Abstract
Background
Acrylamide is a probable human carcinogen formed during cooking of many common foods. Epidemiological studies of acrylamide and breast cancer risk have been null; however, positive associations with ovarian and endometrial cancers have been reported. We studied acrylamide intake and risk of breast, endometrial, and ovarian cancers in a prospective cohort study.
Methods
We assessed acrylamide intake among 88,672 women in the Nurses’ Health Study using food frequency questionnaires administered every four years. Between 1980 and 2006 we identified 6301 cases of invasive breast cancer, 484 cases of invasive endometrial adenocarcinoma, and 416 cases of epithelial ovarian cancer. We used Cox proportional hazards models to study the association between acrylamide and cancer risk.
Results
We found no association between acrylamide intake and breast cancer overall or according to estrogen and progesterone receptor status. We found an increased risk of endometrial cancer among high acrylamide consumers (adjusted relative risk [RR] for highest versus lowest quintile=1.41, 95% CI: 1.01–1.97, p-value for trend=0.03). We observed a non-significant suggestion of increased risk for ovarian cancer overall (RR 1.25, CI: 0.88–1.77, p-trend=0.12), with a significantly increased risk for serous tumors (RR 1.58, CI: 0.99–2.52, p-trend=0.04). Associations did not differ by smoking status.
Conclusions
We observed no association between acrylamide and breast cancer. Risk of endometrial cancer and possibly ovarian cancer was greater among high acrylamide consumers.
Impact
This is the second prospective study to report positive associations with endometrial and ovarian cancers. These associations should be further evaluated to inform public health policy.
Keywords: Acrylamide, diet, breast cancer, endometrial cancer, ovarian cancer
INTRODUCTION
In 2002, Swedish researchers announced the finding of acrylamide in commonly consumed baked and fried foods.(1) Prior to 2002, exposure to acrylamide, which is classified as a probable human carcinogen (2), was thought to come mainly from occupational settings and tobacco use. In foods, acrylamide is formed during high-heat cooking as part of the Maillard or browning reactions. Major sources of acrylamide in the U.S. diet are French fries, potato chips, cold breakfast cereal, coffee, and baked goods and snack foods.(3) In recent years, the food industry has made efforts to reduce the formation of acrylamide during food processing.
In animal tests, acrylamide causes several types of cancers in hormone-sensitive tissues, including mammary tumors in female rats, when administered in drinking water at levels 1000 to 10,000 times higher than typical human dietary exposure.(4, 5) Estimates of the increase in human cancer risk from dietary levels of acrylamide predicted by animal models are low, with relative risks of 1.006–1.05 for the highest versus lowest consumers.(6,7,8) However, the types of tumors seen in animal studies has raised interest in whether dietary acrylamide may increase the risk of breast or reproductive tumors in women.
The association between dietary intake of acrylamide and risk of breast(9–14), endometrial(13,15), and ovarian(9, 10, 13, 16) cancers has been studied in case-control and prospective cohort studies. Results for breast cancer have been null, though one study found a non-statistically significant suggestion of increased risk of estrogen- and progesterone-receptor positive cancers in postmenopausal women.(14) For endometrial cancer, Hogervorst et al.(13) found a suggestion of increased risk overall and a significantly increased risk among never smoking women in the Netherlands Cohort Study. No association was found for endometrial cancer in a cohort of Swedish women.(15) For ovarian cancer, Pelucchi et al.(9) found no association in a hospital-based case-control study in Italy and Switzerland, and Larsson et al.(16) found no association in a prospective study of Swedish women. However, a positive association was seen in the Netherlands Cohort Study.(13) The positive associations with ovarian and endometrial cancer risk in the Netherlands Cohort Study were somewhat surprising, given the previous null results for other cancers, the extremely low relative risks predicted from animal studies, and the inherently imprecise estimates of acrylamide intake based on calculations from consumption of foods.
We used the Nurses' Health Study (NHS) cohort to study the association between dietary acrylamide intake and risk of breast, endometrial, and ovarian cancer in a large population of pre- and postmenopausal U.S. women with periodically updated information on acrylamide intake across more than 20 years of follow-up. Because smoking is a major source of acrylamide exposure, we also studied the association among never-smoking women to isolate the effect of dietary acrylamide exposure.
MATERIALS AND METHODS
Study Population
The Nurses' Health Study (NHS) is a prospective cohort of 121,700 female nurses, aged 30–55 in 1976 when a mailed baseline questionnaire was completed. The cohort is followed with self-administered mailed questionnaires every two years. In 1980, participants asked to complete a 61-item food-frequency questionnaire (FFQ). The FFQ was expanded to 116 items in 1984, and similar FFQs have been administered every two to four years since then.
For this analysis, we included women who completed the 1980 FFQ. Women who reported daily energy intakes less than 500 kcal/day or greater than 3,500 kcal/day were excluded, as were women who left 10 or more food items blank. Women with a previous diagnosis of cancer (except for non-melanoma skin cancer) were excluded. For the endometrial cancer analysis, women with a hysterectomy at baseline were excluded. For the ovarian cancer analysis, women with a bilateral oophorectomy or pelvic irradiation at baseline were excluded. Thus, 88,672 women were included in the breast cancer analysis, 69,019 women in the endometrial cancer analysis, and 80,011 women in the ovarian cancer analysis. Follow-up through June 2006 among women with a 1980 FFQ was 95.0%. This research was approved by the institutional review board of Brigham and Women's Hospital.
Acrylamide Intake Assessment
FFQs were used to assess usual dietary intake over the previous year in 1980, 1984, 1986, 1990, 1994, 1998, and 2002. For each food item a portion size was given and respondents were asked to choose from nine possible frequencies of consumption, from never to six or more servings per day. Daily acrylamide intake was calculated by multiplying the acrylamide content of each food item by its frequency of consumption and then summing across all acrylamide-containing foods.
To best represent long-term diet we used cumulative average acrylamide intake as our main exposure measure. That is, 1980 intake was used for follow-up from 1980–1984, the average of 1980 and 1984 intake was used for follow-up from 1984–1986, the average of 1980, 1984, and 1986 was used for follow-up from 1986–1990, and so on. This exposure measure also reduces random within-person measurement error over time. In secondary analyses we used baseline (1980) acrylamide intake only. In addition, we performed a latency analysis for breast cancer because of the large number of cases. We used our repeated measures of acrylamide intake to analyze the effect of latency time (time from exposure to cancer) by relating each measure of acrylamide intake to breast cancer incidence during specific periods of latency time: 0–4 years, 4–8 years, 8–12 years, and 12–16 years.
We previously described the creation of the acrylamide food composition database and its validity.(17) Briefly, data on acrylamide content of foods were taken from published U.S. FDA data along with additional analyses of U.S. food samples performed for us by the Swedish National Food Administration. Acrylamide values were assigned to over 40 food items including: English muffins/rolls/bagels, breakfast cereal, coffee, decaffeinated coffee, cookies, crackers, dark bread, French fries, muffins, nuts, beans, brownies, cake, candy (with chocolate and/or nuts), chocolate, chowder, donuts, fried breaded fish, grains (couscous, bulgur, etc.), ice cream, pancakes, pie, processed meats, sweet rolls, tortillas, white bread, wheat germ, frozen yogurt, peanut butter, pizza, popcorn, potato chips, potatoes (baked/mashed/roasted), pretzels, prunes, and sweet potatoes/yams. For breakfast cereal, participants were asked to report which brand of cereal they use the most. This brand was used to calculate acrylamide intake. Commonly consumed cereal brands were analyzed for this study, and for brands without analyzed values, we imputed a value based on cereals with similar grain composition and processing (e.g. puffs, flakes).
