Abstract
Purpose
To evaluate the feasibility of Dose-Painting Intensity-Modulated Radiation Therapy (DP-IMRT) with a hypofractionated regimen to treat nasopharyngeal carcinoma (NPC) with concomitant toxicity reduction.
Materials/Methods
From 10/02–4/07, 25 newly diagnosed NPC patients were enrolled on a prospective trial. DP-IMRT was prescribed to deliver 70.2 Gy using 2.34 Gy fractions to the gross tumor volume for the primary and nodal sites while simultaneously delivering 54 Gy in 1.8 Gy fractions to regions at risk of microscopic disease. Patients received concurrent and adjuvant platin–based chemotherapy similar to the Intergroup 0099 trial.
Results
Patient/disease characteristics: median age 46; 44% Asian; 68% male; 76% WHO III; 20% T1, 52% T2, 16% T3, 12% T4; 20% N0, 36% N1, 36% N2, 8% N3. With median follow-up of 33 months, 3-year local control was 91%, regional control was 91%, freedom from distant metastases was 91%, and overall survival was 89%. The average mean dose to each cochlea was 43 Gy. With median audiogram follow-up of 14 months, only one patient had clinically significant (grade 3) hearing loss. 12% of patients developed temporal lobe necrosis; one patient required surgical resection.
Conclusions
Preliminary findings using a hypofractionated DP-IMRT regimen demonstrated that local control, freedom from distant metastases and overall survival compared favorably to other series of IMRT and chemotherapy. The highly conformal boost to the tumor bed resulted low rates of severe ototoxicity (Grade 3–4). However, the incidence of in-field brain radiation necrosis indicates that 2.34 Gy per fraction is not safe in this setting.
Keywords: Nasopharyngeal cancer, hypofractionated, Dose-painting, Intensity-Modulated Radiation Therapy
INTRODUCTION
Radiotherapy represents the primary treatment modality for patients with nasopharyngeal cancer (NPC). Historically, local control (LC) rates for early stage disease with conventional radiation therapy (RT) range from 64% to 95% but decreased to 44% to 68% for locally advanced disease.(1–4) More modern series with combined modality therapy report LC up to 95%.(5–9) For patients with extensive locoregional disease, the addition of concurrent chemotherapy is a standard recommendation based on a survival benefit.(9) Despite advances, patients with large primary tumors still fail locally within the radiation target volumes. Therefore new methods to improve local control must still be pursued.
Local control for NPC is directly related to the dose and technical accuracy with which the dose is delivered to the target volume.(10–11) However, the large number of critical anatomic structures adjacent to the normal tissue has made high-dose delivery a challenging treatment planning issue. After our experience using three-dimensional conformal boost did not show improved rates of local control in comparison to two-dimensional methods, we began using intensity-modulated radiation therapy (IMRT) treatment plans to deliver the entire treatment.(12) We and other investigators have reported that IMRT provides improved dose distributions over 3D conformal plans with encouraging clinical outcomes and excellent LC.(13–16) In addition, the improved dosimetry obtained with IMRT has resulted in reduced toxicity. The most common and debilitating long-term side effects seen in our NPC patients treated with conventional radiotherapy techniques are xerostomia and hearing loss. Recent studies have demonstrated an improvement in salivary function in patients treated with IMRT.(17–18)
There have been a number of different strategies utilized for dose escalation including brachytherapy(19), stereotactic RT(20), and dose-painting IMRT (DP-IMRT).(21) To address locally advanced disease, we sought to increase the biologically effective dose to the boost volume, and designed a hypofractionated regimen of 2.34 Gy for a total of 70.2 Gy. In this study, we seek to evaluate the feasibility of using DP-IMRT with a hypofractionated regimen plus chemotherapy to treat nasopharyngeal carcinoma with concomitant toxicity reduction. Here we report our preliminary results.
