Abstract
OBJECTIVES
To compare mortality rates among hospitalized injured elders 67 and older across commonly used follow-up periods (e.g., in-hospital, 30-day, 1-year) and to determine the post-injury time after which mortality rates stabilize.
DESIGN
Retrospective analysis of Medicare claims.
SETTING
Oregon and Washington Medicare patients.
PARTICIPANTS
Patients admitted to 171 Oregon and Washington facilities during 2001–2002 with injuries identified by ICD-9 code and followed for one year.
MEASUREMENTS
The primary outcome was mortality in-hospital and at 30, 60, 90, 180, and 365 days. We also evaluated Kaplan Meier survival curves and daily mortality rates post-admission. The rate of change (slope) in the survival curves and daily mortality rates were analyzed to select the time point after which mortality rates were no longer decreasing.
RESULTS
There were 32,135 injured elders hospitalized over the 2-year period, with a median age of 82 (interquartile range 77–88). Cumulative mortality in-hospital and at 30, 60, 90, 180, and 365 days was: 4.1%, 9.7%, 13.6%, 16.1%, 21.3%, and 28.4%, respectively. Mortality rates stabilized by six months post-injury, with 89% of the change occurring within 60 days. While serious injuries, medical comorbidities, and pre-injury nursing facility residence were all associated with higher mortality, they did not affect the pattern of mortality after injury.
CONCLUSION
In-hospital mortality greatly underestimates post-discharge mortality among injured elders, as a substantial portion of persons die shortly after discharge from the hospital. Mortality rates appear to stabilize by six months after injury, although 60-day post-admission follow-up captures most of the excess daily mortality rate.
Keywords: Geriatrics, Injuries, Trauma, Outcome assessment
INTRODUCTION
Injuries in the elderly are a common cause of morbidity and mortality. Although mortality is a crude measure of global function and health, it is easily categorized and represents a very commonly assessed outcome in research and clinical care. However, a preferred follow-up period after injury for assessing mortality remains unclear. Follow-up periods used in previous geriatric injury studies are highly variable, ranging from the in-hospital period up to six years post-injury.1,2 Commonly used follow-up periods include in-hospital, 30 days, 60 days, 90 days, six months, and one year.3,4 Selection of the follow-up period is often based on convenience, availability of data, available resources, costs, and other factors. Many existing sources of data (e.g., administrative hospital discharge databases and most trauma registries) only track clinical outcomes during the acute hospital stay. Mortality estimates based solely on the in-hospital period might underestimate the significance of injuries, because the early mortality after discharge is larger than the observed hospital mortality.5 For researchers, comparisons of in-hospital mortality may be heavily biased by factors unrelated to either injury severity or quality of care. These may include provider practices, regional standards of care, insurance status, social circumstances (e.g., living alone), hospital availability of a case manager, and access to skilled nursing and rehabilitation facilities.
An ideal duration for follow-up will balance the missed injury-associated mortality seen when patients are followed for too short a period with the increased costs, resources, and proportion of patients lost-to-follow-up seen with longer surveillance. Assessment of the duration of follow-up after which there is minimal change in the mortality rate would provide a guideline for future geriatric injury research, assist in measuring the effect of interventions, and better define the high-risk period following injury for increased clinical vigilance.
The objective of this study was to compare mortality differences among hospitalized injured elders across commonly used follow-up periods (e.g., in-hospital, 30-day, 1-year) and to determine the post-injury time point after which daily mortality rates have minimal change.
METHODS
Study Design
This was a retrospective analysis of Medicare claims for patients ages 67 years and older admitted to 171 Oregon and Washington hospitals with any injury diagnosis.
Study Setting and Population
Medicare patients admitted from January 1, 2001 through December 31, 2002 were identified by ICD-9 diagnosis injury codes 800–959, excluding 905–909 (late effects of injury), 930–939 (foreign bodies), and 958 (complications of injury). All patients had at least one full year of data preceding the injury admission available to determine comorbidity burden and were tracked for 1-year following the date of admission. Patients who died prior to hospital admission (on scene or in the emergency department) were excluded. Only patients enrolled in fee-for-service Medicare programs were included, as our datasets did not track utilization by those in managed care programs. The study was approved with a HIPAA Waiver of Consent by the Oregon Health & Science University (OHSU) Institutional Review Board and the Centers for Medicare and Medicaid Services Privacy Board.