We compared FFQ-assessed acrylamide intake with a biomarker of acrylamide intake, hemoglobin adducts of acrylamide and its genotoxic metabolite glycidamide, in a sample of 296 non-smoking women in the Nurses' Health Study II cohort. The correlation was 0.34 (p<0.0001), adjusted for age, energy intake, BMI, and alcohol intake, and corrected for random within-person variation in the adduct measurement.(17)
Assessment of Covariates
Information on smoking, weight, parity, contraceptive use, menopausal status and hormone use, hysterectomy and oophorectomy, family history of cancer, physical activity, use of medications, and medical conditions including cancer, hypertension, and diabetes was collected in biennial questionnaires. Information on height and age at menarche were collected in the 1976 questionnaire. Intakes of total energy and possible nutrient confounders including folate, animal fat, glycemic index, alcohol, and caffeine were calculated from the FFQs using cumulative averages as described above.
Information on family history of ovarian cancer was not collected until 1992, and information on duration of lactation was not collected until 1986, so we were not able to adjust for these potential confounders in the breast or ovarian cancer analyses. However, family history of ovarian cancer reported in 1992, 1996, and 2000 was not associated with acrylamide intake in 1990 (Table 1) or in 1980 (data not shown), and the percent of women who had ever lactated was not associated with acrylamide intake in 1990 (Table 1) or 1980 (data not shown). However, women with higher acrylamide intake had slightly lower total durations of lactation; among those who ever lactated, those in the lowest quintile of intake reported 11 months and those in the highest quintile reported 9 months total. We conducted an analysis of ovarian and breast cancer using 1986 as baseline and found adjustment for history of lactation (ever vs. never lactated or duration of lactation) had no effect on the relative risks (data not shown).
Table 1.
Age standardized characteristics of the study population in 1990*
|
Quintile of calorie-adjusted acrylamide intake |
|||||
|---|---|---|---|---|---|
| Q1 (low) | Q2 | Q3 | Q4 | Q5 (high) | |
| Age in 1990 | 58 | 58 | 57 | 56 | 55 |
| Acrylamide intake (mcg/d) | 9 | 13 | 16 | 19 | 26 |
| Acrylamide by body weight (mcg/kg/d) | 0.13 | 0.20 | 0.24 | 0.30 | 0.42 |
| BMI | 26 | 26 | 26 | 26 | 26 |
| Height (inches) | 64 | 65 | 65 | 65 | 64 |
| Current smokers (%) | 13% | 13% | 15% | 19% | 26% |
| Physical activity (MET-hr/wk) | 17 | 16 | 16 | 15 | 13 |
| Diabetes (%) | 3.6% | 3.0% | 2.8% | 2.8% | 2.4% |
| Hypertension (%) | 19% | 19% | 18% | 16% | 16% |
| Age at menarche <13 (%) | 50% | 48% | 50% | 49% | 48% |
| Nulliparous (%) | 7% | 7% | 6% | 6% | 6% |
| Ever lactated (%) | 55% | 58% | 58% | 56% | 51% |
| Premenopausal (%) | 21% | 23% | 23% | 23% | 23% |
| Age at menopause (among postmenopausal) | 47 | 47 | 47 | 47 | 47 |
| Current PMH use (% among postmenopausal) | 32% | 34% | 35% | 34% | 30% |
| Tubal ligation (%) | 16% | 17% | 17% | 18% | 18% |
| Hysterectomy (simple)* (%) | 31% | 31% | 31% | 31% | 32% |
| Double oophorectomy* (%) | 16% | 16% | 15% | 16% | 16% |
| Family history of breast cancer (%) | 9% | 10% | 10% | 11% | 10% |
| Family history ovarian cancer (%, 1992) | 2.3% | 2.8% | 2.5% | 2.7% | 2.6% |
| History of benign breast disease (%) | 39% | 41% | 41% | 41% | 39% |
| Nutrient Intakes (per day)† | |||||
| Energy intake (kcal) | 1711 | 1776 | 1777 | 1753 | 1674 |
| Alcohol (g) | 5.7 | 5.6 | 5.4 | 5.1 | 4.6 |
| Total fat (g) | 56 | 56 | 57 | 58 | 60 |
| Animal fat (g) | 34 | 32 | 32 | 32 | 33 |
| Trans fat (g) | 2.4 | 2.6 | 2.7 | 2.9 | 3.3 |
| Carbohydrates (g) | 195 | 197 | 197 | 196 | 192 |
| Glycemic index | 52 | 52 | 53 | 53 | 53 |
| Folate (mcg) | 447 | 431 | 422 | 410 | 389 |
| Caffeine (mg) | 155 | 214 | 261 | 321 | 421 |
| Intakes of high acrylamide foods (serv/day) | |||||
| Coffee | 1.0 | 1.8 | 2.2 | 2.8 | 3.4 |
| Breakfast cereal | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 |
| French fries | 0.01 | 0.03 | 0.04 | 0.06 | 0.11 |
| Potato chips | 0.04 | 0.1 | 0.1 | 0.1 | 0.2 |
| Potatoes (baked, roasted, mashed) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Baked goods‡ | 0.5 | 0.7 | 0.8 | 0.8 | 0.8 |
Note: Data (except for age) were directly standardized to the age distribution of the entire cohort. Means or percentages are shown as indicated.
Characteristics of the population for breast cancer analysis is shown. Women were censored at the time of double oophorectomy for the ovarian cancer analysis, and women were censored at the time of hysterectomy for the endometrial cancer analysis.
All nutrients except alcohol are adjusted for total energy intake using the residual method.
Cookies, brownies, donuts, cake, sweet rolls, pie.
Ascertainment of Cancer Cases
Biennial follow-up questionnaires were used to identify newly diagnosed cases of breast, endometrial, and ovarian cancer. When participants reported a cancer diagnosis, we asked for confirmation of the diagnosis and permission to obtain relevant medical records.
For breast cancer, pathology reports confirmed 98% of self-reported breast cancers, thus all self-reported cancers were included in the analysis. Information on estrogen and progesterone receptor status was obtained from pathology reports and was available for 72% of cases. A recent validation study in this cohort found that pathology reports provide accurate information on estrogen receptor status.(18) Cases of carcinoma-in-situ were not included.
For endometrial cancer, we included cases of invasive adenocarcinoma confirmed by medical records. For ovarian cancer we included cases of invasive and borderline epithelial cancers confirmed by medical records. Information on histological type and subtype was taken from pathology reports. A validation study comparing pathology reports to a standardized review of slides in 215 ovarian cancer cases from the cohort found a concordance of 83% for histological subtype and 98% for invasiveness. Only cases of endometrial and ovarian cancers confirmed by medical records were included in the analysis to ensure that cases met our histological criteria.
Deaths were documented by responses to questionnaires by family members, by the postal service, or through the National Death Index.
Statistical Analysis
For the breast cancer analysis, each participant contributed person-time from the return of the 1980 questionnaire until the first occurrence of: cancer diagnosis (breast or other non-melanoma cancer), death, or June 1, 2006. For endometrial cancer, women were followed until the first occurrence of: cancer diagnosis (endometrial or other non-melanoma cancer), death, hysterectomy, or June 1, 2006. For ovarian cancer, women were followed until the first occurrence of: cancer diagnosis (ovarian or other non-melanoma cancer), death, bilateral oophorectomy, pelvic irradiation, or June 1, 2006. For each analysis, participants were divided into quintiles based on their acrylamide intake. Acrylamide intake was adjusted for total energy intake using the residual method, and quintiles were created using these energy-adjusted intakes.(19) Relative risks (RRs) of breast cancer were calculated as the incidence rate for a given quintile of consumption divided by the rate in the lowest quintile.
