Abstract
Background
Improved diagnostic, predictive, and prognostic biomarkers for pancreatic ductal adenocarcinoma (PDAC) are urgently needed. Platelet factor 4 (PF4) has been proposed as a diagnostic biomarker for PDAC. We assessed the diagnostic and prognostic potential of serum PF4 levels in PDAC patients.
Methods
Serum PF4 levels were determined by ELISA in an initial cohort of 62 PDAC patients, 62 healthy control subjects, and 34 chronic pancreatitis (ChPT) patients. A second validation set consisted of 71 PDAC patients. Linear regression models were used to relate PF4 to class, gender, age, stage, platelet count, and diagnosis. Survival analyses were performed using univariate and multivariate Cox models.
Results
In the initial cohort, serum PF4 levels distinguished PDAC from ChPT patients (P = 0.011), but not healthy control subjects (P = 0.624). In PDAC patients, high serum PF4 level significantly predicted decreased survival independent of all covariates examined (P < 0.01). The prognostic relationship of serum PF4 levels remained significant in the validation set. Venous thromboembolism (VTE) occurred in 20% of the 133 PDAC patients. The VTE risk was higher in subjects with elevated PF4 levels (P = 0.009).
Conclusions
Serum PF4 is shown for the first time to be prognostic for survival in PDAC patients. High PF4 is associated with an increased risk for the development of VTE. Impact: Serum PF4 levels may be useful for patient stratification and to direct treatment options in patients with pancreatic cancer including anticoagulation prophylaxis. The relationship between high PF4 levels and poorer outcomes requires further study.
Keywords: PF4, prognosis, VTE, DVT, PE, chronic pancreatitis, ELISA
Introduction
Although pancreatic cancer comprises only 3% of estimated new cancer cases, it is the fourth most common cause of cancer mortality in the United States (1). The five year survival rate for patients with PDAC is only about 5% for all stages, with a survival rate of about 20% in patients with local disease and 2% in patients with distant metastases (2). PDAC typically develops with few symptoms and only a minority of cases is diagnosed at an early stage. When symptoms such as weight loss, abdominal pain and jaundice do occur, distant metastases are often present, precluding treatment for cure. Surgery remains the only potentially curative treatment, but most patients recur and succumb to the disease despite resection. Optimal treatment strategies for patients with pancreatic cancer are still evolving and largely target the malignancy, although therapies that treat tumor-driven complications can decrease morbidity and prolong survival. Venous thromboembolism, for example, is highly associated with PDAC and preventative strategies are evolving (3, 4). Neoadjuvant treatment has the potential to improve outcome for patients undergoing surgical resection (5) and postoperative adjuvant therapy has been shown to improve survival and delay development of recurrent disease after resection (6). However, only relatively primitive consensus selection criteria for these therapies have been developed. Thus, there is a need for biomarkers that will: 1) allow for early identification of patients with pancreatic cancer while the disease is treatable for cure, 2) help differentiate patients with PDAC from those with chronic pancreatitis to ensure correct treatment, 3) optimally select patients for neoadjuvant therapy, surgical resection, and post-operative adjuvant therapy, and 4) predict outcome more accurately than stage alone.
Using mass spectrometry-based serum peptide profiling, a recent publication identified PF4 as a potential biomarker for pancreatic cancer (7). This study showed that PF4 was significantly lower in serum from PDAC patients as compared to healthy controls, while PF4 was statistically significantly higher in serum from patients with acute pancreatitis compared to PDAC. These results were verified using ELISA to measure PF4 serum levels. In the current study, we proposed to validate the results of the previous study by determining if serum PF4 could distinguish between pancreatic cancer patients and healthy control subjects in an independent cohort. We extended the previous study by assessing if serum PF4 levels could distinguish between PDAC and chronic pancreatitis. We further assessed PF4 as a prognostic factor in predicting survival in patients with pancreatic cancer. Finally, we assessed a possible cause of decreased survival associated with elevated PF4 levels.
