Abstract
Objective
To determine if mycophenolate mofetil (MMF) diminished skin and muscle disease activity in children with juvenile dermatomyositis (JDM) permitting decrease in corticosteroid dosage.
Methods
Retrospective data review for 50 JDM children identified the following: 38 (76%) girls, 39 (78%) white, 10 (20%) Hispanic, 1 (2%) black, mean age 12.2 ± 5.0 years who had been given MMF for 12 months. MMF dose and frequency, type of infection, white blood cell count (WBC), corticosteroid dose and validated disease activity score (DAS), (sub scores for skin [DAS-S], muscle [DAS-M]), were obtained.
Results
Twelve months after start of MMF, mean DAS-S decreased from 5.24 ± 0.29 to 3.72 ± 0.29 (p=0.001) and DAS-M dropped from 2.44 ± 0.39 to 1.17 ± 0.28 (p=0.002). Prednisone dose decreased from 0.39 mg/kg/day ± 0.06 mg to 0.23mg/kg/day ± 0.02 mg (p=0.0001), with resumption of linear growth (p=0.008). The WBC/lymphocyte count was unchanged over 12 months on MMF. Infection rate was assessed in a subset of 26 children with JDM followed for 12 months before start of MMF and compared with ensuing 12 months on MMF. There was no significant difference between pretreatment and first 6 months of MMF (p=0.44), but infection rate dropped in months 7–12 (p=0.001).
Conclusions
MMF appears to be worthy of consideration as an additional therapeutic modality for treatment of children with JDM. These data suggest that use of MMF decreases skin and muscle disease activity and is steroid sparing. MMF appears to be well tolerated, but patients should be monitored for infection.
Juvenile dermatomyositis (JDM) is the most common pediatric inflammatory myopathy, with an incidence of 3.2 cases/million children/year in the US (1). Children present with a characteristic rash, which includes heliotrope discoloration of the eyelids and Gottron’s papules on extensor surfaces, and symmetrical proximal muscle weakness. The primary lesion in JDM is a systemic small vessel vasculopathy (2), which visibly progresses with delay in the institution of effective immunosuppressive therapy (3), and is reflected in gene expression profiles of muscle from untreated children with definite/probable diagnosis of JDM (4). Gene expression profiles of muscle (5, 6, 7) as well as peripheral blood from adults (8, 9) and children (10) with dermatomyositis have also demonstrated up-regulation of Type-1 interferon (IFN)-inducible genes, as well as increased interferon-alpha (IFN-α) activity in sera of untreated children with JDM (11). The gene expression profiles from children with JDM share features with gene expression profiles obtained from examination of blood from patients with lupus (12). Although distinct clinical entities, some of the commonality of gene expression suggests there may be similarities in the pathophysiology between these diseases and that treatment modalities may overlap as well.
The specific approach to therapy for JDM is controversial, but corticosteroids are the cornerstone of treatment (13). The numerous side effects of corticosteroids, particularly the negative impact on growth in children, as well as lack of adequate clinical response to this regimen in some patients, has prompted a search for alternative immunosuppressant therapy for children with active symptoms of JDM. Mycophenolate mofetil (MMF) is established as an effective treatment for lupus nephritis and other systemic lupus manifestations in both adults (14) and children (15). Furthermore, adult dermatomyositis patients were reported to have symptomatic improvement with MMF therapy (16, 17). Initially approved for the prevention of organ transplantation rejection, (18) MMF has been employed in the management of a range of other autoimmune diseases (19).
The mechanism of action of MMF has been studied and has shown that hepatic metabolism converts MMF to the active moiety, mycophenolic acid (MPA). MPA selectively inhibits de novo purine metabolism by reversibly binding to inosine monophosphate dehydrogenase. By depleting guanosine and deoxyguanosine nucleotides in T and B lymphocytes, it inhibits their proliferation, antibody production, and prevents leukocyte binding to endothelial cells (20). Our experience of the response of children with JDM to MMF was assessed by comparing clinical and laboratory information at the time of start of MMF therapy with that at the 6 and 12 month follow-up visits.