We used Cox proportional hazards models to adjust for other risk factors. To control as finely as possible for confounding by age, calendar time, and any possible two-way interactions between these two time scales, we stratified the analysis jointly by age in months at the start of each follow-up period and calendar year of the current questionnaire cycle. For multivariable models, we considered different possible confounders for each cancer based on possible risk factors previously identified for that cancer. For breast cancer we adjusted for: smoking (never, past <25 cig/d, past 25+ cig/d, current <25 cig/d, current 25+ cig/d), BMI (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25 to <30, 30+), height (quartiles), joint menopausal status/age at menopause/PMH use (premenopausal, uncertain status, postmenopausal and age at menopause <45, 45–52, or >52 and PMH use never, former, current <5 years, or current 5+ years), joint parity and age at first birth (nulliparous, parity 1–2 and age at first birth <25, parity 1–2 and age at first birth 25-<30, parity 1–2 and age at first birth 30+, parity 3–4 and age at first birth <25, parity 3–4 and age at first birth 25-<30, parity 3–4 and age at first birth 30+, parity 5+ at age at first birth <25, parity 5+ and age at first birth 25+) family history of breast cancer (yes/no), benign breast disease (yes/no), age at menarche (<13, 13, 14+), physical activity (≤18 met-h/wk, >18 met-h/wk), folate (quintiles), glycemic index (quintiles), animal fat intake (quintiles), alcohol (continuous, g/day), and energy intake (continuous, kcal/d). We also considered intake of caffeine, vegetable fat, saturated fat, trans fat, carbohydrates, and glycemic load as possible confounders; however these were not included in the final models as they had negligible effects on the relative risk estimates or standard errors for acrylamide.
For endometrial cancer we adjusted for: smoking (see categories for breast cancer), BMI (<20, 20-<21, 21-<22, 22-<23, 23-<24, 24-<25, 25-<27, 27-<29, 29-<30, 30-<32, 32-<35, 35-<40, 40+), age at menarche (<13, 13, 14+), joint menopausal status/age at menopause/PMH use (see categories for breast cancer), parity (nulliparous, 1–2, 3–4, 5+), oral contraceptive use (never, 0–3 years of use, >3-<5 years, 5+ years of use), high blood pressure (yes/no), diabetes (yes/no), physical activity (≤18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), and energy intake (continuous, kcal/d). Because of the strong association between BMI and endometrial cancer risk we adjusted for BMI more finely than in the breast or ovarian cancer models. We also considered age at first birth, height, aspirin use, and intake of total fat, animal fat, carbohydrates, glycemic load, calcium, alcohol, and red meat as possible confounders; however these were not included in the final models as they had negligible effects on the relative risk estimates or standard errors for acrylamide.
For ovarian cancer we adjusted for: smoking (see categories for breast cancer), BMI (see categories for breast cancer), parity (see categories for endometrial cancer), oral contraceptive use (see categories for endometrial cancer), menopausal status and PMH use (premenopausal, uncertain status, postmenopausal and never used PMH, postmenopausal and former PMH, postmenopausal and current PMH use), tubal ligation (yes/no), physical activity (≤18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), and energy intake (continuous, kcal/d). We also considered height, age at menarche, hysterectomy status, age at menopause, age at first birth, and intake of alcohol, folate, vitamin A, vitamin C, alpha-carotene, lycopene, saturated fat, trans fat, and milk as possible confounders; however these were not included in the final models as they had negligible effects on the relative risk estimates or standard errors for acrylamide.
The SAS Proc PHREG was used for all analyses, and the Anderson-Gill data structure was used to handle time-varying covariates efficiently. All covariates except height and age at menarche were updated in each questionnaire cycle when new data was available. To test for a linear trend across quintiles of intake we modeled acrylamide as a continuous variable using the median intake for each quintile.
Because smoking is an important source of acrylamide, we stratified our analyses by smoking status to isolate the effect of dietary acrylamide among never smoking women. We also stratified by menopausal status, alcohol intake (< or ≥ 10 g/day), and BMI (< or ≥ 25 kg/m2). We stratified by menopausal status because risk factors for pre- and postmenopausal cancers may differ. We stratified by alcohol intake and BMI because these factors may affect the activity of CYP2E1, the enzyme which metabolizes acrylamide to glycidamide; we previously found that alcohol intake and BMI were both significantly correlated with hemoglobin adducts of acrylamide independent of dietary acrylamide intake.(17) We created interaction terms between the stratification variables and quintile of acrylamide intake and tested the significance of interactions using likelihood ratio tests to compare the models without an interaction term to those with an interaction term.
To examine whether the observed associations could be attributed to acrylamide in general or to some other component of some high-acrylamide food items we modeled quintiles of acrylamide intake simultaneously with quintiles of intake of each major acrylamide-contributing food to see the effect on associations of acrylamide intake and cancer risk.
All tests of statistical significance are two-sided.
RESULTS
From 1980 through June 2006 we documented 6301 cases of invasive breast cancer, 484 cases of invasive endometrial adenocarcinoma, and 416 cases of epithelial ovarian cancer. The mean age of cases was 61 years for breast cancer (range 35–84), 61 for endometrial cancer (range 39–81), and 59 for ovarian cancer (range 39–81).
Characteristics of the study population by quintile of calorie-adjusted acrylamide intake in 1990 are shown in Table 1. Estimated acrylamide intake ranged from a mean of 9 mcg/day in the lowest quintile to 26 mcg/day in the highest quintile. Adjusting for age, women with higher dietary acrylamide intakes were more likely to smoke, were less physically active, less likely to have diabetes or hypertension, and less likely to use postmenopausal hormones. High acrylamide intakes were associated with somewhat lower intakes of alcohol, animal fat, and folate and somewhat higher intakes of trans fat, carbohydrates, and caffeine. Intake of high-acrylamide foods including coffee, breakfast cereal, French fries, potato chips, and baked goods increased across quintiles of acrylamide consumption.
The contribution of different foods to acrylamide intake in the cohort is shown in Figure 1. The major sources of acrylamide were: coffee (20%), breakfast cereal (15%), French fries (12%), potato chips (7%), pretzels (6%), potatoes (baked/roasted/mashed) (6%), and other baked goods and snack foods. The major contributors to acrylamide intake were also the major contributors to between-person variation in acrylamide intake in this population (data not shown).
Figure 1.
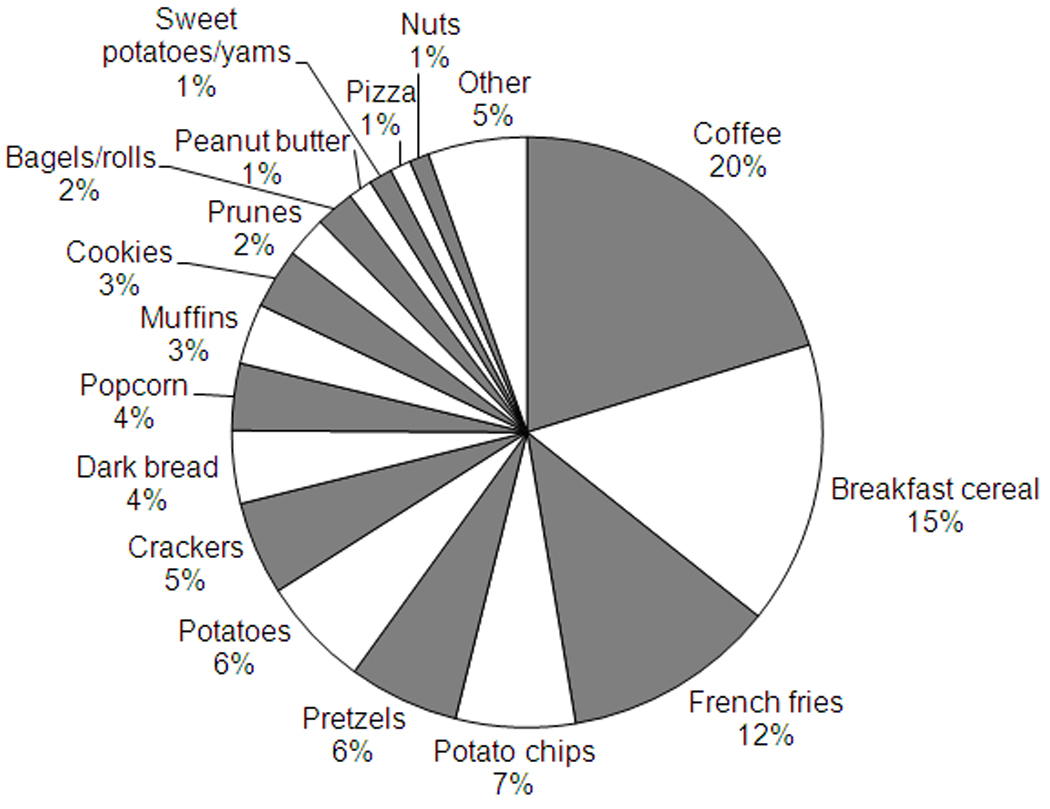
Food contributors to acrylamide intake, 1998
The association between acrylamide intake and cancer risk is shown overall and among never smokers in Table 2. No association was seen between acrylamide intake and breast cancer risk in either the age-adjusted or fully adjusted models. The adjusted relative risk of breast cancer for the highest versus lowest quintile of intake was 0.95 (95% CI: 0.87–1.03, p-trend=0.50) overall and 0.89 (95% CI: 0.78–1.02, p-trend=0.18) among never smoking women.
