Dear Editors,
Brzustowicz and colleagues (2004) identified significant linkage disequilibrium between schizophrenia and markers within the gene encoding nitric oxide synthase 1 (neuronal; NOS1) adaptor protein (NOS1AP; also termed carboxyl-terminal PDZ ligand of nNOS or CAPON). Quantitative real-time PCR (qRT-PCR) analysis of mRNA from human postmortem dorsolateral prefrontal cortex further revealed that expression of the short isoform of the NOS1AP gene (NOS1AP-S) is significantly increased in patients with schizophrenia (Xu, et al., 2005). More recently, the group also identified a functional risk allele within NOS1AP and showed that this change increased NOS1AP mRNA expression in a cell culture system (Wratten, et al., 2009). Despite these recent reports establishing linkage between NOS1AP and schizophrenia, little is known about NOS1AP protein expression in the brains of affected patients.
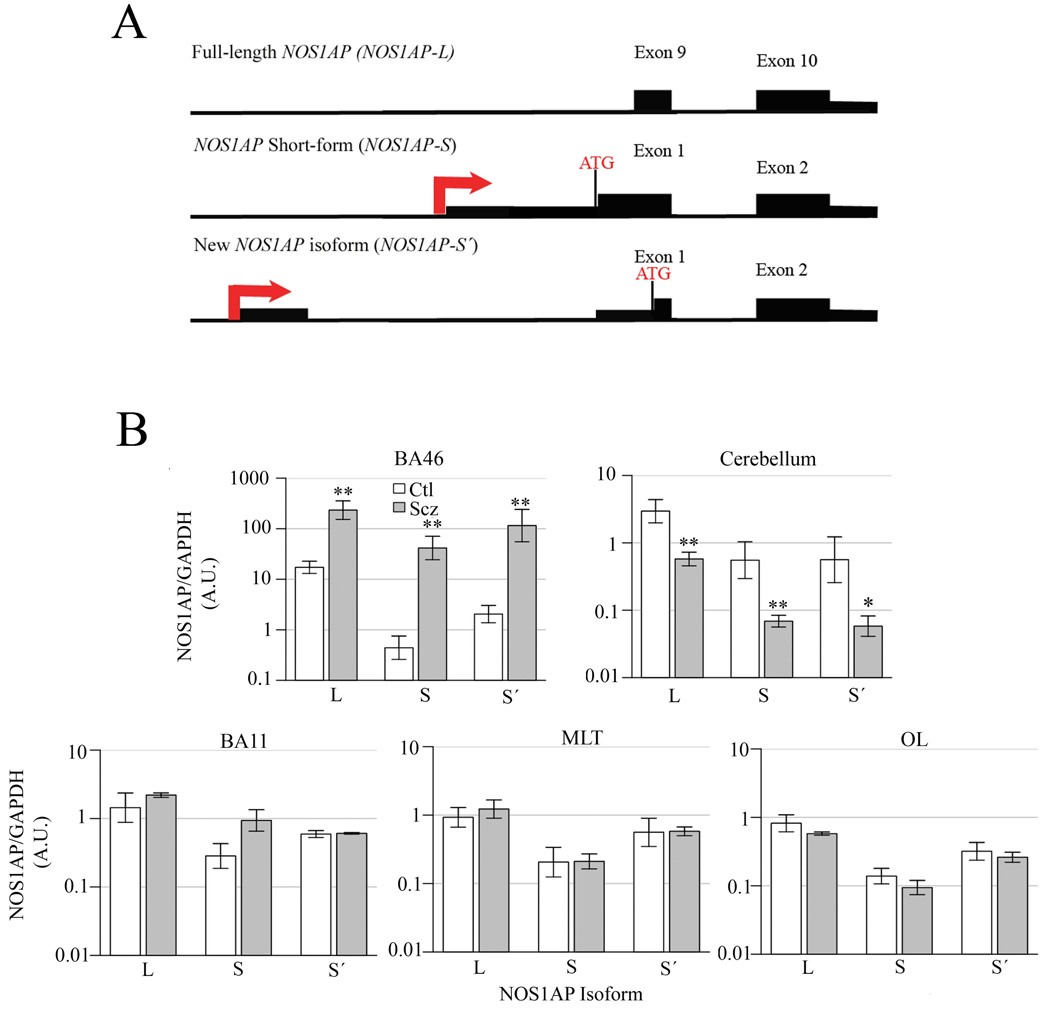
Previous reports described two distinct NOS1AP isoforms: full-length NOS1AP-L (10 exons, ~75kD) and NOS1AP-S, a C-terminal specific transcript that encodes only the PDZ domain (Jaffrey, et al., 1998; Xu, et al., 2005). We have now identified a novel isoform, NOS1AP-S’ (Figure 1A), in mouse and human tissue using qRT-PCR (data not shown). To evaluate the expression levels of these three NOS1AP isoforms in human brain tissue, postmortem samples from Brodmann’s Area (BA) 46, BA11, Medial Temporal Lobe (MTL), Occipital Lobe (OL), and cerebellum of unaffected patients and those with schizophrenia were obtained from the Human Brain and Spinal Fluid Resource Center (Los Angeles, CA) and subjected to Western blotting with normalization to GAPDH or actin, as previously described (Xu, et al., 2005). Investigators were blinded to all subject information until after statistical analysis. The logarithms of the normalized values for subjects with schizophrenia and unaffected control patients within the same brain region were compared using the standard t-test. Correction for testing of multiple expression levels was made using permutations of case/control labels. Secondary examination of linear models with other covariates was based on the AICc model selection criterion (Burnham, 2002). The L (p = 0.0067; reported p-values