METHODS AND MATERIALS
Study Objectives/Patient Eligibility
Between 10/2002 and 4/2007, 25 NPC patients were enrolled on our institutional review board protocol (IRB# 02-077A), “Phase I/II Study of Dose-Painting using Intensity Modulated Radiation Therapy Plus Chemotherapy in Patients with Stage II-IVB Nasopharyngeal Carcinoma.” Patients with previously untreated stages II-IVB NPC, with a Karnofsky performance status (KPS) ≥ 60, who met criteria for blood counts and other tests (ie, WBC ≥ 3,000/mm3; platelets ≥ 100,000 mm3; serum creatinine ≤ 1.5 mg/dl) were eligible. Patients younger than 18 years old or with active cancer other than non-melanoma skin cancer or carcinoma in-situ of the cervix were excluded. Pretreatment evaluations consisted of a history, physical, dental, nutritional, audiogram, and laboratory studies. Magnetic resonance imaging (MRI) of the nasopharynx/neck was required unless there was a contraindication. Positron emission tomography (PET) was performed on all patients as a staging modality. Additional tests to evaluate the extent of disease included chest x-ray, liver function tests, alkaline phosphatase, lactate dehydrogenase and when indicated liver scans if there was a suspicion of metastasis. The disease was staged according to the 1997 American Joint Committee on Cancer Staging.(22) All patients signed written informed consents.
The primary objective was to test the feasibility of hypofractionated DP-IMRT with chemotherapy. Other objectives were to determine rates of ototoxicity and xerostomia, LC, regional control, distant metastasis (DM), and overall survival (OS).
Treatment
Patients were immobilized in the supine position with custom Aquaplast masks, and target localization was accomplished using computed tomography (CT) images. Fusion of MRI and/or PET with the planning CT images was performed to aid in the delineation of the gross tumor volume (GTV). GTVg included the primary disease and nodes greater than 1 cm diameter or nodes with necrotic centers. A 1 cm margin was added to the GTVg to define the planning target volume (PTV), PTVg, to include biologic and technical uncertainties. The margin was applied in all dimensions except posterior where a 5 mm margin was used because there is less biological uncertainty at the bones of the skull. PTVm was defined by PTVg which included potential routes of NPC spread plus bilateral retropharyngeal and cervical lymph nodal regions for all patients with a 5 mm margin for technical uncertainties. Normal structures of interest outlined in three dimensions included the globes, optic nerves, optic chiasm, brainstem, spinal cord, cochlea, parotid gland, submandibular glands, oral cavity and larynx.
RT was delivered using a DP-IMRT technique. PTVg received 70.2 Gy in 2.34 Gy/fraction, and PTVm received 54 Gy in 1.8 Gy/fraction over 30 days. Plans were normalized and dose was prescribed such that at least 95% of the PTV’s encompassing involved and electively irradiated sites of disease received at least 70 Gy and 54 Gy, respectively. In addition, the dose received by 99% of the GTV was not less than 70 Gy. Parotid glands were limited to a mean dose of 26 Gy when possible, and the cochlea were limited by a maximum dose of 60 Gy when possible, both without compromising PTV or GTV coverage.
Based on results of Intergroup 0999 and according to our previously published experience, patients received planned concurrent cisplatin chemotherapy 100 mg/m2 i.v. on Weeks 1 and 4 of radiotherapy, plus up to 3 planned adjuvant cycles of cisplatin 100 mg/m2 i.v. and 5-fluorouracil 1000 mg/m2/day by continuous i.v. infusion Days 1–4.(9) For adjuvant therapy, carboplatin-based treatment could be substituted if the risk of cisplatin toxicity was deemed excessive (hearing loss or kidney dysfunction). Guidelines for dose modifications were specified in the protocol.
Follow-Up/Statistical Analysis
Planned patient assessment posttreatment included physical examination with fiberoptic nasopharyngoscopy every 3 months for the first year of follow-up, every 4 months in the second year, every 6 months in the third-fifth years annually and thereafter. A follow-up CT or MRI scan of the nasopharynx and neck plus a PET scan was performed approximately every 6 months from the completion of radiation therapy for the first 2 years. Afterward, an annual CT or MRI scan was obtained. A suspected recurrence was histologically proven unless a biopsy would cause an excessive risk of injury to the patient. To assess for DM, a chest radiograph and liver function tests were performed annually. Patients were classified as progression free as long as they remain alive without local, regional, or distant recurrences.
