Abstract
Background
Listeriosis occurs mainly in persons at extremes of age and with immunocompromising conditions. It is believed that most cases of listeriosis are acquired in the community. A cluster of listeriosis in hospitalized patients prompted the present investigation.
Methods
Case series of listeriosis, from 21 August 2006 to 01 June 2007, in a hospital in the city of Rio de Janeiro, Brazil.
Results
Six patients with Listeria monocytogenes infection were identified: 5 during hospitalization and one at a day-clinic. By the time the infection was diagnosed, five patients had been in the hospital for a mean of 9 days. All patients were elderly (median age: 80 years) and had immunocompromising conditions. Five (83%) patients died. Four patients developed bloodstream infections, 3 caused by serotype 1/2b. Two had peritonitis, one caused by serotype 3b and another by serotype 1/2b. Four L. monocytogenes isolates belonged to a single PFGE genotype, suggesting a common source. An epidemiological investigation pointed to the hospital kitchen as the possible contamination.
Conclusions
Data suggest a healthcare associated outbreak of listeriosis and highlight the importance of developing guidelines for prevention and treatment of healthcare associated foodborne diseases, especially in hospitals with immunocompromised adult patients.
Introduction
Listeria monocytogenes is a Gram-positive, facultative intracellular bacterium, widely present in the environment and an important cause of zoonosis, especially in herd animals.(1;2) This microorganism causes invasive infection with high mortality,(3;4) mainly in persons at extremes of age and with immunocompromising conditions.(5-7) It is believed that most cases of listeriosis are foodborne and acquired in the community.(8-10) However, although uncommon, healthcare associated (HA) listeriosis can occur in adult and newborn patients due to consumption of contaminated food or cross-infection, usually as meningitis, bacteremia or enterocolitis.(11-15)
A main concern about HA-listeriosis is that L. monocytogenes is naturally resistant to many of the antibiotics prescribed empirically for the treatment of HA infection (HAI), such as third and fourth-generation cephalosporin,(16;17) and possibly, carbapenems.(18) Thus, the occurrence of HA-listeriosis in adults may be followed by incorrect empirical therapy and a poor clinical outcome.
On 21 August 2006, one patient was diagnosed with bloodstream infection by L. monocytogenes at the Hematology Ward of Hospital A in Rio de Janeiro, Brazil. During the first 6 months of 2007, four additional hospitalized patients with listeriosis were identified at the same hospital, which prompted the present investigation.
Methods
Setting
The study was conducted at a 465-bed adult public Hospital A with a 34-bed day-clinics for patients with AIDS and hematologic diseases, and for hemodialysis. The food offered to inpatients and day-clinic patients is prepared in the hospital kitchen. Each ward has a specific area where the meals are sent directly from the kitchen before distribution to patients.
Study design and population
A case-patient was defined as the isolation of L. monocytogenes from a normally sterile site between 21 August 2006 through 01 June 2007. The records after January 2002 at the microbiology laboratory were reviewed for previous cases.
Case-finding and data collection
The isolation of L. monocytogenes from blood and peritoneal fluid with symptoms of infection was defined as bloodstream infection and peritonitis, respectively. Death was classified as related to listeriosis if it occurred within three days of infection diagnosis and without other identifiable causes. Neutropenia was defined by a neutrophil cell count ≤ 500 cells/mm3. Patient-cases were detected by daily review of microbiological records performed as part of active surveillance for HAI. The following information were retrospectively collected for each patient with listeriosis by medical chart review: demographic information; underlying diseases; neutrophil cell count; date of admission, listeriosis onset, culture results and outcome; type of infection and outcome; antibiotic used for therapy; admission to or day-clinic care in the University Hospital within the 3 months previous to admission and type of nutrition (oral, enteral and parenteral) during admission. Detailed data about the type of food consumed could not be collected because of insufficient information in medical charts and nutritional records for either the time prior to admission or during hospital stay. In addition, most (83%) of the case-patients had already died at the time of investigation. Although specifically enquired, no changes could be documented in the kitchen environment, staff and food sources. However, there were no records to confirm this information.