Materials and Methods
Serum samples were obtained from an initial 158 subjects, including 62 patients with histologically or cytologically confirmed PDAC, 62 healthy control subjects, and 34 chronic pancreatitis patients. A second group of 71 patients with confirmed PDAC were chosen as a validation cohort. PDAC subjects were selected based on certainty of the diagnosis of PDAC by pathologic evaluation, availability of complete clinical information, including cause of death, and adequate pretreatment blood samples. Three cases were censored in survival analyses due to causes of death other than cancer progression. Healthy control subjects were chosen to gender-match and age-approximate the PDAC cases and were obtained from two sources. First, adults accompanying index patients to clinic visits were approached and screened. Second, excess sera from de-identified healthy controls were obtained from a large reference laboratory managed by the University of Utah Department of Pathology. Subject characteristics are summarized in Table 1.
Table 1.
Subject characteristics
| Subject Group | Number of Cases in Initial Cohort | Median Age (Range) | Number of Cases in Validation Cohort | Median Age (Range) | |
|---|---|---|---|---|---|
| Pancreatic Ductal Adenocarcinoma | |||||
| Total | 62 | 68 (42 – 89) | 71 | 65 (44 – 87) | |
| Female | 28 | 70 (43 – 89) | 32 | 69.5 (44 – 87) | |
| Male | 34 | 65 (42 – 86) | 39 | 62 (47 – 82 ) | |
| Stage IA | 1 | 3 | |||
| Stage IB | 4 | 5 | |||
| Stage IIA | 8 | 4 | |||
| Stage IIB | 18 | 18 | |||
| Stage III | 10 | 12 | |||
| Stage IV | 21 | 29 | |||
| Healthy Control | |||||
| Total | 62 | 67.5 (42 – 94) | |||
| Female | 28 | 69 (43 – 94) | |||
| Male | 34 | 65 (43 – 83) | |||
| Chronic Pancreatitis | |||||
| Total | 34 | 48.5 (29 – 81) | |||
| Female | 16 | 47.5 (30 – 63) | |||
| Male | 18 | 49.5 (29 – 81) | |||
PDAC cases were further stratified by stage, extent of metastatic dissemination (“class”) and treatment. In statistical models, stage IA, IB, and IIA cases were combined into a single group due to the paucity of these early stage cases in the cohort. For the purposes of this discussion, stratification based on “class” refers to either node negative (N0), node positive (N1), or presence of distant metastases (M1), independent of node status. The patient was considered to have treatment expected to affect survival if he or she had received either surgical resection and/or at least one full course of chemotherapy and/or radiation.
Blood was collected prior to the patient receiving treatment, separated into the serum component, and frozen in aliquots for subsequent analysis. Serological PF4 measurements were performed by ELISA (Asserchrom, Stago, New Jersey, USA). The samples were diluted 1:2,500 and PF4 levels measured according to the manufacturer’s recommendations. Absorbance readings were compared to the calibration curve and the dilution factor was accounted for by multiplying each value by 2.5 (2500/1000) to generate values in kU/ml. Stratified Kaplan-Meier curves were used to plot survival and compute median survival. The Cox model and logrank test were used for formal survival analysis. Linear regression models were used to relate PF4 to class, gender, age, stage, platelet count, and diagnosis. P-values < 0.05 were considered significant. All statistical analyses were performed using the R version 2.8.0 statistical software (8).
Clinical charts of the 133 patients with PDAC were reviewed to determine the incidence of (VTE) after the date of the pretreatment serum sample and included pulmonary embolism (PE), deep vein thrombosis (DVT), splenic, mesenteric, and portal VTE. All VTE diagnoses were confirmed through imaging such as computed tomography or Doppler ultrasound. Platelet counts taken within one day of the research blood draw were also abstracted for each PDAC case.
All studies were performed with the approval of the Institutional Review Board at the University of Utah.
Results
PF4 as a Diagnostic Biomarker
To assess diagnostic capabilities, serum PF4 levels were first measured in an initial cohort consisting of PDAC, chronic pancreatitis, and healthy control subjects. The median PF4 serum level was 12.0 kU/ml (0.4 – 20.4, range) in healthy control subjects, 11.1 kU/ml (1.3 – 25.8) in PDAC patients, and 8.9 kU/ml (1.2 – 22.1) in chronic pancreatitis patients. The difference between PF4 serum levels in patients with PDAC and the control group was not statistically significant (P = 0.62). However, the difference between PF4 serum levels in patients with chronic pancreatitis was significantly lower compared to either PDAC (P = 0.011) or the control group (P = 0.003).
