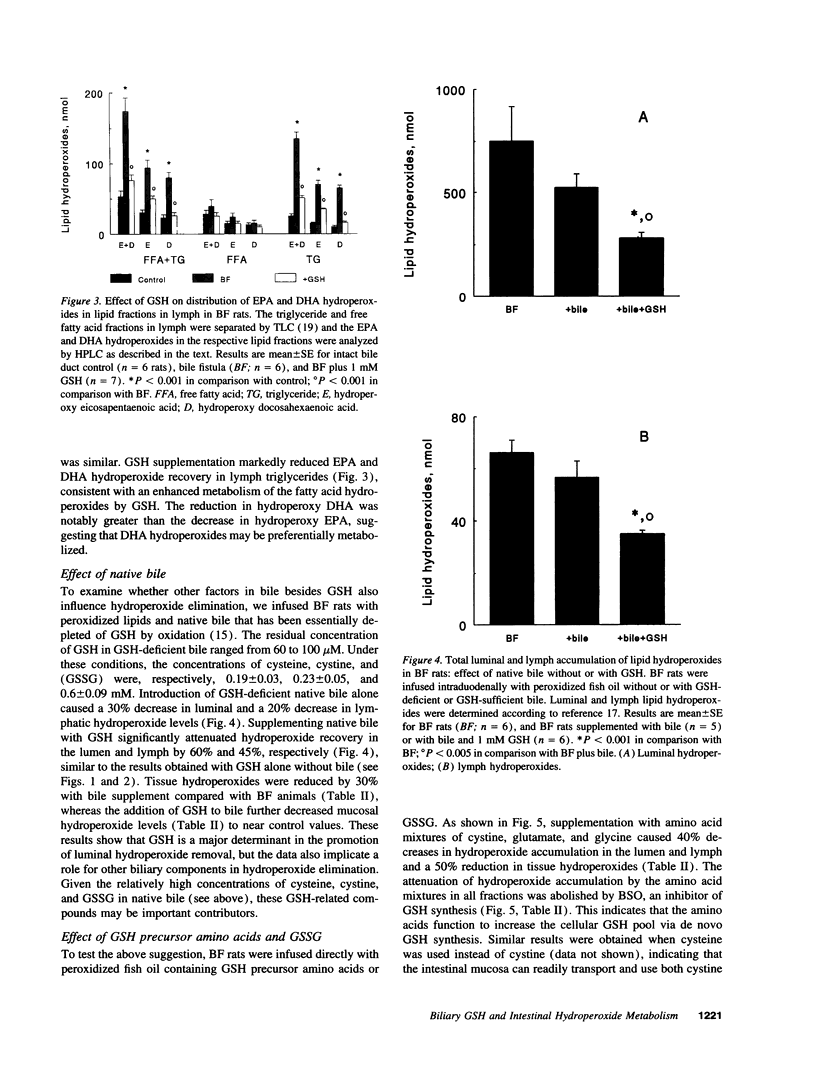
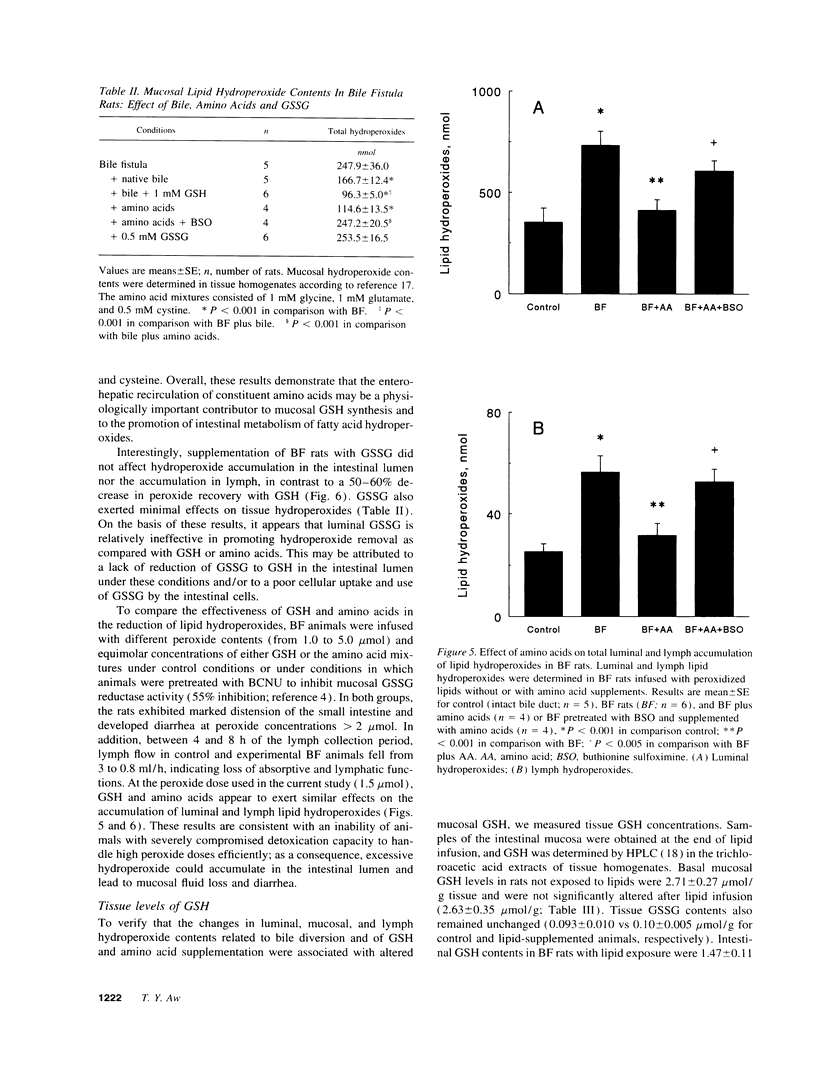
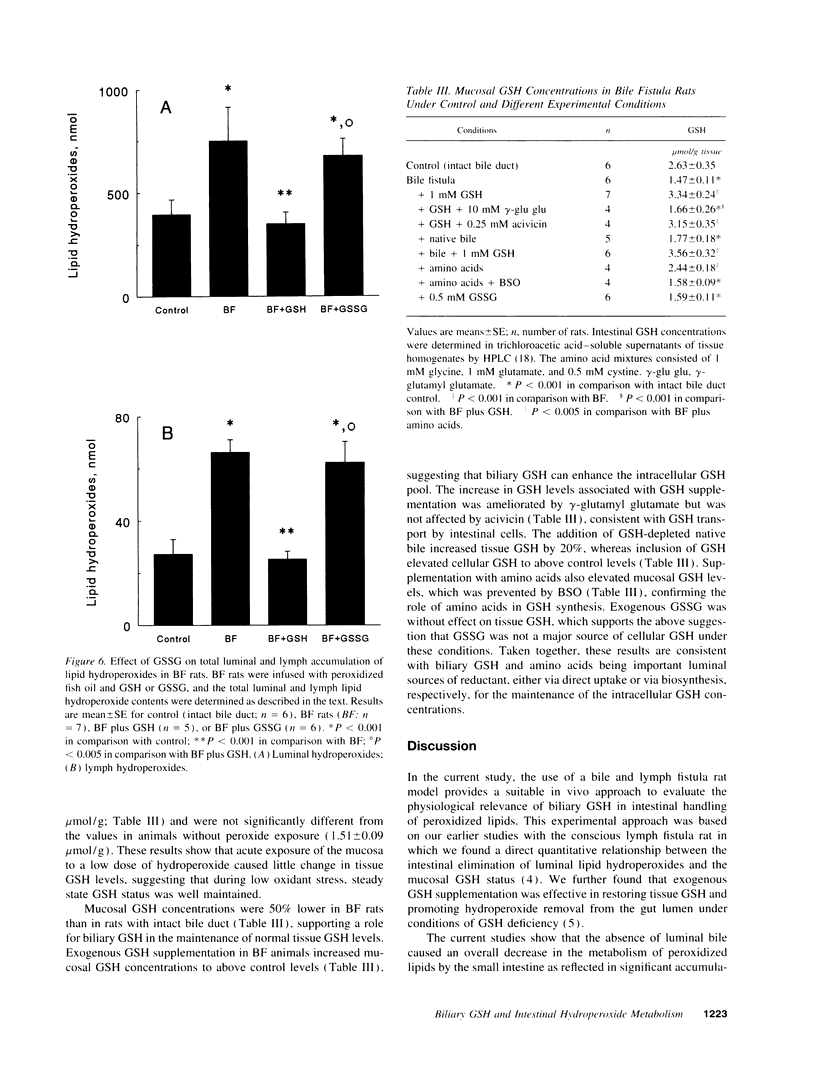
Abstract
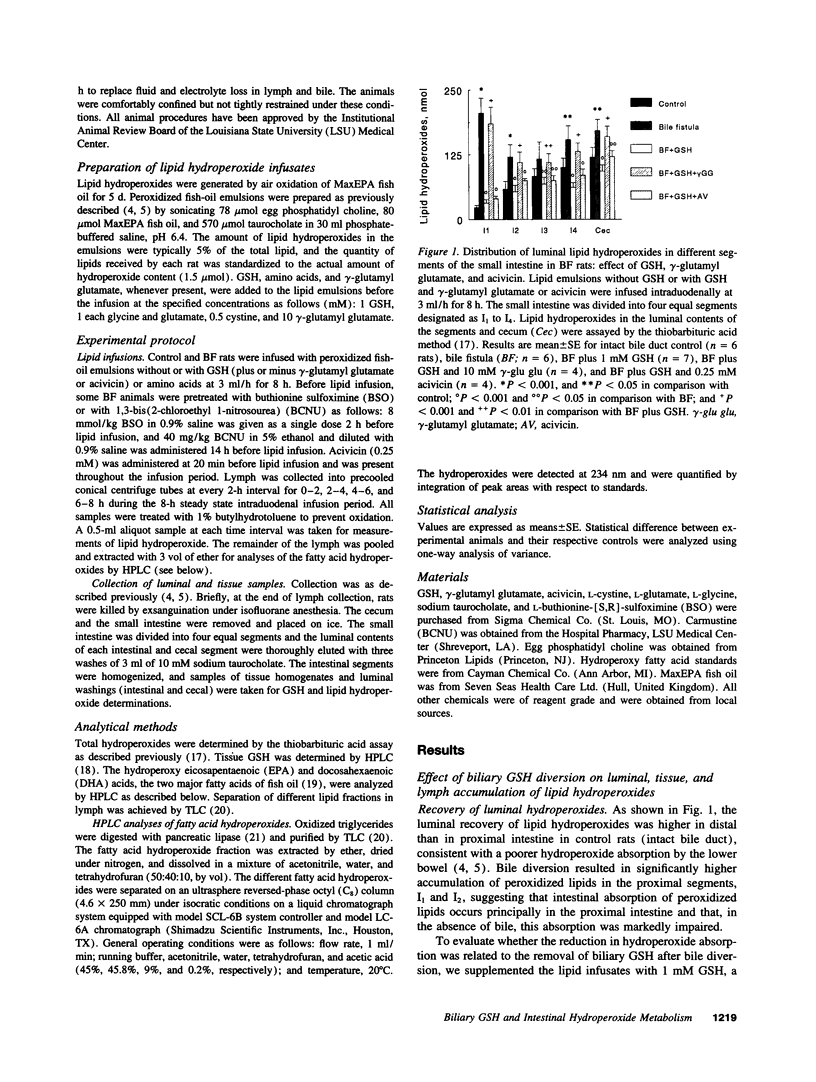
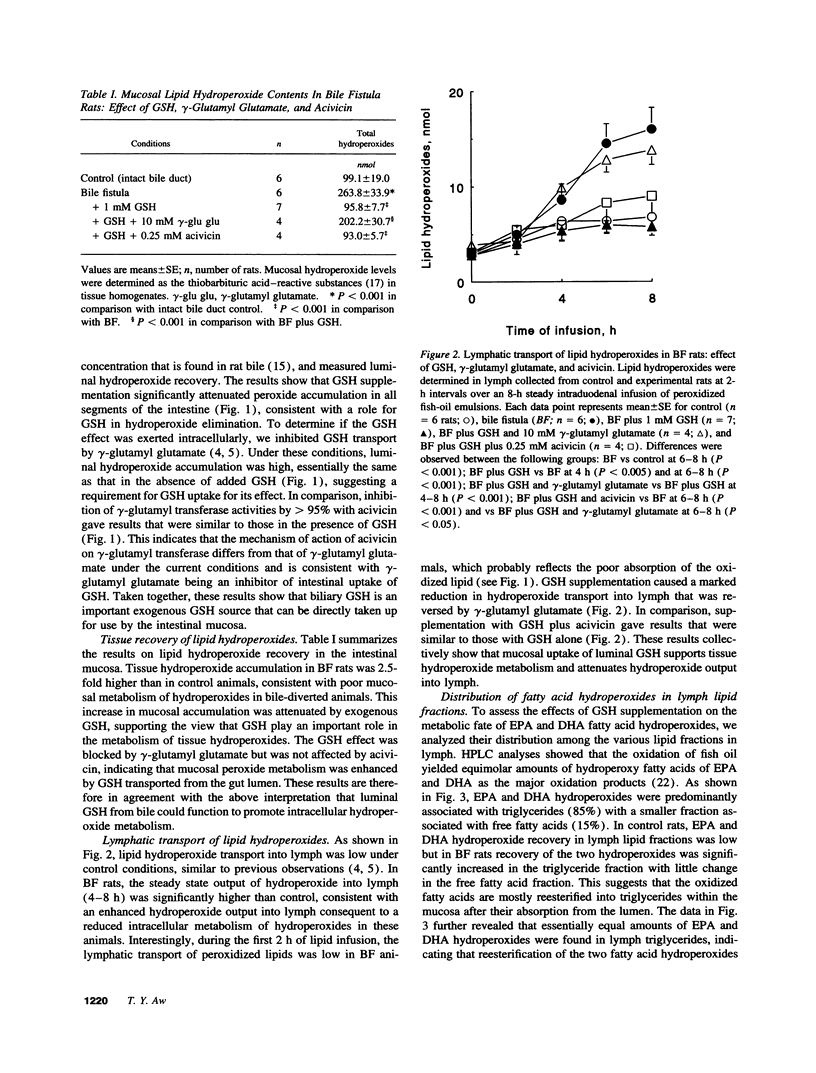
We previously found that exogenous GSH enhances mucosal GSH and promotes lipid hydroperoxide metabolism by rat small intestine (AW, T. Y., and M. W. WIlliams, 1992. Am. J. Physiol. 263:G665-G672). In this study, we have developed an in vivo bile and lymph fistula rat model to test the hypothesis that biliary GSH is an important luminal source of GSH. Peroxidized fish oil was infused into the proximal intestine, and hydroperoxide accumulation in lumen, mucosa, and lymph was determined. Diversion of bile decreased mucosal GSH and increased hydroperoxide accumulation in all fractions. Supplementation with GSH, but not with GSSG, increased tissue GSH and attenuated hydroperoxide accumulation (50-60%), consistent with enhancement of hydroperoxide removal by exogenous GSH. Addition of native bile deficient in GSH, but not cysteine, cystine, or GSSG, decreased luminal and lymph hydroperoxide levels by 20-30%. Amino acid supplementation concurrently attenuated hydroperoxide recoveries in these fractions by 30-40% and increased mucosal GSH by 40%, indicating a role for biliary amino acids in hydroperoxide elimination. The effect of amino acids was abolished by buthionine sulfoximine, confirming their role in GSH biosynthesis. Collectively, the results demonstrate that bile is a