Abstract
In addition to hemorrhagic cystitis, Fanconi Syndrome is a serious clinical side effect during ifosfamide (IFO) therapy. Fanconi syndrome is a generalized dysfunction of the proximal tubule which is characterized by excessive urinary excretion of glucose, phosphate, bicarbonate, amino acids and other solutes excreted by this segment of the nephron including L-carnitine. Carnitine is essential cofactor for β-oxidation of long-chain fatty acids in the myocardium. IFO therapy is associated with increased urinary carnitine excretion with subsequent secondary deficiency of the molecule. Cardiac abnormalities in IFO-treated cancer patients were reported as isolated clinical cases. This study examined whether carnitine deficiency and oxidative stress, secondary to Fanconi Syndrome, provoke IFO-induced cardiomyopathy as well as exploring if carnitine supplementation using Propionyl-L-carnitine (PLC) could offer protection against this toxicity. In the current study, an animal model of carnitine deficiency was developed in rats by D-carnitine-mildronate treatment Adult male Wistar albino rats were assigned to one of six treatment groups: the first three groups were injected intraperitoneally with normal saline, D-carnitine (DC, 250 mg/kg/day) combined with mildronate (MD, 200 mg/kg/day) and PLC (250 mg/kg/day), respectively, for 10 successive days. The 4th, 5th and 6th groups were injected with the same doses of normal saline, DC-MD and PLC, respectively for 5 successive days before and 5 days concomitant with IFO (50 mg/kg/day). IFO significantly increased serum creatinine, blood urea nitrogen (BUN), urinary carnitine excretion and clearance, creatine phosphokinase isoenzyme (CK-MB), lactate dehydrogenase (LDH), intramitochondrial acetyl-CoA/CoA-SH and thiobarbituric acid reactive substances (TBARS) in cardiac tissues and significantly decreased adenosine triphosphate (ATP) and total carnitine and reduced glutathione (GSH) content in cardiac tissues. In carnitine-depleted rats, IFO induced dramatic increase in serum creatinine, BUN, CK-MB, LDH, carnitine clearance and intramitochondrial acetyl-CoA/CoA-SH, as well as progressive reduction in total carnitine and ATP in cardiac tissues. Interestingly, PLC supplementation completely reversed the biochemical changes-induced by IFO to the control values. In conclusion, data from the present study suggest that: Carnitine deficiency and oxidative stress, secondary to Fanconi Syndrome, constitute risk factors and should be viewed as mechanisms during development of IFO-induced cardiotoxicity. Carnitine supplementation, using PLC, prevents the development of IFO-induced cardiotoxicity through antioxidant signalling and improving mitochondrial function.
Key words: ifosfamide, Fanconi Syndrome, carnitine deficiency, cardiotoxicity, D-carnitine, mildronate, propionyl-L-carnitine
Introduction
Ifosfamide (IFO) is an oxazaphosphorine alkylating agent which is commonly used in cancer chemotherapy and immunosuppressive protocols.1 Unfortunately, the optimal clinical usefulness of IFO is severely limited by a high incidence of nephrotoxicity in the form of Fanconi Syndrome especially in children.2,3 It is well documented that IFO is a prodrug that must be biotransformed by hepatic cytochrome P450 system to produce the active alkylating species, isophosphoramide mustard (IPM) and the urotoxic metabolite, acrolein, the main cause of hemorrhagic cystitis.1,3 IFO also undergoes N-dechloroethylation pathway to produce the toxic metabolites, 2 and 3-dechloroethylated compounds as well as