PATIENTS AND METHODS
Patients
The medical records of all children diagnosed with definite/probable JDM at Children’s Memorial Hospital Pediatric Rheumatology clinic were reviewed. Children with overlap syndrome were excluded. We included all patients in this retrospective analysis who had received MMF for a minimum of 12 months, n=50. The Institutional Review Board approved this study (IRB #2002–11762).
Indications for use of MMF
The major indication for the addition of MMF to the therapeutic regimen was lack of the child’s response to previous interventions. This is documented in Table 1 as duration from onset of symptoms to start of MMF, mean 3.6 (range 0.2–16.2) years, as well as duration of symptoms from onset of first symptom to initial treatment, mean 2.8 years (range 0.1–15.2). Children with JDM who had already been treated, 32/50, were referred from other centers, and they had a much more varied background of previous therapy. After evaluation, the initial therapy at our center is fairly standard and was used in 46/50 (92%) patients, under the direction of a single physician (LMP). IVMP at 30 mg/kg, was administered daily on three consecutive days when possible, followed by IVMP given once to three times a week, with oral prednisone at 0.5 mg/kilo on non-IVMP days, until the child’s indicators of immune activation had normalized. In addition, the following was used: methotrexate at a minimum of 15 mg/meter square, vitamin D to achieve a blood level in the therapeutic range (>35ng/mL), 1 gm of folic acid and a proton pump inhibitor. Hydroxychloroquine was also administered on a less consistent basis and usage of sunscreen with a minimum of SPF 40 was urged. In the remaining four untreated patients with a severe rash at presentation, MMF was begun as part of the initial therapy.
Table 1.
Demographics and Clinical Characteristics
| n (%) | Age at MMF Start median yrs (min, max) | DUD* median mths (min, max) | Onset to MMF Start median yrs (min, max) | Dx** to MMF Start median yrs (min, max) | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 38 (76) | 12.3 (4.6, 23.2) | 4.5 (1.3, 113.8) | 3.4 (0.2, 16.2) | 2.8 (0.1, 15.2) |
| Male | 12 (24) | 12.9 (4.2, 17.1) | 8.5 (1.1, 22.3) | 3.6 (1.1, 10.5) | 2.6 (0.2, 10.3) |
| Race | |||||
| White | 39 (78) | 12.9 (4.2, 23.2) | 4.5 (1.3, 113.9) | 4.1 (0.2, 16.2) | 3.2 (0.1, 15.2) |
| Hispanic | 10 (20) | 11.4 (4.6, 16.5) | 6.4 (1.1, 22.3) | 2.4 (1.1, 10.5) | 2.0 (0.2, 11.3) |
| Black | 1 (2) | 7.1 (7.1, 7.1) | 9.4 (9.4, 9.4) | 1.8 (1.8, 1.8) | 1.0 (1.0, 1.0) |
| Total | 50 (100) | 12.6 (4.2, 23.2) | 5.0 (1.1, 113.8) | 3.6 (0.2, 16.2) | 2.8 (0.1, 15.2) |
DUD = Duration of Untreated Disease
Dx = Diagnosis
Assessments
MMF effectiveness was assessed by comparing clinical and laboratory information at the time of start of MMF therapy with that at the 6 and 12 month follow-up visits. All eligible patients who had been followed for at least 12 months were included in the analyses. For analysis of the infection rate, the number and type of infections occurring in 26/50 patients followed by our clinic for the 12 months before the start of MMF therapy was compared with the number and type of infections in the 12 months after MMF had been initiated.
Validated DAS (21), including skin and muscle involvement subscores, were calculated for patients at onset and follow-up visits. All JDM patients were started on an initial dose of 20mg/kg of MMF divided twice a day. Data on MMF dosing and adverse events attributed to MMF, including types of infection and frequency, were obtained. Additional medications prescribed for JDM, including corticosteroid dose and frequency, were recorded at start of MMF therapy and at 6 and 12 month follow-up visits.