Table 2.
Relative risk (and 95% confidence interval) of breast, endometrial, and ovarian cancers by quintile of calorie-adjusted acrylamide intake, 1980–2006
| Quintile of calorie-adjusted acrylamide intake |
||||||
|---|---|---|---|---|---|---|
| Q1 (low) | Q2 | Q3 | Q4 | Q5 (high) | p-value | |
| Median intake (mcg/d) | 8.7 | 12.7 | 15.7 | 19.0 | 25.1 | for trend |
| BREAST CANCER | ||||||
| Num. cases | 1282 | 1280 | 1331 | 1287 | 1121 | |
| Person-years | 405,391 | 404,518 | 404,349 | 404,769 | 405,644 | |
| Age-adjusted RR* | 1.00 | 0.94 (0.87–1.02) | 0.99 (0.92–1.07) | 0.99 (0.91–1.07) | 0.94 (0.87–1.02) | 0.36 |
| Multivariable RR† | 1.00 | 0.93 (0.86–1.01) | 0.98 (0.91–1.06) | 0.98 (0.90–1.06) | 0.95 (0.87–1.03) | 0.50 |
| Never smokers only | ||||||
| Num. cases | 652 | 610 | 582 | 516 | 392 | |
| Person-years | 210,666 | 198,085 | 187,783 | 171,665 | 151,532 | |
| Age-adjusted RR* | 1.00 | 0.94 (0.84–1.05) | 0.96 (0.86–1.08) | 0.96 (0.85–1.08) | 0.90 (0.80–1.03) | 0.21 |
| Multivariable RR† | 1.00 | 0.91 (0.81–1.02) | 0.93 (0.83–1.05) | 0.94 (0.84–1.06) | 0.89 (0.78–1.02) | 0.18 |
| ENDOMETRIAL CANCER | ||||||
| Num. cases | 88 | 100 | 106 | 102 | 88 | |
| Person-years | 277,776 | 277,073 | 276,839 | 277,409 | 277,789 | |
| Age-adjusted RR* | 1.00 | 1.07 (0.80–1.43) | 1.17 (0.88–1.56) | 1.15 (0.86–1.54) | 1.12 (0.83–1.52) | 0.41 |
| Multivariable RR‡ | 1.00 | 1.12 (0.83–1.50) | 1.31 (0.97–1.77) | 1.35 (0.99–1.84) | 1.41 (1.01–1.97) | 0.03 |
| Never smokers only | ||||||
| Num. cases | 53 | 47 | 59 | 56 | 42 | |
| Person-years | 144,400 | 135,320 | 128,534 | 116,601 | 102,813 | |
| Age-adjusted RR* | 1.00 | 0.93 (0.63–1.39) | 1.30 (0.89–1.90) | 1.37 (0.93–2.01) | 1.31 (0.86–2.00) | 0.06 |
| Multivariable RR‡ | 1.00 | 0.97 (0.64–1.46) | 1.35 (0.90–2.02) | 1.47 (0.97–2.24) | 1.43 (0.90–2.28) | 0.04 |
| OVARIAN CANCER | ||||||
| Num. cases | 87 | 75 | 95 | 81 | 78 | |
| Person-years | 246,187 | 245,523 | 245,327 | 245,667 | 246,155 | |
| Age-adjusted RR* | 1.00 | 0.85 (0.62–1.16) | 1.10 (0.81–1.47) | 0.95 (0.70–1.29) | 1.00 (0.73–1.37) | 0.78 |
| Multivariable RR§ | 1.00 | 0.93 (0.68–1.29) | 1.29 (0.94–1.76) | 1.17 (0.84–1.64) | 1.25 (0.88–1.77) | 0.12 |
| Never smokers only | ||||||
| Num. cases | 40 | 38 | 31 | 24 | 23 | |
| Person-years | 126,888 | 118,416 | 112,475 | 101,849 | 91,082 | |
| Age-adjusted RR* | 1.00 | 1.03 (0.65–1.63) | 0.92 (0.57–1.48) | 0.82 (0.49–1.37) | 0.92 (0.54–1.55) | 0.53 |
| Multivariable RR§ | 1.00 | 1.17 (0.72–1.88) | 1.04 (0.63–1.74) | 1.11 (0.63–1.94) | 1.19 (0.66–2.15) | 0.63 |
Age-adjusted models are adjusted for age in months and calendar year.
Breast cancer multivariable models additionally adjusted for: smoking (never, past <25 cig/d, past 25+ cig/d, current <25 cig/d, current 25+ cig/d), BMI (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25 to <30, 30+), height (quartiles), menopausal status/age at menopause/PMH use (premenopausal, uncertain status, postmenopausal: age at menopause <45, 45–52, or >52 AND PMH use never, former, current <5 years, or current 5+ years), parity and age at first birth (nulliparous, parity 1–2 and age first birth <25, parity 1–2 age first birth 25-<30, parity 1–2 age first birth 30+, parity 3–4 age first birth <25, parity 3–4 age first birth 25-<30, parity 3–4 age first birth 30+, parity 5+ age first birth <25, parity 5+ age first birth 25+) family history of breast cancer (yes/no), benign breast disease (yes/no), age at menarche (<13, 13, 14+), physical activity (<=18 met-h/wk, >18 met-h/wk), folate, glycemic index, and animal fat intake (quintiles), alcohol (continuous, g/day), energy intake (continuous, kcal/d).
Endometrial cancer multivariable models additionally adjusted for: smoking (see categories for breast cancer), BMI (<20, 20-<21, 21-<22, 22-<23, 23-<24, 24-<25, 25-<27, 27-<29, 29-<30, 30-<32, 32-<35, 35-<40, 40+), age at menarche (<13, 13, 14+), menopausal status/age at menopause/PMH use (see categories for breast cancer), parity (nulliparous, 1–2, 3–4, 5+), oral contraceptive use (never, 0–3 years of use, >3-<5 years, 5+ years of use), high blood pressure (yes/no), diabetes (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d).
Ovarian cancer multivariable models additionally adjusted for: smoking (see categories for breast cancer), BMI (see categories for breast cancer), parity (see categories for endometrial cancer), oral contraceptive use (see categories for endometrial cancer), menopausal status and PMH use (premenopausal, uncertain status, postmenopausal and never used PMH, postmenopausal and former PMH, postmenopausal and current PMH use), tubal ligation (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d).
We found an increased risk of endometrial cancer among women with the highest acrylamide intakes after adjustment for confounders, in particular smoking, BMI, and caffeine. The adjusted RR for those in the highest versus lowest quintile was 1.41 (95% CI: 1.01–1.97, p-trend=0.03). The association was similar among never smoking women (RR 1.43, 95% CI: 0.90–2.28, p-trend=0.04). Adjustment for caffeine strengthened the association with endometrial cancer in the overall group, though not among never smokers. Adjusting for all covariates except caffeine, the adjusted RR for the highest versus lowest quintile was 1.28 (95% CI: 0.94–1.75, p-trend=0.08) overall and 1.49 (95% CI: 0.97–2.29, p-trend=0.01) among never smokers.
We observed a non-statistically significant suggestion of an increased risk of ovarian cancer overall after adjustment for confounders, in particular caffeine intake. The adjusted RR was 1.25 (95% CI: 0.88–1.77, p-trend=0.12), with a similar association among never smoking women (RR 1.19, 95% CI: 0.66–2.15, p-trend=0.63).
