Abstract
The balance between oxidation and antioxidation is believed to be critical in maintaining healthy biological systems. Under physiological conditions, the human antioxidative defense system including e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione (GSH) and others, allows the elimination of excess reactive oxygen species (ROS) including, among others superoxide anions (O2.-), hydroxyl radicals (OH.), alkoxyl radicals (RO.) and peroxyradicals (ROO.). However, our endogenous antioxidant defense systems are incomplete without exogenous originating reducing compounds such as vitamin C, vitamin E, carotenoids and polyphenols, playing an essential role in many antioxidant mechanisms in living organisms. Therefore, there is continuous demand for exogenous antioxidants in order to prevent oxidative stress, representing a disequilibrium redox state in favor of oxidation. However, high doses of isolated compounds may be toxic, owing to prooxidative effects at high concentrations or their potential to react with beneficial concentrations of ROS normally present at physiological conditions that are required for optimal cellular functioning. This review aims to examine the double-edged effects of dietary originating antioxidants with a focus on the most abundant compounds, especially polyphenols, vitamin C, vitamin E and carotenoids. Different approaches to enrich our body with exogenous antioxidants such as via synthetic antioxidants, diets rich in fruits and vegetables and taking supplements will be reviewed and experimental and epidemiological evidences discussed, highlighting that antioxidants at physiological doses are generally safe, exhibiting interesting health beneficial effects.
Key words: antioxidants, reactive oxygen species, oxidative stress, double-edged effects, fruits and vegetables, supplements, physiological doses, high doses
Introduction
Humans live in the presence of various ubiquitous environmental stressors including UV radiation, microbes, allergens and various pollutants such as increased ozone, cigarette smoke and polycyclic aromatic hydrocarbons, which can amplify the generation of reactive oxygen species (ROS) in the body.1–5 ROS can be defined as intermediate oxygen carrying metabolites with or without an unpaired electron, comprising oxyradicals (i.e., oxygen-centered free radicals) such as superoxide anions (O2•-), hydroxyl radicals (OH•), alkoxyl radicals (RO•) and peroxyradicals (ROO•) and non-radicals such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl) and singlet oxygen (1O2), able to oxidize other components and turning them into free radicals, often causing a chain reaction leading to the formation of numerous new radicals.6–9 Prominent radicals that may be formed in vivo include both relatively stable radicals such as the urate radical (UrH•-), the ascorbyl radical (Asc•-), the vitamin E radical (VE•) and phenoxyl radicals (Phl•), and reactive radicals encompassing carbon-centered free radicals [e.g., lipid radicals (L•)] and sulphur-centered radicals [e.g., glutathiyl radicals (GS•)], which, in aerobic medium, can result in species with higher oxidative potential [such as lipid peroxyl radicals (LOO•), lipid alkoxyl radicals (LO•) and thiyl radicals (GSOO•, GSO• and GSO2OO-)].7,10 Radical chain reaction typically continues until the system becomes anaerobic or the substrate [e.g., membrane fatty acids (LH)] is depleted; however the chain reaction can be stopped when two radicals form non-radical products or by the presence of chain-breaking antioxidants (e.g., vitamin E and polyphenols).7,10,11
Physical stressors such as acute aerobic, anaerobic and intense exhaustive exercise can result in excessive reactive oxygen production.12–14 In this regard, the superoxide radical (O2•-), resulting from monoelectronic reduction of oxygen, is considered to be the precursor of ROS including OH-, RO-, ROO- and H2O2.9 For instance, the superoxide radical (O2•-) can react with nitric oxide (-NO), a nitrogen-centered radical, generating a highly reactive molecule, the peroxynitrite anion (ONOO-), also termed a reactive oxygen and nitrogen species (RONS), able to cause DNA fragmentation and lipid oxidation.7,8,10 Animal experiments have shown that stressful situations such as immobilization stress and sleep deprivation stimulate excessive production of such toxic oxygen metabolites.15,16 Emotional stress and depressed mood are also associated with a massive formation of oxygen free radicals.9,17–21 Overproduction of oxygen-derived radical species can further result from diets excessive in fat and carbohydrates and are relatively deficient in antioxidant vitamins.7,22,23 Other conditions or pathways which may amplify ROS formation favouring oxidative stress include metabolism of alcohol or pharmaceutical agents, therapeutic (x-ray) radiation, hyperthermia, inflammation and iron overload.3,24,25 Therefore, our antioxidant system has to be efficient against these stressful conditions, some of which can routinely occur in our daily lives, avoiding the onset of oxidative stress, constituting a causative or associated risk factor for a number of human diseases including chronic complications such as cardiovascular diseases (CVD),7,26 cancer,7,27 and neurodegenerative diseases,7,28 with probably over 100 associated diseases in total.29 Furthermore, our antioxidant system, which is incomplete without exogenous reducing compounds such as vitamin C, vitamin E, carotenoids and polyphenols, has the role to quench excess oxygen-derived reactive species generated during normal cellular metabolism utilizing molecular oxygen such as during mitochondrial respiration (in which 85% of inhaled oxygen is metabolized) and processes catalyzed by NAD(P)H oxidase and xanthine oxidase.7,9
The following review focuses on the double-edged effects of natural antioxidants. We report several studies showing controversial results of exogenous antioxidants, discussing that the type, dosage and matrix of exogenous antioxidants may be determining factors impacting the balance between beneficial or deleterious effects of these natural compounds.
Evidence of Double-Edged Effects of Exogenous Antioxidants
Definition, necessity and sources.