PF4 as a Prognostic Biomarker
We next examined if PF4 correlated with survival in patients with PDAC. The median PF4 serum value in PDAC subjects, 11 kU/ml, was used as a discrete cut-off in the Cox model. Serum PF4 levels were a significant predictor of survival whether PF4 was used as a continuous (P = 0.031) or discrete (P = 0.035) variable (Table 2). Serum PF4 was inversely related to survival, with higher PF4 levels corresponding to poorer prognosis. We then sought to validate the predictive effect of PF4 in a novel set of 71 patients with histologically or cytologically confirmed PDAC. Again, high PF4 significantly correlated with shorter survival time when using serum PF4 either as a continuous variable (P = 0.030) or as a discrete variable (P = 0.050) at the median threshold of 11 kU/ml identified in the initial cohort (Table 2). In the combined data set of 133 PDAC patients, those with PF4 serum levels below the threshold had a median survival of 389 days while those with above the threshold had a median survival of 254 days (Table 3), a difference of approximately 4.5 months.
Table 2.
Relationship of serum PF4 to overall survival in PDAC subjects
| Predictor | Stratification | Median Survival (days) | 95% CI | P-value* |
|---|---|---|---|---|
| Initial Cohort | ||||
| PF4 | Continuous (N = 62) | 352 | 310 – 411 | 0.031 |
| PF4 | ≤ 11 kU/ml (N = 31) | 401 | 345 – 918 | |
| > 11 kU/ml (N = 31) | 291 | 174 – 400 | 0.035 | |
| Validation Cohort | ||||
| PF4 | Continuous (N = 71) | 283 | 227 – 402 | 0.030 |
| PF4 | ≤ 11 kU/ml (N = 34) | 323 | 275 – 681 | |
| > 11 kU/ml (N = 37) | 235 | 170 – 370 | 0.050 |
Global P-value by log-rank test
Table 3.
Univariate survival analysis
| Predictor | Level | Median Survival (days) | 95% CI | Hazard Ratio | P-value |
|---|---|---|---|---|---|
| PF4 | Continuous (N = 133) | 323 | 283 – 385 | 1.08 | 0.003 |
| PF4* | ≤ 11 kU/ml (N = 65) | 389 | 313 – 467 | 1.00 | --- |
| >11 kU/ml (N = 68) | 254 | 203 – 343 | 1.77 | 0.004 | |
| Age | Continuous (N = 133) | 323 | 283 – 385 | 1.024 | 0.005 |
| Class* | N0 (N = 32) | 401 | 289 – 929 | --- | --- |
| N1 (N = 51) | 402 | 336 – 455 | 1.27 | 0.37 | |
| M1 (N = 50) | 170 | 116 – 314 | 2.85 | <0.0001 | |
| Gender* | Female (N = 60) | 254 | 189 – 367 | --- | --- |
| Male (N = 73) | 374 | 291 – 448 | 0.61 | 0.013 | |
| Platelet Count | Continuous (N = 124) | 323 | 283 – 385 | 0.23 | 0.82 |
| Stage* | IA, I, IIA (N = 27) | 401 | 255 - ∞ | --- | |
| IIB (N = 34) | 402 | 343 – 467 | 1.50 | 0.19 | |
| III (N = 23) | 336 | 283 - ∞ | 1.53 | 0.20 | |
| IV (N = 49) | 170 | 116 – 314 | 3.22 | <0.0001 | |
| Treatment* | No (N = 54) | 283 | 179 – 411 | --- | --- |
| Yes (N = 76) | 343 | 291 – 400 | 0.99 | 0.94 |
Categorical variables were treated as ordered with the first level assigned as the reference
In univariate survival analysis of the combined data set, the presence of distant metastasis (stage IV and its equivalent M1 class), gender, and age were also significant predictors of survival, whereas survival was independent of platelet count or treatment (Table 3). Of the potential adjustment variables, the correlations with PF4 were minimal and only platelet count was significantly related to the PF4 predictor (Table 4). None of the adjustment variables were significantly related to both PF4 levels and survival and the contribution of serum PF4 to survival remained significant when adjusting for platelet counts alone (P = 0.013) or all predictors (P = 0.01) in multivariate models (data not shown).