Criteria for change of medical therapy
In addition to the DAS scores for skin (DAS-S) and muscle (DAS-M), obtained at the initial visit and each visit thereafter, the child with JDM was also assessed using a consistent panel of physical and laboratory tests at each visit. This evaluation included the Childhood Myositis Assessment Score (CMAS), performed by an experienced pediatric physical therapy team. Serologic tests included muscle enzymes (creatine kinase, lactic acid dehydrogenase, and aldolase), complete blood count, neopterin, von Willebrand factor antigen (compared with the normal range for the child’s blood group), and flow cytometry evaluation of peripheral lymphocyte subsets. If the child’s symptoms improved, and the laboratory tests were in the range of normal for age, then the IVMP (30mg/kg with one gram maximum) was slowly decreased in frequency, until it was discontinued, and then the prednisone was decreased by 1 mg/month, if the interval testing showed no reactivation. In contrast, if the child did not respond clinically and/or the laboratory parameters showed continued activation, then other medications were added. The following immunosuppressive therapy was continued when MMF was started: intravenous pulse methylprednisolone (n=15), methotrexate (n=38), hydroxychloroquine (n=19) and IVIG (n=4). During the 12 month course, because 5/50 (10%) children were still symptomatic or had abnormal laboratory values despite MMF in conjunction with the other medications, the additional medications were started/restarted: cyclosporine (n=1/1), IVIG (n=4/1), methotrexate (n=0/5), hydroxychloroquine (n=2/2), and intravenous methylprednisolone (n=2/5). At the 12 month follow-up visit, the following medications were continued to be used as treatments: intravenous pulse methylprednisolone (n=10), methotrexate (n=36) hydroxychloroquine (n=18), while more patients were receiving IVIG (n=8).
Statistical Analysis
We used mean, median, and standard deviation to describe continuous variable (e.g. age, duration of untreated disease, and others) and used proportions to summarize dichotomous variables (e.g. gender, race, etc). Linear mixed models were applied to analyze changes of DAS scores, prednisone dose, height and weight over time. Cochran-Mantel-Haenszel test and Poison regression were applied to assess changes of MMF infection rates over time. Wilcoxon Signed-Rank test was used for pair-wise comparison of prednisone dose, height and weight. Analyses were conducted using SAS 9.3 and 0.05 was used as the criteria for the level of statistical significance.
Results
Patients
The demographics and clinical characteristics for the 50 patients are presented in Table 1. The demographics are similar to previous reports from our center documenting a female bias (76%) with a predominately white (78%) population. At diagnosis, this group had a long duration of untreated disease (median=5 months), preceding the start of conventional therapy, which was given for a mean of 2.8 years before the start of MMF.
Disease activity
After start of MMF, the mean DAS-S decreased from 5.24 ± 0.29 to 4.20 ± 0.28 at 6 months (p=0.003) and 3.72 ± 0.29 at 12 months after start of MMF, (p= 0.001). Correspondingly, the DAS-M dropped from 2.44 ± 0.39 to 1.17 ± 0.28 (p=0.002) after 12 months of therapy, but not at 6 months (Figure 1). When the patients who received IVIG were removed from this analysis, and the data reanalyzed, the conclusions remained essentially the same: the DAS-S significantly decreased over 12 months (p=0.0001), DAS-M also improved (p=0.02). Specifically, compared to data at the start of MMF, the DAS-S was significantly lower at 6 months (p=0.004) and further improved at 12 months (p<0.0001). Similarly, DAS-M was significantly lower at both 6 months (p=0.02), but did not further change between 6 months and 12 months (p=0.96).
Figure 1.