Measurements
The primary data on the patients’ index hospitalization and demographics was released to the investigators by the Centers for Medicare and Medicaid Services from the Medicare Provider Analysis and Review (MEDPAR) file. This file contained up to 9 ICD-9 diagnosis codes. These were used to calculate an ICD-9 based injury severity score (ICISS) for each patient.6 The ICISS is the product of the empirically-derived survival risk ratios of each ICD-9 injury code. Survival risk ratios for each diagnosis factored into the ICISS were derived from the 2002 National Inpatient Sample.7
Injuries were grouped as femoral neck (hip) fractures, other extremity injuries, spinal injuries, head injuries, chest injuries, and other injuries using the method described by Clark et al.8 Hip fractures were defined by an ICD-9 code of 820. Other extremity injuries were defined by ICD-9 codes of 808 (pelvic fractures), 810–819 (upper extremity fractures), 821–829 (lower extremity fractures besides hip fractures), 831–838 (dislocations), 840–845 (sprains and strains), 880–897 (wounds), 912–917 (superficial injuries), 923–924 (contusions), and 927–928 (crush injuries). Spinal injuries were defined by ICD-9 codes 805–806 and 952. Head injuries were defined by ICD-9 codes 800–804 and 850–854. Chest injuries were defined by ICD-9 codes 807, 860–862, 875, and 901. Abdominal injuries were defined by codes 863–868, although this category was not used by Clark et al. A dichotomous measure of major operative intervention was constructed from ICD-9 procedure codes in the MEDPAR file and included any of the following major operative procedures: brain, spine, neck, chest, abdominal, or vascular. Orthopedic surgical procedures were considered separately. Our list of ICD-9 procedure codes constituting major surgery was compiled from six years of Oregon trauma registry data. This list was reviewed by an experienced physician to exclude procedures that would usually be done at the bedside, without the need for the operating room or general anesthesia (e.g., chest tube placement, closed reductions of fractures and dislocations, central venous catheter placement, and sutured laceration repair).
Data for medical comorbidities were obtained from the MEDPAR and Outpatient Medicare Standard Analytical Files for one year prior to the index injury admission. The adaptation of the Charlson comorbidity index devised by Deyo et al was used to convert ICD-9 codes to a comorbidity score.9 This validated system uses weighted scores for 17 diagnoses as well as age to produce a prognostic index for survival. Mortality and date of death were tracked for one year after injury in the Medicare Denominator Standard Analytic File.
Outcomes
Mortality was the primary outcome and was assessed at specific follow-up intervals up to 1 year following the date of the index injury admission. Cumulative mortality was calculated for the following intervals: in-hospital, 30, 60, 90, 180, and 365 days post-admission. Time intervals after injury rather than after discharge were chosen to avoid the variability associated with differing hospital lengths of stay. Hospitalizations at different acute care facilities occurring within two days of each other that did not have a discharge destination code designating them as transfers to an inpatient rehabilitation or intermediate care facility were considered to be inter-hospital transfers and were included in the calculation of hospital stay.
Data Analysis
Descriptive statistics (median, interquartile range [IQR], proportions) were used to characterize the sample. Kaplan Meier survival curves were used to illustrate survival over the follow-up time period. We defined the daily mortality rate as the hazard function, i.e. the number dying on a given day divided by the number alive at the beginning of that day. We considered the earliest point at which the daily mortality rate stopped declining to be the optimum period of follow-up after injury. These points were determined visually by the plot of the daily mortality rates for the overall cohort and for pre-defined subgroups. To minimize the effect of outliers, curves were fit to the data using the locally weighted scatter-plot smoothing (LOWESS) technique.10 The hazard function h(t), which is the probability of death on any given day after injury (t) for those who have survived until that day, was calculated based on the smoothed curve of the daily mortality rate.
While our primary method of assessment was visual inspection of the mortality curves, we also sought to quantify the fall in the daily mortality rate at various time points. We compared the maximum daily mortality rate, which was approximated by the y-intercept of the LOWESS smoothed curve on the day of admission with the final rate to which it settled at one year post-injury. The formula used is shown in the footnote to table 3.
Table 3.
The Percentage Of Elevation In Daily Mortality Rates At Various Time Points Relative To The Rate At One Year Post-Admission For Injured Medicare Patients.
| 30 days | 60 days | 90 days | 180 days | |
|---|---|---|---|---|
|
Overall (n=32,135) |
24.7% | 11.2% | 4.8% | 0.7% |
|
ICISS ≤ 0.9* (n=1,607) |
22.3% | 2.3% | 1.8% | 0% |
|
Charlson ≥3† (n=4,414) |
23.6% | 12.5% | 6.7% | 3.2% |
|
SNF resident prior to Injury‡ (n=1,421) |
52.3% | 16.3% | 9.9% | 5.1% |
|
Hip Fracture (n=11,738) |
31.2% | 12.2% | 5.9% | 0% |
Seriously injured subgroup, defined by ICD-9 based Injury Severity Score (ICISS) ≤ 0.9.
