Abstract
BACKGROUND
Anti-epileptic drugs (AED) are increasingly used in the management of serious mental illness yet their effects on nicotine metabolism have not been studied.
METHODS
This study investigated the effects of 3 AED (carbamazepine, CBZ; oxcarbazepine, OCB or valproic acid, VPA) on nicotine and nicotine metabolite levels in 149 smokers with schizophrenia and bipolar disorder who participated in an afternoon blood draw for nicotine, cotinine, and 3’-hydroxycotinine (3HC). The ratio of 3HC to cotinine was calculated as a marker of CYP2A6 metabolic activity. Eight smokers were taking CBZ, six were taking OCB and 40 were taking VPA.
RESULTS
The 3HC/cotinine ratio was significantly higher in individuals taking CBZ or OCB (combined, n=14) vs. those not taking it (mean 0.993 vs. 0.503; p< 0.001). The cotinine/cigarette per day ratio was significantly lower in individuals taking CBZ or OCB. The 3HC/cotinine ratios were also significantly higher in the subgroup of individuals taking CBZ (n=8) vs. those not taking it. There were no significant differences in nicotine or cotinine levels or 3HC/cotinine ratios in individuals taking VPA vs. those not taking it. We conducted backward stepwise linear regression models to identify predictors of the log transformed 3HC/cotinine ratios. Taking CBZ and number of cigarettes smoked per day were significant determinants of log 3HC/Cotinine.
CONCLUSIONS
CBZ likely induces hepatic metabolism via CYP2A6 and is associated with increased 3HC/Cotinine ratios.
IMPACT
Increased nicotine metabolism in individuals using AED has implications for increased smoking behavior and exposure to more tobacco toxins that warrants further study.
Keywords: nicotine, smoking, carbamazepine, metabolism
1. INTRODUCTION
More than 60 million Americans smoke cigarettes according to the 2007 National Survey on Drug Use and Health (1). Nicotine is essential to maintaining tobacco use and has importance as a pharmaceutical treatment for smoking cessation. When inhaled, nicotine is rapidly delivered to the systemic circulation and once in the bloodstream, distributed extensively to body tissues (2). The plasma half life of nicotine averages about 2 hours with seventy to eighty percent metabolized in the liver by the cytochrome P450 enzyme CYP2A6. Nicotine is metabolized to cotinine, and cotinine is further metabolized to 3’-hydroxycotinine (3HC) primarily by the liver enzyme CYP2A6 (3). To a much lesser degree, nicotine is metabolized by other oxidative (CYP2B6 and CYP2D6) and non-oxidative pathways (2).
Since CYP2A6 enzyme activity is the major determinant of the rate of nicotine metabolism and smokers tends to regulate levels of nicotine in their bodies, it follows that the rate of CYP2A6 activity would influence smoking behavior. Genetic variants in CYP2A6 associated with slower than normal metabolism have been associated with reduced risk of becoming a smoker, smoking fewer cigarettes per day and greater likelihood of quitting (4–6). The ratio of nicotine metabolites of 3’-hydroxycotinine to cotinine (3HC/cotinine, the nicotine metabolite ratio) is a marker of CYP2A6 metabolic activity and a noninvasive measure of the rate of nicotine metabolism that also has implications for smoking behavior (7). Studying phenotypes (i.e. metabolic ratios) is likely to be more informative than studying genotypes for nicotine metabolism because of the relatively low frequency of abnormal alleles in the Caucasian population, and the large variability in CYP2A6 enzyme activity even among people with normal (wild type) CYP2A6 genes (8).
Smoking is more prevalent in people with serious forms of mental illnesses, such as schizophrenia and bipolar disorder, than in the general population (9–11). The effect of medications used in the management of serious mental illness on nicotine metabolism and smoking behavior has not been well studied. Smokers with schizophrenia have no differences in 3HC/cotinine ratios compared to control smokers, indicating that schizophrenia per se does not affect the ability to metabolize nicotine at CYP2A6 (12). Although these smokers are usually taking antipsychotic medications, it is unlikely that drug interactions from antipsychotic medications impacted on these results given the lack of clinically significant interactions at CYP2A6 (13–15). Oral contraceptive medications are one of few medications known to increase nicotine metabolism at CYP2A6 (16). Anti-epileptic drugs (AED) are of particular concern with respect to drug interactions because of their potential to induce many hepatic cytochrome P450 enzymes. AEDs are commonly used for conditions other than epilepsy including bipolar disorder, neuropathic pain and migraine prophylaxis (17). Adjunctive treatment with lithium or an AED is a commonly used strategy for managing aggression or mood in schizophrenia and current estimates are that at least 50% of patients with schizophrenia receive treatment with these mood stabilizers (18–19).