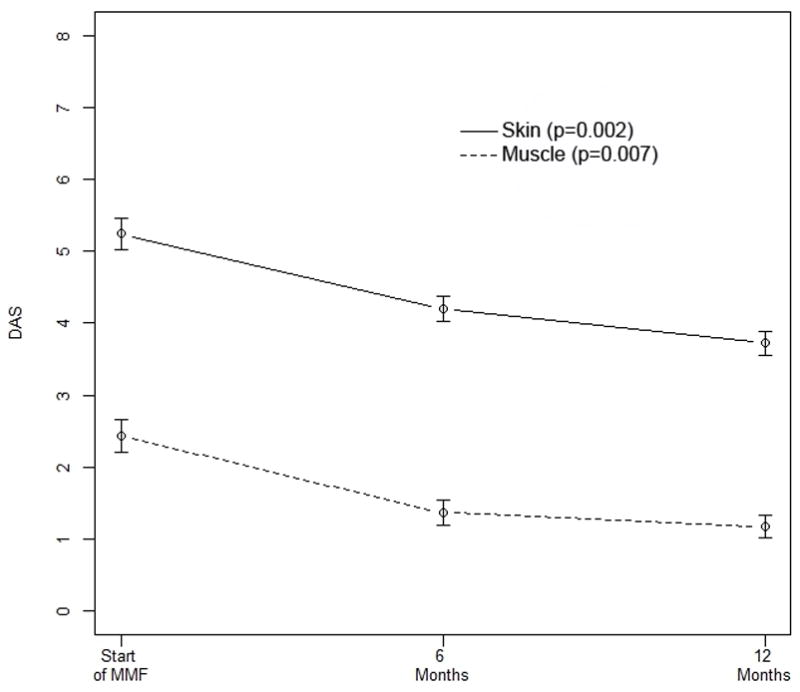
DAS-S and DAS-M are significantly improved over 12 months of MMF therapy, with p-values of 0.002 and 0.007, respectively. The DAS-S is also significantly lower at six months (p=0.003) compared to the values before the start of MMF. Similarly, DAS-M was significantly lower at both 6 months (p=0.02), but did not further change between 6 months and 12 months (p=0.96). Bars show the mean and SEM.
Medication requirements
The prescribed dose of prednisone (mg/kg/day) at MMF follow-up at both 6 and 12 months was significantly lower than that at the start of MMF therapy, dropping from 0.39 ± 0.06 mg/kg to 0.34 ± 0.05 mg/kg after 6 months of MMF (p=0.044) to 0.23mg/kg ± 0.02 (p<0.0001) at 12 months after start of MMF (Figure 2). The decrease in prednisone dose, as recounted in the methods section, was only instituted when all the laboratory data and the child’s disease signs and symptoms were within normal limits.
Figure 2.
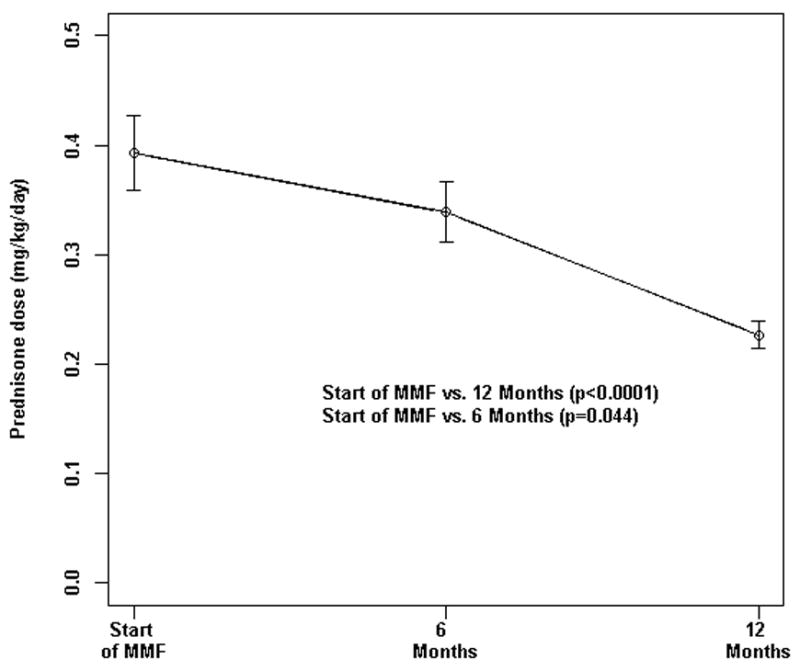
Daily prednisone dose (mg/kg/day) at start of MMF was significantly higher as compared to follow-up visits. P-value* was determined by linear mixed modeling. Bars show the mean and SEM.