rich source of reductant for maintaining mucosal GSH to promote intestinal metabolism of luminal peroxidized lipids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Meister A. Intrahepatic transport and utilization of biliary glutathione and its metabolites. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1246–1250. doi: 10.1073/pnas.83.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw T. Y., Grigor M. R. Effect of intramolecular fatty acid distribution on aspects of triacylglycerol digestion and absorption studied in vitro. Biochim Biophys Acta. 1978 Dec 22;531(3):257–265. doi: 10.1016/0005-2760(78)90207-2. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Williams M. W. Intestinal absorption and lymphatic transport of peroxidized lipids in rats: effect of exogenous GSH. Am J Physiol. 1992 Nov;263(5 Pt 1):G665–G672. doi: 10.1152/ajpgi.1992.263.5.G665. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Clarkson T. W. Sulfobromophthalein inhibition of glutathione and methylmercury secretion into bile. Am J Physiol. 1985 Feb;248(2 Pt 1):G238–G245. doi: 10.1152/ajpgi.1985.248.2.G238. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Jacob R., Boyer J. L. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J Biol Chem. 1986 Jun 15;261(17):7860–7865. [PubMed] [Google Scholar]
- Ballatori N., Truong A. T., Ma A. K., Boyer J. L. Determinants of glutathione efflux and biliary GSH/GSSG ratio in perfused rat liver. Am J Physiol. 1989 Mar;256(3 Pt 1):G482–G490. doi: 10.1152/ajpgi.1989.256.3.G482. [DOI] [PubMed] [Google Scholar]