Pure tone audiograms were obtained pretreatment to assess individual baseline hearing thresholds. Testing was performed in a double-walled audiometric testing suite (IAC 1400 series). Bone and air conduction thresholds (250–8000 Hz and 250–4000 Hz, respectively) were obtained using a Grason-Stadler 1761 clinical audiometer (Grason-Stadler, Madison, WI) with TDH-50 ear phones and MX-51 ear cushions. Posttreatment audiograms were obtained at various intervals after completion of radiotherapy. Sensorineural hearing loss was graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v3.0.(23) Xerostomia and trismus were assessed at each follow-up visit by treating physicians and also graded according to CTCAE v3.0.(23)
The Kaplan-Meier method was used to calculate actuarial rates of LC, regional control, freedom from DM, and OS.(24) Other secondary endpoints included skin toxicity, mucositis, ototoxicity, xerostomia, and trismus. All percentages reported were based on all 25 patients. All time-to-failure endpoints were calculated from the date of diagnosis to the date of failure/last follow-up for patients who did not experience failure. Patients who developed DM were still observed for locoregional failure.
RESULTS
Characteristics of the 25 patients including gender, ethnicity, and tumor histology according to the World Health Organization(25) are outlined in Table 1. Median age was 46 years. All patients received the prescription dose of 70.2 Gy to gross disease or PTVg concurrent with chemotherapy. Only 2 patients did not receive adjuvant chemotherapy: patient refusal (n=1) and thrombocytopenia (n=1). Amongst the 23 patients that received adjuvant platin-based chemotherapy, the mean number of cycles received was 2.8. There was no correlation between the number of adjuvant chemotherapy cycles and metastases-free survival. 52% of patients received adjuvant Carboplatin (vs. Cisplatin) secondary to toxicities.
Table 1.
Patient and disease characteristics of 25 patients treated with DP-IMRT*.
| n | % | |
|---|---|---|
| Gender | ||
| M | 17 | 68% |
| F | 8 | 32% |
| Ethnicity | ||
| Asian | 11 | 44% |
| Caucasian | 8 | 32% |
| Other | 6 | 24% |
| Histology | ||
| WHO† I | 2 | 8% |
| WHO II | 4 | 16% |
| WHO III | 19 | 76% |
| Tumor stage | ||
| T1 | 5 | 20% |
| T2 | 13 | 52% |
| T3 | 4 | 16% |
| T4 | 3 | 12% |
| Nodal stage | ||
| N0 | 5 | 20% |
| N1 | 9 | 36% |
| N2 | 9 | 36% |
| N3 | 2 | 8% |
| Stage group (AJCC‡) | ||
| I | 0 | 0% |
| II | 9 | 36% |
| III | 11 | 44% |
| IVA/B | 5 | 20% |
Abbreviations:
= dose-pointing intensity-modulated radiation therapy;
= World Health Organization;
= American Joint Committee on Cancer.
The median follow-up amongst living patients was 33 months (range 14–77 months). At 3 years, the actuarial rate of LC was 91% (Fig 1.), regional control was 91%, metastases-free survival was 91%, and OS was 89% (Fig. 2). All patients that developed local failures were concurrently diagnosed with regional failures in the oropharynx or neck nodes. The rates of LC, regional control, metastases-free survival and OS compares favorably to our previously reported series of IMRT and chemotherapy.(16) 67% of T4 patients remained disease free at last follow-up.
Fig. 1.
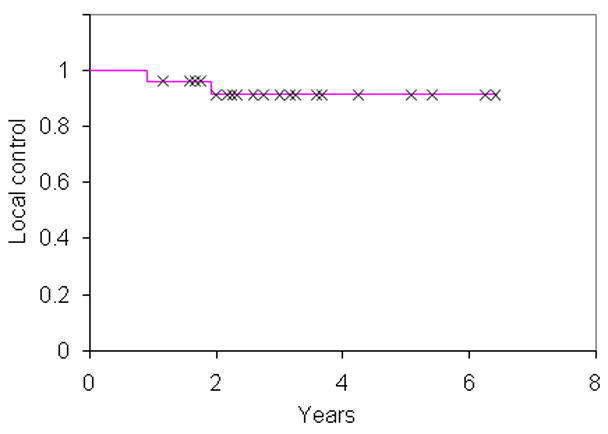
Local control for 25 patients treated with dose-painting intensity-modulated radiation therapy.