To investigate if the cases at Hospital A were part of a widespread problem in the city in Rio de Janeiro, hospital epidemiologists of several other public and private hospitals were contacted and asked for the occurrence of cases.
Environmental investigation and interventions
The Infection Control Team reported the occurrence of listeriosis to the Hospital Risk Management Committee, which, in turn, reported the cases to the State Health Surveillance Agency. The hospital kitchen and food storage areas were examined for sanitary conditions and contamination by listeria. Simultaneously to the investigation of potential sources, the following measures were adopted to prevent new cases: reinforcement of food hygiene and environmental cleaning routines, retraining of food handlers and cleaning team on proper hygienic techniques, increase in the number of the cleaning team staff, substitution of damaged utensils and kitchen equipment, and separation of raw food, fresh cheese and cut fruit from prepared meals. Finally, ampicillin was included in the empirical therapy of HAI for some of the patients with hematologic diseases and immunosuppressive conditions.
Microbiological analysis
Clinical bacterial isolates were obtained during the routine investigation of infectious episodes. Blood and peritoneal fluid samples were cultured into Bactec bottles and bacterial identification was performed by standard biochemical tests.(19) A microbiological survey for contamination of listeria was performed in May 2007, when 45 samples were collected: 33 from kitchen environment and 12 from food items (raw and prepared). Environmental specimens were sampled with saline moistened swabs. About 25g of food samples were homogenized into 25 mL of Listeria Enrichment Broth (LEB, Oxoid) with listeria selective supplement (Oxoid) containing cycloheximide, acriflavine and nalidixic acid.(20) After incubation at 30°C for 24 hours, 10μL aliquots of LEB cultures were smeared onto PALCAM agar plates (Difco) and selective Chromoplate agar for listeria (Merck) and incubated at 30°C for 48 hours.(21) Suspect colonies were identified by biochemical analysis (22) and PCR with hly gene and 23S rRNA primers.(23)
Strain typing
Serotype determinations were performed with polyclonal cross-absorbed antisera(24) and PCR typing.(25) Genotyping was performed by pulsed field gel electrophoresis (PFGE) with ApaI (Roche) restriction enzyme.(26) L. monocytogenes strain LM1 and Listeria innocua strain LI of our laboratory collection of animal isolates were used as internal controls. Interpretation of DNA banding patterns was performed by visual inspection and computer assisted analysis using the software GelCompar II version 4.5 (Applied Maths, Kortrijk, Belgium).
Results
From 21 August 2006 to 01 June 2007, 6 patients hospitalized at the Hospital A were diagnosed to have listeriosis. No additional cases were found by review of the microbiology records after January 2002, and no more cases occurred until the end of November, 2009. No other hospitals in Rio de Janeiro reported cases. Therefore, the cases were restricted to Hospital A only from August 2006 to June 2007. During this time-period, there were 11,320 admissions, with a cumulative incidence of 0.53 listeriosis case-patients per 1,000 admissions. By the time the infection was diagnosed, 5 patients had been in the hospital for a mean of nine days (range: 7-14 days). The sixth patient had symptoms of listeriosis at the time of hospital admission, but had undergone chemotherapy in the hematology day-clinic during the previous 3 months.
All case-patients were elderly (median age: 80 years, range: 63-92 years) and had immunosuppressive conditions (Table I). Three patients had bloodstream infections following diarrhoea, 1 patient had bloodstream infection and 2 had peritonitis with no other identifiable infections. Unfortunately, stool samples from none of these patients were cultured. Five patients were empirically treated with third or fourth-generation cephalosporins, according to the hospital guidelines for antimicrobial use. One patient did not receive any empirical antimicrobial agents. Once culture results were available, 2 patients had the therapy changed to ampicillin, 1 patient remained with ceftriaxone and 3 patients had already died. The median length of hospital stay was 15 days (range: 1-25 days). Five (83%) patients died, including those treated with ampicillin. Four (67%) deaths were related to listeriosis: 3 patients died within 3 days of infection onset, and 1 patient died 10 days after listeriosis onset and repeated isolation of L. monocytogenes, without another identifiable cause.