Owing to the fundamental role of antioxidants in human life and health, and their general popularity due to increased media attention, the demand for these compounds by the general public has been recently increasing. Antioxidants have been defined as substances that, when present at low concentrations compared to an oxidizable compound (e.g., DNA, proteins, lipids or carbohydrates), delay or prevent oxidative damage caused by the presence of ROS.30,31 ROS at high doses become deleterious, exhibiting pathophysiological actions, whereas, at low doses they may be beneficial for normal physiological actions (reviewed in ref. 7, 26, 32 and 33). Exogenous antioxidants play a key role in this delicate equilibrium between oxidation and antioxidation in living systems.7,9,34,35
Our antioxidant defense system includes endogenous (enzymatic and non-enzymatic) antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione (GSH), among others and exogenous antioxidants such as vitamin C, vitamin E, carotenoids and polyphenols, with the diet being the main source (Table 1).9,34,36,37 Endogenous and exogenous antioxidants act interactively (e.g., synergistically) to maintain or re-establish redox homeostasis, such as during the regeneration of vitamin E by glutathione (GSH) or vitamin C to prevent lipid peroxidation processes,7 which can affect membrane fluidity and damage membrane proteins by e.g., inactivating receptors, enzymes and ion channels, even disrupting membrane integrity resulting eventually in cell death.17 Catechins might prevent the consumption of vitamin E by scavenging hydrophilic radicals near membrane surfaces, whereas vitamin E scavenges lipid peroxyl radicals (LOO-) as hydrogen donor to stop free radical chain reactions (chain-breaking antioxidant).11 The intake of complete foods rich in naturally-occurring antioxidants, including nutrients (e.g., vitamins) and phytochemicals (e.g., polyphenols) has been widely recommended by many health organizations, such as within the “five a day campaign.”38 Indeed, humans are not capable of synthesizing these antioxidant compounds de novo; and plant food (e.g., apples, plums, bananas, tomatoes, potatoes, onions, broccolis, etc.,) constitutes the natural source of these antioxidants.9,34,36,37,39 In addition to their natural occurrence in foods, fortification, supplementation with isolated components and intake of synthetic antioxidant additives such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tert-butyl hydroquinone (TBHQ) and propyl, octyl and dodecyl gallates (used initially to protect and to preserve the nutritional quality and to increase shelf-life of processed foods)40,41 constitute further sources of antioxidants.
Table 1.
Human antioxidant defense systems include endogenous (enzymatic and non-enzymatic) and exogenous antioxidants, with the diet being the main exogenous source
| Antioxidant defense system | |
| Endogenous antioxidants | Exogenous antioxidants |
Enzymatic antioxidants
|
Prinicipal dietary antioxidants from fruits, vegetables and grains
|
| Non-enzymatic antioxidants (principal intracellular reducing agents) Glutathione (GSH), uric acid, lipoic acid, NADPH, coenzyme Q, albumin, bilirubin | |
and their glucosides.
In vitro evidence.
In vitro studies have highlighted the cytoprotective activity of plant food constituents such as polyphenols and mixtures and their preventive effects against oxidative stressinduced cell death.42–46 Thus, although the antioxidant activity of phytochemicals is well recognized,7,9,34,35 they can also display prooxidant activities under certain conditions, such as at high doses or in the presence of metal ions.47–50 The prooxidant or antioxidant activity intimately depends on their concentration. In this regard, recent studies employing cell models have highlighted the prooxidative activity of several polyphenols already known as antioxidants such as quercetin, catechins including epicatechin and epigallocatechin-3-gallate (EGCG) and gallic acid.50–55 For example, at high doses, it has been demonstrated that quercetin (50 µM) can potentiate superoxide radical (O2•-) generation within isolated mitochondria and cultured cells.51 In another study, the antioxidant activity of quercetin was observed only at low doses (0.1–20 µM) while higher concentrations (>50 µM) decreased cell survival and viability, thiol content, total antioxidant capacity and activities of SOD, CAT and glutathione S-transferase.52 It has also been demonstrated that flavonoids (quercetin and fisetin) at low concentrations (10–25 µM) protect rat H4IIE cells against H2O2-induced cytotoxicity, DNA strand breaks and apoptosis, whereas high concentrations (50–250 µM) caused cytotoxicity, DNA damage and apoptosis.50 It was also shown that flavonoids at high concentrations can generate ROS by autoxidation (e.g., myricetin and quercetagetin) and redox-cycling (e.g., quercetin).56–59
In addition to the concentration of antioxidants, the presence of metal ions has been reported to play an important role. It was revealed that EGCG in the presence of transition metals causes oxidative damage to isolated and cellular DNA.53 Dietary antioxidants such as phenolics can display prooxidant activities in the presence of metal ions owing to their reducing capacity and forming chelates, such as with the transition metals iron and copper, which are important properties of these compounds in plants.3,47,48,53 The mechanism of the antioxidative action of natural compounds is considered as primary when antioxidants act directly on free radicals (-R-) by a scavenging process characterized by the donation of hydrogen atoms (resulting in the formation of -RH) or electrons (resulting in the formation of -R-).60–62 It is secondary, when the antioxidants absorb UV radiation or intervene in anti-oxidation processes as chelators of transition metal ion catalysts, act as deactivators of singlet oxygen (1O2) or convert hydroperoxides (ROOH) to non-radical species.60–63 However, the strong reducing power of antioxidants may also affect metal ions, especially Fe3+ and Cu2+, increasing their ability to form highly reactive hydroxyl radical concentrations, potentially harmful radicals, originating from peroxides via the Fenton (2) reaction.7,10
| (1) |
| (2) |
Such conditions could be problematic in organisms overloaded by iron as in the case of hemochromatosis,7 a disease characterized by increased iron absorption and storage from the diet. As a consequence, the metal chelating activity of several phenolics may result in the reduction of the prooxidant capacity of metal ions, however, phenolics may also act as prooxidants by chelating metals in a manner that maintains or increases their catalytic activity.48 In vitro, it has been shown that the pH influences oxidoreductions of phenolic compounds, suggesting that the pH of biological tissues could impact antioxidant/prooxidant activities of phenolics and their chelating activity. For example, a decrease in pH causes a reduced chelating effect of phenolics toward iron, possibly due to increased solubility of the complexes.48,64 The effect of pH could however be different for various phenolics. While at pH 7.4 certain phenolics have displayed prooxidant activities which at lower pH (5.8) were reported to possess antioxidant properties (e.g., γ-resorcyclic acid), others have exhibited antioxidant activities (e.g., hydrobenzoic acid).48,64
Antioxidant phenolics, when scavenging free radicals, can form less reactive phenoxyl radicals, which are stabilized by delocalization of unpaired electrons around the aromatic ring.11 However, even though these radicals are relatively stable, they can also display prooxidant activities inducing cellular damage (reviewed in ref. 53). It is well established that one of the chemopreventive mechanisms of polyphenols (or fruits and vegetables rich in antioxidants) against cancer development is the inhibition of initiation, the first step of carcinogenesis occurring following oxidative DNA damage leading to mutagenesis.27,65,66 In a recent review, the prooxidant activity of individual dietary polyphenols and their ability to induce mitochondrial dysfunction and consequently apoptosis has been suggested as a possible anticancer mechanisms.53
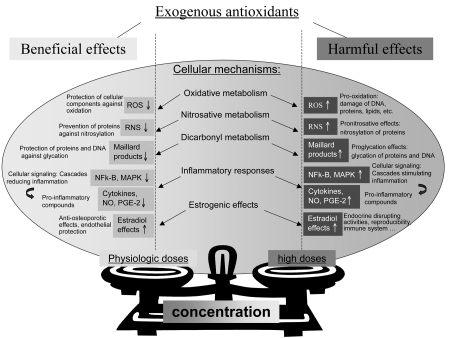