High co-morbidity subgroup, defined by a Charlson co-morbidity score ≥3.
Skilled nursing facility (SNF) residents prior to injury.
We also analyzed mortality rates and patterns in predefined sub-groups: seriously injured patients, those with a high comorbidity burden, and residents in skilled nursing facilities prior to admission. We defined seriously injured patients as those with an ICISS of 0.9 or less, a definition used in previous trauma studies.11 We defined patients with an elevated comorbidity burden as those with a Charlson score of ≥ 3. Skilled nursing facility (SNF) residence prior to injury was defined by a hospitalization with an indicator as a SNF in the MEDPAR file within 90 days preceding the index hospitalization.12
Database management and analysis was performed using SAS v.9.1 (SAS Institute, Cary, NC). LOWESS smoothing curves were generated using Robust Fit (University of St. Andrews, Fife, Scotland). Figures were created using Microsoft Excel 2007 (Microsoft, Redmond, WA).
RESULTS
There were 32,135 injured elders admitted to 171 hospitals over the two-year period, of whom 4.1% died during the initial hospital stay and 28.4% died within one year of the date of admission. Characteristics of the patient population and details of their injury hospitalization are described in Table 1. Median age at the time of the injury hospitalization was 82 years (IQR 77–88) and the median ICISS was 0.97 (IQR 0.96–0.98). Median length of hospital stay was 4 days (IQR 3–6, range 0–109). Hip fractures were the most common injury (36.5% of patients).
Table 1.
Characteristics of Hospitalized Injured Medicare Patients In Oregon And Washington (N=32,135).
| n | ||
|---|---|---|
| Male | 10,382 | 32.3% |
| Age at time of injury (median, IQR) | 82 | 77–88 |
| Age ranges | ||
| 67–69 | 1,814 | 5.6% |
| 70–79 | 9,869 | 30.7% |
| 80–89 | 14,559 | 45.3% |
| 90–99 | 5,704 | 17.7% |
| 100–104 | 189 | 0.6% |
| White | 30,993 | 96.4% |
| Charlson co-morbidity index (median, IQR) | 1 | 0–2 |
| 0 | 15,042 | 46.8% |
| 1–2 | 12,679 | 39.4% |
| ≥3 | 4,414 | 13.7% |
| Skilled nursing facility resident prior to injury | 1,421 | 4.4% |
| Index injury hospitalization | ||
| Length of stay (median days, IQR) | 4 | 3–6 |
| Inter-hospital transfer after admission | 771 | 2.4% |
| ICD-9 based Injury Severity Score (ICISS, median, IQR) | 0.97 | 0.96–0.98 |
| Serious injury (ICISS ≤0.9) | 1,607 | 5.0% |
| Any major surgery | 18,447 | 57.4% |
| Major non-orthopedic surgery | 1,528 | 4.8% |
| Injuries* | ||
| Hip fracture | 11,738 | 36.5% |
| Other extremity injury | 12,751 | 39.7% |
| Spine injury | 2,278 | 7.1% |
| Head injury | 2,383 | 7.4% |
| Chest injury | 1,960 | 6.1% |
| Abdominal injury | 538 | 1.7% |
Categories of injury were not exclusive. Patients could have had more than one injury.
Cumulative mortality in-hospital and at fixed time points after injury are described in Table 2. Mortality in-hospital (4.1%) substantially underestimated mortality relative to 30 days from the initial date of admission (9.7%) and longer follow-up periods. Mortality for all the subgroups (seriously injured, high-comorbidity burden, and SNF residents prior to injury admission) was substantially higher than for the overall cohort at all time points.
Table 2.