are nominal), S´ (p = 0.0082) and S (p = 0.0041) isoforms were increased in BA46 of patients with schizophrenia (Figure 1B) at a nominal significance level, although only the increase in the S isoform was significant (p<0.05) under permutation-based multiple testing adjustment. These data are consistent with previous reports strongly implicating this region in the etiology of schizophrenia (Barch, 2005; Bunney and Bunney, 2000; Xu, et al., 2005). The L (p = 0.0031), S (p = 0.0060), and S´ (p = 0.0156) isoforms were decreased in cerebellum of affected individuals (Figure 1B), although only the decrease of the L isoform was significant under adjustment. While some reports have indicated that schizophrenia may affect the cerebellum, the results are not as consistently observed as in other regions, namely the prefrontal cortex (Avila, et al., 2002; Kapoor, et al., 2006). There were no significant differences in NOS1AP expression between control and affected patients in BA11, the MTL, or the OL (Figure 1B). Additional analysis of NOS1AP expression reveals that no significant changes were evident in BA11, the MTL, or the OL of patients with schizophrenia versus those who are unaffected. While some studies have reported a role for BA11 in schizophrenia, others find no changes in expression of NMDA receptor pathway proteins (Toro and Deakin, 2005).
Figure 1.
(A) NOS1AP isoforms. The intron/exon boundaries and the predicted transcriptional (arrow) and translational (ATG) start sites for the different NOS1AP isoforms are illustrated. The new NOS1AP isoform is characterized by a unique 5’ exon and transcriptional start site. The new short form protein (NOS1AP-S’) is predicted to be ~18kD and is a truncated version of full-length NOS1AP (NOS1AP-L) but includes a carboxyl-terminal PDZ-binding domain. (B) NOS1AP expression. Densitometry analysis of postmortem brain tissue samples of BA46 (Ctl=4, Scz=3), cerebellum (Ctl=6, Scz=6), BA11 (Ctl=5, Scz=5), MTL (Ctl=5, Scz=5), and OL (Ctl=6, Scz=6) from control (n=6) and affected (n=6) individuals diagnosed with schizophrenia via immunoblotting for all three isoforms of NOS1AP. * p<0.05 (nominal) and ** p<0.01 (nominal). Similar results were drawn from immunoblots normalized to actin (data not shown).