It is worth noting that beneficial or harmful effects of natural compounds may also occur independently from their (anti-) oxidative properties e.g., as a result of the activation of particular cellular pathways including inflammatory processes, nitrogen and dicarbonyl metabolisms for which a close relation exists (Fig. 1).7,67–69 Indeed, inflammation, nitrosative stress (resulting from excessive production of reactive nitrogen species) and carbonyl stress (resulting from excessive accumulation of reactive dicarbonyl compounds) may exacerbate or provoke oxidative stress and vice versa.3,7,10,17,67,70 Besides the biphasic effects of antioxidants on oxidative metabolism, it has also been reported that natural compounds can display double-edged effects on inflammatory reactions. For example, β-carotene at low doses exhibited antioxidant71 and anti-inflammatory72 properties in human HL-60 cells, whereas, at high doses prooxidant activity71 and pro-inflammatory effects72 [an increase in the production of pro-inflammatory mediators tumor necrosis factor-α (TNFα) and interleukin-8 (IL-8)] have been reported. To the best of our knowledge, there is still no work reporting that antioxidants, in certain cases as described above, can provoke nitrosative stress or carbonyl stress despite that these stresses may result from oxidative stress disturbances; however, their protective potentials on theses stresses have been demonstrated.73,74 Other activities of natural compounds might result in beneficial as well as harmful effects such as due to the estrogen-like activity of isoflavones, as they are able to bind to s-estradiol receptors (Fig. 1).75,76 Independently of their antioxidant activity, polyphenols are also able to exert modulatory effects in cells, resulting in outcomes depending on the activated pathways among others, e.g., by interacting with intracellular signaling cascades (e.g., the nuclear factor kappa B (NFκB) and the mitogen-activated protein kinase (MAPK)) or by binding to the ATP-binding sites of a large number of proteins, including mitochondrial ATPase, calcium plasma membrane ATPase, protein kinase A, protein kinase C and topoisomerase (reviewed in ref. 69). It also has been revealed that some antioxidants (e.g., quercetin and naringenin) are able to inhibit certain cytochrome P450 enzymes (CYP1A1 and CYP3A4, respectively) involved in the bioactivation of chemical carcinogens,77 constituting another proposed chemopreventive mechanism of polyphenols against cancer development including lung cancer.78 In addition, it has been demonstrated that polyphenols (e.g., chlorogenic acid, EGCG and rutin) at pharmacological (non-nutritional) doses could interact with GABAA receptors or modulate neurotransmitters (e.g., serotonin and noradrenaline), resulting in interesting pharmacologic properties on the central nervous system including anti-anxiety and antidepressant activities.9,79
Figure 1.
Double-edged effects of exogenous antioxidants on cellular responses including oxidative, nitrosative and dicarbonyl metabolisms and other pathways such as inflammatory processes depending potentially on their concentrations: physiologic doses leading to beneficial effects whereas high doses may result in harmful effects.
In vivo evidence: animal experiments, epidemiological data and human intervention trials.
In animal experiments, it has been demonstrated that long-term intake of some natural food items such as apple,80 olive oil81 and honey82 reversed several side effects associated with aging including brain oxidative stress,80,81 cognitive deterioration82 and anxiety.80–82 Transgenic mice having vitamin E deficiency in the brain suffered from oxidative stress in this vital organ, developing anxious behavior without abnormalities in the locomotor performance.83 It was also demonstrated that isolated polyphenols (e.g., quercetin, rutin and epigallocatechin-3-gallate) are able to reverse oxidative stress toxicity induced by certain conditions (e.g., pharmacological treatment, ischemia-reperfusion) in rat models.84,85 Individual antioxidants from plant foods have also displayed cytoprotective activities and interesting pharmacological properties in rodents such as antidepressant and anxiolytic effects, among others.9,79,86
The potential adverse effects of exogenous antioxidants on consumer health has first concerned synthetic antioxidants including BHA and BHT, following their carcinogenity and toxicity at higher doses in rodents87,88 and monkeys,88 possibly resulting from prooxidative properties at higher concentrations. Surprisingly, it has also been reported that BHA displayed anticarcinogenic activity against various carcinogens in animal models.89–91 This disagreement could be explained by the doses administered and, perhaps, the duration of the treatment. In certain parts of the world, humans have consumed daily doses of BHA and BHT of ca. 0.1 mg/kg.88 An LD50 of ca. 2,000 mg/kg of these synthetic antioxidants has been reported for most animals,88 raising the question on the toxicity of these additives on human health at chronic exposure. Interestingly, high concentrations of antioxidants including BHT and BHA in food items, can also increase spoilage of food items, rather then result in prolonged shelf-life due to pro-oxidant activities.92 As a consequence, tendencies emerged to replace synthetic antioxidants in foods and pharmaceutical preparations by natural antioxidants, due to presumably increased safety and higher acceptance by the consumer.41
With respect to humans, many of the health beneficial functions of dietary ingredients, including antimutagenicity, anticarcinogenity and anti-aging, among others, have been discussed in relation to their antioxidant properties.27,35,66 Epidemiological investigations have played a key role in investigating the preventive action of diets rich in naturally occurring antioxidants on disease development and progression.78,93–99 Indeed, regular consumption of fruits and vegetables has been shown to be inversely associated with lower mortality, presumably due to the protection offered by plant foods against the development of chronic human diseases related to oxidative stress such as cancer or CVD.78,93–100 Even though more recent, prospective studies such as results of the EPIC study indicate that these retrospectively obtained results, at least with respect to cancer, might have been somewhat overestimated, still significant reduction of consumption of fruits and vegetables on e.g., colorectal cancer was found.101 However, it has been hypothesized that specific food items e.g., apples and onions confer protection against lung cancer and coronary heart disease (Table 2).78,98,99 Epidemiologists have postulated that the health beneficial effects of apple and onion against lung cancer may be attributed to few or even individual components, such as quercetin, owing to its potent chemopreventive activity against carcinogens in vitro77 and in in vivo animal studies.102,103
It is interesting to note that in a prospective cohort study monitoring human volunteers for several years (8–14 years), a reduction (albeit being non-significant) of the risk to develop coronary heart disease by intake of fruit and vegetables has been noticed for persons consuming more than 4 servings/d, and that this protection was more pronounced (and significant) in persons with a high consumption of fruits and vegetables (≥8 servings/d).104 In addition, the pooled meta-analysis of eight prospective studies showed a negative relation between higher consumption of fruits and vegetables, and stroke risk (ischaemic and haemorrhagic stroke).105 Based on these results, it was concluded that consumption of more than 5 servings/d of fruits and vegetables causes a more pronounced reduction in strokes than with 3–5 portions/d,105 a recommended portion being somewhat vaguely defined as 80–100 g. However, the average fruit and vegetable intake in most developed countries is only about 3 servings/d.105
Nevertheless, there is increasing evidence that the observed associated health advantageous effects of plant food consumption may not be attributable to a specific compound, but rather to the whole fruit and vegetable, following additive or synergist actions of complex mixtures of phytochemicals and nutrients.27,106 While earlier epidemiological and observational studies have suggested that increased carotenoid intake can go along with decreased risk of developing certain types of cancer, such as digestive tract cancer,107 or lung cancer108,109 and decreased risk of markers of CVD,100 many individual supplementation trials in humans failed to result in observed health beneficial effects or even suggested that antioxidant compounds can be toxic under certain conditions such as at high doses or when synergistic compounds are lacking. For example, supplementing β-carotene alone (20 mg/day),110 β-carotene and retinol (30 mg/d β-carotene and 25,000 IU retinyl palmitate)111 over several years increased the lung cancer incidence in smokers. The same was observed in asbestos workers.112
However, it has also to be stated that some supplementation trials employing β-carotene, especially in healthy subjects, did not find increased mortality due to cancer,113 or found even decreased overall mortality due to decreased incidence of cancer114 (Table 2). Long-term supplementation studies with natural sources of antioxidants are virtually non-existing; however, short-term supplementation studies employing natural sources of antioxidants such as carotenoids demonstrated decreased oxidative stress markers and improved blood lipids.115,116
Table 2.