Baseline acrylamide intake from the 1980 FFQ was not associated with any of the three cancers. The adjusted relative risk for the top versus bottom quintile was 0.95 (95% CI: 0.88–1.03, p-trend=0.10) for breast cancer, 1.12 (95% CI: 0.80–1.55, p-trend=0.31) for endometrial cancer, and 0.98 (95% CI: 0.70–1.36, p-trend=0.78) for ovarian cancer. Acrylamide intake from the expanded 1984 FFQ, with follow-up through 2006, was also not associated with the risk of any of the cancers (results not shown).
In the latency analysis, acrylamide intake was not associated with breast cancer risk for any latency period. The adjusted relative risks comparing the top to bottom quintiles were 1.04 (95% CI: 0.95–1.13, p-trend=0.45) for 0–4 years latency (5459 cases), 1.02 (0.93–1.12, p-trend=0.37) for 4–8 years (4843 cases), 1.09 (0.98–1.20, p-trend=0.17) for 8–12 years (4173 cases), and 0.99 (0.89–1.11, p-trend=0.88) for 12–16 years (3294 cases).
There were no statistically significant interactions between acrylamide intake and menopausal status or BMI and risk of any of the three cancers (Table 3). However, for both endometrial and ovarian cancers, statistically significant trends were seen only among those with BMI less than 25. For endometrial cancer, the adjusted RR for the highest versus lowest quintile was 2.51 (95% CI: 1.32–4.77, p-trend=0.004) among those with BMI less than 25. For ovarian cancer, the adjusted RR for the highest versus lowest quintile was 1.84 (95% CI: 1.14–2.97, p-trend=0.01) for those with BMI less than 25. There was no suggestion of modification of the acrylamide-cancer risk association according to alcohol intake for breast, endometrial, or ovarian cancers (results not shown). Also, acrylamide intake was not associated with risk of breast cancers characterized by hormone receptor status (Table 4). Acrylamide was also not associated with risk of breast cancers characterized by hormone receptor status among never smoking women (results not shown).
Table 3.
Relative risk (and 95% confidence interval) of breast, endometrial, and ovarian cancers by quintile of calorie-adjusted acrylamide intake according to menopausal status and BMI, 1980–2006
| Quintile of calorie-adjusted acrylamide intake |
p-value | p-value for | |||||
|---|---|---|---|---|---|---|---|
| Q1 (low) | Q2 | Q3 | Q4 | Q5 (high) | for trend | interaction | |
| BY MENOPAUSAL STATUS | |||||||
| BREAST CANCER | |||||||
| Postmenopausal, N cases | 1051 | 1079 | 1115 | 1012 | 822 | ||
| Multivariable RR† | 1.00 | 0.92 (0.84–1.00) | 0.97 (0.89–1.06) | 0.93 (0.85–1.01) | 0.93 (0.84–1.02) | 0.22 | |
| Premenopausal, N cases | 165 | 141 | 148 | 201 | 227 | 0.27 | |
| Multivariable RR† | 1.00 | 0.96 (0.76–1.21) | 0.96 (0.76–1.21) | 1.17 (0.94–1.46) | 1.07 (0.87–1.33) | 0.23 | |
| ENDOMETRIAL CANCER | |||||||
| Postmenopausal, N cases | 74 | 87 | 96 | 88 | 65 | ||
| Multivariable RR‡ | 1.00 | 1.11 (0.80–1.53) | 1.36 (0.98–1.88) | 1.38 (0.98–1.94) | 1.29 (0.89–1.89) | 0.11 | |
| Premenopausal, N cases | 12 | 12 | 9 | 11 | 21 | 0.15 | |
| Multivariable RR‡ | 1.00 | 1.09 (0.46–2.62) | 1.00 (0.39–2.58) | 0.85 (0.32–2.26) | 2.27 (0.96–5.40) | 0.05 | |
| OVARIAN CANCER | |||||||
| Postmenopausal, N cases | 68 | 56 | 71 | 61 | 52 | ||
| Multivariable RR§ | 1.00 | 0.90 (0.62–1.30) | 1.30 (0.91–1.87) | 1.19 (0.81–1.76) | 1.16 (0.76–1.78) | 0.28 | |
| Premenopausal, N cases | 14 | 16 | 16 | 19 | 25 | 0.30 | |
| Multivariable RR§ | 1.00 | 1.10 (0.51–2.41) | 1.48 (0.69–3.17) | 1.32 (0.61–2.86) | 1.63 (0.76–3.46) | 0.19 | |
| BY BMI | |||||||
| BREAST CANCER | |||||||
| BMI < 25, N cases | 636 | 629 | 614 | 593 | 541 | ||
| Multivariable RR† | 1.00 | 0.96 (0.85–1.07) | 0.96 (0.85–1.07) | 0.95 (0.84–1.06) | 0.92 (0.81–1.03) | 0.17 | |
| BMI ≥ 25, N cases | 646 | 651 | 717 | 694 | 580 | 0.60 | |
| Multivariable RR† | 1.00 | 0.92 (0.82–1.02) | 1.01 (0.90–1.13) | 1.01 (0.90–1.13) | 0.97 (0.86–1.09) | 0.87 | |
| ENDOMETRIAL CANCER | |||||||
| BMI < 25, N cases | 20 | 32 | 38 | 40 | 31 | ||
| Multivariable RR‡ | 1.00 | 1.70 (0.94–3.09) | 2.08 (1.15–3.77) | 2.41 (1.32–4.38) | 2.51 (1.32–4.77) | 0.004 | |
| BMI ≥ 25, N cases | 68 | 67 | 68 | 62 | 57 | 0.20 | |
| Multivariable RR‡ | 1.00 | 0.92 (0.64–1.32) | 1.07 (0.74–1.54) | 0.99 (0.67–1.46) | 1.08 (0.72–1.64) | 0.62 | |
| OVARIAN CANCER | |||||||
| BMI < 25, N cases | 44 | 47 | 48 | 38 | 52 | ||
| Multivariable RR§ | 1.00 | 1.27 (0.82–1.97) | 1.56 (1.00–2.43) | 1.47 (0.92–2.36) | 1.84 (1.14–2.97) | 0.01 | |
| BMI ≥ 25, N cases | 44 | 29 | 42 | 43 | 29 | 0.45 | |
| Multivariable RR§ | 1.00 | 0.66 (0.40–1.08) | 1.01 (0.64–1.61) | 0.95 (0.58–1.54) | 0.84 (0.49–1.44) | 0.86 | |
Breast cancer multivariable models adjusted for: age in months, calendar year, smoking (never, past <25 cig/d, past 25+ cig/d, current <25 cig/d, current 25+ cig/d), BMI (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25 to <30, 30+), height (quartiles), parity and age at first birth (nulliparous, parity 1–2 and age first birth <25, parity 1–2 age first birth 25-<30, parity 1–2 age first birth 30+, parity 3–4 age first birth <25, parity 3–4 age first birth 25-<30, parity 3–4 age first birth 30+, parity 5+ age first birth <25, parity 5+ age first birth 25+) family history of breast cancer (yes/no), benign breast disease (yes/no), age at menarche (<13, 13, 14+), physical activity (<=18 met-h/wk, >18 met-h/wk), folate, glycemic index, and animal fat intake (quintiles), alcohol (continuous, g/day), energy intake (continuous, kcal/d). Postmenopausal model adjusted for joint age at menopause and PMH use variable (age at menopause <45, 45–52, or >52 AND PMH use never, former, current <5 years, or current 5+ years). BMI models adjusted for joint menopausal status, age at menopause, and PMH use variable (premenopausal, uncertain status, postmenopausal: age at menopause <45, 45–52, or >52 AND PMH use never, former, current <5 years, or current 5+ years).
Endometrial cancer multivariable models adjusted for: age in months, calendar year, smoking (see categories for breast cancer), BMI (<20, 20-<21, 21-<22, 22-<23, 23-<24, 24-<25, 25-<27, 27-<29, 29-<30, 30-<32, 32-<35, 35-<40, 40+), age at menarche (<13, 13, 14+), parity (nulliparous, 1–2, 3–4, 5+), oral contraceptive use (never, 0–3 years of use, >3-<5 years, 5+ years of use), high blood pressure (yes/no), diabetes (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d). Postmenopausal model adjusted for joint age at menopause and PMH use variable (see categories for breast cancer). BMI models adjusted for joint menopausal status, age at menopause, and PMH use variable (see categories for breast cancer).