Cumulative Mortalities and Daily Mortality Rates (hazard function) At Pre-Defined Time Points After Admission.
| In-hospital | 30 days | 60 days | 90 days | 180 days | 365 days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Mortality | ||||||||||||
|
Overall (n=32,135) |
1,331 | 4.1% | 3,127 | 9.7% | 4,382 | 13.6% | 5,176 | 16.1% | 6,832 | 21.3% | 9,122 | 28.4% |
|
ICISS ≤ 0.9* (n=1,607) |
351 | 21.8% | 437 | 27.2% | 503 | 31.3% | 530 | 33.0% | 584 | 36.3% | 648 | 40.3% |
|
Charlson score ≥3† (n=4,414) |
295 | 6.7% | 693 | 15.7% | 959 | 21.7% | 1,142 | 25.9% | 1,511 | 34.2% | 1,949 | 44.2% |
|
SNF resident before injury‡ (n=1,421) |
81 | 5.7% | 236 | 16.6% | 337 | 23.7% | 387 | 27.2% | 507 | 35.7% | 635 | 44.7% |
|
Hip Fracture (n=1 1,738) |
424 | 3.6% | 1,205 | 10.3% | 1,733 | 14.8% | 2,069 | 17.6% | 2,731 | 23.2% | 3,586 | 30.6% |
| Daily Mortality Rate (hazard function) | ||||||||||||
| Overall | 0.00203 | 0.00115 | 0.00082 | 0.00060 | 0.00057 | |||||||
| ICISS ≤ 0.9 | 0.00383 | 0.00136 | 0.00127 | 0.00097 | 0.00104 | |||||||
|
Charlson score ≥3 |
0.00301 | 0.00202 | 0.00150 | 0.00120 | 0.00091 | |||||||
|
SNF resident before injury |
0.00404 | 0.00213 | 0.00179 | 0.00154 | 0.00127 | |||||||
| Hip Fracture | 0.00224 | 0.00129 | 0.00098 | 0.00061 | 0.00069 | |||||||
The daily mortality rate was calculated as the number dying on a given day divided by the number alive at the beginning of that day.
Seriously injured subgroup, defined by ICD-9 based Injury Severity Score (ICISS) ≤ 0.9.
High co-morbidity subgroup, defined by a Charlson co-morbidity score ≥3.
Skilled nursing facility (SNF) residents prior to injury.
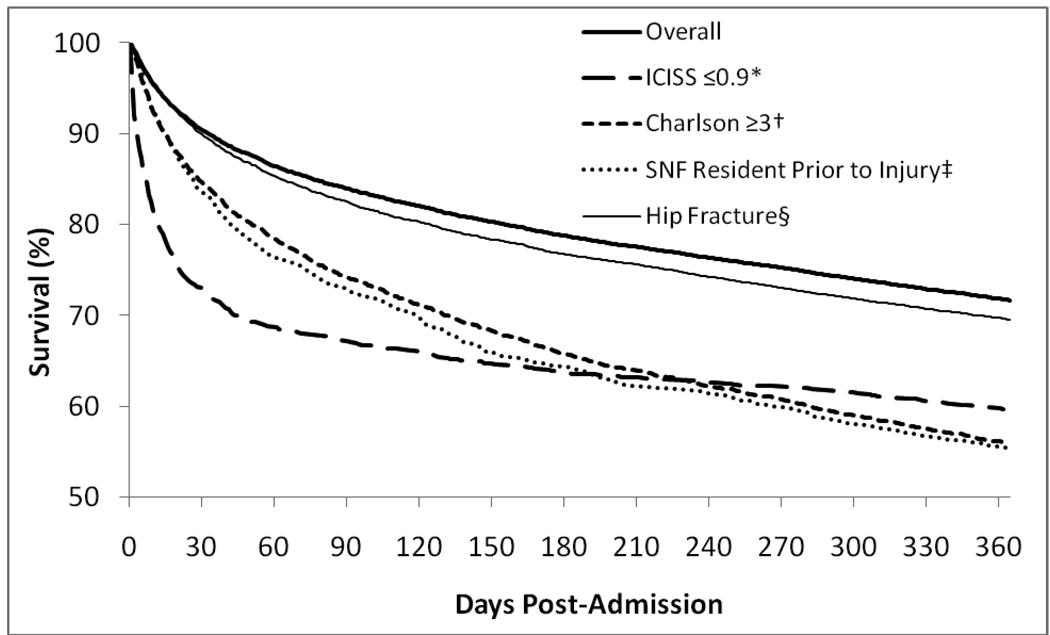
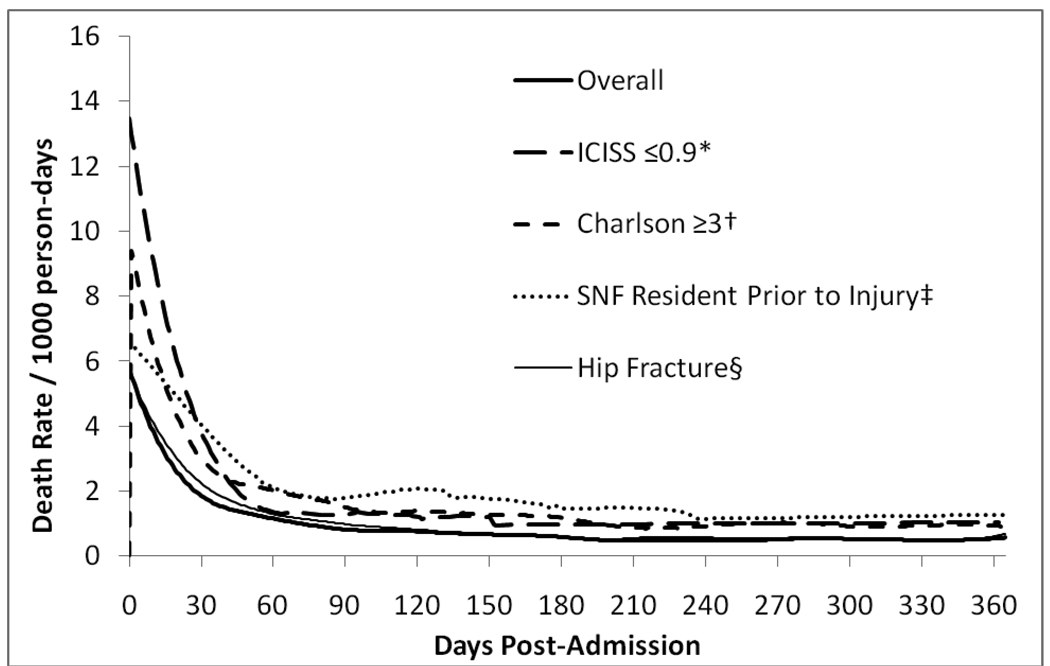
Kaplan-Meier survival curves are shown in Figure 1 for the entire cohort and subgroups. Figure 2 represents the same information as plots of the daily mortality rate on each day following the date of admission. This plot could also be described as the smoothed hazard estimate × 1000. Patients with serious injuries, high comorbidity burden, nursing facility residents and those with hip fractures all showed higher mortality than the overall cohort; however, the pattern of mortality was similar among all groups. In each case, the daily mortality rate (i.e., the instantaneous slope of the survival curve