Compilation of data from epidemiological (retrospective and prospective) investigations and human intervention trials highlighting the role of the diet (fruits and vegetable, supplements) on human diseases or biomarkers of health
| Epidemiological and dietary intervention studies employing plant foods | References | Supplementation | References |
Retrospective epidemiological data:
|
Knekt et al.98,99 & Le Marchand78 |
Human intervention trials with supplements:
|
Reviewed by Goralczyk,138 |
| Reviewed by Peto et al.136 | #Beta-Carotene and Retinol Efficacy Trial111 | ||
| Reviewed by Lee et al.27 | *The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group110 | ||
Prospective cohort epidemiological trials:
|
Joshipura et al.104 | ||
| He et al.105 | Leppälä et al.139 | ||
Human dietary intervention trials:
|
Reviewed by Halliwell65 | Prieme et al.117 | |
| Podmore et al.118 | |||
| Reviewed by Lairon137 | Blot et al.114 |
Nevertheless, the absence of beneficial activities of individual antioxidants, and even toxic effects8,27,73,106,117,118 may be explained by the dose-dependent behavior these components exhibit outside their natural matrix, highlighting the important properties of complex mixtures such as of whole foods containing essential elements (vitamins, minerals), dietary fiber and non-nutrient phytochemicals including flavonoids, phenolic acids, several carotenoids, and many more. Several studies have shown that supplementation with isolated forms of vitamin C, vitamin E or β-carotene had no beneficial effects.8,73,106 For example, supplementing diets of 30 healthy individuals with high doses of vitamin C (500 mg/d) caused an increase of oxidative damage in the DNA from lymphocytes, suggesting prooxidative effects at elevated doses.118 In another study, supplementation with vitamin E and vitamin C failed to reduce oxidative DNA damage in smokers.117 In contrast, some studies on healthy human volunteers consuming fruits and vegetables rich in vitamin C decreased levels of oxidative DNA damage65 (Table 2). It has been suggested that at physiological conditions, the antioxidative properties of vitamin C outweigh its possible prooxidant activity.3,118 Human trials and in vitro studies showed that oxidative stress causes a rapid depletion of vitamin C and vitamin E.3,119 Vice versa, deficiency of vitamin E has also shown to provoke oxidative stress disturbances in transgenic rats.83 Synergistic actions between vitamin C and vitamin E therefore appear important in their preventive activity against lipid peroxidation.7
Another example showing the importance of dosing on health concerns EGCG, a dietary antioxidant existing in green tea, marketed also in other preparations owing to its proposed preventive activity against oxidative stress. It has been demonstrated that EGCG at pharmacological doses (30 and 60 mg/kg) abolishes anxiety in mice;120 at 150 mg/kg however this tea polyphenol caused death to mice (100% mortality) in less than 24 h, presumably due to its high hepatotoxicity noticed above 100 mg/kg.54 Among green tea catechins, it has been revealed that, at higher doses, the most cytotoxic was EGCG, which is also the most abundant tea catechin.54 Interestingly, despite green tea being viewed as a healthy drink with chemopreventive potential against cancer development,66 tea, when consumed very frequently (>1 l/d), has been associated with increased incidence of esophageal cancer in some countries such as northern Iran or India, even though this has been discussed to be due to consumption of hot tea,121,122 further more, green tea has been shown to be able to produce H2O2 in the mouth cavity.123
In general, antioxidants when delivered as dietary supplements contain isolated (synthetic or concentrated) compounds in concentrated form. For example, a typical vegetarian diet contains 20 times less quercetin than a single dose of many supplements of this antioxidant available on the market.53 High, isolated concentrations of carotenoids, EGCG and vitamin C are also common (Table 3). Carotenoid supplements for example mostly contain β-carotene, lycopene or lutein and xeaxanthin, and contain often the manifold of a typical daily intake. Unfortunately, for many dietary antioxidants, no upper tolerable intake level (UL) has been established, with exception for some vitamins.124,125 While carotenoids have been taken also for its vitamin A activity and against macular degeneration,36,126,127 especially lycopene has been marketed as an antioxidant.128 Negative effects of taking high amounts of lycopene, also from diets, have been hypothesized to cause skin alterations and contribute to adverse effects such as abdominal problems (French Food Safety Agency AFFSA, www.afssa.fr/Documents/NUT2004sa0336.pdf).
Table 3.
Examples of antioxidant concentration in fruits, vegetables and in supplement preparations available on the market
| Dietary antioxidants | Rich dietary sources | Concentration in foods (mg/100 g) | Concentration in supplements*** (mg/capsule) |
| Vitamin C | bell pepper, citrus fruits140,141 | 10–170 | 100–1000 |
| Quercetin | apples, onions140 | 4–46 | 100–800 |
| Carotenoids | leafy vegetables, plums, tomatoes, watermelon, carrots140,141 | 0.2–10 | 5–15 |
| EGCG | green tea142 | 5–450# | 25–360 |
| Selenium* | fish (dairy producs, potato, rice)140,141 | 1–150* | 0.07–0.20 |
| Vitamin E | fish, meat, leafy vegetables140,141 | 0.2–10 | 400 IU** |
| Isoflavonoids | soy, beans, peanuts140,143 | 0.1–155 | 50–150 |
*µg/100 g
mg/cup (ca. 225 mL of tea beverage)
1 IU alpha tocopherol = 0.667 mg
internet data.