Table I.
Characteristics and clinical data of patients with listeriosis
| Characteristic | Case-patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Gender | Female | Male | Female | Male | Female | Male |
| Age (years) | 86 | 74 | 62 | 67 | 92 | 90 |
| Underlying condition | Lymphoma | Liver cirrhosis (Child's class C) |
Lymphoma | Liver cirrhosis (Child's class B) |
Cardiac disease | Cardiac disease |
| Reason for admission | Febrile neutropenia | Liver failure and gastrointestinal bleeding | Intestinal obstruction | Liver failure | Heart failure and pneumonia | Heart failure |
| Ward of admission | Hematology | Internal medicine | Hematology | Internal medicine | Internal medicine | Internal medicine |
| Type of listeriosis | Primary bacteremia | Primary peritonitis | Secundary bacteremia | Primary peritonitis | Secondary bacteremia | Secondary bacteremia |
| Symptoms | Fever | Fever | Diarrhea, abdominal pain and fever | Fever | Diarrhea, abdominal pain and fever | Diarrhea and fever |
| Listeria serotype | NA | 3b | 1/2b | 1/2b | 1/2b | 1/2b |
| Presence of neutropenia | Yes | No | Yes | No | No | No |
| Type of nutrition | Oral | Oral | Oral | Oral | Oral/Enteral | Oral |
| Time (days) from | ||||||
| admission to listeriosis onset | 0* | 10 | 9 | 14 | 8 | 7 |
| listeriosis onset to culture result | 5 | 4 | 9 | 2 | 7 | 4 |
| listeriosis onset to outcome | 1 | 1 | 26 | 10 | 10 | 3 |
| Antibiotic therapy | ||||||
| Empirical | Cefepime | Ceftriaxone | Cefepime plus metronidazole | Ceftriaxone | Cefepime | None |
| After culture result | ** | ** | Ampicillin | Ceftriaxone | Ampicillin | ** |
| Patient outcome | Death | Death | Death | Discharge | Death | Death |
NA: isolate not available;
patient had undergone chemotherapy in the hematologic day-clinic during the previous three months;
patient died before culture result was available
Microbiological analysis and strain typing
A total of 8 clinical L. monocytogenes isolates were obtained from the 6 case-patients. However, the isolate obtained in 2006 was not saved. Therefore, 7 clinical isolates from 5 case-patients were available for further studies: 5 obtained from blood cultures of 3 patients and 2 from peritoneal fluid cultures of 2 patients. Listeria was not recovered from the environmental or food samples. Biochemical and PCR identification confirmed all clinical isolates as L. monocytogenes. The 5 blood isolates and a peritoneal fluid isolate belonged to serotype 1/2b; the other peritoneal fluid isolate belonged to serotype 3b. PFGE banding patterns of serotype 1/2b isolates formed a cluster with at least 95% similarity; the serotype 3b isolate belonged to a different PFGE genotype (Fig 1). As all the isolates typed were epidemiologically related, this was a polyclonal outbreak.
Fig 1.
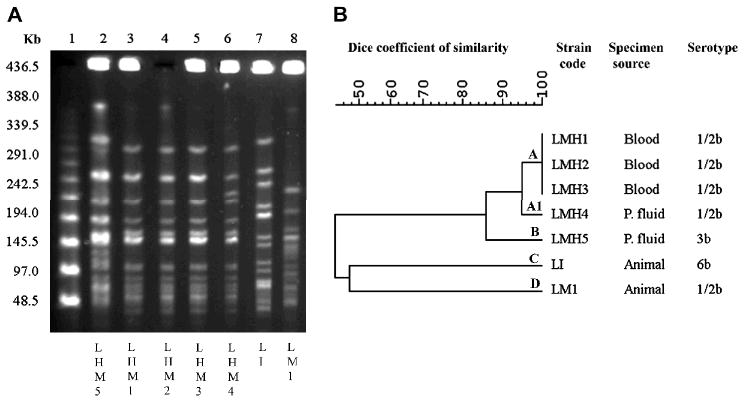
(A) PFGE profiles of ApaI-digested chromosomal DNA of five L. monocytogenes isolates recovered from case-patients. Lines 1 and 9, molecular size markers; lines 2, genotype B isolate from case-patient; lanes 3, 4 and 5, genotype A isolates from case-patients; line 6, genotype A1 isolate from case-patient; lines 7, genotype C isolate (L. monocytogenes) from animal; line 8, genotype D isolate (L. innocua) from animal. (B) Dendrogram resulting from computer-assisted analysis of the profiles shown in panel A. P. fluid: peritoneal fluid.