Ovarian cancer multivariable models adjusted for: age in months, calendar year, smoking (see categories for breast cancer), BMI (see categories for breast cancer), parity (see categories for endometrial cancer), oral contraceptive use (see categories for endometrial cancer), tubal ligation (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d). Postmenopausal model adjusted for PMH use (never, former, or current). BMI models adjusted for joint menopausal status and PMH use variable (premenopausal, uncertain status, postmenopausal and never PMH use, postmenopausal and former PMH use, and postmenopausal and current PMH use).
Table 4.
Relative risk (and 95% confidence interval) of breast cancer and ovarian cancer subtypes by quintile of calorie-adjusted acrylamide intake
| Quintile of cum. avg. acrylamide intake, mcg/day |
p-value | |||||
|---|---|---|---|---|---|---|
| Q1 (low) | Q2 | Q3 | Q4 | Q5 (high) | for trend | |
| BREAST CANCER | ||||||
| ER+/PR+, N cases | 520 | 594 | 606 | 580 | 505 | |
| Multivariable RR† | 1.00 | 0.98 (0.87–1.10) | 0.99 (0.88–1.12) | 0.99 (0.87–1.11) | 0.99 (0.87–1.13) | 0.99 |
| ER+/PR-, N cases | 136 | 161 | 173 | 148 | 119 | |
| Multivariable RR† | 1.00 | 1.09 (0.87–1.38) | 1.20 (0.95–1.51) | 1.08 (0.85–1.37) | 1.04 (0.80–1.34) | 0.88 |
| ER-/PR+, N cases | 28 | 23 | 20 | 35 | 32 | |
| Multivariable RR† | 1.00 | 0.78 (0.45–1.37) | 0.66 (0.37–1.20) | 1.18 (0.70–1.98) | 1.09 (0.63–1.87) | 0.35 |
| ER-/PR-, N cases | 178 | 159 | 175 | 174 | 153 | |
| Multivariable RR† | 1.00 | 0.86 (0.69–1.07) | 0.94 (0.76–1.17) | 0.95 (0.77–1.19) | 0.88 (0.70–1.11) | 0.52 |
| OVARIAN CANCER | ||||||
| Invasive, N cases | 72 | 66 | 88 | 71 | 66 | |
| Multivariable RR‡ | 1.00 | 0.98 (0.69–1.38) | 1.45 (1.03–2.03) | 1.28 (0.89–1.83) | 1.31 (0.89–1.92) | 0.09 |
| Borderline, N cases | 14 | 9 | 7 | 9 | 12 | |
| Multivariable RR‡ | 1.00 | 0.74 (0.31–1.77) | 0.57 (0.22–1.51) | 0.74 (0.29–1.87) | 0.99 (0.40–2.46) | 0.92 |
| Serous, N cases | 40 | 46 | 54 | 52 | 53 | |
| Multivariable RR‡ | 1.00 | 1.11 (0.71–1.73) | 1.43 (0.92–2.22) | 1.39 (0.88–2.20) | 1.58 (0.99–2.52) | 0.04 |
| Serous Invasive, N cases | 33 | 39 | 48 | 44 | 43 | |
| Multivariable RR‡ | 1.00 | 1.14 (0.70–1.86) | 1.59 (0.99–2.57) | 1.52 (0.92–2.51) | 1.67 (0.99–2.81) | 0.04 |
Breast cancer models adjusted for: age in months, calendar year, smoking (never, past <25 cig/d, past 25+ cig/d, current <25 cig/d, current 25+ cig/d), BMI (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25 to <30, 30+), height (quartiles), parity and age at first birth (nulliparous, parity 1–2 and age first birth <25, parity 1–2 age first birth 25-<30, parity 1–2 age first birth 30+, parity 3–4 age first birth <25, parity 3–4 age first birth 25-<30, parity 3–4 age first birth 30+, parity 5+ age first birth <25, parity 5+ age first birth 25+) family history of breast cancer (yes/no), benign breast disease (yes/no), age at menarche (<13, 13, 14+), physical activity (<=18 met-h/wk, >18 met-h/wk), folate, glycemic index, and animal fat intake (quintiles), alcohol (continuous, g/day), energy intake (continuous, kcal/d). Postmenopausal model adjusted for joint age at menopause and PMH use variable (age at menopause <45, 45–52, or >52 AND PMH use never, former, current <5 years, or current 5+ years).
Ovarian cancer models adjusted for: age in months, calendar year, smoking (see categories for breast cancer), BMI (see categories for breast cancer), parity (nulliparous, 1–2, 3–4, 5+), oral contraceptive use (never, 0–3 years of use, >3-<5 years, 5+ years of use), menopausal status and PMH use (premenopausal, uncertain status, postmenopausal and never used PMH, postmenopausal and former PMH, postmenopausal and current PMH use), tubal ligation (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d).
For ovarian cancer, the association was stronger for serous ovarian tumors, with an adjusted RR of 1.58 (95% CI: 0.99–2.52, p-trend=0.04, 245 cases) and for serous invasive tumors (RR 1.67, 95% CI: 0.99–2.81, p-trend=0.04, 207 cases) (Table 4). Serous tumors were the only subtype of ovarian cancer with enough cases to examine separately.
We studied the association between acrylamide and risk of endometrial and ovarian cancers while adjusting for the major acrylamide-contributing foods to see if the observed associations might be due to some other component of high-acrylamide foods. The association between quintile of acrylamide intake and endometrial cancer risk was essentially unchanged when additionally adjusted for coffee, breakfast cereal, French fries, or potato chips, the top four food contributors to acrylamide intake (Table 5). The association between quintile of acrylamide and ovarian cancer was essentially unchanged when additionally adjusted for coffee, breakfast cereal, or French fries. However, the association was attenuated when adjusted for potato chip intake (RR 1.01, 95% CI: 0.69–1.49, p-trend=0.82). Potato chip intake was significantly associated with ovarian cancer risk in a model without acrylamide intake (RR for highest versus lowest quintile of potato chip intake = 1.54, 95% CI: 1.14–2.09, p-trend=0.001); however the potato chip association was no longer signficant when also adjusted for acrylamide (RR for highest versus lowest quintile of potato chip intake = 1.27, 95% CI: 0.89–1.80, p-trend=0.24). This may be because simultaneously adjusting for acrylamide and potato chips substantially reduces the variation in both. None of the other major acrylamide-contributing foods were significantly independently associated with risk of either endometrial or ovarian cancer.
Table 5.