Tolerability of MMF
Overall, patients tolerated MMF well and there were no serious adverse events attributed to MMF between onset and follow-up visit. None of our children stopped MMF because of toxicity. We used as an inclusion criteria for this study “12 months of therapy” in order to try to identify adverse reactions to MMF. Toxicity to MMF was not reported during the course of therapy, at the level of 20 mg/kg divided BID in the setting of the other drug therapy as noted. Two children reported some abdominal discomfort which resolved when the dose of MMF was lowered.
Infection rate
Forty-one out of 50 (82%) patients experienced 132 infections during the study period. The incidence of infections was 0.25/month before start of MMF, 0.21 infections per month in the first six months of MMF therapy, and 0.12 infections per month in the next six months (7–12 months). There was not a significant difference between the pretreatment and the first 6 months after the start of MMF therapy (p=0.44), but in the second 6 months (7–12) the infection rate was significantly lower than in the pretreatment data (p=0.001). The majority of these infections were viral upper respiratory infections (53%), followed by sinusitis and otitis media in 8.3% and 5.3%, respectively. Additional infections occurring in less than 5% of patients included herpes zoster, conjunctivitis, central line infection, thrush, dental abscess, skin abscess, infected calcification, urinary tract infection, strep throat and mononucleosis. No serious infection requiring hospitalization related to MMF occurred during the study period, and none of the JDM children developed leucopenia or B cell abnormalities as measured by flow cytometry (data not shown).
Growth parameters
Using the method of least square means to compile the data for height and weight, the mean height of the children when MMF was started was 146.25 cm ± 3.69, and by 12 months later, their height had increased to 149.51 cm ± 3.69, p=0.016, while the mean weight increased as well, from 50.42 kg ± 3.31 to 53.25 kg ± 3.81, p=0.005.
Discussion
This is the first report that describes pediatric patients with JDM treated with MMF and followed for one year after start of therapy. The rationale for the use of MMF in this patient population was based on the finding that, like pediatric systemic lupus erythematosus (SLE) in which MMF has been effective (15), children with JDM have a documented a florid Type-1 interferon induced gene expression pattern (5, 8, 10). In addition, both patients with SLE and JDM have increased serum IFN-α activity as determined by the WISH reporter cell assay (11, 24). After the pharmacokinetics and safety data for MMF had been obtained in children (23), MMF was reported to be an effective therapy for children with SLE (15). Some of the symptoms of JDM share similarities with SLE. For example, both have a vasculitic malar rash, although the SLE band test is not present in skin from children with JDM. Disease resistance to conventional therapy in JDM is characterized by persistence of skin involvement, often after the musculoskeletal symptoms normalize (13). Active skin disease at any level of severity in children with JDM has been associated with calcinosis development. A goal of the therapy is to control cutaneous inflammation, which may minimize this devastating complication (25).
In 2000, Gelber et al (26) described four adult patients with severe cutaneous disease related to dermatomyositis and improvement in skin manifestations following MMF. Subsequent case reports of adults with dermatomyositis, polymyositis, and myositis associated with connective tissue disease have suggested control of muscle inflammation by MMF (17, 26–31). It is difficult to make direct comparisons of these studies given the different outcome measures utilized in the investigations, but improvement in weakness, creatine kinase (CK) or overall disease activity was not uncommon. Similarly, in our cohort we demonstrate a significant decrease in disease activity of both skin and muscle inflammation at follow-up. We did not report CK levels because children with long disease duration greater than four months are more likely to have muscle enzymes that are in the range of normal values (32).
Recent reviews (33, 34) of the treatment of dermatomyositis include a discussion of MMF, and the authors report that they find MMF useful as a second choice adjunct treatment after methotrexate. Given the improvement in both skin and muscle DAS in our patients, we concur that MMF may be a beneficial option in children with JDM not responding to traditional therapy with corticosteroid and methotrexate. Since IVIG had been found to potentiate the effect of MMF (31), some of the more resistant cases received this therapy as well. In addition to disease activity improvement, we found that children receiving MMF were able to decrease the dose of corticosteroids. This finding is comparable with reports in adults with idiopathic inflammatory myopathy (16, 17, 27–31, 34). In children, there is even greater concern to minimize corticosteroid exposure given their deleterious impact on growth and bone mineral accretion. We had demonstrated that prior to corticosteroid exposure; children with JDM had reduced lumbar spine bone mineral density (35), which could be intensified by the long term administration of corticosteroids.