chloroacetaldehyde (CAA), a substance that decomposed to give another potential toxic compound thiodiaglycolic acid (TDGA). Earlier and recent studies reported that IFO therapy is associated with severe nephrotoxicity.4,5 It has been reported that local formation and accumulation of CAA in human kidney is the major cause of IFO-induced nephrotoxicity.6–8 In isolated rat kidney mitochondria, Nissim et al. reported that CAA inhibits NADH: ubiquinone oxidoreductase which disrupts oxidative phosphorylation, leading to multiple metabolic abnormalities, including elevation of NADH, decrease pyruvate dehydrogenase reaction and TCA cycle.7 Hence, despite the co-administration of mesna which has low reactivity with CAA, treatment with IFO is associated with severe proximal tubular dysfunction in the form of Fanconi Syndrome, which is characterized by excessive urinary excretion of glucose, phosphate, bicarbonate, amino acids and other solutes handled by this segment of the nephron including L-carnitine.9,10
L-Carnitine is an endogenous compound that plays an important physiological role in the transfer of long-chain fatty acids across the inner membrane of mitochondria for their β-oxidation and energy production.11 Kidney plays an important role in keeping the homeostasis of carnitine by conserving 95% of the filtered carnitine.12 The tubular reabsorption is compromised in IFO-treated patients suffering from nephrotoxicity thus, high renal loss of the compound persisted during the treatment.13 In addition, it is well documented that cachectic cancer patients are especially at risk for carnitine deficiency due to decreased intestinal absorption and increased renal losses.14–18 It has been reported that IFO therapy is associated with increased secretion of carnitine derivatives in the urine with subsequent secondary deficiency of the molecule.13 It is well documented that CAA and TDGA, the two major toxic metabolites of IFO, inhibit the oxidation of long-chain fatty acids (carnitine-dependent) but not medium chain-fatty acids (carnitine-independent) indicating that these compounds either sequester carnitine or inhibit long-chain fatty acid oxidation by inhibition of carnitine palmitoyl transferase-I (CPT I).6,19 Although kidney is the main organ responsible for endogenous synthesis of L-carnitine, up to date in the literature, we could not find any study investigating the effects of IFO on renal handling of carnitine and its metabolic consequences on the myocardium under condition of carnitine depletion and supplementation. Therefore, this study has been initiated to investigate the effects of the standard IFO-induced Fanconi Syndrome regimen on serum, urine and cardiac carnitine levels in normal and carnitine depleted rats and its relationship to IFO-induced cardiotoxicity. The second aim was to gain insights into the possibility of mechanism-based protection of the heart by PLC against side effects of IFO.
Results
Table 1 shows the effects of IFO on serum creatinine and BUN in PLC-supplemented and carnitine-depleted rats. IFO resulted in a highly significant 233 and 155%, increase in serum creatinine and BUN, respectively as compared to control. Treatment with DC-MD for 5 days before and 5 days concomitant with IFO resulted in a significant 53 and 102% increase in the levels of serum creatinine and BUN, respectively as compared to IFO alone. Interestingly, administration of PLC 5 days before and 5 days concomitant with IFO resulted in complete reversal of the increases in serum creatinine and BUN to the control values.
Table 1.