Discussion
Reports from convenience samples suggest L. monocytogenes is widely disseminated in several food products in Brazil.(27-29) However, the occurrence of outbreaks or the endemicity of listeriosis in Brazil is largely unknown because this is not a notifiable disease in the country. In the present study, a cluster of listeriosis in hospitalized patients is characterized for the first time in Brazil. The evidence that this cluster represented an outbreak is as follows: 1) the cases clustered in time over a 10-month period in one hospital, 2) no cases were identified in any of the other hospitals in the same city during the same time period, 3) 5 of 6 isolates belonged to the same serotype (1/2b), 4) all of the 1/2b serotype strains shared a PFGE pattern with 95% similarity. Potential limitations of the present study are that previous patients with listeria could have occurred in the hospital but not diagnosed. However, neither the microbiology techniques nor the guidelines for collection of clinical specimens for microbiology analysis have changed at least in the previous two years. In addition, as there is no official notification system in the city, we cannot be sure there were no further cases in the city.
The classification of listeriosis as a HAI is usually difficult, since the incubation period of the infection is extremely variable, ranging from a few hours to 70 days.(30;31) We speculate that these cases were hospital-associated because all patients became symptomatic after seven days of admission or had received food in the day-clinic of Hospital A in the previous 3 months.
The possible vehicle of listeria probably originated in the hospital kitchen, since the cases occurred in the different wards of Hospital A and stopped after an intervention in the kitchen. In addition, other hospitals in the city did not report the occurrence of listeriosis, even when specifically inquired, which supported the hypothesis of a common source at the Hospital A. As expected, all cases occurred in patients with predisposing conditions. Three case-patients had bacteremia due to L. monocytogenes serotype 1/2b preceded by diarrhoea, which suggests the bloodstream infections were secondary to gastroenteritis. In fact, outbreaks of gastroenteritis caused by L. monocytogenes serotype 1/2b were previously associated with ingestion of contaminated food.(32;33) Two patients in the present case-series developed primary peritonitis, a condition rarely described and most of the time associated with chronic liver disease.(34;35) Likewise, patients with peritonitis in the present report had cirrhosis. These infections were caused by serotype 1/2b and, surprisingly, serotype 3b. Detection of clinical L. monocytogenes serotype 3b isolates has been rarely reported by surveillance systems and publications in medical reference databases.(36-38) In Brazil, two studies performed by one of the authors included a considerable number of samples from humans, animals, food and the environment from different regions, between 1969 and 2000.(39;40) L. monocytogenes isolates were obtained from more than 3,000 samples, with a predominance of serotypes 4b and 1/2a. Serotype 3b was found only once, from seafood, and serotype 1/2b was found in less than 4% of the specimens, all from humans. The finding of a patient with peritonitis by serotype 3b might indicate intake of heavily-contaminated food or increased virulence of the serotype.
Since listeriosis in hospitalized adult patients is unusual, this diagnosis was not immediately considered for the patients involved in the present outbreak. As a consequence, five patients received empirical therapy inappropriate for L. monocytogenes.(41;42) Mortality related to listeriosis was high and mostly related to bacteremia, as described previously for this pathogen even in patients with correct treatment.(43)
A major concern about HA-listeriosis is the possibility of emergence of multirresistant strains: listeria is already naturally resistant to several antimicrobial agents. Indeed, ampicillin resistance has been previously described in a clinical L. monocytogenes isolate due to a transferable plasmid.(44;45)
Despite active surveillance for HAI have been performed for several years in Hospital A, it does not include foodborne diseases. Descriptive data about the present listeriosis outbreak suggest that a common source of contamination was located in the hospital kitchen. These data highlight the importance by Infection Control Team to be alert about foodborne diseases and the quality of food offered to the patients. Protocols developed for prevention and treatment of HAI in adult patients should include infections caused by L. monocytogenes, mainly in those hospitals for populations with immunosuppressive conditions.