Increased susceptibility to infection associated with the use of MMF was highlighted in a report by Rowin et al. (36) in which 3 of 10 patients with dermatomyositis developed opportunistic infections and resultant death in one patient. This is in contrast to our study, which did report infection as the most common side effect, but none of the patients had a serious infection requiring hospitalization. A study of patients with renal transplant receiving MMF as maintenance immunosuppression demonstrated no mortality from infection (18). Among adults with myositis receiving MMF, life-threatening infections were not reported (16, 17, 27–31, 34, 37–38). Gastrointestinal side effects can be associated with MMF, but in our cohort, only one child developed vomiting while taking the liquid form of MMF, which stopped when tablets were used. Caution in female patients of child-bearing potential is warranted, as MMF is contraindicated in pregnancy (39). Fortunately, none of the subjects in our cohort became pregnant and we advise practitioners to counsel female patients accordingly. A very rare but serious complication of MMF therapy, mentioned in a report of B cell lymphomas in three patients with SLE, was the occurrence of primary central nervous system lymphoma (PCNSL) in a single patient with dermatomyositis treated with MMF and methotrexate, which spontaneously resolved with discontinuation of both drugs (40). It is clear that because of the lack of controlled trials in patients with myositis, the role of MMF remains to be confirmed.
There are several limitations to this study: first is the retrospective study design; and second, is the lack of a concurrent control group of JDM, closely matched for age and disease severity, who had not been given MMF. Despite these limitations, we report the results of 50 pediatric patients treated with MMF, which is a robust sample size, given that JDM is a rare disease. We do not report therapeutic drug monitoring of MMF, as this remains controversial, even in the transplant literature. Further case-control pharmacokinetic studies in adult and pediatric subjects with myositis should be considered to better understand the role of therapeutic drug monitoring in this patient population.
In conclusion, MMF appears to be worthy of consideration as an additional therapeutic modality for the treatment of children with JDM. This study suggests that both skin and muscle manifestations respond to MMF in children with JDM, but the skin inflammation, which is often resistant to therapy, responds especially well. Therefore, MMF is steroid-sparing and offers an alternative therapy as well as minimizing corticosteroid exposure in children with JDM. MMF appears to be well-tolerated, but clinicians should judiciously monitor patients for infectious and hematologic complications. Randomized, controlled trials of MMF in patients with JDM would help to establish its role in the management of this often chronic and devastating disease.
Acknowledgments
Supported in part by: NIH/NIAMS grant R01-AR48289 (LMP), Macy’s Miracle Foundation (LMP), and Cure JM Foundation (LMP, KRS).