Table 4.
Correlation between serum PF4 and covariates
| Covariate | Method | Correlation | N | P-value |
|---|---|---|---|---|
| Age | Pearson | −0.090 | 133 | 0.30 |
| Class | Spearman | 0.108 | 133 | 0.22 |
| Gender | Spearman | −0.071 | 133 | 0.42 |
| Platelet Count* | Pearson | 0.196 | 124 | 0.02 |
| Stage | Spearman | 0.125 | 133 | 0.15 |
| Treatment† | Spearman | 0.056 | 130 | 0.52 |
Nine subjects with missing platelet count were not included
Three subjects with missing treatment information were not included
Relationship of Serum PF4 Levels and VTE in PDAC Patients
Given the potential coagulation promoting function of PF4, the possibility that serum PF4 levels could predict increased risk for VTE was tested in our combined cohort. Overall, 27 of 133 PDAC subjects (20.3%) developed VTE. Cross tabulation of VTE and serum PF4 levels demonstrated a dramatic shift in the proportion of cases that went on to develop VTE in the high PF4 group (Figure 1) and indicated a 2.7 fold increased risk for development of VTE in cases with pretreatment serum PF4 levels greater than 11 kU/ml. Using PF4 as a continuous variable also indicated a significant correlation between pretreatment PF4 levels and the development of VTE (P = 0.009 by logistic regression). In univariate analysis, development of VTE was not significantly related to platelet count (P = 0.28) and PF4 remained a significant predictor of VTE development in multivariate analyses (Table 5).
Figure 1.
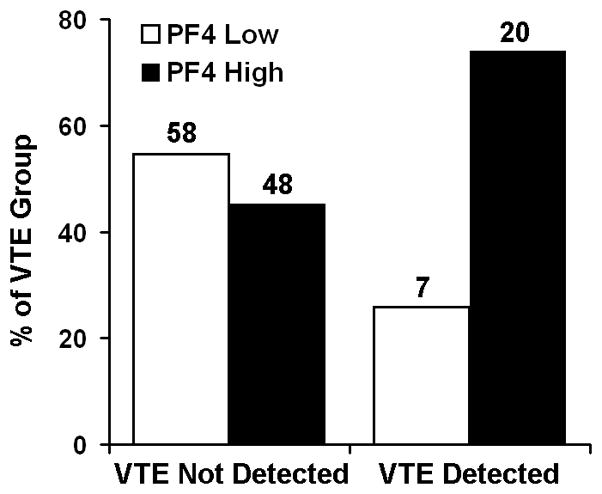
High serum PF4 correlates with the development of VTE. PDAC patients were divided into two groups based on the detection of VTE. Each group was subdivided based on pretreatment serum PF4 levels using the 11 kU/ml threshold. Numbers above each bar represent the sample size for each group. A significantly higher proportion of patients with VTE had elevated pretreatment PF4 serum levels (P = 0.009 by Fisher’s Exact Test).
Table 5.