Acknowledgments
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Pesquisa and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro of Brazil, and the Fogarty International Center Program in Global Infectious Diseases Research Training Program (D43 TW006563-07) of the National Institutes of Health in the USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Jones D. The place of Listeria among gram-positive bacteria. Infection. 1988;16 2:S85–S88. doi: 10.1007/BF01639727. [DOI] [PubMed] [Google Scholar]
- 2.Chasseignaux E, Toquin MT, Ragimbeau C, Salvat G, Colin P, Ermel G. Molecular epidemiology of Listeria monocytogenes isolates collected from the environment, raw meat and raw products in two poultry- and pork-processing plants. J Appl Microbiol. 2001 Nov;91(5):888–99. doi: 10.1046/j.1365-2672.2001.01445.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennion JR, Sorvillo F, Wise ME, Krishna S, Mascola L. Decreasing listeriosis mortality in the United States, 1990-2005. Clin Infect Dis. 2008 Oct 1;47(7):867–74. doi: 10.1086/591131. [DOI] [PubMed] [Google Scholar]
- 4.Suarez MM, Bautista RM, Almela M, Soriano A, Marco F, Bosch J, et al. Listeria monocytogenes bacteremia: analysis of 110 episodes. Med Clin (Barc) 2007 Jul 7;129(6):218–21. doi: 10.1157/13107920. [DOI] [PubMed] [Google Scholar]
- 5.Kruszyna T, Walsh M, Peltekian K, Molinari M. Early invasive Listeria monocytogenes infection after orthotopic liver transplantation: case report and review of the literature. Liver Transpl. 2008 Jan;14(1):88–91. doi: 10.1002/lt.21428. [DOI] [PubMed] [Google Scholar]
- 6.Tsai SH, Chu SJ, Wu CP, Wang NC. Listerial meningitis in a patient with undiagnosed acquired immunodeficiency syndrome: ampicillin should be added to the empirical antibiotic coverage. Emerg Med J. 2006 Sep;23(9):e50. doi: 10.1136/emj.2006.036152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radice C, Munoz V, Castellares C, Casanova M, Serrano D, Carrion R, et al. Listeria monocytogenes meningitis in two allogeneic hematopoietic stem cell transplant recipients. Leuk Lymphoma. 2006 Aug;47(8):1701–3. doi: 10.1080/10428190600648135. [DOI] [PubMed] [Google Scholar]
- 8.Mead PS, Dunne EF, Graves L, Wiedmann M, Patrick M, Hunter S, et al. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol Infect. 2006 Aug;134(4):744–51. doi: 10.1017/S0950268805005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food--10 states, 2006. MMWR Morb Mortal Wkly Rep. 2007 Apr 13;56(14):336–9. [PubMed] [Google Scholar]
- 10.Schlech WF, III, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, et al. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–6. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 11.Graham JC, Lanser S, Bignardi G, Pedler S, Hollyoak V. Hospital-acquired listeriosis. J Hosp Infect. 2002 Jun;51(2):136–9. doi: 10.1053/jhin.2002.1234. [DOI] [PubMed] [Google Scholar]
- 12.Shetty A, McLauchlin J, Grant K, O'Brien D, Howard T, Davies EM. Outbreak of Listeria monocytogenes in an oncology unit associated with sandwiches consumed in hospital. J Hosp Infect. 2009 Aug;72(4):332–6. doi: 10.1016/j.jhin.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Hof H, Lampidis R, Bensch J. Nosocomial listeria gastroenteritis in a newborn, confirmed by random amplification of polymorphic DNA. Clin Microbiol Infect. 2000 Dec;6(12):683–6. doi: 10.1046/j.1469-0691.2000.00111.x. [DOI] [PubMed] [Google Scholar]
- 14.Hof H, Lampidis R. Retrospective evidence for nosocomial Listeria infection. J Hosp Infect. 2001 Aug;48(4):321–2. doi: 10.1053/jhin.2001.0999. [DOI] [PubMed] [Google Scholar]