in figure 1) had settled to the same rate seen at one year post-injury by six months. This stabilization at 180 days is shown numerically in Table 2, in which the smoothed daily mortality rate (the hazard function) for the overall cohort is 0.00060 at 180 days post-injury and decreases only slightly to 0.00057 at 365 days. Most of the change occurred by 60 days. The seriously injured subgroup experienced a higher daily mortality rate in the early period following injury, extending through 90 days, but with a later cumulative mortality similar to the other subgroups.
Figure 1. Kaplan-Meier Curves For Post-Injury Survival Among Hospitalized Medicare Patients (n = 32,135).
*Seriously injured subgroup, defined by ICD-9 based Injury Severity Score (ICISS) ≤ 0.9 (n=1,607). †High co-morbidity subgroup, defined by a Charlson co-morbidity score ≥3 (n=4,414). ‡Skilled nursing facility (SNF) residents prior to injury (n=1,421). §Hip fracture (n=11,738).
Figure 2. Daily Mortality Rates Beginning With Admission For Hospitalized Injured Medicare Patients (n=32,135).
The daily mortality rate was calculated as the number dying on a given day divided by the number alive at the beginning of that day, divided by 1000 for clarity. These rates could also be described as the smoothed hazard estimates × 1000. *Seriously injured subgroup, defined by ICD-9 based Injury Severity Score (ICISS) ≤ 0.9 (n=1,607). †High co-morbidity subgroup, defined by a Charlson co-morbidity score ≥3 (n=4,414). ‡Skilled nursing facility (SNF) residents prior to injury (n=1,421). §Hip fracture (n=11,738).
Table 3 quantifies the degree to which the daily mortality rate was elevated compared to where it settled at one year post-injury. For the full cohort, the daily mortality rate at 60 days had fallen to within 11.2% of the mortality rate at one year; the rate at 90 days had fallen to within 4.8%. Subgroups of patients with a high comorbidity burden, skilled nursing facility residents, and those with hip fractures displayed similar patterns of mortality. Seriously injured patients displayed a more rapid decline of mortality rate (i.e., most patients dying following injury did so earlier than other subgroups and the full cohort), with 60-day follow-up capturing all except 2.3% of the excess daily mortality rate increase compared with one-year.
