As such, multiple courses of corticosteroids are not infrequent among persons with chronic gout. Additionally, patients can experience breakthrough flares while initiating urate-lowering therapy despite NSAID or colchicine prophylaxis. With the increasing prevalence and incidence of gout12, 13 and frequent coexistent comorbidities,14 physicians are increasingly faced with individuals for whom few options are available for management of their acute attacks. There is, therefore, a clinical need for additional acute gout treatment options for those who have contraindications to, are intolerant of, or are refractory to existing therapies.
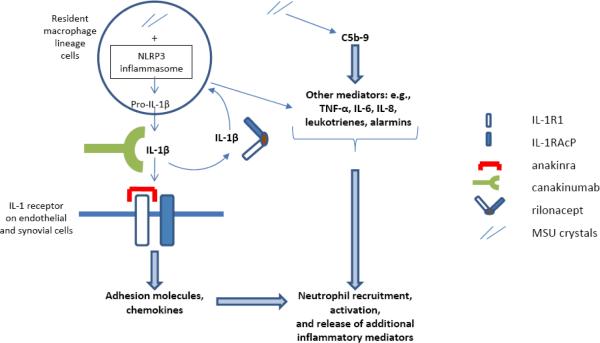
With the recognition that monosodium urate (MSU) crystals can directly activate the NLRP3 inflammasome, a key regulator of the pro-inflammatory cytokine interleukin-1β (IL-1β),15 gout therapy has begun to focus on the potential efficacy of IL-1 blockade. The strategy of IL-1 targeted therapy was first demonstrated to be effective in patients with cryopyrin-associated periodic syndromes (CAPS) in whom spontaneous mutations of NLRP3, which encodes the NLRP3 (cryopyrin) protein, are associated with excessive release of IL-1β.16 Three IL-1 antagonists, anakinra, rilonacept, and canakinumab, each with different compositions and mechanisms of action (Table 1), have been demonstrated to provide rapid relief of symptoms in CAPS.16–18 For gout, the extent to which such targeted therapy may be successful likely depends on the scenario in which the therapy is being used (Figure 1). For IL-1 antagonism to be maximally effective in the treatment of acute gout, it would likely have to be given very early on in the course of an attack to abort the downstream effects of IL-1β and other events in the inflammatory cascade, i.e., ideally before other cytokines are produced, against which IL-1 antagonists have no activity. For chronic active gouty arthritis, with chronic synovitis and/or frequently recurring, clustered, or non-resolving flares, the inflammatory processes described (see Figure 1 text) are likely ongoing with potentially persistent IL-1β release along with the other inflammatory mediators. Thus, IL-1 antagonism may only partially suppress the ongoing inflammatory processes and, by extension, symptoms in this setting. In contrast, prophylaxis with an IL-1 antagonist during initiation of urate-lowering therapy in the absence of chronic active gout may be predicted to have the greatest potential to suppress gouty inflammation by providing sufficient, steady-state IL-1 inhibitory activity in the synovium and its microvasculature, thereby more efficiently counteracting any newly secreted IL-1β that may arise and blunting the start of the gouty inflammatory cascade. Whether these three IL-1 antagonists should be expected to have equivalent efficacy in gout given their different compositions and mechanisms of action is not known. For example, each of the anti-TNFα agents is efficacious in rheumatoid arthritis, whereas in inflammatory bowel disease, only the anti-TNFα monoclonal antibodies are efficacious while etanercept, a soluble TNF receptor, is not.19
Table 1.
Composition, mechanism of action, and specificity of IL-1 antagonists
| Agent | Composition | Mechanism of Action and Specificity |
|---|---|---|
| Anakinra | Recombinant IL-1Ra | Binds to IL-1R1, blocking IL-1β and IL-1α |
| Canakinumab | Fully human monoclonal antibody with IL-1β specificity | Neutralizes IL-1β |
| Rilonacept | IL-1 Trap: dimeric fusion protein composed of extracellular domains of human IL-1R1 and IL-1RAcP linked to Fc portion of IgG1 | Neutralizes IL-1β and IL-1α |
Abbreviations: IL – interleukin; IL-1Ra – interleukin-1 receptor antagonist; IL-1 Trap – interleukin-1 target-related affinity profiling; IL-1R1 – interleukin-1 receptor 1; IL-1RAcP – interleukin-1 receptor accessory protein
Figure 1. Mechanisms of inflammation and IL-1 antagonism in acute gout.