Carbamazepine (CBZ), used in the treatment of bipolar disorder, has a very high potential for drug interactions. CBZ is a potent inducer of CYP3A4 and other oxidative enzyme systems in the liver, and it increases glucuronyltransferase activity. This results in the acceleration of the metabolism of many drugs including warfarin, tricyclic antidepressants, antipsychotics, oral contraceptives, glucocorticoids, cyclosporin, theophylline, chemotherapeutic agents and cardiovascular drugs (20). Carbamazepine also induces the metabolism of other anticonvulsants, particularly valproic acid (21).
Oxcarbazepine (OCB), approved for use in the US in 2000, is a keto-derivative of carbamazepine has largely inactive metabolites. It was developed specifically to reduce the side effects and medication interactions associated with CBZ (22). OCB is metabolized through reduction and conjugation (glucuronidation) and unlike many other antiepileptic drugs, its metabolism is not induced or inhibited via the cytochrome P-450 system (23). Few clinically significant drug interactions with OCB have been reported although OCB can however induce CYP3A4 and CYP3A5, leading to reduction in levels and effectiveness of oral contraceptives (24,25). OCB is generally used to treat the same conditions as CBZ and is often preferred since it is better tolerated by patients and causes fewer rashes (26).
Valproic acid (VPA) was approved for use as anticonvulsant in the United States in 1978 and treats a variety of seizure disorders. Since the 1990s use of valproate increased rapidly for psychiatric indications and was FDA approved for treatment of mania in 1996. Various forms of the chemical (divalproex sodium or valproic acid) dissociate to the active valproate ion in the gastrointestinal tract and divalproex is the most commonly prescribed formulation (27). Valproic acid (VPA) is highly protein bound and metabolized primarily via mitochondrial oxidation with less than 20% of the dose eliminated by other oxidative mechanisms (28).
The aim of this study was to investigate the association between 3 commonly used AED, carbamazepine, oxcarbazepine and valproic acid, on nicotine and nicotine metabolite levels in smokers with schizophrenia and bipolar disorder.
2. METHODS
This study consists of an analysis of serum nicotine and nicotine metabolite levels in smokers with mental illness taking various psychotropic medications.
2.1. Subjects
The sample includes 65 smokers with schizophrenia (SCZ) who participated in quit smoking studies or studies of nicotine intake that required them to have blood sampling for nicotine and are described elsewhere in greater detail (12). The sample also includes consecutively enrolled smokers from an ongoing study of nicotine intake and smoking topography in smokers with schizophrenia or bipolar disorder (36 SCZ; 48 bipolar, BPD) that is currently underway.
Subjects had to be currently enrolled in mental health treatment and stable on psychiatric medications in order to participate. All subjects had their diagnosis of schizophrenia or bipolar disorder confirmed with the Structured Clinical Interview for DSM (SCID; 29) and smoked more than 8 cigarettes per day (cpd). Individuals with a diagnosis of schizoaffective disorder were excluded. Seriously cognitively impaired patients were also excluded and subjects were required to score 24 or higher on the Folstein Mini Mental Status Examination (30) to be eligible
Any subject using NRT (nicotine gum, patch, inhaler, nasal spray, lozenge), clonidine, bupropion, nortriptyline or varenicline was excluded. We excluded subjects using tobacco products other than cigarettes as well as anyone who was pregnant, since this is associated with accelerated metabolism of nicotine and cotinine (31). All subjects were required to bring their own cigarettes in for testing procedures. Analyses were performed using data from these 149 smokers (101 SCZ; 48 BPD). The study was approved by the UMDNJ-Robert Wood Johnson Medical School Institutional Review Board (IRB).
2.2. Procedures
After signing the consent forms, subjects completed an assessment battery including a smoking history and assessment of their current tobacco use (measured as cigarettes per day, CPD), demographic and medication questionnaire, and the Fagerstrom Test for Nicotine Dependence (FTND; 32). Baseline expired carbon monoxide (CO) was measured using an EC-50 Smokerlyzer (Bedfont Scientific, NJ). All medications were recorded and antipsychotic medication dose was converted to chlorpromazine equivalents (CPZ; 33). Use and dose (mg) of AED drugs was recorded. All subjects participated in an afternoon blood draw for nicotine, cotinine, and 3HC on a usual smoking day, taken approximately two minutes after smoking one of their own cigarettes. For subjects enrolled in the quit smoking study this was done prior to setting the quit date. Serum was frozen at − 20 °C for later analysis. Specimens were sent to the Clinical Pharmacology Laboratory at the University of California, San Francisco for analysis of nicotine, cotinine and 3-hydroxycotinine, which were quantified liquid chromatography-mass spectrometry (7).