Fig. 2.
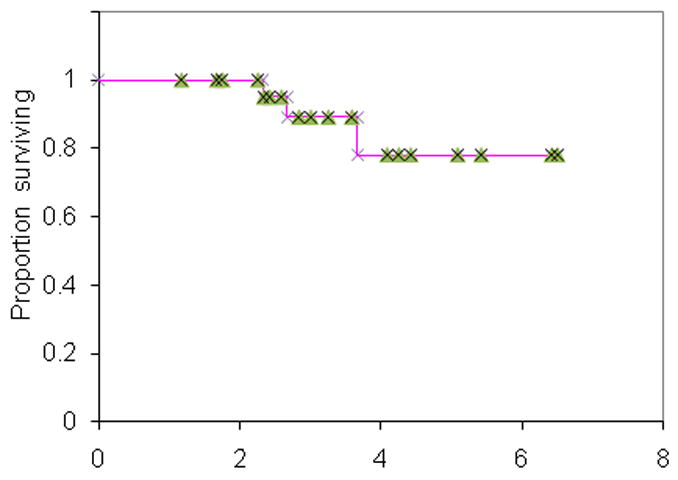
Overall survival for 25 patients treated with dose-painting intensity-modulated radiation therapy.
There have been 2 patients with both concurrent local and regional recurrences at 11 and 23 months, stage T4N2 and T1N2 respectively. Both local failures were within the PTVg and received 70 Gy. Of note, the patient with T1N2 disease had imaging highly suggestive of locoregional failure however pathology was negative for local recurrence. She was concurrently diagnosed with biopsy-proven metastatic disease. Based on imaging, she was counted as a local failure for the purposes of this study.
There have been two patients who developed metastatic disease, one to liver and the second to bone at 14 and 23 months, respectively. All patients who developed local or distant failures went on to die of disease, representing all 3 deaths in the study.
Grade 3 and 4 mucositis were experienced in 36% and 4% of patients, respectively. Eighteen patients (72%) had PEG tubes placed electively before or during treatment for nutritional support. 16% of patients developed grade 3 or 4 skin toxicity. No other grade 4 acute toxicities were noted. All living patients are now PEG free.
All patients received audiograms before treatment and after with a median follow-up of 14 months (range 3–62 months). The results are depicted in Fig 3. No patients had Grade 4 hearing loss, and only 1 patient had Grade 3 hearing loss where therapeutic intervention such as a hearing aid might be warranted. For this patient, dosimetry to the cochlea was within specified dose limit constraints: mean cochlea dose 41 Gy, maximum cochlea dose 51 Gy. Dosimetric analysis was performed on all patients (50 ears). The average mean dose to the cochlea was 43 Gy (range 34–71 Gy). Xerostomia was assessed in all patients during follow-up. 76% of patients developed Grade 1 xerostomia at a median follow-up of 33 months; no patients developed Grade 2 or greater xerostomia. These results are depicted in Fig 4. The average mean dose to the parotid was 29 Gy. Mean dose was limited to ≤26 Gy in 66% of parotid glands without sacrificing PTV coverage. Trismus was also assessed during follow-up, and only 3 patients (12%) developed grade 1 trismus. One patient developed a radiation-induced symptomatic twelfth nerve cranial neuropathy.
Fig 3.
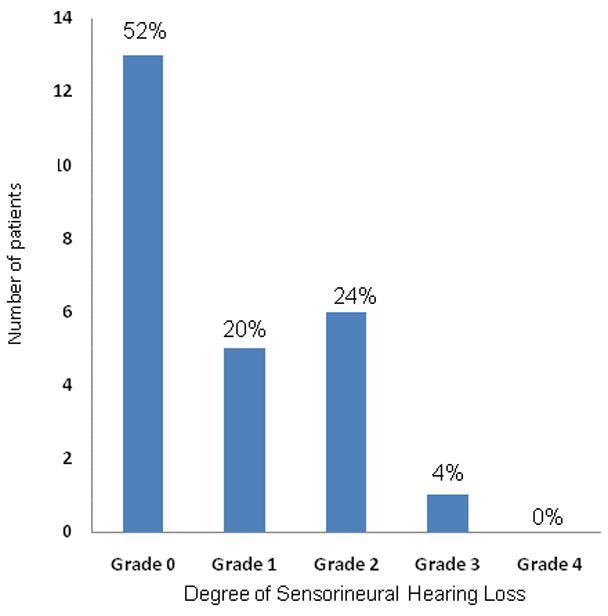
Degree of sensorineural hearing loss with a median follow-up of 14 months after intensity modulated radiation therapy for 50 ears in 25 patients, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events v3.0.(23)
Fig 4.