Effect of ifosfamide (IFO), propionyl-l-carnitine (PLC), D-carnitine-mildronate (DC-MD) and their combination on serum creatinine and blood urea nitrogen (BUN) in rats
| Treatment groups | Serum creatinine (mg/dl) | BUN (mg/dl) |
| Control | 0.57 ± 0.04 | 52 ± 1.43 |
| DC-MD | 0.57 ± 0.04 | 60 ± 2.34 |
| PLC | 0.54 ± 0.04 | 46 ± 3.18 |
| IFO | 1.9 ± 0.22* | 133 ± 8.92* |
| IFO plus DC-MD | 2.9 ± 0.24*$ | 277 ± 16.5*$ |
| IFO plus PLC | 0.94 ± 0.15# | 57 ± 1.43# |
Rats were randomly divided into six different groups of 10 animals each: Control, D-carnitine-mildronate (DC-MD, carnitine-depleted group), pLC (carnitine supplemented group), IFO, DC-MD plus IFO and PLC plus IFO. Carnitine depletion was induced in rats by daily intraperitoneal injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days. Carnitine supplementation was induced in rats by daily intraperitoneal injection of PLC (250 mg/kg/day) for 10 successive days. Fanconi syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days. IFO-carnitine depleted rats were given the same doses of DC-MD for 5 days before and 5 days concomitant with IFO. IFO-carnitine supplemented rats were given the same doses of PLC for 5 days before and 5 days concomitant with IFO. At the end of the treatment protocol, serum creatinine and BUN, indices of nephrotoxicity, were measured in serum. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
The effects of IFO on urinary carnitine excretion and carnitine clearance in PLC-supplemented and carnitine-depleted rats are shown in Table 2. Administration of IFO (50 mg/kg/day) for 5 days resulted in 2- and 2.3-folds increase in carnitine excretion and clearance, respectively, as compared to the control group. Treatment of carnitine-depleted rats with IFO resulted in a significant increase in both excretion and clearance of carnitine, as compared to IFO alone. Carnitine supplementation by daily administration of PLC for 5 days before and 5 days concomitant with IFO resulted in 1.8- and 0.8-folds increase in carnitine excretion and clearance, respectively, as compared to the control values. Administration of DC-MD for 10 successive days resulted in a significant 4- and 10.5-folds increase as compared to the control group.
Table 2.
Effect of ifosfamide (IFO), propionyl-l-carnitine (PLC), D-carnitine-mildronate (DC-MD) and their combination on urinary carnitine excretion and carnitine clearance in rats
| Treatment groups | Urinary carnitine excretion (µmol/day) | Carnitine clearance (ml/min) × 10−2 |
| Control | 0.53 ± 0.06 | 0.85 ± 0.14 |
| DC-MD | 2.78 ± 0.50* | 9.75 ± 2.66* |
| PLC | 10.06 ± 0.88* | 6.97 ± 066* |
| IFO | 1.61 ± 0.11 | 3.50 ± 0.78* |
| IFO plus DC-MD | 4.17 ± 0.68*#$ | 9.39 ± 1.42*# |
| IFO plus PLC | 4.53 ± 1.79*# | 4.52 ± 2.14* |
Rats were randomly divided into 6 different groups of 10 animals each: Control, D-carnitine-mildronate (DC-MD, carnitine-depleted group), PLC (carnitine supplemented group), IFO, DC-MD plus IFO and PLC plus IFO. Carnitine depletion was induced in rats by daily intraperitoneal injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days. Carnitine supplementation was induced in rats by daily intraperitoneal injection of PLC (250 mg/kg/day) for 10 successive days. Fanconi Syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days. IFO-carnitine depleted rats were given the same doses of DC-MD for 5 days before and 5 days concomitant with IFO. IFO-carnitine supplemented rats were given the same doses of PLC for 5 days before and 5 days concomitant with IFO. At the end of the treatment protocol, carnitine excretion and clearance were measured in 24 hours urine. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
Table 3 shows the effects of IFO on the activity of serum CK-MB and LDH, in PLC-supplemented and carnitine-depleted rats. Administration of IFO resulted in a significant 49 and 79% increase in serum CK-MB and LDH, respectively, as compared to the control group. Treatment with either PLC (carnitine-supplemented rats) or combined DC-MD (carnitine-depleted rats) for 10 successive days showed non-significant changes. Treatment with DC-MD for 5 days before and 5 days concomitant with IFO resulted in a significant 54% increase in the activity of CK-MB and 12% elevation in LDH as compared to IFO alone. Interestingly, administration of PLC for 5 days before and 5 days after IFO resulted in a complete reversal of IFO-induced increase in serum CK-MB and LDH to the control values.
Table 3.