Impact of Antioxidants on the Double-Edged Effect of ROS
In addition to the different effects antioxidants could exhibit in vivo depending on their present concentration, the doubleedged effects of oxygen metabolites is also recognized and well documented.7,33 The redox state of a cell and its oscillation determines its cellular functioning (reviewed in ref. 7 and 33). At low doses, ROS possess a crucial role in many physiological functions such as cellular signaling, gene expression, the regulation of immune responses and fostering antioxidative defense mechanisms.7,10,32,33 For example, it was demonstrated that at least 40 various genes can be activated by H2O2 in mammalian cells.32 The balance between oxidant production and antioxidant protection is believed to be critical in maintaining healthy biological systems. Therefore, antioxidants at high doses could, despite acting as prooxidants, also disrupt the redox balance following their potential to interact with ROS present at physiological concentrations required for optimal cellular functioning, leading to cellular dysfunction.33 This assumption was reinforced by findings showing that transgenic animals overexpressing antioxidant enzyme systems (e.g., SOD and GPx) display abnormalities in function, including overexpression of certain genes such as immediate early genes (IEGs)129 and certain proteins.25 GPx overexpression in transgenic mice for example resulted in their development into a thermosensitive phenotype, suggesting a dysfunction in thermoregulation.25
At high concentrations, ROS are toxic compounds leading to lipid peroxidation and the oxidation of other sensitive biomolecules such as proteins and DNA.7,17,32 When this situation occurs, cells enter an oxidative stress state, characterized by the disequilibrium between oxidant production and antioxidant protection in favor of the former.7,17 Oxidative stress can cause cellular dysfunction by e.g., inducing changes in gene expression, protein expression, cellular signaling, membrane fluidity, potentially resulting in cell death.7,17 Dietary antioxidants play a key role in reinforcing our antioxidant system to eliminate the excess of oxygen metabolites. An interactive and often synergistic action occurs between endogenous and exogenous antioxidants to maintain a balance between oxidation and antioxidation.7 It has been estimated that concentrations of antioxidant micronutrients such as vitamin C, vitamin E and carotenoids range between high micromolar and low millimolar levels in human plasma and organs, while polyphenol concentrations are in the high nanomolar to low micromolar range.130 However, polyphenols have been reported to be more efficient than vitamin C against oxidative stress at tissue levels.130 In this respect, it has been suggested that phenolics are among the most active substances from natural sources, displaying a variety of health-promoting properties such as cytoprotective, antibacterial, antiviral, anti-aging, antiinflammatory, antiallergenic, antimutagenic, vasodilatory, anxiolytic, antidepressant and cognitive enhancing effects.9,35 Polyphenols including phenolic acids and flavonoids are the most abundant class of antioxidant phytochemicals, existing in fruits and vegetables in concentrations around up to several 100 mg/100 g,131 and thereby constituting the major class of antioxidants derived from the diet, with estimated intakes in westernized countries around 0.4–1.0 g/d and capita.132,133 It is noteworthy however that the total amounts of antioxidative constituents present in the food matrix may not be completely extractable by the gastrointestinal (GI) tract, depending on several parameters, such as complexation by the food matrix or the presence of potential inhibitors of absorption. For carotenoids, for example, these factors have been summarized in the mnemonic term SLAMENGHI,36,134 (comprising factors species, molecular linkage, amount compounds, matrix effects, effectors of absorption and bioconversion, nutrient status of host, genetic factors, host-related factors, interaction of all factors). In the GI tract, once nutrients and phytochemicals are present in soluble and bioaccessible form, they may be taken up by the epithelium and exert their antioxidant activity.135 However, to be bioactive in other organs, additional factors of bioavailability such as absorption by the gut mucosa, transport to their place of action, formation of phase I and phase II metabolites and excretion do play a role.
Conclusion
The balance between oxidation and antioxidation (redox balance) is critical in maintaining a healthy biological system.7,9,17 In cellular redox state, the double-edged effect does not only concern ROS, but also antioxidants. Physiologic doses of exogenous antioxidants are required to maintain or re-establish redox homeostasis.7,34 However, high doses of exogenous antioxidants may disrupt redox balance. Considering epidemiological studies and trials on humans taking antioxidant compounds, it is evident that the health benefits of phytochemicals and nutrients were observed predominantly when being consumed within their natural food matrices (fruits, vegetables, grain, etc.). Compounds within plant foods may therefore be considered as being more safe and healthy compared to isolated, high doses, such as present in supplements. Two main factors seem to be predisposing for the beneficial activities of plant foods: (1) the general low concentration of nutrients and non-nutrients in these natural food matrices and (2) the additive or synergistic actions of complex mixture profiles of phytochemicals and nutrients. Supplementation approaches do generally not take into account both aspects, which could explain the controversial results observed in supplementation studies.
Acknowledgements
The present project is supported by the National Research Fund, Luxembourg and cofunded under the Marie Curie Actions of the European Commission (FP7-COFUND).