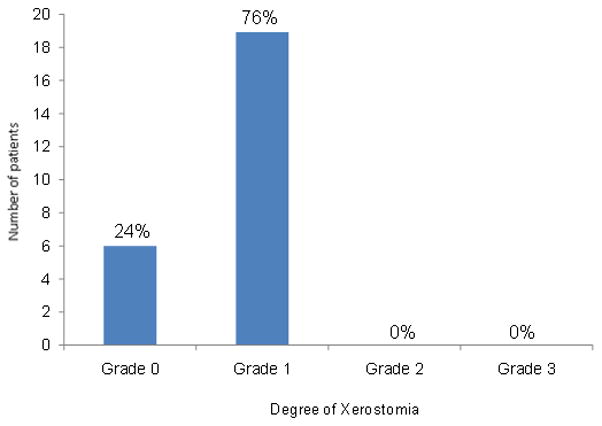
Degree of xerostomia with a median follow-up of 33 months for 25 patients, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events v3.0.(23)
3 patients (12%) developed temporal lobe radiation necrosis. Median time to detection after radiation was 24 months (range 19–48 months). Two of the cases developed in areas that received the prescription dose of 70.2 Gy because of intracranial PTVg extension as depicted in Fig 5; the third case occurred in an area of the temporal lobe adjacent to the PTVg. One patient was symptomatic; one required surgical resection. 67% of patients with T4 disease developed temporal lobe necrosis. In comparison, there were no cases of temporal lobe necrosis in our IMRT series in which there were a higher percentage of patients with intracranial extension utilizing a regimen of 2.12 Gy per fraction for a total of 70 Gy.(16)
Fig 5.
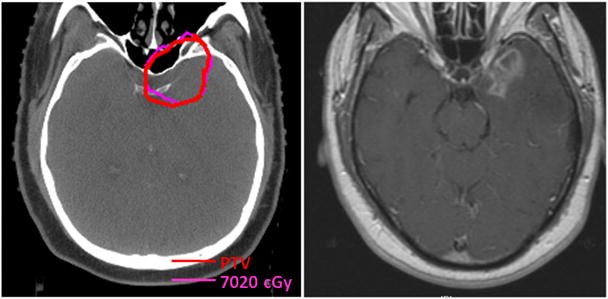
Axial CT*-based planning slice (left) through the PTV† of a patient with intracranial tumor extension who developed temporal lobe necrosis (MRI‡ right) in a region that received prescription dose.
Abbreviations: * = Computed tomography; † = Planning target volume; ‡ = Magnetic resonance imaging.
DISCUSSION
Local control in the nasopharynx correlates with both stage, dose, and technical accuracy with which the dose is delivered to the target volume.(1, 26) In our updated IMRT series, we noted the absence of local failure among stage T1//T2 disease.(16) However, for locally advanced tumors, local failures occurred within the GTV suggesting that a more effective dose may be required to improve current local control rates. This could be achieved by pure dose escalation or altered fraction schemes that yield a higher biologically equivalent dose. Building on our experience with DP-IMRT, we developed a 2.34 Gy per fraction for a total of 70.2 Gy treatment regimen. Based on a linear-quadratic equation with an alpha/beta ratio of 10 for tumor response, this resulted in a 5.3% increase in the biologic equivalent dose to the boost volume; the 2 Gy per fraction equivalent of 72.2 Gy.
Preliminary findings using a hypofractionated DP-IMRT regimen demonstrated that LC, regional control, freedom from DM and OS compared favorably to other IMRT and chemotherapy series.(15–16, 27) In comparison to our updated IMRT series, a local and regional failure developed in a T1 staged patient. Our second concurrent local and regional failure occurred in a patient with extensive T4 disease. The 2 other patients with T4 disease remain free of disease at last follow-up suggesting that they might have benefited from the hypofractionated approach. However, both have developed temporal lobe necrosis. One of the patients was symptomatic and required surgical resection; the second patient was asymptomatic.