DISCUSSION
In this study, we demonstrate substantial differences in mortality among Medicare patients hospitalized with injuries depending on the follow-up period used. In-hospital mortality, a very commonly used outcome measure in injury research, was less than half the 30-day mortality (following date of admission), suggesting that a substantial portion of hospitalized injured elders will die shortly after being discharged from the hospital. These findings also demonstrate significant limitations with geriatric injury studies using in-hospital mortality as the primary outcome measure. Our results also demonstrate that the change in mortality rates over time appears to stabilize by six months after injury, suggesting that this time period represents the preferred duration of follow-up, although 60 days may be a reasonable proxy, particularly for seriously injured patients.
Previous studies have shown the inadequacy of relying upon in-hospital mortality as a primary outcome measure. Jencks et al demonstrated that conclusions about outcomes for elders admitted in different regions of the country for four medical diagnoses differed based on whether in-hospital or 30-day mortality was measured.13 A study of injured elders by Gorra et al demonstrated similar variation between regions based on when the mortality outcome was measured.14 Mullins et al found that in an all-ages cohort of admitted injured patients, substantially more patients died within 30 days of being discharged from the hospital alive than died in-hospital.5 This effect was most prominent among elderly patients, particularly those identified as having a cause of death related to trauma. Our study reproduces this finding of many deaths closely following discharge in an elderly population.
This balance between in-hospital and early post-discharge mortality may be affected by factors at the patient, provider, and community level. Patient level factors include disease processes which may be incompletely treated and more or less appropriate for palliation outside the acute care hospital, family resources for home care, and end-of-life preferences. Provider-level factors may include trauma center designation, availability of case managers, and provider thoughts about futile care. Community-level factors may include established regional treatment practices and the availability of skilled nursing and rehabilitation facilities. Recognizing the multi-dimensional causes of variation in hospital stays, we believe our results and those of others support evaluating outcomes at fixed intervals after injury when possible.
Given the agreement of our findings with previous authors that in-hospital mortality is a suboptimal outcome measure for injured elders, we sought to find the shortest appropriate fixed follow-up period after injury. One approach would have been to follow patients until the mortality rate for the injured cohort returned to that seen in the general population. This approach is unworkable, however, as previous studies by Gubler et al and McGwin et al both showed that mortality rates for injured Medicare patients never returned to baseline, even through five to six years of follow-up.1,2 Another approach would have been to follow patients through the episode of care related to their injury, linking claims that appeared to be temporally and anatomically related, as done by Lestina et al for a cohort of injured managed care patients.15 The limitation of this approach to the issue of mortality assessment is that episodes of care will be quite different for patients with fatal versus non-fatal injuries. Our approach was to consider the period after injury during which the daily mortality rate (hazard function) was elevated as the high-risk period through which surveillance should continue.
We found that the daily mortality rate did not change from 180 to 365 days post-injury. These results are consistent with Gubler’s et al and McGwin et al’s findings. While neither author reported exactly how long their initial period of declining mortality rates persisted, examination of survival curves from both studies suggests that it was less than six months after injury. Our results were also consistent with those of Wunsch et al, who found that while cumulative mortality in hospitalized Medicare patients was elevated at three years of follow-up when compared with matched non-hospitalized controls, this effect was most pronounced in the first six months after hospitalization.16 This effect was greatest in those who required mechanical ventilation in an intensive care unit.
Although our daily mortality rates had stabilized by six months post-injury, 60 days appeared to be an acceptable alternative follow-up interval, with 89% of the fall in daily mortality rate accounted for by 60 days. Even greater capture of post-injury changes in mortality rates would be achieved by extending follow-up to 90 days, which would capture 95% of the excess daily mortality rate. Both in-hospital and 30-day mortality provide incomplete follow-up periods, as the daily mortality rate continued to fall within the 30–60 day window.
To assess applicability to a more seriously injured population, such as that seen in trauma registries, we analyzed the subgroup with an ICISS ≤ 0.9. The seriously injured experienced the highest early mortality as well as the earliest decline to a steady daily rate of mortality after injury. While this decline was not so precipitous that we would advocate using 30-day follow-up as an ideal measure in a seriously injured population, 60-day surveillance should be reliable. We used prior nursing home residence as another marker of poorer baseline functional and medical status; this group also displayed similar results of an overall elevated cumulative mortality but a similar pattern of decline in the mortality rate. This finding suggests that these conclusions can be applied equally to studies involving community dwelling or institutionalized elders.