Multivariate Analysis: PF4 predicting VTE
| Model : PF4 + Platelet only | Model : All predictors | ||||
|---|---|---|---|---|---|
| Predictor | Level | Estimate† | P-value | Estimate† | P-value |
| PF4 | Continuous | 0.132 | 0.024 | 0.14 | 0.027 |
| Platelet Count | Continuous | 0.0003 | 0.87 | −0.0001 | 0.94 |
| Age | Continuous | --- | --- | 0.011 | 0.61 |
| Class | N0* | --- | --- | --- | --- |
| N1 | --- | --- | −0.88 | 0.39 | |
| M1 | --- | --- | −1.15 | 0.99 | |
| Gender | Female* | --- | --- | --- | --- |
| Male | --- | --- | −0.079 | 0.97 | |
| Stage | IA, IB or IIA* | --- | --- | --- | --- |
| IIB | --- | --- | 0.62 | 0.62 | |
| III | --- | --- | 0.90 | 0.36 | |
| IV | --- | --- | 1.52 | 0.99 | |
| Treatment | No* | --- | --- | --- | --- |
| Yes | --- | --- | 0.0008 | 1.00 | |
Categorical variables were treated as ordered with the first level assigned as the reference
Estimates are natural log odds
Discussion
In our patient cohort, serum PF4 levels did not accurately distinguish between PDAC patients and normal healthy subjects, a finding contradictory to previously published results (7). The reasons behind this discrepancy are not immediately evident, although in both studies, relatively small sample size and large group variances could have led to spurious differences. Institutional effects in which either self-selection or referral bias leads to differences in patient characteristics in the two institutions could also be a contributing factor in the discrepant findings. Serum PF4 levels did distinguish chronic pancreatitis patients from normal control subjects and PDAC patients in our cohort, suggesting serum PF4 levels may have some diagnostic utility, particularly if combined in a panel with other biomarkers. Clinically, discrimination between chronic pancreatitis and PDAC is a typical diagnostic dilemma.
Importantly, our data demonstrates that serum PF4 level is a promising prognostic factor in patients with PDAC. In our cohort, PF4 was a robust predictor of survival independent of other predictors such as stage, class, and treatment. Since PF4 is stored in and released by platelets, it is possible that the changes in PF4 levels merely reflected changes in platelet counts. Indeed, in our cohort, serum PF4 levels and platelet counts were directly correlated (Table 4). However, the correlation was low (0.196) indicating that platelet counts could explain only about 4% of the variance in PF4 levels. Furthermore, platelet counts were not significantly related to survival (Table 3). These results suggest that PF4 either directly participates in or is an indicator of an underlying process occurring in some patients leading to poorer survival. PF4 is a CXC chemokine (CXCL4) present in alpha-granules of platelets at concentrations approximately 20,000 times higher than in the plasma (9, 10). The exact function of PF4 in normal physiology and in cancer is unknown. It is known, however, that PF4 is released upon platelet activation and binds to heparin-like molecules with high affinity, thus neutralizing heparin’s anticoagulant activity, suggesting a pro-coagulative function (11). Further, PF4-heparin complexes have been implicated as the target antigens in heparin-induced thrombocytopenia with thrombosis (12). PF4 is also one of a number of chemokines that have been variably found to either augment platelet activity and aggregation or fully initiate platelet adhesion, activation, and aggregation (13).
A pro-coagulative role for PF4 offers one possible explanation for the link between high serum PF4 levels and reduced survival. Pancreatic cancer cells are believed to activate platelets and other pro-coagulation factors, often leading to clinical manifestations such as DVT, PE, and disseminated intravascular coagulation (14). Development of VTE is a common complication of malignancy (4) and is associated with decreased survival (15, 16). In a study of 21 advanced pancreatic cancer patients, median survival was 8 months in those patients with VTE as compared to 21 months for those without VTE (17). Thus, the presence of VTE seemingly indicates a worse prognosis in an already dismal form of cancer.
Consistent with previously reported incidence rates (3, 18), 20% of PDAC case developed VTE in our cohort. The increased risk for development of VTE in cases with high pretreatment serum PF4 suggests that PF4 predicts the onset of VTE in PDAC patients. The significance of this relationship persisted in multivariate analysis (Table 5) indicating that the link between PF4 and VTE could not be explained by differences in the covariates. Both surgery and chemotherapy are known to increase the incidence of VTE (4, 19, 20), while the pro-coagulant response is activated by radiotherapy (19). Of note, the multivariate model accounted for potential changes in VTE due to whether or not the patient received surgical, chemotherapeutic, or radiation treatment. The correlation between high PF4 levels and development of VTE may provide at least a partial explanation for the link between PF4 and survival rates and suggests a method for identification of patients that may benefit from prophylactic anti-coagulation therapy.