The accelerated regimen did not result in increased acute toxicities of the skin, mucous membranes, or salivary glands indicating that treatment was well tolerated. Furthermore, no patients required significant treatment breaks nor did any patient require their chemotherapy to be held during radiation. Previously, a Phase I dose escalation trial without concurrent chemotherapy indicated that 2.36 Gy per fraction for a total of 70.8 Gy was the maximal tolerable dose delivered to the GTV while using a simultaneous integrated boost for head and neck cancers.(21) Dose escalation to fractions of 2.46 Gy was not tolerated for more than 10–15 treatments.
Mean dose to the cochlea and concurrent chemotherapy have been established as known risk factors for ototoxicity.(28) The highly conformal boost to the tumor bed resulted in low rates of severe (Grade 3–4) cochlea toxicity despite the hypofractionated approach with concurrent cisplatin-based chemotherapy. Limiting the maximum dose to the cochlea to 60 Gy resulted in only one patient developing clinically significant hearing loss (grade 3). This compares favorably to our IMRT study, which resulted in 15% grade 3 hearing loss.(16) Additionally, the accelerated regimen did not result in increased rates of xerostomia as no patients developed Grade 2 toxicity, defined as significant oral intake alteration.
Concerning was the incidence of in-field temporal lobe radiation necrosis. 12% of patients treated on the protocol developed radiation necrosis; one requiring surgery. In contrast, standard fractionation in our previous IMRT series did not result in any cases.(16) Of the patients that developed temporal lobe necrosis, 67% had intracranial extension suggesting that volume of brain tissue irradiated might be a risk factor when a hypofractionated dose regimen is utilized. Dose constraints to the temporal lobes were not specified in this protocol and the PTV was not modified to limit dose to the temporal lobes but such measures should be considered to reduce the risk of temporal lobe necrosis. Because of proximity to the middle cranial fossa, NPC is unique among head and neck tumors for a relatively high risk of temporal lobe necrosis.(29) A recent study looking at factors affecting the risk of temporal lobe necrosis identified the dose per fraction, total dose, and overall treatment time as significant risk factors.(30) In a recent study analyzing the major late toxicities after conformal radiation for nasopharyngeal cancer, no cases of temporal lobe necrosis were observed in 171 patients treated by RT alone (70 Gy) and 22 patients treated with an additional brachytherapy boost. However, the rate of temporal lobe necrosis increased to 8.3% at 5 years among the 33 patients given a 5 Gy stereotactic boost.(28) This risk was also noted in another study where a stereotactic boost of 7–15 Gy was administered in a single fraction after external beam RT to 66 Gy.(20) At a median follow-up of 3.4 years, the incidence of temporal lobe necrosis was 12%. Taken together, these results suggest that the therapeutic margin for nasopharyngeal carcinoma is very narrow.
Preliminary findings using a hypofractionated DP-IMRT with chemotherapy demonstrate excellent LC, regional control, freedom from DM and OS. The highly conformal boost to the tumor bed resulted in acceptable acute toxicity and low rates of severe cochlear toxicity. However, the incidence of in-field radiation necrosis in our study and in other reports, indicate that 2.34 Gy per fraction is not safe in this setting and a lower dose per fraction should be used.
Acknowledgments
This study was supported by NCI grant 3P01CA59017. Preliminary results were presented at the 51st annual meeting of the American Society for Radiation Oncology (ASTRO), November 4th, 2009 Chicago, Illinois. The authors have no further disclaimers or conflicts of interest.
We would like to thank Amy S. Budnick for her help analyzing the audiograms.