2.3. Statistical Analysis
Independent sample t-tests or Mann-Whitney Test (for continuous variables) and Chi-square tests (for categorical variables) were used to compare baseline differences between smokers with schizophrenia and smokers with bipolar disorder on their socio-demographic, smoking history and clinical variables. For all other analyses, groups with SCZ and BPD were combined in order to evaluate effects of medications. Subjects taking all forms of valproic acid were consolidated into one group (VPA). Analysis of variance (ANOVA) was used to compare nicotine, exhaled CO and cpd in smokers taking and not taking AED. Ratios of 3HC/cotinine were calculated for all subjects. Since cotinine values and 3HC/cotinine ratios are not normally distributed, we used the Kruskal-Wallis test for comparisons of these means. Analyses were adjusted for gender and race since these have been shown to affect nicotine metabolism (2).
We conducted backward stepwise linear regression models to identify predictors of the log transformed 3HC/cotinine ratios. The variables entered into the model included age, gender, race, education, body mass index (BMI), cigarettes per day, expired carbon monoxide, time of blood draw, FTND score, and smoking mentholated cigarettes. Antipsychotic medication type (typical versus atypical), antipsychotic dose (CPZ equivalents), taking CBZ (vs. not taking), taking VPA (vs. not taking), taking OCB (vs. not taking) and diagnosis group (SCZ vs. BPD) were also included in the regression model. The criterion for eliminating variables from the model was set at p value greater than or equal to 0.10. We also examined the correlation between the 3HC/cotinine ratio and dose in mg of AED (CBZ-OCB, VPA) using Spearman’s correlation coefficient. All analyses were performed using SPSS 16.0 (Chicago, IL, USA) and SAS 9.1 (Cary, NC, USA).
3. RESULTS
3.1. Comparisons of Smokers with Schizophrenia versus Bipolar Disorder
Smokers with SCZ (n=101) were compared to smokers with BPD (n=48) on smoking and demographic variables including age, ethnicity, gender, cigarettes per day (cpd) smoked, expired CO at baseline, years smoked, total FTND score and age of first smoking (See Table 1). Smokers with SCZ were older (44 vs. 37 years, p<0.001), smoked more cpd (24.7 vs. 20.1, p<0.05) and had a higher FTND score (6.8 vs. 5.8, p<0.01) compared to BPD smokers.
Table 1.
Smokers with Schizophrenia or Bipolar Disorder
| Smokers with Schizophrenia (n=101) |
Smokers with Bipolar Disorder (n=48) |
p-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Cigarettes Per Day* | 24.7 (12.1) | 20.1 (7.6) | 0.019 |
| Baseline CO (ppm)* | 23.9 (11.3) | 18.8 (9.9) | 0.009 |
| FTND* | 6.8 (1.9) | 5.8 (2.1) | 0.002 |
| Age of first smoking | 14.4 (5.5) | 14.8 (4.6) | 0.697 |
| Age* | 44.3 (9.8) | 37.1 (12.1) | <0.001 |
| Past Quit Attempts | 2.9 (2.6) | 3.3 (4.2) | 0.591 |
| Ethnicity* | Count (%) | Count (%) | 0.036 |
| African-American | 32 (31.7) | 6 (12.5) | |
| Caucasian | 62 (61.4) | 34 (70.8) | |
| Asian | 3 (3.0) | 1 (1.0) | |
| Hispanic | 2 (2.0) | 4 (8.3) | |
| Other | 2 (2.0) | 3 (6.3) | |
| Gender | Count (%) | Count (%) | 0.978 |
| Male | 65 (64.4) | 31 (64.6) | |
| Female | 36 (35.6) | 17 (35.4) | |
| Taking Antipsychotic medication* | 101 (100.0) | 34 (70.8) | <0.001 |
| Use of Atypical Antipsychotic | 91 (90.1) | 32 (94.1) | 0.476 |
| Taking Antiepileptic (AED) | 31 (30.7) | 21 (43.8) | 0.118 |