Effect of ifosfamide (IFO), propionyl-l-carnitine (PLC), D-carnitine-mildronate (DC-MD) and their combination on serum creatine phosphokinase isoenzyme (CK-MB) and lactate dehydrogenase (LDH) in rats.
| Treatment groups | CK-MB (U/L) | LDH (U/L) |
| Control | 353.60 ± 8.06 | 344.47 ± 19.60 |
| DC-MD | 464.67 ± 27.43 | 440.40 ± 23.96 |
| PLC | 308.40 ± 19.87 | 348.40 ± 25.83 |
| IFO | 547.01 ± 21.40* | 616.80 ± 23.20* |
| IFO plus DC-MD | 733.80 ± 35.10*$ | 695.60 ± 40.01*$ |
| IFO plus PLC | 351.42 ± 21.80# | 432.60 ± 23.60# |
Rats were randomly divided into 6 different groups of 10 animals each: Control, D-carnitine-mildronate (DC-MD, carnitine-depleted group), PLC (carnitine supplemented group), IFO, DC-MD plus IFO and PLC plus IFO. Carnitine depletion was induced in rats by daily intraperitoneal injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days. Carnitine supplementation was induced in rats by daily intraperitoneal injection of PLC (250 mg/kg/day) for 10 successive days. Fanconi Syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days. IFO-carnitine depleted rats were given the same doses of DC-MD for 5 days before and 5 days concomitant with IFO. IFO-carnitine supplemented rats were given the same doses of PLC for 5 days before and 5 days concomitant with IFO. At the end of the treatment protocol, CK-MB and LDH, indices of cardiotoxicity, were measured in serum. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
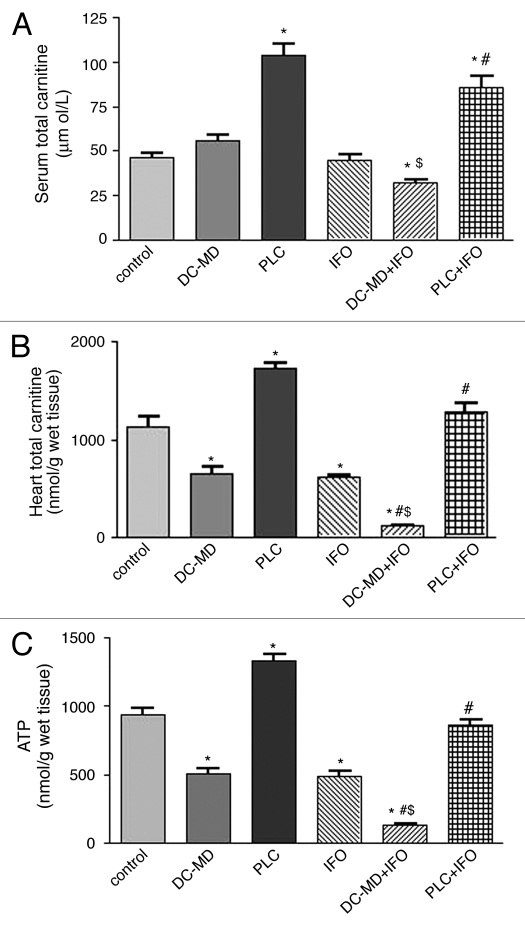
Figure 1 shows the effects of IFO on the levels of total carnitine in serum (Fig. 1A), total carnitine in cardiac tissues (Fig. 1B) and ATP concentration in cardiac tissues (Fig. 1C) from PLC-supplemented and carnitine-depleted rats. Administration of PLC for 10 successive days resulted in a significant 126% increase in serum total carnitine, whereas, administration of DC-MD for 10 successive days resulted in a non-significant change as compared to the control group. Treatment with IFO resulted in non significant changes in serum carnitine level as compared to the results of the control group. Treatment with DC-MD for 5 days before and 5 days concomitant with IFO decreased serum carnitine levels as compared to the results of the control, IFO and DC-MD groups. Interestingly, daily administration of PLC for 5 days before and 5 days concomitant with IFO resulted in a significant 87 and 91% increase in serum carnitine as compared to the results of the control and IFO groups, respectively.
Figure 1.