Our data show an alteration of three NOS1AP isoforms in specific regions of the brain for patients diagnosed with schizophrenia. Initially identified in rat, NOS1AP plays a role in the inhibition of glutamate neurotransmission via disruption of NOS1 binding to Postsynaptic Density Protein-95 and -93. This results in uncoupling of NOS1 from the NMDA receptor, and ultimately, inhibition of receptor function (Brzustowicz, 2008; Jaffrey, et al., 1998; Xu, et al., 2005). These data suggest a role for NOS1AP in glutamate receptor hypofunction and manifestation of schizophrenia.
Acknowledgments
Funding for this study was provided by NIAAA grant K25 AA015346 (to S.B.), NARSAD Young Investigator Award (to J.H.M), NIMH grant R01 MH062440 and the NARSAD/Staglin Family Music Festival Schizophrenia Research Award (to L.M.B.), and NARSAD 2007 Toulmin Independent Investigator Award and NSF grant IBN-0548543 and IBN-0919747 (to B.L.F). M.L.P. was supported by the IGERT Program on Biointerfaces -NSF grant DGE-0333196 and Louis Bevier Dissertation Fellowship. Human postmortem tissue specimens were obtained from the Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Healthcare Center, 11301 Wilshire Blvd, Los Angeles, CA 90073, which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society, and the Department of Veterans Administration. We thank Dr. Erik Charych for initial Western blotting studies using actin as a control.
Role of funding source
None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Hadzimichalis and Previtera performed experiments, analyzed results, and wrote the paper. Moreau, Li, Lee, Dulencin, and Matteson performed experiments. Buyske analyzed results and wrote the paper. Millonig, Brzustowicz, and Firestein designed experiments, analyzed results, and wrote the paper.
Conflict of interest
None.
Contributor Information
Norell M. Hadzimichalis, Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ, USA.
Michelle L. Previtera, Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ, USA.
Michael P. Moreau, Department of Genetics, Rutgers University, Piscataway, NJ, USA
Bo Li, Department of Neuroscience and Cell Biology and Center for Advanced Biotechnology and Medicine, UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ, USA.
Gum Hwa Lee, Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ, USA.
Anna M. Dulencin, Department of Neuroscience and Cell Biology and Center for Advanced Biotechnology and Medicine, UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ, USA
Paul G. Matteson, Center for Advanced Biotechnology and Medicine, UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ, USA
Steven Buyske, Department of Statistics and Biostatistics and Department of Genetics, Rutgers University, Piscataway, NJ, USA.
James H. Millonig, Department of Genetics, Rutgers University, Piscataway, NJ, USA; Department of Neuroscience and Cell Biology, and Center for Advanced Biotechnology and Medicine, UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ, USA
Linda M. Brzustowicz, Department of Genetics, Rutgers University, Piscataway, NJ, USA
Bonnie L. Firestein, Department of Cell Biology and Neuroscience, Rutgers University 604 Allison Road, Piscataway, NJ 08854-8082, USA
References
- Avila MT, Weiler MA, Lahti AC, Tamminga CA, Thaker GK. Effects of ketamine on leading saccades during smooth-pursuit eye movements may implicate cerebellar dysfunction in schizophrenia. Am J Psychiatry. 2002;159(9):1490–1496. doi: 10.1176/appi.ajp.159.9.1490. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Brzustowicz LM. NOS1AP in schizophrenia. Curr Psychiatry Rep. 2008;10(2):158–163. doi: 10.1007/s11920-008-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Burnham KPaA, David R. Model selection and multimodel inference: a practical information-theoretic approach. New York, New York: Springer-Verlag; 2002. [Google Scholar]
- Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20(1):115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Lim KS, Cheng A, Garrick T, Kapoor V. Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1) Brain Res. 2006;1106(1):205–210. doi: 10.1016/j.brainres.2006.05.082. [DOI] [PubMed] [Google Scholar]
- Toro C, Deakin JFW. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophrenia research. 2005;80(2–3):323–330. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wratten NS, Memoli H, Huang Y, Dulencin AM, Matteson PG, Cornacchia MA, Azaro MA, Messenger J, Hayter JE, Bassett AS, et al. Identification of a schizophrenia-associated functional noncoding variant in NOS1AP. Am J Psychiatry. 2009;166(4):434–441. doi: 10.1176/appi.ajp.2008.08081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM. Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med. 2005;2(10):e263. doi: 10.1371/journal.pmed.0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]