Relative risk (and 95% confidence interval) of endometrial and ovarian cancers by quintile of calorie-adjusted acrylamide intake additionally adjusting for high-acrylamide foods, 1980–2006
| Quintile of calorie-adjusted acrylamide intake |
p-value | |||||
|---|---|---|---|---|---|---|
| Q1 (low) | Q2 | Q3 | Q4 | Q5 (high) | for trend | |
| ENDOMETRIAL CANCER | ||||||
| Num. cases | 88 | 100 | 106 | 102 | 88 | |
| Person-years | 277,776 | 277,073 | 276,839 | 277,409 | 277,789 | |
| Multivariable RR† | 1.00 | 1.12 (0.83–1.50) | 1.31 (0.97–1.77) | 1.35 (0.99–1.84) | 1.41 (1.01–1.97) | 0.03 |
| MV RR + coffee§ | 1.00 | 1.08 (0.80–1.46) | 1.26 (0.93–1.72) | 1.31 (0.95–1.80) | 1.37 (0.97–1.94) | 0.05 |
| MV RR + cereal§ | 1.00 | 1.09 (0.81–1.47) | 1.27 (0.94–1.72) | 1.31 (0.95–1.79) | 1.37 (0.98–1.93) | 0.05 |
| MV RR + French fries§ | 1.00 | 1.16 (0.85–1.58) | 1.41 (1.02–1.95) | 1.51 (1.06–2.16) | 1.69 (1.11–2.57) | 0.01 |
| MV RR + potato chips§ | 1.00 | 1.11 (0.82–1.49) | 1.29 (0.94–1.75) | 1.32 (0.95–1.83) | 1.37 (0.95–1.97) | 0.06 |
| OVARIAN CANCER | ||||||
| Num. cases | 87 | 75 | 95 | 81 | 78 | |
| Person-years | 246,187 | 245,523 | 245,327 | 245,667 | 246,155 | |
| Multivariable RR‡ | 1.00 | 0.93 (0.68–1.29) | 1.29 (0.94–1.76) | 1.17 (0.84–1.64) | 1.25 (0.88–1.77) | 0.12 |
| MV RR + coffee§ | 1.00 | 0.93 (0.67–1.29) | 1.29 (0.94–1.78) | 1.18 (0.83–1.65) | 1.24 (0.87–1.78) | 0.15 |
| MV RR + cereal§ | 1.00 | 0.92 (0.67–1.27) | 1.26 (0.92–1.73) | 1.15 (0.82–1.62) | 1.23 (0.86–1.76) | 0.15 |
| MV RR + French fries§ | 1.00 | 0.90 (0.64–1.25) | 1.24 (0.88–1.74) | 1.15 (0.78–1.68) | 1.29 (0.83–1.99) | 0.14 |
| MV RR + potato chips§ | 1.00 | 0.90 (0.65–1.24) | 1.18 (0.85–1.63) | 1.02 (0.72–1.46) | 1.01 (0.69–1.49) | 0.82 |
Endometrial cancer multivariable models adjusted for: age in months, calendar year, smoking (never, past <25 cig/d, past 25+ cig/d, current <25 cig/d, current 25+ cig/d), BMI (<20, 20-<21, 21-<22, 22-<23, 23-<24, 24-<25, 25-<27, 27-<29, 29-<30, 30-<32, 32-<35, 35-<40, 40+), age at menarche (<13, 13, 14+), menopausal status/age at menopause/PMH use (premenopausal, uncertain status, postmenopausal: age at menopause <45, 45–52, or >52 AND PMH use never, former, current <5 years, or current 5+ years), parity (nulliparous, 1–2, 3–4, 5+), oral contraceptive use (never, 0–3 years of use, >3-<5 years, 5+ years of use), high blood pressure (yes/no), diabetes (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d).
Ovarian cancer multivariable models adjusted for: age in months, calendar year, smoking (see categories for endometrial cancer), BMI (<18.5, 18.5 to <20, 20 to <22.5, 22.5 to <25, 25 to <30, 30+), parity (see categories for endometrial cancer), oral contraceptive use (see categories for endometrial cancer), menopausal status and PMH use (premenopausal, uncertain status, postmenopausal and never used PMH, postmenopausal and former PMH, postmenopausal and current PMH use), tubal ligation (yes/no), physical activity (<=18 met-h/wk, >18 met-h/wk), caffeine intake (quintiles), energy intake (continuous, kcal/d).
Adjusted as in multivariable model and additionally adjusted for intake of given food in quintiles (for acrylamide quintile analysis) or as a continuous variable using the median intake for each quintile (for acrylamide p-value for trend analysis).
DISCUSSION
In our large, prospective cohort study among women, we found no association between acrylamide intake and risk of breast cancer. However, we observed an increased risk of endometrial cancer overall and among never smoking women, and a suggestion of increased risk of ovarian cancer overall, with a significantly increased risk for invasive and serous ovarian tumors.
The lack of association between acrylamide intake and breast cancer risk is in line with findings from previous prospective studies based on FFQs, including two cohorts of Swedish women(10,12), the Netherlands Cohort Study(13), and the Nurses' Health Study II(11). A separate report from the Netherlands Cohort Study (14) did find a suggestion of increased risk of estrogen- and progesterone-receptor positive in postmenopausal women; however, neither the relative risks for the top quintiles nor the p-values for linear trend were statistically signifcant. These results appear to be in conflict with a nested case-control study of hemoglobin-acrylamide adducts and breast cancer risk in a Danish cohort.(20) This study reported a positive association between biomarker levels and ER+ tumors. However, the meaning of these results with respect to dietary acrylamide intake is unclear, as the association was statistically significant only among smokers, who are exposed to much higher levels of acrylamide through smoking than diet. Our observation of no association between acrylamide intake and risk of breast cancer for different latency periods from 0–4 years to 12–16 years adds to the generally null findings for breast cancer.
Our findings for endometrial and ovarian cancer are similar to those of Hogervorst et al. in the Netherlands Cohort Study.(13) They found a significantly increased risk of ovarian cancer overall and a significantly increased risk of endometrial cancer among never smoking women. Only invasive ovarian epithelial tumors were included in their analysis, while we included both borderline and invasive ovarian epithelial tumors in our main analysis; we did see a stronger association for invasive tumors alone. Larsson et al. found no association between acrylamide intake and risk of invasive epithelial ovarian cancer(16) or endometrial cancer(15) in the Swedish Mammography Cohort; however, the range of intakes in this population was smaller than in our population or in the Netherlands Cohort Study.
Our relative risks are somewhat weaker than those observed in the Netherlands Cohort Study. It may be that acrylamide intake is measured with less error in a Dutch population where Dutch spiced cake is the major contributor to variation in acrylamide intakes. In our cohort, much variation in acrylamide intakes came from coffee, cold breakfast cereal, French fries, and potato chips. Therefore acrylamide intake from our FFQ may be subject to more measurement error due to the large variation in acrylamide content of these foods between different brands and even between different batches of the same brand. For example, the U.S. FDA found acrylamide concentrations ranging from 117 to 1030 micrograms per kilogram in 29 samples of fast-food French fries from nine different restaurants. In seven samples of fries from different McDonald's locations, the content ranged from 155 to 497 micrograms per kilogram.(21)
The wide variation in acrylamide content of foods combined with the fact that our FFQ was not specifically designed to assess cooking methods and food preparation techniques makes misclassification of acrylamide intake the major limitation in our study. It is likely that this measurement error is non-differential with respect to cancer outcomes, so we would expect it to dilute observed associations. Given that we did see associations between acrylamide and endometrial cancer and invasive and serous ovarian tumors in spite of this measurement error, the true associations may be greater than the observed relative risks of 1.3 to 1.4 for the highest quintile of intake.
Confounding by other components of acrylamide-rich foods or by factors associated with acrylamide intake is also a possibility. We tried to account for possible confounding as much as possible by adjusting for known and suspected risk factors for each cancer. Adjustment for caffeine intake was important for both the endometrial and ovarian cancer analyses. When caffeine intake was included in the models for endometrial cancer, the association with acrylamide intake was strengthened and the p-value for linear trend became statistically significant. An inverse association between caffeine or coffee intake and endometrial cancer has been suggested in several case-control studies,(22–26) but this association has not been examined in prospective studies. For ovarian cancer, caffeine was the main confounder. Caffeine and caffeinated coffee have previously been shown to be inversely related to ovarian cancer in this cohort.(26) It is possible that different components of coffee have opposite effects on endometrial or ovarian cancer risk; the effects of acrylamide with and without adjustment for caffeine intake merit investigation in future studies.
Strengths of our prospective analysis include good sample sizes for all three cancers, which allowed us to examine never-smoking women alone, to evaluate breast cancer and ovarian cancer subtypes, and to examine different latency periods for breast cancer. Our multiple FFQs allowed us to assess intake over an extended period, 26 years of follow-up, and to use cumulative average intake to reduce measurement error in the FFQs, which is particularly important in assessing acrylamide intake.