| AED Type | Count (%) | Count (%) | 0.516 |
| Carbamazepine (CBZ) | 3 (3.0) | 3 (6.3) | |
| Oxcarbazepine (OCB) | 3 (3.0) | 3 (6.3) | |
| Valproic Acid (VPA) | 24 (23.8) | 14 (29.2) | |
| VPA and CBZ | 1 (1.0) | 1 (2.1) | |
| Mean (SD) | Mean (SD) | ||
| Chlorpromazine (CPZ) equivalents* | 593.5 (500.6) | 281.9 (428.6) | <0.001 |
| CBZ or OCB daily dose (mg) | 367.14 (297.0) | 735.7 (816.9) | 0.681 |
| VPA daily dose (mg) | 1162.5 (678.3) | 1416.7 (894.7) | 0.332 |
| Serum Nicotine levels (ng/mL) | 29.2 (12.7) | 26.8 (13.6) | 0.300 |
| Serum Cotinine levels (ng/mL)* | 377.8 (191.0) | 322.4 (166.4) | 0.046^ |
| Serum 3-Hydroxycotinine (ng/mL) | 166.8 (81.7) | 150.6 (140.6) | 0.699^ |
| 3HC/Cotinine Ratio* | 0.49 (0.36) | 0.68 (0.50) | 0.003^ |
| Serum Cotinine + 3-Hydroxycotinine (ng/mL) | 513.7 (246.5) | 460.1 (205.5) | 0.168 |
Denotes significance at p< 0.05
Mann-Whitney Test
Although there were no significant differences in serum nicotine concentrations between SCZ and BPD groups, respectively (mean nicotine 29.2 vs. 26.8 ng/mL; p= NS), there were differences for serum cotinine (mean cotinine 377.8 vs. 322.4 ng/mL; p=0.046) and 3HC/cotinine ratios (0.49 vs. 0.68, p<0.01; Table 1). Seventy one percent of BPD smokers were taking antipsychotic medications compared to all of the SCZ smokers and most were taking the newer, second generation atypical antipsychotics (90 SCZ vs. 94% BPD, NS). Mean CPZ equivalents were higher for SCZ compared to BDP (593.5 vs. 281.9 mg, p<0.001), indicating higher doses of antipsychotic medication in the SCZ group. Forty-four percent of BPD smokers were taking AED compared to 31% of SCZ smokers (NS) and the frequency of use of carbamazepine (CBZ), oxcarbazepine (OCB), valproic acid (VPA) was not different between groups (Table 1). Two subjects (one BPD, one SCZ) were taking both VPA and CBZ simultaneously.
3.2 Mean serum levels of nicotine and nicotine metabolites in subjects taking carbamazepine
For these analyses, smokers with SCZ and BPD were combined in order to evaluate effects of medications and analyses were adjusted for diagnosis group. For most analyses except where noted, subjects taking carbamazepine and oxcarbazepine were consolidated into one group (CBZ-OCB). A total of fourteen smokers in the sample were taking CBZ or OCB (7 SCZ, 7 BPD). Clinical characteristics of these smokers is detailed in Table 2. There were no significant differences in serum nicotine (mean nicotine 26.4 vs. 28.6 ng/mL; p= NS) in individuals taking CBZ-OCB vs. those not taking it (Table 3). Differences in cotinine levels in individuals taking CBZ-OCB, however, approached significance (mean cotinine 268.2 vs. 369.5 ng/mL; p=0.067) and the cotinine/cpd ratio was significantly lower in individuals taking CBZ-OCB (11.1 vs. 16.1; p<0.05). The 3HC/cotinine ratios were significantly higher in individuals taking CBZ-OCB vs. those not taking it (mean 0.993 vs. 0.503; p<0.001). The 3HC/cotinine ratios were also significantly higher in individuals taking CBZ-OCB in the subgroups of smokers with BPD or SCZ (Figure 1). These results were also not changed when we repeated these analyses controlling for gender and race. We ran a 2×2 ANOVA to test for the possible interaction effect between diagnostic group and taking CBZ-OCB. There was a main effect for taking CBZ, but no effect for group and no interaction effect.
Table 2.