Effect of ifosfamide (IFO) on the levels of total carnitine in serum (A), total carnitine in cardiac tissues (B) and ATP concentration in cardiac tissues (C) from propionyl-l-carnitine (PLC)-supplemented and carnitine-depleted rats. Rats were randomly divided into 6 different groups of 10 animals each: Control, D-carnitine-mildronate (DC-MD, carnitine-depleted group), PLC (carnitine supplemented group), IFO, DC-MD plus IFO and PLC plus IFO. Carnitine depletion was induced in rats by daily intraperitoneal injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days. Carnitine supplementation was induced in rats by daily intraperitoneal injection of PLC (250 mg/kg/day) for 10 successive days. Fanconi Syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days. IFO-carnitine depleted rats were given the same doses of DC-MD for 5 days before and 5 days concomitant with IFO. IFO-carnitine supplemented rats were given the same doses of PLC for 5 days before and 5 days concomitant with IFO. At the end of the treatment protocol, total carnitine was measured in serum whereas ATP and total carnitine were measured in cardiac tissues. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
On the other hand, IFO alone resulted in a significant 45% decrease in total carnitine level in cardiac tissues, while administration of DC-MD alone caused a significant 42% decrease as compared to the control group. Combined treatment with IFO and DC-MD resulted in a significant 90, 82 and 80% decrease in the total carnitine level in cardiac tissues as compared to the control, DC-MD and IFO groups, respectively. Moreover, challenging with IFO alone significantly decreased ATP levels by 52% in cardiac tissues as compared to control. Carnitine-depleted rats showed significant 45% reduction in cardiac ATP levels, while combining modality also lowered the ATP content by about 86, 74 and 72% compared to either control, DC-MD and IFO given alone, respectively. Interestingly, carnitine supplementation by daily administration of PLC to animals receiving IFO resulted in a complete reversal of IFO-induced decrease in total carnitine and ATP levels in cardiac tissues to the control values.
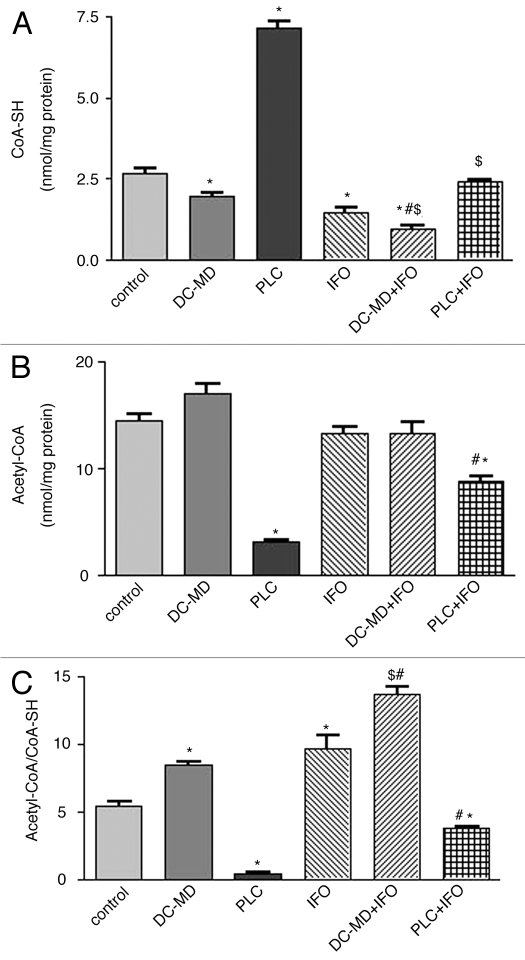
Figure 2 shows the effects of IFO on the level of CoA-SH (Fig. 2A), acetyl-CoA (Fig. 2B) and acetyl-CoA/CoA-SH (Fig. 2C) in heart mitochondria isolated from carnitine depleted and supplemented rats. Treatment with previously mentioned regimen of IFO and DC-MD resulted in a significant 46 and 26% decrease in CoA-SH level and a significant 79 and 57% increase in acetyl-CoA/CoA-SH ratio as compared to the control and IFO groups, respectively. Administration of IFO to carnitine-depleted rats resulted in a significant 64 and 33% decrease in CoA-SH level in isolated rat heart mitochondria, thus raising the ratio to 52 and 41% as compared to the results of the control and IFO groups, respectively. Interestingly, daily administration of PLC to IFO-treated rats resulted in a complete reversal of IFO-induced changes in CoA-SH and aceyl-CoA/CoA-SH level in mitochondria to the control values.