Footnotes
Conflicts of Interest Notification: The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chu AM, Flynn MB, Achino E, et al. Irradiation of nasopharyngeal carcinoma: correlations with treatment factors and stage. Int J Radiat Oncol Biol Phys. 1984;10:2241–2249. doi: 10.1016/0360-3016(84)90229-3. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe RT, Goffinet DR, Bagshaw MA. Carcinoma of the nasopharynx. Eighteen years’ experience with megavoltage radiation therapy. Cancer. 1976;37:2605–2612. doi: 10.1002/1097-0142(197606)37:6<2605::aid-cncr2820370607>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Mesic JB, Fletcher GH, Goepfert H. Megavoltage irradiation of epithelial tumors of the nasopharynx. Int J Radiat Oncol Biol Phys. 1981;7:447–453. doi: 10.1016/0360-3016(81)90129-2. [DOI] [PubMed] [Google Scholar]
- 4.Bailet JW, Mark RJ, Abemayor E, et al. Nasopharyngeal carcinoma: treatment results with primary radiation therapy. Laryngoscope. 1992;102:965–972. doi: 10.1288/00005537-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:142–151. doi: 10.1016/j.ijrobp.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–6975. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 8.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 10.Marks JE, Bedwinek JM, Lee F, et al. Dose-response analysis for nasopharyngeal carcinoma: an historical perspective. Cancer. 1982;50:1042–1050. doi: 10.1002/1097-0142(19820915)50:6<1042::aid-cncr2820500604>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Vikram B, Mishra UB, Strong EW, et al. Patterns of failure in carcinoma of the nasopharynx: I. Failure at the primary site. Int J Radiat Oncol Biol Phys. 1985;11:1455–1459. doi: 10.1016/0360-3016(85)90332-3. [DOI] [PubMed] [Google Scholar]
- 12.Wolden SL, Zelefsky MJ, Hunt MA, et al. Failure of a 3D conformal boost to improve radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2001;49:1229–1234. doi: 10.1016/s0360-3016(00)01588-1. [DOI] [PubMed] [Google Scholar]
- 13.Xia P, Fu KK, Wong GW, et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:329–337. doi: 10.1016/s0360-3016(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 14.Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys. 2003;56:145–157. doi: 10.1016/s0360-3016(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 15.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 16.Wolden SL, Chen WC, Pfister DG, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64:57–62. doi: 10.1016/j.ijrobp.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 17.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 18.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Teo PM, Leung SF, Lee WY, et al. Intracavitary brachytherapy significantly enhances local control of early T-stage nasopharyngeal carcinoma: the existence of a dose-tumor-control relationship above conventional tumoricidal dose. Int J Radiat Oncol Biol Phys. 2000;46:445–458. doi: 10.1016/s0360-3016(99)00326-0. [DOI] [PubMed] [Google Scholar]
- 20.Hara W, Loo BW, Jr, Goffinet DR, et al. Excellent local control with stereotactic radiotherapy boost after external beam radiotherapy in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2008;71:393–400. doi: 10.1016/j.ijrobp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Lauve A, Morris M, Schmidt-Ullrich R, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: II--clinical results. Int J Radiat Oncol Biol Phys. 2004;60:374–387. doi: 10.1016/j.ijrobp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Fleming ID, Cooper J, Henson DE, editors. AJCC cancer staging manual. 5. Philadelphia: Lippincott-Raven Publishers; 1997. [Google Scholar]
- 23.Williams J, Chen Y, Rubin P, et al. The biological basis of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:182–188. doi: 10.1016/S1053-4296(03)00045-6. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan E, Meir P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.International histological classification of tumors. Histological classification of upper respiratory track tumors. World Health Organization. 1978;19:32. [Google Scholar]
- 26.Fu KK. Prognostic factors of carcinoma of the nasopharynx. Int J Radiat Oncol Biol Phys. 1980;6:523–526. doi: 10.1016/0360-3016(80)90071-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AW, Ng WT, Hung WM, et al. Major late toxicities after conformal radiotherapy for nasopharyngeal carcinoma-patient- and treatment-related risk factors. Int J Radiat Oncol Biol Phys. 2009;73:1121–1128. doi: 10.1016/j.ijrobp.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Glass JP, Hwang TL, Leavens ME, et al. Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer. 1984;54:1966–1972. doi: 10.1002/1097-0142(19841101)54:9<1966::aid-cncr2820540930>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee AW, Kwong DL, Leung SF, et al. Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys. 2002;53:75–85. doi: 10.1016/s0360-3016(02)02711-6. [DOI] [PubMed] [Google Scholar]