One limitation of our study is that, as a retrospective review, the occurrence of VTE may have been underestimated. The prevalence of VTE in the patient cohort was established only if detected in the course of patient care and was not systematically assessed prospectively in each case. Furthermore, the contribution of VTE to cause of death was not addressed. That VTE, specifically PE, contributes to fatality was indicated in an autopsy series of 441 PDAC cases that demonstrated a 42% incidence of PE at the time of death, with PE listed as the immediate cause of death in 34% of cases (21). Prospective studies designed to address these shortcomings will be required to adequately establish the value of PF4 for predicting the development of life-threatening thromboembolic events.
In summary, our study identified serum PF4 as a strong independent predictor of survival and correlated with the development of VTE in PDAC patients. Serum PF4 levels may prove to be a valuable adjunct in identifying patients that may benefit from prophylactic anti-thrombotic therapy. Serum PF4 levels may help distinguish PDAC patients from those with ChPT.
Acknowledgments
This work was supported in part by research grants from the National Institutes of Health (R03 CA115225 to SJM and P30CA042014 to the Huntsman Cancer Institute for support of core facilities). K.E.P was supported in part by a Ruth L. Kirschstein National Research Service Award from the National Institutes of Health (T35HL07744).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Compton CC, Mulvihill SJ. Prognostic factors in pancreatic carcinoma. Surg Oncol Clin N Am. 1997;6:533–54. [PubMed] [Google Scholar]
- 3.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 4.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 5.Raut CP, Evans DB, Crane CH, Pisters PW, Wolff RA. Neoadjuvant therapy for resectable pancreatic cancer. Surg Oncol Clin N Am. 2004;13:639–61. ix. doi: 10.1016/j.soc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 7.Fiedler GM, Leichtle AB, Kase J, et al. Serum peptidome profiling revealed platelet factor 4 as a potential discriminating peptide associated with pancreatic cancer. Clin Cancer Res. 2009;15:3812–9. doi: 10.1158/1078-0432.CCR-08-2701. [DOI] [PubMed] [Google Scholar]
- 8.R-project.org [Internet] Vienna, Austria: Institute for Statistics and Mathematics; [updated 2010 May 31; cited 2010 July 15]. Available from: http://www.r-project.org. [Google Scholar]
- 9.Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res. 2009 doi: 10.1016/j.thromres.2009.11.023. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Maurer AM, Zhou B, Han ZC. Roles of platelet factor 4 in hematopoiesis and angiogenesis. Growth Factors. 2006;24:242–52. doi: 10.1080/08977190600988225. [DOI] [PubMed] [Google Scholar]
- 11.Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci U S A. 1993;90:3574–7. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiral J, Bridey F, Dreyfus M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68:95–6. [PubMed] [Google Scholar]
- 13.Al-Mondhiry H. beta-Thromboglobulin and platelet-factor 4 in patients with cancer: correlation with the stage of disease and the effect of chemotherapy. Am J Hematol. 1983;14:105–11. doi: 10.1002/ajh.2830140202. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–63. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 16.Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Invest. 2009;27 (Suppl 1):63–74. doi: 10.1080/07357900802656681. [DOI] [PubMed] [Google Scholar]
- 17.Zawin MCC, Rahman Z, et al. Multi-row detector CT detection of subclinical visceral and pulmonary thrombosis in advanced pancreatic cancer is an adverse prognostic indicator. Proc Am Soc Clin Oncol. 2003;22 abstract 1419. [Google Scholar]
- 18.Thodiyil PA, Kakkar AK. Variation in relative risk of venous thromboembolism in different cancers. Thromb Haemost. 2002;87:1076–7. [PubMed] [Google Scholar]
- 19.Byrne M, Reynolds JV, O’Donnell JS, et al. Long-term activation of the pro-coagulant response after neoadjuvant chemoradiation and major cancer surgery. Br J Cancer. 102:73–9. doi: 10.1038/sj.bjc.6605463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine MN. Prevention of thrombotic disorders in cancer patients undergoing chemotherapy. Thromb Haemost. 1997;78:133–6. [PubMed] [Google Scholar]
- 21.Ogren M, Bergqvist D, Wahlander K, Eriksson H, Sternby NH. Trousseau’s syndrome - what is the evidence? A population-based autopsy study. Thromb Haemost. 2006;95:541–5. doi: 10.1160/TH05-10-0694. [DOI] [PubMed] [Google Scholar]