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/12858
References
- 1.Halliwell B. Oxygen radicals: a commonsense look at their nature and medical importance. Med Biol. 1984;62:71–77. [PubMed] [Google Scholar]
- 2.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 3.Splettstoesser WD, Schuff-Werner P. Oxidative stress in phagocytes—“the enemy within”. Microsc Res Tech. 2002;57:441–455. doi: 10.1002/jemt.10098. [DOI] [PubMed] [Google Scholar]
- 4.Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006;26:427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- 5.Maiese K. Environmental stimulus package: Potential for a rising oxidative deficit. Oxid Med Cell Longev. 2009;2:179–180. doi: 10.4161/oxim.2.4.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rammal H, Bouayed J, Soulimani R. A direct relationship between aggressive behavior in the resident/intruder test and cell oxidative status in adult male mice. Eur J Pharmacol. 2010;627:173–176. doi: 10.1016/j.ejphar.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Phagocyte-derived reactive species: salvation or suicide? Trends Biochem Sci. 2006;31:509–515. doi: 10.1016/j.tibs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Bouayed J. Polyphenols: a potential new strategy for the prevention and treatment of anxiety and depression. Curr Nutr Food Sci. 2010;6:13–18. [Google Scholar]
- 10.Delattre J, Beaudeux JL, Bonnefont-Rousselot D. Radicaux libres et stress oxydant. Aspects biologiques et pathologiques. Éditions Médicales internationales. 2005:1–492. [Google Scholar]
- 11.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 12.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29:245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 13.Rosa EF, Takahashi S, Aboulafia J, Nouailhetas VL, Oliveira MG. Oxidative stress induced by intense and exhaustive exercise impairs murine cognitive function. J Neurophysiol. 2007;98:1820–1826. doi: 10.1152/jn.01158.2006. [DOI] [PubMed] [Google Scholar]
- 14.Vollaard NB, Shearman JP, Cooper CE. Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Med. 2005;35:1045–1062. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Goyal R, Prakash A. Possible GABAergic mechanism in the protective effect of allopregnenolone against immobilization stress. Eur J Pharmacol. 2009;602:343–347. doi: 10.1016/j.ejphar.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Singh A. Possible involvement of GABAergic mechanism in protective effect of melatonin against sleep deprivation-induced behaviour modification and oxidative damage in mice. Fundam Clin Pharmacol. 2009;23:439–448. doi: 10.1111/j.1472-8206.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid Med Cell Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol. 2007;564:146–149. doi: 10.1016/j.ejphar.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 19.Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008;22:1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Rammal H, Bouayed J, Younos C, Soulimani R. The impact of high anxiety level on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharmacol. 2008;589:173–175. doi: 10.1016/j.ejphar.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Maiese K. High anxiety: Recognizing stress as the stressor. Oxid Med Cell Longev. 2009;2:61–62. doi: 10.4161/oxim.2.2.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloomer RJ, Fisher-Wellman KH. Systemic oxidative stress is increased to a greater degree in young, obese women following consumption of a high fat meal. Oxid Med Cell Longev. 2009;2:19–25. doi: 10.4161/oxim.2.1.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Chan AC, Chow CK, Chiu D. Interaction of antioxidants and their implication in genetic anemia and their implication in genetic anemia. Proc Soc Exp Biol Med Biol. 1999;22:274–282. doi: 10.1177/153537029922200310. [DOI] [PubMed] [Google Scholar]
- 25.Mirochnitchenko O, Palnitkar U, Philbert M, Inouye M. Thermosensitive phenotype of transgenic mice overproducing human glutathione peroxidases. Proc Natl Acad Sci USA. 1995;92:8120–8124. doi: 10.1073/pnas.92.18.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009;2:259–269. doi: 10.4161/oxim.2.5.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KW, Lee HJ, Lee CY. Vitamins, phytochemicals, diets and their implementation in cancer chemoprevention. Crit Rev Food Sci Nutr. 2004;44:437–452. doi: 10.1080/10408690490886674. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 29.Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid Med Cell Longev. 2009;2:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 32.Grune T. Oxidants and antioxidative defense. Hum Exp Toxicol. 2002;21:61–62. doi: 10.1191/0960327102ht210oa. [DOI] [PubMed] [Google Scholar]
- 33.Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 34.Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biehler E, Bohn T. Methods for assessing aspects of carotenoid bioavailability. Curr Nutr Food Sci. 2010;6:44–69. [Google Scholar]
- 37.André CM, Larondelle Y, Evers D. Dietary antioxidants and oxidative stress from a human and plant perspective: A review. Curr Nutr Food Sci. 2010;6:2–12. [Google Scholar]
- 38.5 a day: The Department of Health 2008. US department of Health. http://www.5aday.nhs.uk/topTips/default.html.
- 39.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 40.Jadhav SJ, Nimbalkar SS, Kulkarni AD, Madhavi DL. Lipid oxidation in biological and food systems. In: Madhavi DL, Deshpande SS, Salunkhe DK, editors. Food antioxidants: technological, toxicological and health perspectives. New York: Marcel Dekker Inc.; 1995. [Google Scholar]
- 41.Moure A, Cruz JM, Franco D, Manuel Dominguez J, Sineiro J, Dominguez H, Nunez MJ, Parajo JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. [Google Scholar]
- 42.Heo HJ, Choi SJ, Choi SG, Shin DH, Lee JM, Lee CY. Effects of banana, orange and apple on oxidative stress-induced neurotoxicity in PC12 cells. J Food Sci. 2006;69:357–360. doi: 10.1111/j.1750-3841.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 43.Heo HJ, Kim DO, Choi SJ, Shin DH, Lee CY. Apple phenolics protect in vitro oxidative stress-induced neuronal cell death. J Food Sci. 2004;69:357–360. [Google Scholar]
- 44.Heo HJ, Lee CY. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J Agric Food Chem. 2005;53:1984–1989. doi: 10.1021/jf048616l. [DOI] [PubMed] [Google Scholar]
- 45.Romier-Crouzet B, Van De Walle J, During A, Joly A, Rousseau C, Henry O, Larondelle Y, Schneider YJ. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem Toxicol. 2009;47:1221–1230. doi: 10.1016/j.fct.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Romier B, Van De Walle J, During A, Larondelle Y, Schneider YJ. Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br J Nutr. 2008;100:542–551. doi: 10.1017/S0007114508966666. [DOI] [PubMed] [Google Scholar]
- 47.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55:396–398. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 49.Raza H, John A. Green tea polyphenol epigallocatechin-3-gallate differentially modulates oxidative stress in PC12 cell compartments. Toxicol Appl Pharmacol. 2005;207:212–220. doi: 10.1016/j.taap.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Watjen W, Michels G, Steffan B, Niering P, Chovolou Y, Kampkotter A, et al. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. J Nutr. 2005;135:525–531. doi: 10.1093/jn/135.3.525. [DOI] [PubMed] [Google Scholar]