Reference List
- 1.Mendez EP, Lipton RB, Dyer A, Ramsey-Goldman R, Roftcher P, Bowyer S, et al. Incidence of Juvenile Dermatomyositis (JDM) 1995–98: results from the NIAMS Registry. Arthritis Care Res. 2003;49:300–5. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- 2.Banker BQ, Victor M. Dermatomyositis (systemic angiopathy) of childhood. Medicine. 1966;45:261–89. doi: 10.1097/00005792-196607000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Christen-Zaechs S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Juvenile dermatomyositis: Persistent association of nailfold capillaroscopy changes and skin involvement over 36 months with duration of untreated disease. Arthritis Rheum. 2008;58:571–6. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 5.Chen YW, Shi R, Geraci N, Shrestha S, Gordish-Dressman H, Pachman LM. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunol. 2008;9:43. doi: 10.1186/1471-2172-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan DA, Krasnoselska I, et al. Expression profiling in DQA1*0501 children with juvenile dermatomyositis: A novel model of pathogenesis. J Immunol. 2002;168:4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Dimachkie MM, Xiong M, Tan FK, Arnett FC. cDNA microarrays reveal distinct gene expression clusters in idiopathic inflammatory myopathies. Med Sci Monit. 2004;10(7):BR191–BR197. [PubMed] [Google Scholar]
- 8.Baechler EC, Bauer JW, Slattery CA, Ortmann WA, Espe KJ, Novitzke J, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med. 2007;13(1–2):59–68. doi: 10.2119/2006-00085.Baechler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56(11):3784–92. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor KA, Abbot KA, Sabin B, Kuroda M, Pachman LM. MxA gene expression in juvenile dermatomyositis peripheral blood mononuclear cells: association with muscle involvement. Clin Immunol. 2006;120(3):319–25. doi: 10.1016/j.clim.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-alpha activity in juvenile dermatomyositis: Associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60(6):1815–24. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371(9631):2201–12. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 14.Roland M, Barbet C, Paintaud G, Magdelaine-Beuzelin C, Diot E, Halimi JM, Lebranchu Y, Nivet H, Buchler M. Mycophenolate mofetil in patients with systemic lupus erythematosus: a prospective pharmacokinetic study. Lupus. 2009;18(5):441–447. doi: 10.1177/0961203308098631. [DOI] [PubMed] [Google Scholar]
- 15.Falcini F, Capannini S, Martini G, La Torre F, Vitale A, Mangiantini F, Nacci F, Cerinic MM, Cimaz R, Zulian F. Mycophenolate mofetil for the treatment of juvenile onset SLE: a multicenter study. Lupus. 2009;18(2):139–143. doi: 10.1177/0961203308094999. [DOI] [PubMed] [Google Scholar]
- 16.Edge JC, Outland JD, Dempsey JR, Callen JP. Mycophenolate mofetil as an effective corticosteroid-sparing therapy for recalcitrant dermatomyositis. Arch Dermatol. 2006;142(1):65–9. doi: 10.1001/archderm.142.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Majithia V, Harisdangkul V. Mycophenolate mofetil (CellCept): an alternative therapy for autoimmune inflammatory myopathy. Rheumatology (Oxford) 2005;44(3):386–9. doi: 10.1093/rheumatology/keh499. [DOI] [PubMed] [Google Scholar]
- 18.Gozdowska J, Urbanowicz A, Baczkowska T, Pazik J, Matlosz B, Cieciura T, Szmidt J, Chmura A, Durlik M. Safety and tolerance of sodium mycophenolate in patients after renal transplantation--an obsertional study. Transplant Proc. 2009;41(8):3016–18. doi: 10.1016/j.transproceed.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 19.Iaccarino L, Rampudda M, Canova M, Della LS, Sarzi-Puttinic P, Doria A. Mycophenolate mofetil: what is its place in the treatment of autoimmune rheumatic diseases? Autoimmun Rev. 2007;6(3):190–195. doi: 10.1016/j.autrev.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 21.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with Juvenile Dermatomyositis (JDM): Reliability and validity evidence. Arthritis Care Res. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 22.Huber AM, Feldman BM, Rennebohm RM, Hicks JE, Lindsley CB, Perez MD, et al. Validation and clinical significance of the Childhood Myositis Assessment Scale for assessment of muscle function in the juvenile idiopathic inflammatory myopathies. Arthritis Rheum. 2004;50(5):1595–1603. doi: 10.1002/art.20179. [DOI] [PubMed] [Google Scholar]