In acute gout, phagocytosed MSU crystals activate the NLRP3 inflammasome, leading to caspase-1 activation, which in turn leads to cleavage of pro-IL-1β and secretion of mature IL-1β. IL-1β can induce further production of IL-1β (as well as many other inflammatory mediators) and further activation of synovial lining cells and phagocytes. MSU crystals also induce many other inflammatory cytokines (e.g., TNF-α, IL-6, IL-8, alarmins such as S100A8/A9) by both IL-1-dependent and IL-1 independent mechanisms, and MSU crystal-induced C5b-9 terminal complement pathway activation is an important driver of experimental acute gout. IL-1β must bind to both IL-1R1 and IL-1RAcP for signal transduction to occur. In endothelial cells, IL-1β appears to be a major trigger for altered adhesion molecule and chemokine expression, which, together with the other inflammatory events, results in neutrophil recruitment that drives the initiation of gouty inflammation. IL-1α, which is not depicted here, is expressed as a plasma membrane-bound molecule that also binds the IL-1 receptor. The role of IL-1α in experimental and clinical gout has not yet been defined via direct study. Anakinra binds to IL-1R1, blocking IL-1β and IL-1α; rilonacept acts as a soluble receptor, comprised of both IL-1R1 and IL-1RAcP fused to the Fc portion of IgG1, neutralizing IL-1β as well as IL-1α; canakinumab, a monoclonal antibody with IL-1β specificity, neutralizes IL-1β only. See text for discussion of potential role of IL-1 antagonism in acute gout, chronic active gout, and prophylaxis during urate-lowering therapy initiation.
Abbreviations: IL – interleukin; IL-1R1 – interleukin-1 receptor 1; IL-1RAcP – interleukin-1 receptor accessory protein; MSU – monosodium urate; NLRP – nucleotide binding oligomerization domain (NOD)-like receptor protein; TNF-α – tumor necrosis factor-alpha
Anakinra, a recombinant IL-1 receptor antagonist, was shown to have potential in an open-label pilot study of 10 gout patients, some of whom had acute gout and others who had chronic tophaceous gout, including two in whom anakinra was administered while initiating allopurinol therapy.20 Rilonacept (IL-1 trap), which is a soluble receptor-Fc fusion protein that inhibits both IL-1α and IL-1β and acts as a soluble decoy receptor, has been studied to varying degrees for acute gout, chronic active gout, and gout flare prophylaxis during urate-lowering therapy initiation. Preliminary data regarding rilonacept's efficacy in acute gout indicates it failed to significantly improve pain relative to a standard regimen of indomethacin.21 In a small 10-subject study of chronic active gouty arthritis, significant improvement in pain over baseline levels while on placebo was demonstrated, with pain recurrence after discontinuation of therapy, suggesting that IL-1 blockade was responsible for symptom relief.22 Finally, in a study of gout flare prophylaxis, 83 participants were randomized to rilonacept versus placebo for 16 weeks during initiation of allopurinol, with a significant reduction in gout flares.23 In a preliminary report of this study, which ultimately included 241 participants, there was an 80% reduction in gout flares for the 160mg dose, and a 73% reduction for the 80mg dose at 16 weeks compared with placebo.21 The efficacy of rilonacept compared with a standard prophylactic regimen that would be typically given during initiation of urate-lowering therapy is not known.
So et al report in this issue24 the efficacy of canakinumab, a fully human monoclonal antibody with IL-1β selective activity, in acute gout. In this single-blind Phase 2 dose-finding study, 200 participants who could not take NSAIDs or colchicine due to contraindications or intolerances were randomized to 5 single subcutaneous doses of canakinumab (28–29 participants per arm) versus a single intramuscular dose of triamcinolone acetonide 40mg (57 participants) plus appropriate placebos. The investigators report that all canakinumab doses were more effective than the single triamcinolone dose for reducing pain in acute gout, with the 150mg dose showing the greatest efficacy. Additionally, at 8 weeks post-baseline, those in the 150mg canakinumab arm had a 94% lower risk of recurrent gout flare (based on patient-reported signs and symptoms) compared with the triamcinolone arm.