Hip fractures comprised more than a third of our cohort. Our cumulative mortalities (Table 2) are similar to those reported by Brauer et al in a recent observational study of a nationwide Medicare sample.17 Our 30-day mortality of 10.3% is within the range she reported for 2002 of 6% for women and 12% for men. Our one-year mortality of 30.6% was also similar to her estimates of 23% for women and 35% for men. This supports our conclusion that in-hospital mortality is a poor marker of overall outcomes for the hip-fracture population as well.
Previous studies have demonstrated the influence of medical co-morbidities on trauma outcomes. In a case-control study of adult trauma patients of all ages, Morris et al showed significantly more comorbidities in those who died versus surviving controls matched by age and injury severity.18 Hollis et al demonstrated increased odds of death in injured patients with medical co-morbidities when stratified by age, although this effect was only sustained in mildly and moderately injured subsets.19 We compared the group with a high co-morbidity burden (Charlson score ≥3) to the entire cohort. This group had a substantially higher mortality at most time points, supporting the conclusion of Hollis et al that pre-existing medical conditions influence trauma outcomes. While overall mortality was higher among those with comorbidities, the pattern and timing of their deaths after injury were similar to the overall cohort, again supporting a stable daily mortality rate within a follow-up interval between 60 days and six months. This subgroup exhibited survival curves and daily mortality rates similar to the prior skilled nursing facility residents.
Our findings have important implications for both the researcher and the geriatrician. For the researcher, they show that studies using in-hospital mortality will be heavily biased by factors influencing whether death occurs in-hospital or shortly after discharge. Investigators should make every effort to identify and control for these factors, define mortality outcomes at a 60-day to six-month time-point after injury, or use outcomes based on survival time rather than dichotomous measures of mortality. For clinicians, our findings highlight the need for greater planning for post-discharge care to prevent post-discharge death when possible. Knowing these survival patterns will help geriatricians counsel their patients on treatment plans that agree with their end of life preferences.
Limitations
Our cohort consisted of elders hospitalized with injury diagnoses. Therefore, injured elders who did not survive to hospital admission or who were discharged home from an outpatient setting (i.e. the emergency department or clinic) were not included in the sample. Inclusion of these patients may have changed our findings, although it is uncommon for an elder at high-risk to be released without at least a period of observation in the hospital. Another piece of information missing from our data was mechanism of injury. While the dataset did contain ICD-9 mechanism of injury codes (e-codes), these were reported in only 29% of cases, and were therefore considered to be unreliable. Furthermore, we are forced to assume that the injury occurred on or just before the date of admission.
While mortality is an easily defined outcome, it does not tell the full story of the sequelae of geriatric injury. Even injuries which are not fatal may have significant effects on functional outcomes (e.g., return to independent living, ability to ambulate, self-sufficiency with activities of daily living). We did not examine non-mortality or functional outcomes, as reliable markers of function were not available in the database. Discharge disposition (e.g., to an inpatient rehabilitation facility or skilled nursing facility) was inconsistently reported. Similarly, the presence or absence of nursing facility bills may be unreliable, as Medicare only pays for inpatient rehabilitation and skilled nursing facilities, but not for other types of institutions for the elderly. These are inherent limitations of outcomes research based on administrative billing databases. A strength of Medicare data is its accuracy in reporting the date of death, as it is based on daily reports from the Social Security Administration.
Using a regional dataset may also limit generalizability. For example, elders in the Pacific Northwest are racially homogenous and may not be comparable to elder populations in other regions of the US. Another limitation is the lack of data from the 19% of the Medicare entitled population covered by Medicare managed care organizations, a percentage that is even higher in the West.8 These patients may have different baseline characteristics than the fee-for-service Medicare population which constituted our sample.
CONCLUSION
In-hospital mortality underestimates injury mortality among elders, as our results suggest that many injured elders die shortly after discharge from an acute care hospital. Mortality rates appear to stabilize by six months after the initial date of admission, although 60-day follow-up will capture most of the increased mortality rate, especially in the most seriously injured. For clinicians, these time intervals represent periods requiring particular vigilance for complications and adverse outcomes from injury. For researchers, these follow-up intervals may serve as guidelines in the design of future geriatric injury studies.
ACKNOWLEDGMENTS
This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Sponsor’s role: The Oregon Clinical and Translational Research Institute (OCTRI) provided educational programs for Dr. Fleischman’s research training but had no direct role in this project.
Footnotes
Presentations: An abstract of this project was presented at the 2009 Society for Academic Emergency Medicine Annual Meeting in New Orleans, LA (May 17, 2009).