- 23.Filler G, Hansen M, LeBlanc C, Lepage N, Franke D, Mai I, et al. Pharmacokinetics of mycophenolate mofetil for autoimmune disease in children. Pediatr Nephrol. 2003 May;:445–9. doi: 10.1007/s00467-003-1133-1. [DOI] [PubMed] [Google Scholar]
- 24.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007 Sep;:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pachman LM, Veis A, Stock S, Abbott K, Vicari F, Patel P, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis Rheum. 2006;54(10):3345–50. doi: 10.1002/art.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelber AC, Nousari HC, Wigley FM. Mycophenolate mofetil in the treatment of severe skin manifestations of dermatomyositis: a series of 4 cases. J Rheumatol. 2000;27(6):1542–5. [PubMed] [Google Scholar]
- 27.Schneider C, Gold R, Schafers M, Toyka KV. Mycophenolate mofetil in the therapy of polymyositis associated with a polyautoimmune syndrome. Muscle Nerve. 2002;25(2):286–8. doi: 10.1002/mus.10026. [DOI] [PubMed] [Google Scholar]
- 28.Pisoni CN, Cuadrado MJ, Khamashta MA, Hughes GR, D’Cruz DP. Mycophenolate mofetil treatment in resistant myositis. Rheumatology (Oxford) 2007;46(3):516–8. doi: 10.1093/rheumatology/kel336. [DOI] [PubMed] [Google Scholar]
- 29.Caramaschi P, Volpe A, Carletto A, Bambara LM, Biasi D. Long-standing refractory polymyositis responding to mycophenolate mofetil: a case report and review of the literature. Clin Rheumatol. 2007;26(10):1795–6. doi: 10.1007/s10067-006-0526-5. [DOI] [PubMed] [Google Scholar]
- 30.Bandelier C, Guerne PA, Genevay S, Finckh A, Gabay C. Clinical experience with mycophenolate mofetil in systemic autoimmune conditions refractory to common immunosuppressive therapies. Swiss Med Wkly. 2009;139(3–4):41–6. doi: 10.4414/smw.2009.12441. [DOI] [PubMed] [Google Scholar]
- 31.Danieli MG, Calcabrini L, Calabrese V, Marchetti A, Logullo F, Gabrielli A. Intravenous immunoglobulin as add on treatment with mycophenolate mofetil in severe myositis. Autoimmun Rev. 2009;9(2):124–7. doi: 10.1016/j.autrev.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148(2):247–53. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Iorizzo LJ, III, Jorizzo JL. The treatment and prognosis of dermatomyositis: an updated review. J Am Acad Dermatol. 2008;59(1):99–112. doi: 10.1016/j.jaad.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 34.Hengstman GJ, van den Hoogen FH, van Engelen BG. Treatment of the inflammatory myopathies: update and practical recommendations. Expert Opin Pharmacother. 2009;10(7):1183–90. doi: 10.1517/14656560902913815. [DOI] [PubMed] [Google Scholar]
- 35.Rouster-Stevens KA, Langman CB, Price HE, Seshadri R, Shore RM, Abbott K, et al. RANKL:osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis. Arthritis Rheum. 2007;56(3):977–83. doi: 10.1002/art.22433. [DOI] [PubMed] [Google Scholar]
- 36.Rowin J, Amato AA, Deisher N, Cursio J, Meriggioli MN. Mycophenolate mofetil in dermatomyositis: is it safe? Neurology. 2006;66(8):1245–7. doi: 10.1212/01.wnl.0000208416.32471.c0. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Chu SH, Wei TY, Yen TH, Chiang YJ, Wu CT, et al. Does mycophenolate mofetil increase the incidence of infections in stable renal transplant recipients initially treated with a two-drug regimen? Transplant Proc. 2004;36(7):2122–3. doi: 10.1016/j.transproceed.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Marie I, Hachulla E, Cherin P, Hellot MF, Herson S, Levesque H, et al. Opportunistic infections in polymyositis and dermatomyositis. Arthritis Rheum. 2005;53(2):155–65. doi: 10.1002/art.21083. [DOI] [PubMed] [Google Scholar]
- 39.Ostensen M, Lockshin M, Doria A, Valesini G, Meroni P, Gordon C, et al. Update on safety during pregnancy of biological agents and some immunosuppressive anti-rheumatic drugs. Rheumatology (Oxford) 2008;47(Suppl 3):iii28–iii31. doi: 10.1093/rheumatology/ken168. [DOI] [PubMed] [Google Scholar]
- 40.Tsang HH, Trendell-Smith J, Wu AK, Mok MY. Diffuse large B-cell lymphoma of the central nervous system in mycophenolate mofetil-treated patients with systemic lupus erthematosus. Lupus. 2009 Nov 6; doi: 10.1177/0961203309347921. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