Characteristics of Patients Taking CBZ or OCB
| Group | Age/ Gender |
Race | Cigarettes per Day |
FTND Score |
Baseline CO (ppm) |
3HC/COT Ratio |
Daily Dose (mg) |
Medication | Other Medications | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SCZ | 46/M | CAUCASIAN | 40 | 10 | 13 | 1.56 | 900 | CBZ | Acetominophen/hydrocodone, Carisoprodol, Celecoxib, Citalopram, Clonazepam, Haloperidol decanoate, Quetiapine, Trihexyphenidyl |
| 2 | SCZ | 50/M | AFRICAN AMERICAN | 15 | 6 | 11 | 0.31 | 600 | OCB | Atorvastatin, Benztropine, Metformin, Paroxetine, Risperidone |
| 3 | SCZ | 24/M | CAUCASIAN | 10 | 4 | 11 | 0.47 | 300 | OCB | Fluoxetine, Olanzapine |
| 4 | SCZ | 48/F | AFRICAN AMERICAN | 10 | 5 | 25 | 2.64 | 200 | CBZ | Benztropine, Divalproex Sodium, Quetiapine, Risperidone |
| 5 | SCZ | 44/F | AFRICAN AMERICAN | 50 | 10 | 47 | 0.61 | 400 | CBZ | Atorvastatin, Risperidone |
| 6 | SCZ | 28/M | CAUCASIAN | 20 | 5 | 40 | 0.56 | 250 | OCB | Hydrochlorothiazide, Risperidone, Sertraline |
| 7 | SCZ | 46/F | AFRICAN AMERICAN | 15 | 2 | 24 | 0.48 | 200 | CBZ | Lithium, Paroxetine, Quetiapine |
| 8 | BPD | 50/M | CAUCASIAN | 20 | 2 | 6 | 0.32 | 300 | OCB | Atenolol, Celecoxib, Hydrochlorothiazide, Lisinopril, Lorazepam |
| 9 | BPD | 24/M | CAUCASIAN | 30 | 9 | 12 | 0.43 | 250 | CBZ | Atorvastatin, Calcium, Divalproex Sodium, Risperidone |
| 10 | BPD | 43/M | OTHER | 18 | 7 | 15 | 1.04 | 1200 | OCB | Fluticasone/Salmeterol, Hydroxyzine, Quetiapin, Zolpidem |
| 11 | BPD | 23/M | CAUCASIAN | 20 | 6 | 26 | 1.49 | 600 | CBZ | Lamotrigine, Levothyroxine, Paliperidone, Trazadone |
| 12 | BPD | 28/M | CAUCASIAN | 30 | 8 | 21 | 2.17 | 2400 | OCB | Alprazalam, Benztropine, Buprenorphine/Naloxone, Cetirizine, Doxepin, Pantoprazole, Propranolol, Ramelteon, Ziprasidone |
| 13 | BPD | 40/M | AFRICAN AMERICAN | 18 | 1 | 12 | 0.58 | 200 | CBZ | Clonidine |
| 14 | BPD | 38/F | CAUCASIAN | 30 | 8 | 15 | 1.23 | 200 | CBZ | Clonazepam, Fluoxetine, Risperidone, Simvastatin |
Table 3.
Nicotine, Cotinine, and 3HC Levels and 3HC/Cotinine Ratios in Smokers Taking and Not Taking AED
| Cigarettes per Day¥ |
Exhaled CO¥ (ng/mL) |
Serum Nicotine¥ (ng/mL) |
Serum CotinineΨ (ng/mL) |
Cotinine/ CPD RatioΨ |
Serum 3HCΨ (ng/mL) |
3HC/ Cotinine RatioΨ |
Sum of Serum Cotinine + 3HCΨ (ng/mL) |
||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) | Mean (SD) |
||
| CBZ or OCB Use€ | |||||||||
| Yes | 14 | 23.3 (11.5) | 19.9 (11.7) | 26.4 (14.6) | 268.2 (155.7) | 11.1 (7.9)* | 147.1 (61.0) | 0.993 (0.733)** | 415.9 (177.9) |
| No | 135 | 23.2 (11.0) | 22.5 (11.0) | 28.6 (12.9) | 369.5 (185.4) | 16.1 (10.2) | 136.4 (79.8) | 0.503 (0.349) | 504.8 (238.8) |
| Valproic Acid Use$$ | |||||||||
| Yes | 40 | 23.7 (10.9) | 22.6 (10.2) | 28.5 (13.1) | 389.8 (195.5) | 16.4 (9.5) | 160.9 (85.7)* | 0.622 (0.567) | 550.7 (246.3) |
| No | 109 | 23.1 (11.1) | 22.2 (11.5) | 28.3 (13.0) | 349.1 (180.4) | 15.3 (10.4) | 128.7 (73.6) | 0.508 (0.324) | 476.6 (228.2) |
Adjusted for Group (SCZ, BPD);
Adjusted for CBZ/OCB use and Group (SCZ, BPD)
Analysis of Variance (ANOVA)
Kruskal-Wallis test
p<0.001;
p<0.01;
p<0.05
Figure 1.
3HC/Cotinine Ratios in Smokers Taking CBZ (N=14) Vs. Those Not Taking It (N= 135) in Total Sample and Mental Health Subgroups
We also repeated these analyses to examine the same effects in subgroups of smokers taking CBZ (n=8) or taking OCB (n=6). CBZ use was still associated with significant differences in 3HC/cotinine and cotinine/cpd ratio, however OCB was not.