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Ross J. Fleischman: Principal investigator on this project. Primarily responsible for concept and design, analysis, interpretation and drafting the manuscript. Annette L. Adams: Responsible for study concept, acquiring data, data cleaning, and revision of manuscript.
Jerris R. Hedges Responsible for study concept, acquiring data, and revision of manuscript.
O. John Ma: Responsible for interpretation of data and revision of manuscript.
Richard J. Mullins: Responsible for interpretation of data and revision of manuscript.
Craig D. Newgard: Fellowship mentor for Dr. Fleischman. Involved in study concept, data acquisition, analysis, interpretation, and manuscript revision.
All authors approved the final version as submitted.
REFERENCES
- 1.Gubler KD, Davis R, Koepsell T, et al. Long-term survival of elderly trauma patients. Arch Surg. 1997;132:1010–1014. doi: 10.1001/archsurg.1997.01430330076013. [DOI] [PubMed] [Google Scholar]
- 2.McGwin G, Melton SM, May AK, et al. Long-term survival in the elderly after trauma. J Trauma. 2000;49:470–476. doi: 10.1097/00005373-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Clark DE, DeLorenzo MA, Lucas FL, et al. Epidemiology and short-term outcomes of injured medicare patients. J Am Geriatr Soc. 2004;52:2023–2030. doi: 10.1111/j.1532-5415.2004.52560.x. [DOI] [PubMed] [Google Scholar]
- 4.Shyu YI, Liang J, Wu C, et al. Interdisciplinary Intervention for Hip Fracture in Older Taiwanese: Benefits Last for 1 Year. J Gerontol. 2008;63:92–97. doi: 10.1093/gerona/63.1.92. [DOI] [PubMed] [Google Scholar]
- 5.Mullins RJ, Mann NC, Hedges JR, et al. Adequacy of hospital discharge status as a measure of outcome among injured patients. JAMA. 1998;279:1727–1731. doi: 10.1001/jama.279.21.1727. [DOI] [PubMed] [Google Scholar]
- 6.Osler T, Rutledge R, Deis J, et al. ICISS: An international classification of disease-9 based injury severity score. J Trauma. 1996;41:380–388. doi: 10.1097/00005373-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 7.2002 NIS Introduction. [Accessed March 25, 2008];Agency for Healthcare Research and Quality (online) Available at: www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2002.jsp.
- 8.Clark DE, DeLorenzo MA, Lucas FL, et al. Injuries among older americans with and without medicare. Am J Public Health. 2005;95:273–278. doi: 10.2105/AJPH.2003.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical co-morbidity index for use with icd-9-cm administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Hen I, Sakov A, Kafkafi N, et al. The dynamics of spatial behavior: How can robust smoothing techniques help? J Neurosci Methods. 2004;133:161–172. doi: 10.1016/j.jneumeth.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Diggs BS, Mullins RJ, Hedges JR, et al. Proportion of seriously injured patients admitted to hospitals in the US with a high annual injured patient volume: A metric of regionalized trauma care. J Am Coll Surg. 2008;206:212–219. doi: 10.1016/j.jamcollsurg.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen-Oghalai TU, Kuo YF, Zhang DD, et al. Discharge setting for patients with hip fracture: Trends from 2001 to 2005. J Am Geriatr Soc. 2008;56:1063–1068. doi: 10.1111/j.1532-5415.2008.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data. JAMA. 1988;260:2240–2246. [PubMed] [Google Scholar]
- 14.Gorra AS, Clark DE, Mullins RJ, et al. Regional variation in hospital mortality and 30-day mortality for injured medicare patients. World J Surg. 2008;32:954–959. doi: 10.1007/s00268-007-9410-y. [DOI] [PubMed] [Google Scholar]
- 15.Lestina DC, Miller TR, Smith GS. Creating injury episodes using medical claims data. J Trauma. 1998;45:565–569. doi: 10.1097/00005373-199809000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Wunsch H, Guerra C, Barnato AE, et al. Three-year outcomes for medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 17.Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the united states. JAMA. 2009;302:1573–15579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JA, MacKenzie EJ, Edelstein SL. The effect of preexisting conditions on mortality in trauma patients. JAMA. 1990;263:1942–1946. [PubMed] [Google Scholar]
- 19.Hollis S, Lecky F, Yates DW, et al. The effect of pre-existing medical conditions and age on mortality after injury. J Trauma. 2006;61:1255–1260. doi: 10.1097/01.ta.0000243889.07090.da. [DOI] [PubMed] [Google Scholar]