- 51.De Marchi U, Biasutto L, Garbisa S, Toninello A, Zoratti M. Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: A demonstration of the ambivalent redox character of polyphenols. Biochim Biophys Acta. 2009;1787:1425–1432. doi: 10.1016/j.bbabio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int. 2007;31:1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 54.Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Sergediene E, Jonsson K, Szymusiak H, Tyrakowska B, Rietjens IM, Cenas N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett. 1999;462:392–396. doi: 10.1016/s0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 56.Gaspar J, Rodrigues A, Laires A, Silva F, Costa S, Monteiro MJ, et al. On the mechanisms of genotoxicity and metabolism of quercetin. Mutagenesis. 1994;9:445–449. doi: 10.1093/mutage/9.5.445. [DOI] [PubMed] [Google Scholar]
- 57.Metodiewa D, Jaiswal AK, Cenas N, Dickancaite E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med. 1999;26:107–116. doi: 10.1016/s0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 58.Hodnick WF, Kung FS, Roettger WJ, Bohmont CW, Pardini RS. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids. A structure-activity study. Biochem Pharmacol. 1986;35:2345–2357. doi: 10.1016/0006-2952(86)90461-2. [DOI] [PubMed] [Google Scholar]
- 59.Ochiai M, Nagao M, Wakabayashi K, Sugimura T. Superoxide dismutase acts as an enhancing factor for quercetin mutagenesis in rat-liver cytosol by preventing its decomposition. Mutat Res. 1984;129:19–24. doi: 10.1016/0027-5107(84)90118-0. [DOI] [PubMed] [Google Scholar]
- 60.Gordon MH. The development of oxidative rancidity in foods. In: Pokorny J, Yanishlieva N, Gordon MH, editors. Antioxidants in food: practical applications. Cambridge: Woodhead Publishing Limited; 2001. [Google Scholar]
- 61.Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London: Elsevier; 1990. [Google Scholar]
- 62.Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–1418. [Google Scholar]
- 63.Maisuthisakul P, Gordon MH, Pongsawatmanit R, Suttajit M. Enhancing the oxidative stability of rice crackers by addition of the ethanolic extract of phytochemicals from Cratoxylum formosum Dyer. Asia Pac J Clin Nutr. 2007;16:37–42. [PubMed] [Google Scholar]
- 64.Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med. 1997;22:861–870. doi: 10.1016/s0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- 65.Halliwell B. Effect of diet on cancer development: is oxidative DNA damage a biomarker? Free Radic Biol Med. 2002;32:968–974. doi: 10.1016/s0891-5849(02)00808-0. [DOI] [PubMed] [Google Scholar]
- 66.Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–421. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 67.Yamagishi SI, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev. 2010;3:101–108. doi: 10.4161/oxim.3.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Thornalley PJ. Unease on the role of glyoxalase 1 in high-anxiety-related behaviour. Trends Mol Med. 2006;12:195–199. doi: 10.1016/j.molmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Palozza P, Serini S, Torsello A, Boninsegna A, Covacci V, Maggiano N, et al. Regulation of cell cycle progression and apoptosis by beta-carotene in undifferentiated and differentiated HL-60 leukemia cells: possible involvement of a redox mechanism. Int J Cancer. 2002;97:593–600. doi: 10.1002/ijc.10094. [DOI] [PubMed] [Google Scholar]
- 72.Yeh SL, Wang HM, Chen PY, Wu TC. Interactions of beta-carotene and flavonoids on the secretion of proinflammatory mediators in an in vitro system. Chem Biol Interact. 2009;179:386–393. doi: 10.1016/j.cbi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 74.Dkhar P, Sharma R. Effect of dimethylsulphoxide and curcumin on protein carbonyls and reactive oxygen species of cerebral hemispheres of mice as a function of age. Int J Dev Neurosci. 28:351–357. doi: 10.1016/j.ijdevneu.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res. 2003;17:845–869. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- 76.Mennen LI, Walker R, Bennetau-Pelissero C, Scalbert A. Risks and safety of polyphenol consumption. Am J Clin Nutr. 2005;81:326–329. doi: 10.1093/ajcn/81.1.326S. [DOI] [PubMed] [Google Scholar]
- 77.Obermeier MT, White RE, Yang CS, Effects of bioflavonoids on hepatic P450 activities. Xenobiotica. 1995;25:575–584. doi: 10.3109/00498259509061876. [DOI] [PubMed] [Google Scholar]
- 78.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–160. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 79.Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci. 2007;262:77–84. doi: 10.1016/j.jns.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 80.Viggiano A, Monda M, Turco I, Incarnato L, Vinno V, Viggiano E, et al. Annurca apple-rich diet restores longterm potentiation and induces behavioral modifications in aged rats. Exp Neurol. 2006;199:354–361. doi: 10.1016/j.expneurol.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Pitozzi V, Jacomelli M, Zaid M, Luceri C, Bigagli E, Lodovici M, et al. Effects of dietary extra-virgin olive oil on behaviour and brain biochemical parameters in ageing rats. Br J Nutr. 2010;103:1674–1683. doi: 10.1017/S0007114509993655. [DOI] [PubMed] [Google Scholar]
- 82.Chepulis LM, Starkey NJ, Waas JR, Molan PC. The effects of long-term honey, sucrose or sugar-free diets on memory and anxiety in rats. Physiol Behav. 2009;97:359–368. doi: 10.1016/j.physbeh.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 83.Desrumaux C, Risold PY, Schroeder H, Deckert V, Masson D, Athias A, et al. Phospholipid transfer protein (PLTP) deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J. 2005;19:296–297. doi: 10.1096/fj.04-2400fje. [DOI] [PubMed] [Google Scholar]
- 84.Kebieche M, Lakroun Z, Lahouel M, Bouayed J, Meraihi Z, Soulimani R. Evaluation of epirubicininduced acute oxidative stress toxicity in rat liver cells and mitochondria and the prevention of toxicity through quercetin administration. Exp Toxicol Pathol. 2009;61:161–167. doi: 10.1016/j.etp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Itagaki S, Oikawa S, Ogura J, Kobayashi M, Hirano T, Iseki K. Protective effects of quercetin-3-rhamnoglucoside (rutin) on ischemia-reperfusion injury in rat small intestine. Food Chem. 2010;118:426–429. [Google Scholar]
- 86.Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R. The antioxidant effect of plums and polyphenolic compounds against H2O2-induced oxidative stress in mouse blood granulocytes. J Med Food. 2009;12:861–868. doi: 10.1089/jmf.2008.0165. [DOI] [PubMed] [Google Scholar]
- 87.Ito N, Fukushima S, Hagiwara A, Shibata M, Ogiso T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst. 1983;70:343–352. [PubMed] [Google Scholar]
- 88.Branen AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc. 1975;52:59–63. doi: 10.1007/BF02901825. [DOI] [PubMed] [Google Scholar]
- 89.King MM, McCay PB, Kosanke SD. Comparison of the effect of butylated hydroxytoluene on N-nitrosomethylurea and 7,12-dimethylbenz[a]-anthracene-induced mammary tumors. Cancer Lett. 1981;14:219–226. doi: 10.1016/0304-3835(81)90147-6. [DOI] [PubMed] [Google Scholar]
- 90.Wattenberg LW, Sparnins VL. Inhibitory effects of butylated hydroxyanisole on methylazoxymethanol acetate-induced neoplasia of the large intestine and on nicotinamide adenine dinucleotide-dependent alcohol dehydrogenase activity in mice. J Natl Cancer Inst. 1979;63:219–222. [PubMed] [Google Scholar]
- 91.Sydor WJ, Lewis KF, Yang CS. Effects of butylated hydroxyanisole on the metabolism of benzo(a)pyrene by mouse lung microsomes. Cancer Res. 1984;44:134–138. [PubMed] [Google Scholar]
- 92.Akoh CC, Min DB. Food lipids: chemistry, nutrition and biotechnology. 3rd ed. Boca Raton: CRC Press/Taylor & Francis Group; 2008. [Google Scholar]