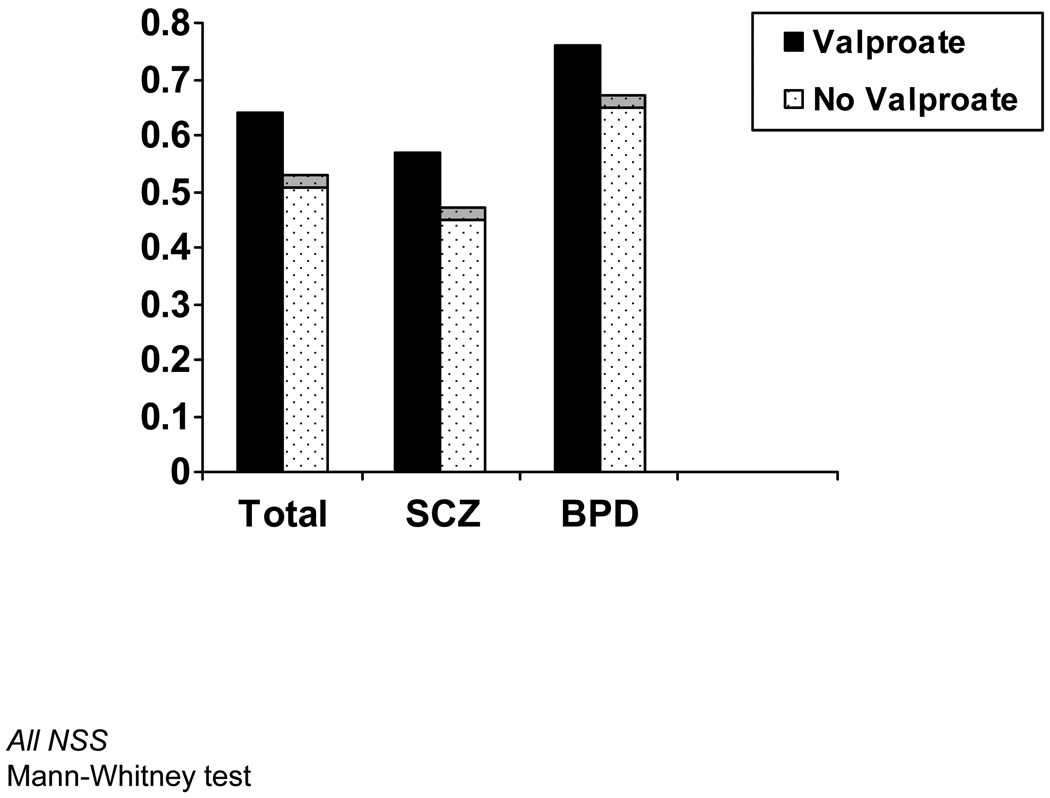
3.3 Mean serum levels of nicotine and nicotine metabolites in subjects taking valproate
Forty smokers in the sample were taking VPA (24 SCZ, 14 BPD). There were no significant differences in nicotine (mean nicotine 28.5 vs. 28.3 ng/mL; p=NS) or cotinine levels (mean cotinine 389.8 vs. 349.1 ng/mL; p=NS) in individuals taking VPA vs. those not taking it. This was true for the total group as well as within the diagnostic subgroups (Figure 2). There were also no differences in 3HC/cotinine ratios (mean 0.62 versus 0.51; F-stat=3.368, p=0.069) although values of the 3HC metabolite were higher in the VPA group (mean 160.9 vs. 128.7; p=0.026). All of the VPA analyses were adjusted for group and concurrent use of CBZ. Results were not changed when we repeated these analyses removing the 2 individuals using both VPA and CBZ and not adjusting for CBZ (See Table 3). Results were also not changed when we repeated these analyses adjusting for gender and race.
Figure 2.
3HC/Cotinine Ratios in Smokers Taking VPA (N=40) Vs. Those Not Taking It (N= 109) in Total Sample and Mental Health Subgroups
3.4 Predictors of 3HC/Cotinine Ratio
Backward stepwise linear regression analyses were conducted to identify the predictors of the log transformed 3HC/Cotinine Ratio. Taking CBZ (B=0.596; SE B=0.228, p=0.011), and number of cigarettes smoked per day (B=0.025; SE B=0.009; p< 0.01), were found to be significant determinants of log 3HC/Cotinine (See Table 4).
Table 4.