Figure 2.
Effect of ifosfamide (IFO), propionyl-l-carnitine (PLC), D-carnitine-mildronate (DC-MD) and their combination on the levels of CoA-SH (A), acetyl-CoA (B) and acetyl-CoA/CoA-SH (C) in isolated rat heart mitochondria. Rats were randomly divided into 6 different groups of 10 animals each: Control, D-carnitine-mildronate (DC-MD, carnitine-depleted group), PLC (carnitine supplemented group), IFO, DC-MD plus IFO and PLC plus IFO. Carnitine depletion was induced in rats by daily intraperitoneal injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days. Carnitine supplementation was induced in rats by daily intraperitoneal injection of PLC (250 mg/kg/day) for 10 successive days. Fanconi Syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days. IFO-carnitine depleted rats were given the same doses of DC-MD for 5 days before and 5 days concomitant with IFO. IFO-carnitine supplemented rats were given the same doses of PLC for 5 days before and 5 days concomitant with IFO. At the end of the treatment protocol, rat hear mitochondria was isolated for measurement of CoA-SH, acetyl-CoA and CoA-SH/acetyl-CoA ratio. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
Table 4 shows the effects of IFO on oxidative stress biomarkers, TBARS and GSH in cardiac tissues. IFO produced GSH nadir amounted to 60% and dramatically elevated the TBARS levels in cardiac tissues by 80% compared to control group. Repeated administration of DC-MD followed by IFO resulted in a significant 29 and 223% increase in the level of GSH in cardiac tissues as compared to the control and IFO groups, respectively. On the other hand, the treatment modality resulted in a significant 44% decrease in the level of TBARS in cardiac tissues as compared to IFO group. Pre-treatment with PLC followed by IFO challenge thereafter resulted in a complete reversal of IFO-induced increase in TBARS and decrease in GSH level in cardiac tissues to the control values.
Table 4.
Effect of ifosfamide (IFO), propionyl-l-carnitine (pLC), D-carnitine-mildronate (DC-MD) and their combination on the levels of thiobarbituric acid reactive substances (TBARS) and reduced glutathione (GSH) in rat cardiac tissues.
| Treatment groups | TBARS (nmol/g wet tissue) | GSH (µmol/g wet tissue) |
| Control | 249.60 ± 14.9 | 0.70 ± 0.02 |
| DC-MD | 118.01 ± 14.7* | 1.02 ± 0.09 |
| PLC | 113.21 ± 9.7* | 1.23 ± 0.14* |
| IFO | 449.14 ± 33.9* | 0.280 ± 0.03* |
| IFO plus DC-MD | 249.10 ± 33.8*#$ | 0.90 ± 0.08$ |
| IFO plus PLC | 243.60 ± 24.8# | 0.89 ± 0.09# |
Rats were randomly divided into 6 different groups of 10 animals each: Control, D-carnitine-mildronate (DC-MD, carnitine-depleted group), PLC (carnitine supplemented group), IFO, DC-MD plus IFO and PLC plus IFO. Carnitine depletion was induced in rats by daily intraperitoneal injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days. Carnitine supplementation was induced in rats by daily intraperitoneal injection of PLC (250 mg/kg/day) for 10 successive days. Fanconi Syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days. IFO-carnitine depleted rats were given the same doses of DC-MD for 5 days before and 5 days concomitant with IFO. IFO-carnitine supplemented rats were given the same doses of PLC for 5 days before and 5 days concomitant with IFO. At the end of the treatment protocol, TBARS and GSH, oxidative stress biomarkers, were measured in rat cardiac tissues. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test. Data are presented as mean ± S.E.M. (n = 10). *, # and $ indicate significant change from control, IFO and DC-MD respectively, at p < 0.05 using ANOVA followed by Tukey-Kramer as a post ANOVA test.