- Bergstedt S. E., Hayashi H., Kritchevsky D., Tso P. A comparison of absorption of glycerol tristearate and glycerol trioleate by rat small intestine. Am J Physiol. 1990 Sep;259(3 Pt 1):G386–G393. doi: 10.1152/ajpgi.1990.259.3.G386. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cepinskas G., Kvietys P. R., Aw T. Y. Omega 3-lipid peroxides injure CaCo-2 cells: relationship to the development of reduced glutathione antioxidant systems. Gastroenterology. 1994 Jul;107(1):80–86. doi: 10.1016/0016-5085(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Fernández-Checa J. C., Takikawa H., Horie T., Ookhtens M., Kaplowitz N. Canalicular transport of reduced glutathione in normal and mutant Eisai hyperbilirubinemic rats. J Biol Chem. 1992 Jan 25;267(3):1667–1673. [PubMed] [Google Scholar]
- Funk M. O., Isacc R., Porter N. A. Preparation and purification of lipid hydroperoxides from arachidonic and gamma-linolenic acids. Lipids. 1976 Feb;11(2):113–117. doi: 10.1007/BF02532660. [DOI] [PubMed] [Google Scholar]
- GLENNER G. G., FOLK J. E. Glutamyl peptidases in rat and guinea pig kidney slices. Nature. 1961 Oct 28;192:338–340. doi: 10.1038/192338a0. [DOI] [PubMed] [Google Scholar]
- Geiger P. G., Thomas J. P., Girotti A. W. Lethal damage to murine L1210 cells by exogenous lipid hydroperoxides: protective role of glutathione-dependent selenoperoxidases. Arch Biochem Biophys. 1991 Aug 1;288(2):671–680. doi: 10.1016/0003-9861(91)90250-m. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Jones D. P. Transepithelial transport of glutathione in vascularly perfused small intestine of rat. Am J Physiol. 1987 May;252(5 Pt 1):G607–G613. doi: 10.1152/ajpgi.1987.252.5.G607. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Wierzbicka G. T., Bowman B. B., Aw T. Y., Jones D. P. Fate of dietary glutathione: disposition in the gastrointestinal tract. Am J Physiol. 1990 Oct;259(4 Pt 1):G530–G535. doi: 10.1152/ajpgi.1990.259.4.G530. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Wierzbicka G. T., Sillau A. H., Bowman B. B., Jones D. P. Bioavailability of dietary glutathione: effect on plasma concentration. Am J Physiol. 1990 Oct;259(4 Pt 1):G524–G529. doi: 10.1152/ajpgi.1990.259.4.G524. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. The mechanism of biliary secretion of reduced glutathione. Analysis of transport process in isolated rat-liver canalicular membrane vesicles. Eur J Biochem. 1983 Aug 15;134(3):467–471. doi: 10.1111/j.1432-1033.1983.tb07590.x. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Coates R. J., Flagg E. W., Eley J. W., Block G., Greenberg R. S., Gunter E. W., Jackson B. Glutathione in foods listed in the National Cancer Institute's Health Habits and History Food Frequency Questionnaire. Nutr Cancer. 1992;17(1):57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N., Eberle D. E., Petrini J., Touloukian J., Corvasce M. C., Kuhlenkamp J. Factors influencing the efflux of hepatic glutathione into bile in rats. J Pharmacol Exp Ther. 1983 Jan;224(1):141–147. [PubMed] [Google Scholar]