While this is a promising study suggesting efficacy of canakinumab for acute gout and prophylaxis against flares, and addresses an important clinical need among those who are refractory to or have contraindications to NSAIDs and/or colchicine, further exploration is required to understand the clinical significance of these findings. First, a key aspect to optimal management of acute gout is to institute therapy as quickly as possible (see above and Figure 1). To be eligible for this particular study, participants had to be within 5 days of the beginning of an acute flare, and could not have taken NSAIDs, corticosteroids, or other IL-1 antagonists for an unspecified time prior to screening, or colchicine 24 hours prior to screening. It's unclear how generalizable this sample is as most patients with gout will have started to take medication for an acute attack within 5 days of its onset. The study's primary endpoint was evaluated at 72 hours post-dose. Given that gout flares are naturally self-limited, with attacks often subsiding within 7–10 days of onset, the information regarding how long participants were experiencing symptoms prior to onset is important to interpret whether any of the improvement in pain 72 hours post-dose would be expected to coincide with the natural spontaneous resolution of the flare. It is also difficult to evaluate comparability between groups at baseline without this information, as well as details about management of the current attack prior to study entry.
The primary outcome, the canakinumab dose that demonstrated a decrease in pain at 72 hours post-dose that was equivalent to the triamcinolone 40mg dose, could not be determined because the authors state that all doses of canakinumab were associated with numerically less pain at that time, although only the 150mg dose was statistically significantly different than the triamcinolone dose. Additionally, based on their predictive statistical modeling, only the 90mg and 150mg doses appeared to be statistically significant. Regardless of statistically significant differences, the important question is that of clinical significance.
To understand whether this is a clinically relevant result, one must ask whether the comparator regimen, a single 40mg dose of intramuscular triamcinolone, is appropriate. Although the authors state that this route of corticosteroid administration and dose of triamcinolone is standard in many of the countries in which the study was conducted, this particular regimen's efficacy has not been studied in a rigorous manner. As mentioned above, the two prior studies that evaluated intramuscular triamcinolone in acute gout evaluated a 60mg dose, with administration of a repeat dose as necessary.11 Whether a 40mg triamcinolone dose has equivalent efficacy to a 60mg dose in acute gout is not known. That 28.6% of the triamcinolone arm used oral prednisolone or prednisone as rescue medication suggests that this single dose of triamcinolone may not have been sufficient for management of acute gout. Thus, the comparator drug regimen in this study does not appear to be optimal for evaluating the efficacy of canakinumab in comparison with an already-demonstrated efficacious treatment. Even so, the study provides valuable additional support to the potential for IL-1 blockade in the management of acute gout, gouty inflammation, and gout prophylaxis, appreciably improving the pain experience in acute gout at 72 hours, reducing CRP at 7 days, and reducing the risk for recurrent flares at 8 weeks. Whether it's better than a standard recommended regimen for acute gout is not clear. In terms of gout flare prophylaxis, the investigators report in another study that all doses of canakinumab reduced the risk for flare at 16 weeks compared with colchicine 0.5mg daily (odds ratios 0.20–0.39) during allopurinol initiation.25 Interestingly, colchicine blocks MSU crystal-induced IL-1β generation upstream of NLRP3 inflammasome activation.15
Consideration of the convenience of whether an agent is a daily, weekly, or once every 8 weekly injection is premature at the present time. For clinicians, especially in this era of comparative effectiveness, the key question is whether targeted IL-1 blockade is any more effective for acute gout than the available standard therapies. For patients in whom even a short course of systemic corticosteroids (or local therapy) is not possible, this class of drug may be an alternative. However, none of these studies in gout have been large enough or long enough to determine important serious adverse events such as infection risk. Further, cost issues are not inconsequential. Any new therapy for acute gout must not only demonstrate efficacy compared with reasonably effective current options, but ideally cost-effectiveness as well. Arguably, had a higher or repeated dose of corticosteroid been used in the current study, the two might well have been equivalent, leaving little clinical reason to choose a drug of higher cost and uncertain safety. The various settings in which these drugs may be useful (acute gout, chronic active gout, and/or prophylaxis during urate-lowering therapy initiation) also require careful consideration and study, with the differences in duration of intended use obviously having different cost implications. At present, rilonacept and canakinumab are FDA-approved for use in CAPS, and anakinra is approved for use in rheumatoid arthritis. Undoubtedly, these are expensive therapies relative to accepted, efficacious ones such as NSAIDs, oral corticosteroids and colchicine, even taking into account the recent price increase of colchicine,26 and they are likely to remain expensive should they be indicated for use in gout. Nevertheless, there is a substantial unmet clinical need among persons with gout in whom standard therapies are not an option or are suboptimal. Further studies of IL-1 antagonists are needed to determine whether therapeutic targeting of this single cytokine may address this need.