Backward Stepwise Linear Regression Analysis for Variables Predicting 3HC/Cotinine Ratios£
| Variable | B | Exp (B) | 95% CI Exp (B) | t statistic | p-value |
|---|---|---|---|---|---|
| Use of CBZ/OCB | |||||
| Yes | 0. 596 | 1.81 | 1.152, 2.854 | 2.619 | 0.011 |
| No | 1.00 | Referent | |||
| Cigarettes per day | 0.025 | 1.025 | 1.007, 1.044 | 2.742 | 0.008 |
Dependent Variable: Log-normal 3HC/Cotinine Ratio
3.5 Effect of AED Dose on 3HC/Cotinine Ratio
Figure 3 shows the correlation between 3HC/Cotinine Ratio and total oral daily dose of CBZ or VPA (in mg). There was a moderate correlation between 3HC/Cotinine Ratio and CBZ dose that approached significance (R2 Linear= 0.485, p=0.08) but no correlation for 3HC/Cotinine Ratio and VPA dose (R2 Linear= 0.048, NSS).
Figure 3.
Correlations between 3HC/Cotinine Ratios and Daily Dose of CBZ or VPA
4. DISCUSSION
The study found significantly higher 3HC/cotinine and cotinine/cpd ratios in smokers with schizophrenia and bipolar disorder taking carbamazepine or oxcarbazepine. The likely mechanism is through induction of CYP2A6 by CBZ and OCB. It is unlikely that this effect was due to mental illness alone since we have previously found nicotine metabolite ratios that are no different from control smokers without mental illness (12). In addition, values for 3HC/cotinine ratios in those not taking CBZ in this study were similar to those previously reported in other samples of non-mentally ill smokers (34,35). Although these smokers were often taking several psychiatric medications simultaneously it is unlikely that the effect was due to these other medications since none are known to have significant effects on CYP2A6 activity and antipsychotic medications were not predictors of nicotine metabolite ratio in the regression analysis. This is the first report of either carbamazepine or oxcarbazepine causing a clinically significant interaction with nicotine metabolism. Although we lacked power in this small sample to detect a significant effect on metabolism from OCB, 3HC/cotinine ratios were still elevated (mean 0.81), suggesting induction of CYP2A6 similar to CBZ and warranting further study.
Increased nicotine metabolite ratios have several implications for smokers taking CBZ or OCB. Oral contraceptives, are known to significantly accelerate nicotine metabolism. A still unanswered question is whether medications such as sex hormones or AED that increase the rate of nicotine metabolism influence either how much a person smokes or how much smoke a person takes in from a cigarette (or both). Although our study did not show evidence of higher CO values in subjects taking CBZ-OCB, more detailed measurements of CO or topography would support the hypothesis that rapid metabolizers are smoking cigarettes more intensively.
CYP2A6 gene variants associated with higher rates of nicotine metabolism and higher clearance of nicotine (4,36) are associated with increased smoking behaviors. Fast metabolizers are more prone to develop nicotine dependence and also smoke more compared to slow metabolizers (37). Fast metabolizers may also have greater difficulty quitting smoking and increased severity of abstinence symptoms. CYP2A6 is also the enzyme responsible for the metabolic activation of procarcinogenic compounds (like nitrosamines) that cause lung cancer (38). Thus persons with higher levels of CYP2A6 activity (i.e., fast metabolizers) may also be at higher risk for tobacco-caused cancer.
Greater CYP2A6 activity, as indicated by a higher nicotine metabolite ratio and higher cotinine/cpd ratio, would be expected to result in a lower serum nicotine level for a given nicotine dose. If the serum nicotine level is the same in faster and slower metabolizers, the faster metabolizers must be taking in more nicotine (and more tobacco smoke) than slower metabolizers. Since smoking-caused disease is related to intake of tobacco smoke, a person with CBZ-induced rapid metabolism of nicotine who is compensating by inhaling more smoke is likely to be at greater risk of disease.
A limitation of this study is that we examined only one pathway for nicotine and cotinine metabolism. While the majority of the clearance of nicotine proceeds via oxidative metabolism, primarily via CYP2A6, to cotinine, there are other metabolic pathways that must be considered that might not be reflected by the 3HC/cotinine ratio. For example some nicotine may be metabolized by CYP2B6 (Hukkanen et al. 2005). Both nicotine and cotinine are metabolized to glucuronides, mediated by UGT enzymes.
Further research is needed to examine the question of whether CBZ or OCB influences either smoking behavior or intake of tobacco smoke from cigarettes in groups of smokers who may or may not have mental illness. As this is also the first report of nicotine metabolites in bipolar disorder it is possible that differences seen in this group (compared to SCZ) are also due to medication effects since diagnostic group was not predictive of faster metabolism although this also warrants further evaluation.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH076672 to JMW) and the National Institute on Drug Abuse (K23-DA140090 to JMW and DA12393 to NLB).