- Kowalski D. P., Feeley R. M., Jones D. P. Use of exogenous glutathione for metabolism of peroxidized methyl linoleate in rat small intestine. J Nutr. 1990 Sep;120(9):1115–1121. doi: 10.1093/jn/120.9.1115. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Smith C. V., Hughes H., Mitchell J. R. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984 Jan;73(1):124–133. doi: 10.1172/JCI111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookhtens M., Maddatu T. Mechanism of changes in hepatic sinusoidal and biliary glutathione efflux with age in rats. Am J Physiol. 1991 Oct;261(4 Pt 1):G648–G656. doi: 10.1152/ajpgi.1991.261.4.G648. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Shan X., Aw T. Y., Shapira R., Jones D. P. Oxygen dependence of glutathione synthesis in hepatocytes. Toxicol Appl Pharmacol. 1989 Nov;101(2):261–270. doi: 10.1016/0041-008x(89)90275-5. [DOI] [PubMed] [Google Scholar]
- Szewczuk A., Milnerowicz H., Polosatov M. V., Sobiech K. A. Immunofluorescent localization of gamma-glutamyl transferase in rat and bovine tissues. Acta Histochem. 1980;66(1):152–159. doi: 10.1016/s0065-1281(80)80090-0. [DOI] [PubMed] [Google Scholar]
- Tso P., Kendrick H., Balint J. A., Simmonds W. J. Role of biliary phosphatidylcholine in the absorption and transport of dietary triolein in the rat. Gastroenterology. 1981 Jan;80(1):60–65. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]