- 15.Winter CH, Brockmann SO, Sonnentag SR, Schaupp T, Prager R, Hof H, et al. Prolonged hospital and community-based listeriosis outbreak caused by ready-to-eat scalded sausages. J Hosp Infect. 2009 Oct;73(2):121–8. doi: 10.1016/j.jhin.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Troxler R, von G A, Funke G, Wiedemann B, Stock I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin Microbiol Infect. 2000 Oct;6(10):525–35. doi: 10.1046/j.1469-0691.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- 17.Vicente MF, Perez-Daz JC, Baquero F, Angel de PM, Berenguer J. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob Agents Chemother. 1990 Apr;34(4):539–42. doi: 10.1128/aac.34.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stepanovic S, Lazarevic G, Jesic M, Kos R. Meropenem therapy failure in Listeria monocytogenes infection. Eur J Clin Microbiol Infect Dis. 2004 Jun;23(6):484–6. doi: 10.1007/s10096-004-1135-3. [DOI] [PubMed] [Google Scholar]
- 19.Murray RP, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology. 9th. Washington, DC: American Society for Microbiology; 2007. [Google Scholar]
- 20.Lee WH, McClain D. Improved Listeria monocytogenes selective agar. Appl Environ Microbiol. 1986 Nov;52(5):1215–7. doi: 10.1128/aem.52.5.1215-1217.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catão R. Listeria spp., coliformes totais e fecais e E. coli no leite cru e pasteurizado de uma indústria de laticícios, no estado da Paraíba (Brasil) In: Ceballos B, editor. Ciênc.Tecnol.Aliment. 3. Vol. 21. 2001. pp. 281–287. [Google Scholar]
- 22.Rocourt J, Schrettenbrunner A, Seeliger HP. Biochemical differentiation of the “Listeria monocytogenes” (sensu lato) genomic groups. Ann Microbiol (Paris) 1983 Jan;134A(1):65–71. [PubMed] [Google Scholar]
- 23.Hudson JA, Lake RJ, Savill MG, Scholes P, McCormick RE. Rapid detection of Listeria monocytogenes in ham samples using immunomagnetic separation followed by polymerase chain reaction. J Appl Microbiol. 2001 Apr;90(4):614–21. doi: 10.1046/j.1365-2672.2001.01287.x. [DOI] [PubMed] [Google Scholar]
- 24.Seeliger HP, Hohne K. Serotyping of Listeria monocytogenes and related species. Methods Microbiol. 1979;13:31–49. [Google Scholar]
- 25.Borucki MK, Call DR. Listeria monocytogenes serotype identification by PCR. J Clin Microbiol. 2003 Dec;41(12):5537–40. doi: 10.1128/JCM.41.12.5537-5540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves LM, Swaminathan B. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol. 2001 Apr 11;65(1-2):55–62. doi: 10.1016/s0168-1605(00)00501-8. [DOI] [PubMed] [Google Scholar]
- 27.Hofer E, Reis CM, Hofer CB. Serovars of Listeria monocytogenes and related species isolated from human clinical specimens. Rev Soc Bras Med Trop. 2006 Jan;39(1):32–7. doi: 10.1590/s0037-86822006000100006. [DOI] [PubMed] [Google Scholar]
- 28.Hofer E, Ribeiro R, Feitosa DP. Species and serovars of the genus Listeria isolated from different sources in Brazil from 1971 to 1997. Mem Inst Oswaldo Cruz. 2000 Sep;95(5):615–20. doi: 10.1590/s0074-02762000000500005. [DOI] [PubMed] [Google Scholar]
- 29.Hofer CB, Melles CE, Hofer E. Listeria monocytogenes in renal transplant recipients. Rev Inst Med Trop Sao Paulo. 1999 Nov;41(6):375–7. doi: 10.1590/S0036-46651999000600008. [DOI] [PubMed] [Google Scholar]
- 30.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007 Aug;9(10):1236–43. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Ooi ST, Lorber B. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis. 2005 May 1;40(9):1327–32. doi: 10.1086/429324. [DOI] [PubMed] [Google Scholar]