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: Results from the 2007 National Survey on Drug Use and Health: National Findings. 2008 (NSDUH Series H-34, DHHS Publication No. SMA 08-4343)
- 2.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL. The human pharmacology of nicotine. Res Adv Alcohol Drug Probl. 1986;9:1–51. [Google Scholar]
- 4.Rao Y, Hoffmann E, Zia M, et al. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- 5.Gu DF, Hinks LJ, Morton NE, Day IN. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet. 2000;64:383–390. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- 6.Tyndale RF, Pianezza ML, Sellers EM. A common genetic defect in nicotine metabolism decreases risk for dependence and lowers cigarette consumption. Nicotine Tob Res. 1999;1:63–67. doi: 10.1080/14622299050011831. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower Metabolism and Reduced Intake of Nicotine from Cigarette Smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 10.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 12.Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.DeVane CL. Principles of Pharmacokinetics and Pharmacodynamics. In: Schatzberg AF, Nemeroff CB, editors. American Psychiatric Publishing Textbook of Psychopharmacology. 3rd ed. Arlington, Va: American Psychiatric Publishing; 2004. pp. 129–145. [Google Scholar]
- 14.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 377–530. [Google Scholar]
- 15.Monostory K, Hazai E, Vereczkey L. Inhibition of cytochrome P450 enzymes participating in p-nitrophenol hydroxylation by drugs known as CYP2E1 inhibitors. Chem Biol Interact. 2004;147:331–340. doi: 10.1016/j.cbi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Johannessen Landmark C, Larsson PG, Rytter E, Johannessen SI. Antiepileptic drugs in epilepsy and other disorders-A population-based study of prescriptions. Epilepsy Res. 2009;87:31–39. doi: 10.1016/j.eplepsyres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother. 2009;9:55–71. doi: 10.1586/14737175.9.1.55. [DOI] [PubMed] [Google Scholar]
- 19.Citrome L, Levine J, Allingham B. Changes in use of valproate and other mood stabilizers for patients with schizophrenia from 1994 to 1998. Psychiatr Serv. 2000;51:634–638. doi: 10.1176/appi.ps.51.5.634. [DOI] [PubMed] [Google Scholar]
- 20.Spina E, Pisani F, Perucca E. Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet. 1996;31:198–214. doi: 10.2165/00003088-199631030-00004. [DOI] [PubMed] [Google Scholar]
- 21.Díaz RA, Sancho J, Serratosa J. Antiepileptic drug interactions. Neurologist. 2008;14:55–65. doi: 10.1097/01.nrl.0000340792.61037.40. [DOI] [PubMed] [Google Scholar]
- 22.Wang PW, Ketter TA. Pharmacokinetics of mood stabilizers and new anticonvulsants. Psychopharmacol Bull. 2002;36:44–66. [PubMed] [Google Scholar]
- 23.Schmidt D, Elger CE. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav. 2004;5:627–635. doi: 10.1016/j.yebeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Fattore C, Cipolla G, Gatti C, et al. Induction of ethinylestradiol and levonorgestrel metabolism by oxcarbazepine in healthy women. Epilepsia. 1999;40:783–787. doi: 10.1111/j.1528-1157.1999.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 25.Johannessen Landmark C, Patsalos PN. Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev Neurother. 2010;10:119–140. doi: 10.1586/ern.09.136. [DOI] [PubMed] [Google Scholar]
- 26.Beydoun A. Safety and efficacy of oxcarbazepine: results of randomized, double-blind trials. Pharmacotherapy. 2000;20:152–158. doi: 10.1592/phco.20.12.152s.35254. [DOI] [PubMed] [Google Scholar]
- 27.Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 28.Clinical Pharmacology of Divalproex. USA: Abbott Laboratories Inc; [accessed 9/2/09]. www.rxabbott.com/pdf/depakote.pdf. [Google Scholar]
- 29.Spitzer RL, Williams JBW. Biometrics Research Department. 722 West 168 Street, New York 10032: New York State Psychiatric Institute; 1985. Structured Clinical Interview for DSM-III-R. [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 33.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 34.Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3'hydroxycotinine to cotinine in plasma and urine. Pharmacogenet Genomics. 2009;19:388–398. doi: 10.1097/FPC.0b013e32832a404f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnstone E, Benowitz N, Cargill A, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80:319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin Pharmacol Ther. 2008;83:115–121. doi: 10.1038/sj.clpt.6100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:264–274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 38.Hecht SS, Hochalter JB, Villalta PW, Murphy SE. 2'-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor. Proc Natl Acad Sci USA. 2000 Nov 7;97:12493–12497. doi: 10.1073/pnas.220207697. [DOI] [PMC free article] [PubMed] [Google Scholar]