Acrylamide is believed to act mainly through its genotoxic metabolite, glycidamide (27); however, our findings suggest that acrylamide may also have hormonal effects that affect cancer risk. A recent study in male rats found no evidence of an effect of acrylamide on the hypothalamus–pituitary–thyroid axis.(27) However, an earlier study found evidence of effects on both neurotransmitters and circulating testosterone and prolactin levels.(28) The effects of acrylamide on female rats have been less studied, though one study found acrylamide administered in drinking water to female Sprague Dawley rats resulted in significantly lower circulating levels of estradiol and progesterone.(29) In addition, acrylamide has been shown to bind to proteins in vivo, so effects on sex hormone binding proteins are possible.(29) Our finding of a stronger association between acrylamide and endometrial and ovarian cancers among women with BMI less than 25 may provide indirect support for a hormonal mechanism, as endogenous hormone production is lower among leaner postmenopausal women, possibly allowing for outside factors to have a greater impact.
The relative risks for endometrial and ovarian cancer, in both our study and that of Hogervorst et al. (13), are greater than those predicted by linear extrapolation from animal models (6). However, there are many uncertainties in extrapolating from the high doses used in animal studies to the low doses found in human diets and in applying the findings from rodent studies to estimates of human cancer risk. In addition, if there is a hormonal pathway invovled in acrylamide carcinogenesis, as discussed above, it is possible that the results of the animal models may not properly apply to human risks.
The results of our study and several other prospective studies have found no association between acrylamide intake and breast cancer risk, though a small increase in risk with higher intakes cannot be ruled out due to error in measuring acrylamide intake. On the other hand, the results of our study along with those of Hogervorst et al.(13) suggest that acrylamide intake may be associated with risk of endometrial and ovarian cancers. The associations between acrylamide intake, and biomarkers of acrylamide intake, and risk of endometrial and ovarian cancers should be examined in other prospective studies to further inform public policy and consumers.
Acknowledgments
Financial Support: This work was supported by a grant from the National Cancer Institute of the National Institutes of Health (P01 CA087969). KMW is partially supported by a National Cancer Institute/National Institutes of Healh Training Grant (T32 CA09001).
REFERENCES
- 1.Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50:4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 2.IARC. Monographs on the evaluation of carcinogenic risks to humans, vol 60. Lyon, France: International Agency for Research on Cancer; 1994. [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. The 2006 exposure assessment for acrylamide. Available from: http://www.cfsan.fda.gov/~dms/acryexpo.html.
- 4.Johnson KA, Gorzinski SJ, Bodner KM, et al. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol. 1986;85:154–168. doi: 10.1016/0041-008x(86)90109-2. [DOI] [PubMed] [Google Scholar]
- 5.Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide. Fundam Appl Toxicol. 1995;27:95–105. doi: 10.1093/toxsci/27.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dybing E, Sanner T. Risk assessment of acrylamide in foods. Toxicol Sci. 2003;75:7–15. doi: 10.1093/toxsci/kfg165. [DOI] [PubMed] [Google Scholar]
- 7.Hagmar L, Tornqvist M. Inconclusive results from an epidemiological study on dietary acrylamide and cancer. Br J Cancer. 2003;894:774–775. doi: 10.1038/sj.bjc.6601016. (author reply p. 775–776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucci LA, Adami H-O. The role of epidemiology in understanding the relationship between dietary acrylamide and cancer risk in humans. In: Friedman M, Mottram D, editors. Chemistry and Safety of Acrylamide in Food. New York: Springer; 2005. pp. 39–47. [DOI] [PubMed] [Google Scholar]
- 9.Pelucchi C, Galeone C, Levi F, et al. Dietary acrylamide and human cancer. Int J Cancer. 2006;118:467–471. doi: 10.1002/ijc.21336. [DOI] [PubMed] [Google Scholar]
- 10.Mucci LA, Sandin S, Balter K, Adami HO, Magnusson C, Weiderpass E. Acrylamide intake and breast cancer risk in Swedish women. JAMA. 2005;293:1326–1327. doi: 10.1001/jama.293.11.1326. [DOI] [PubMed] [Google Scholar]
- 11.Wilson KM, Mucci LA, Cho E, Hunter DJ, Chen WY, Willett WC. Dietary acrylamide intake and risk of premenopausal breast cancer. Am J Epidemiol. 2009;169:954–961. doi: 10.1093/aje/kwn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson SC, Akesson A, Wolk A. Long-term dietary acrylamide intake and breast cancer risk in a prospective cohort of Swedish women. Am J Epidemiol. 2009;169:376–381. doi: 10.1093/aje/kwn319. [DOI] [PubMed] [Google Scholar]
- 13.Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2304–2313. doi: 10.1158/1055-9965.EPI-07-0581. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen GS, Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. Dietary acrylamide intake and estrogen and progesterone receptor-defined postmenopausal breast cancer risk. Breast Cancer Res Treat. 2009 Dec 1; doi: 10.1007/s10549-009-0642-4. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Hakansson N, Akesson A, Wolk A. Long-term dietary acrylamide intake and risk of endometrial cancer in a prospective cohort of Swedish women. Int J Cancer. 2009;124:1196–1199. doi: 10.1002/ijc.24002. [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Akesson A, Wolk A. Long-term dietary acrylamide intake and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Cancer Epidemiol Biomarkers Prev. 2009;18:994–997. doi: 10.1158/1055-9965.EPI-08-0868. [DOI] [PubMed] [Google Scholar]
- 17.Wilson KM, Vesper HW, Tocco P, et al. Validation of a food frequency questionnaire measurement of dietary acrylamide intake using hemoglobin adducts of acrylamide and glycidamide. Cancer Causes Control. 2009;20:269–278. doi: 10.1007/s10552-008-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins LC, Marotti JD, Baer HJ, Tamimi RM. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst. 2008;100:218–221. doi: 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett WC. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 20.Olesen PT, Olsen A, Frandsen H, Frederiksen K, Overvad K, Tjonneland A. Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer, and Health Study. Int J Cancer. 2008;122:2094–2100. doi: 10.1002/ijc.23359. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. Survey data on acrylamide in food: Individual food products. 2002–2004 Available from: http://www.cfsan.fda.gov/~dms/acrydata.html.
- 22.Petridou E, Koukoulomatis P, Dessypris N, Karalis D, Michalas S, Trichopoulos D. Why is endometrial cancer less common in Greece than in other European Union countries? Eur J Cancer Prev. 2002;11:427–432. doi: 10.1097/00008469-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: A nationwide case-control study in Sweden. Nutr Cancer. 2002;42:25–32. doi: 10.1207/S15327914NC421_4. [DOI] [PubMed] [Google Scholar]
- 24.Hirose K, Niwa Y, Wakai K, Matsuo K, Nakanishi T, Tajima K. Coffee consumption and the risk of endometrial cancer: Evidence from a case-control study of female hormone-related cancers in Japan. Cancer Sci. 2007;98:411–415. doi: 10.1111/j.1349-7006.2007.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koizumi T, Nakaya N, Okamura C, et al. Case-control study of coffee consumption and the risk of endometrial endometrioid adenocarcinoma. Eur J Cancer Prev. 2008;17:358–363. doi: 10.1097/CEJ.0b013e3282f0c02c. [DOI] [PubMed] [Google Scholar]
- 26.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008;112:1169–1177. doi: 10.1002/cncr.23275. [DOI] [PubMed] [Google Scholar]
- 27.Bowyer JF, Latendresse JR, Delongchamp RR, et al. The effects of subchronic acrylamide exposure on gene expression, neurochemistry, hormones, and histopathology in the hypothalamus-pituitary-thyroid axis of male Fischer 344 rats. Toxicol Appl Pharmacol. 2008;230:208–215. doi: 10.1016/j.taap.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Ali SF, Hong J-S, Wilson WE, Uphouse LL, Bondy SC. Effect of acrylamide on neurotransmitter metabolism and neuropeptide levels in several brain regions and upon circulating hormones. Arch Toxicol. 1983;52:35–43. doi: 10.1007/BF00317980. [DOI] [PubMed] [Google Scholar]
- 29.Mannaa F, Abdel-Wahhab MA, Ahmed HH, Park MH. Protective role of Panax ginseng extract standardized with ginsenoside Rg3 against acrylamide-induced neurotoxicity in rats. J Applied Toxicol. 2006;26:198–206. doi: 10.1002/jat.1128. [DOI] [PubMed] [Google Scholar]