- 93.Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Potentially anticarcinogenic secondary metabolites from fruit and vegetables. Oxford: Clarendon Press; 997. pp. 313–329. [Google Scholar]
- 94.Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 95.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 96.Muldoon MF, Kritchevsky SB. Flavonoids and heart disease. Brit Med J. 1996;312:458–459. doi: 10.1136/bmj.312.7029.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acheson RM, Williams DRR. Does consumption of fruit and vegetables protect against stroke? Lancet. 1983;1:1191–1193. doi: 10.1016/s0140-6736(83)92467-4. [DOI] [PubMed] [Google Scholar]
- 98.Knekt P, Jarvinen R, Seppanen R, Hellovaara M, Teppo L, Pukkala E, et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol. 1997;146:223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- 99.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hozawa A, Jacobs DR, Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53:447–455. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, et al. Fruit, vegetables and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. Am J Clin Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 102.Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–1196. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]
- 103.Verma AK, Johnson JA, Gould MN, Tanner MA. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988;48:5754–5458. [PubMed] [Google Scholar]
- 104.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 105.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–326. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 106.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003:517–520. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 107.Franceschi S, Bidoli E, La Vecchia C, Talamini R, D'Avanzo B, Negri E. Tomatoes and risk of digestivetract cancers. Int J Cancer. 1994;59:181–184. doi: 10.1002/ijc.2910590207. [DOI] [PubMed] [Google Scholar]
- 108.Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–383. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- 109.Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET) Cancer Epidemiol Biomarkers Prev. 2003;12:350–358. [PubMed] [Google Scholar]
- 110.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group, author. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 111.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 112.Goodman GE, Thornquist M, Kestin M, Metch B, Anderson G, Omenn GS. The association between participant characteristics and serum concentrations of beta-carotene, retinol, retinyl palmitate and alphatocopherol among participants in the Carotene and Retinol Efficacy Trial (CARET) for prevention of lung cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:815–821. [PubMed] [Google Scholar]
- 113.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 115.Rao AV, Agarwal S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr Cancer. 1998;31:199–203. doi: 10.1080/01635589809514703. [DOI] [PubMed] [Google Scholar]
- 116.Hadley CW, Clinton SK, Schwartz SJ. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J Nutr. 2003;133:727–732. doi: 10.1093/jn/133.3.727. [DOI] [PubMed] [Google Scholar]
- 117.Prieme H, Loft S, Nyyssonen K, Salonen JT, Poulsen HE. No effect of supplementation with vitamin E, ascorbic acid or coenzyme Q10 on oxidative DNA damage estimated by 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion in smokers. Am J Clin Nutr. 1997;65:503–507. doi: 10.1093/ajcn/65.2.503. [DOI] [PubMed] [Google Scholar]
- 118.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559–559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 119.Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med. 1998;24:789–797. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- 120.Vignes M, Maurice T, Lante F, Nedjar M, Thethi K, Guiramand J, et al. Anxiolytic properties of green tea polyphenol (-)-epigallocatechin gallate (EGCG) Brain Res. 2006;1110:102–115. doi: 10.1016/j.brainres.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 121.Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:29–29. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ganesh B, Talole SD, Dikshit R. Tobacco, alcohol and tea drinking as risk factors for esophageal cancer: A case-control study from Mumbai, India. Cancer Epidemiol. 2009;33:431–434. doi: 10.1016/j.canep.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Lambert JD, Kwon SJ, Hong J, Yang CS. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radic Res. 2007;41:850–853. doi: 10.1080/10715760601091659. [DOI] [PubMed] [Google Scholar]
- 124.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 125.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium and carotenoids. J Am Diet Assoc. 2000;100:637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 126.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care. 2002;5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 127.Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology. 2008;115:147–157. doi: 10.1016/j.ophtha.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 128.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 129.Kondo T, Sharp FR, Honkaniemi J, Mikawa S, Epstein CJ, Chan PH. DNA fragmentation and Prolonged expression of c-fos, c-jun and hsp70 in kainic acidinduced neuronal cell death in transgenic mice overexpressing human CuZn-superoxide dismutase. J Cereb Blood Flow Metab. 1997;17:241–256. doi: 10.1097/00004647-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 130.Petti S, Scully C. Polyphenols, oral health and disease: A review. J Dent. 2009;37:413–423. doi: 10.1016/j.jdent.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 131.Meulenberg EP. Phenolics: occurrence and immunochemical detection in environment and food. Molecules. 2009;14:439–473. doi: 10.3390/molecules14010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073–2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 133.Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–1191. [PubMed] [Google Scholar]
- 134.Bohn T. Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci. 2008;4:240–258. [Google Scholar]
- 135.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr. 2005;81:268–276. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 136.Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290:201–208. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- 137.Lairon D. Intervention studies on Mediterranean diet and cardiovascular risk. Mol Nutr Food Res. 2007;51:1209–1214. doi: 10.1002/mnfr.200700097. [DOI] [PubMed] [Google Scholar]
- 138.Goralczyk R. Beta-carotene and lung cancer in smokers: review of hypotheses and status of research. Nutr Cancer. 2009;61:767–774. doi: 10.1080/01635580903285155. [DOI] [PubMed] [Google Scholar]
- 139.Leppala JM, Virtamo J, Fogelholm R, Huttunen JK, Albanes D, Taylor PR, et al. Controlled trial of α-tocopherol and β-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20:230–235. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 140.Souci SW, Fachmann W, Kraut H. Food Composition and Nutrition Tables. Stuttgart: CRC Press; 2000. [Google Scholar]
- 141.Heseker B, Heseker H. Nahrstoffe in Lebensmitteln. Sulzbach: Umschau-Zeitschriftenverlag; 2007. (Ger). [Google Scholar]
- 142.U.S. Department of Agriculture, author. USDA database for the flavonoid content of selected foods. Release 2007. [Google Scholar]
- 143.Bohn T. Isoflavone bioavailability from foods and supplements. Dietary factors impacting utilization Agro Food Industry Hi-Tech. 2010;21:59–62. [Google Scholar]