Acknowledgments
Dr. Neogi is supported by: NIAMS K23AR055127, Arthritis Foundation New Investigator Award, ACR/REF Junior Career Development Award in Geriatrics (T Franklin Williams Scholar Award), Boston Claude D. Pepper Older Americans Independence Center (P30-AG031679)
References
- 1.Neogi T, Hunter DJ, Chaisson CE, Allensworth-Davies D, Zhang YQ. Frequency and Predictors of Inappropriate Management of Recurrent Gout Attacks in a Longitudinal Study. Journal of Rheumatology. 2006;33:104–9. First release Nov 1, 2005. [PubMed] [Google Scholar]
- 2.Kim SY, Choi HK. Gout and quality of life. Journal of Rheumatology. 2009;36:865–8. doi: 10.3899/jrheum.090034. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Saag KG. Suboptimal physician adherence to quality indicators for the management of gout and asymptomatic hyperuricaemia: results from the UK General Practice Research Database (GPRD) Rheumatology. 2005;44:1038–42. doi: 10.1093/rheumatology/keh679. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Annals of the Rheumatic Diseases. 2006;65:1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutaria S, Katbamna R, Underwood M. Effectiveness of interventions for the treatment of acute and prevention of recurrent gout--a systematic review. Rheumatology. 2006;45:1422–31. doi: 10.1093/rheumatology/kel071. [DOI] [PubMed] [Google Scholar]
- 7.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis and Rheumatism. 2010;62:1060–8. doi: 10.1002/art.27327. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger N. Overview of the management of acute gout and the role of adrenocorticotropic hormone. Drugs. 2008;68:407–15. doi: 10.2165/00003495-200868040-00002. [DOI] [PubMed] [Google Scholar]
- 9.Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Annals of Emergency Medicine. 2007;49:670–7. doi: 10.1016/j.annemergmed.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854–60. doi: 10.1016/S0140-6736(08)60799-0. [DOI] [PubMed] [Google Scholar]
- 11.Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008:CD005521. doi: 10.1002/14651858.CD005521.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arromdee E, Michet CJ, Crowson CS, O'Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? Journal of Rheumatology. 2002;29:2403–6. [PubMed] [Google Scholar]
- 13.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. Journal of Rheumatology. 2004;31:1582–7. [PubMed] [Google Scholar]
- 14.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR, Jr., Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Annals of the Rheumatic Diseases. 2005;64:267–72. doi: 10.1136/ard.2004.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 16.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. New England Journal of Medicine. 2006;355:581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis and Rheumatism. 2008;58:2443–52. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 18.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. New England Journal of Medicine. 2009;360:2416–25. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 19.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, et al. TNFalpha blockade in human diseases: mechanisms and future directions. Clinical Immunology. 2008;126:121–36. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Initial Phase 3 Studies Results for Rilonacept in the Prevention of Gout Flares in Patients Initiating Uric Acid-lowering Therapy and the Treatment of Patients in the Midst of an Acute Gout Attack Investor Teleconference June 9, 2010 Accessed June 10, 2010, at http://files.shareholder.com/downloads/REGN/941483174x0x380925/e99d3c78-f180-4597-a98d-fc1ce20abe49/REGN_Rilonacept_Call_Presentation.pdf.
- 22.Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Annals of the Rheumatic Diseases. 2009;68:1613–7. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumacher HR, Sundy JS, Terkeltaub R, Knapp HR, Mellis S, Soo Y, et al. Placebo-Controlled Study of Rilonacept for Gout Flare Prophylaxis During Initiation of Urate-Lowering Therapy. Arthritis and Rheumatism. 2009;60:1096. doi: 10.1002/art.33412. abstract. [DOI] [PubMed] [Google Scholar]
- 24.So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis. Arthritis and Rheumatism. 2010 doi: 10.1002/art.27600. DOI: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger N, Lin HY, De Meulemeester M, Rovensky J, Arulmani U, Krammer G, et al. Efficacy of canakinumab (ACZ885) in the prevention of flares in gout patients initiating allopurinol therapy. Annals of the Rheumatic Diseases. 2010;69:121. abstract. [Google Scholar]
- 26.Kesselheim AS, Solomon DH. Incentives for drug development--the curious case of colchicine. New England Journal of Medicine. 362:2045–7. doi: 10.1056/NEJMp1003126. [DOI] [PubMed] [Google Scholar]