- 32.Dalton CB, Austin CC, Sobel J, Hayes PS, Bibb WF, Graves LM, et al. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997 Jan 9;336(2):100–5. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 33.Salamina G, Dalle DE, Niccolini A, Poda G, Cesaroni D, Bucci M, et al. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol Infect. 1996 Dec;117(3):429–36. doi: 10.1017/s0950268800059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adeonigbagbe O, Khademi A, Karowe M, Gualtieri N, Robilotti J. Listeria monocytogenes peritonitis: an unusual presentation and review of the literature. J Clin Gastroenterol. 2000 Jun;30(4):436–7. doi: 10.1097/00004836-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Toyoshima MT, Apanavicius A, de Matos SA, de Almeida GM, Arai MH. Listeria monocytogenes peritonitis in cirrhotic patients: first description in Brazil. Rev Inst Med Trop Sao Paulo. 2006 Sep;48(5):291–3. doi: 10.1590/s0036-46652006000500010. [DOI] [PubMed] [Google Scholar]
- 36.Nagai T, Ikeda M, Sekiguchi H. A case of meningitis infected with Listeria monocytogenes type 3b. Rinsho Byori. 1986 Mar;34(3):282–6. [PubMed] [Google Scholar]
- 37.Goulet V, Hedberg C, Le MA, de V H. Increasing incidence of listeriosis in France and other European countries. Emerg Infect Dis. 2008 May;14(5):734–40. doi: 10.3201/eid1405.071395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbreth SE, Call JE, Wallace FM, Scott VN, Chen Y, Luchansky JB. Relatedness of Listeria monocytogenes Isolates recovered from selected ready-to-eat foods and listeriosis patients in the United States. Appl Environ Microbiol. 2005 Dec;71(12):8115–22. doi: 10.1128/AEM.71.12.8115-8122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofer E, Reis CM, Hofer CB. Serovars of Listeria monocytogenes and related species isolated from human clinical specimens. Rev Soc Bras Med Trop. 2006 Jan;39(1):32–7. doi: 10.1590/s0037-86822006000100006. [DOI] [PubMed] [Google Scholar]
- 40.Hofer E, Ribeiro R, Feitosa DP. Species and serovars of the genus Listeria isolated from different sources in Brazil from 1971 to 1997. Mem Inst Oswaldo Cruz. 2000 Sep;95(5):615–20. doi: 10.1590/s0074-02762000000500005. [DOI] [PubMed] [Google Scholar]
- 41.Troxler R, von GA, Funke G, Wiedemann B, Stock I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin Microbiol Infect. 2000 Oct;6(10):525–35. doi: 10.1046/j.1469-0691.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- 42.Vicente MF, Perez-Daz JC, Baquero F, ngel de PM, Berenguer J. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob Agents Chemother. 1990 Apr;34(4):539–42. doi: 10.1128/aac.34.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerner-Smidt P, Ethelberg S, Schiellerup P, Christensen JJ, Engberg J, Fussing V, et al. Invasive listeriosis in Denmark 1994-2003: a review of 299 cases with special emphasis on risk factors for mortality. Clin Microbiol Infect. 2005 Aug;11(8):618–24. doi: 10.1111/j.1469-0691.2005.01171.x. [DOI] [PubMed] [Google Scholar]
- 44.Poyart-Salmeron C, Carlier C, Trieu-Cuot P, Courtieu AL, Courvalin P. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet. 1990 Jun 16;335(8703):1422–6. doi: 10.1016/0140-6736(90)91447-i. [DOI] [PubMed] [Google Scholar]
- 45.Tsakris A, Papa A, Douboyas J, Antoniadis A. Neonatal meningitis due to multi-resistant Listeria monocytogenes. J Antimicrob Chemother. 1997 Apr;39(4):553–4. doi: 10.1093/jac/39.4.553. [DOI] [PubMed] [Google Scholar]