Discussion
Recent studies in our laboratory20–24 and others25,26 have demonstrated the progression of cisplatin, carboplatin, cyclophosphamide and gentamicin-induced nephrotoxicity, cardiomyopathy and hepatotoxicity under condition of carnitine deficiency and that carnitine supplementation attenuates these multiple organ toxicity. Although it is well documented that IFO therapy is associated with Fanconi Syndrome,7,9,10 we could not find any study investigating the effects of IFO on renal handling of carnitine and its metabolic consequences under condition of carnitine depletion and supplementation. Taken together, this prompted us to investigate, for the first time, whether carnitine deficiency secondary to IFO-induced Fanconi Syndrome plays a role and should be viewed as a risk factor during development of IFO-induced cardiomyopathy as well as exploring if carnitine supplementation using PLC could offer protection against this toxicity.
Results from this study demonstrated that IFO increased nephrotoxicity indices, serum creatinine (233%) and BUN (155%) which precipitates IFO-induced Fanconi Syndrome. Under similar experimental condition, several studies reported that administration of IFO (50 mg/kg) for 5 successive days is associated with severe nephrotoxicity in the form of Fanconi Syndrome.7,9,10 In carnitine depleted rats, IFO caused progressive increase in BUN and serum creatinine which was parallel to the marked increase in urinary carnitine excretion and clearance (Tables 1 and 2). This effect could be due to carnitine deficiency with subsequent impairment of fatty acid oxidation and shifting metabolism into carnitine-independent or non-lipid energy substrates. This speculation is consistent with data presented by Ahmed, et al. which reported that carnitine supplementation to patients undergoing hemodialysis decreased protein catabolism, thereby, reducing serum concentration of the products of protein catabolism, including BUN and creatinine.27
Cardiotoxicity occurs during therapy with several cytotoxic drugs and may be the dose limiting factor in cancer treatment. It can be responsible for long term side effects causing severe morbidity in surviving cancer patients.28 Although IFO has not been added to the list of antineoplastic therapies associated with major cardiac complications, its cardiotoxicity was reported as isolated clinical cases in cancer patients receiving IFO in combination with other anticancer drugs.29–33 It has been reported that high doses of the alkylating drugs cyclophosphamide and IFO may result in a reversible heart failure and life-threatening arrhythmias.34–36 Cardiac effects reported with IFO therapy include supraventricular arrhythmias,29 ventricular premature contraction, dilated cardiomyopathy33 and severe refractory congestive heart failure.7,9,10 In this context, the current study addressed the possible contribution of carnitine deficiency as a risk factor in IFO-induce cardiotoxicity.
Data presented here demonstrated that IFO increased serum LDH and CK-MB. This increase in cardiac enzymes could be due to IFO-induced generation of reactive oxygen species (ROS) and lipid peroxidation of cardiac membranes with the consequent leakage of these enzymes from damaged myocytes. The contribution of oxidative and nitrosative stress in IFO and cyclophosphamide-induced multiple organ toxicity has been recently reported.36–38 It is well documented that ROS and oxidative stress play an important role in organ dysfunction induced by anticancer drugs.39,40 Increased oxidative stress biomarkers and depletion of enzymatic and non-enzymatic antioxidants have been reported in cardiopulmonary and other metabolic disorders.41 Fascinatingly, administration of PLC for 5 days before and 5 days concomitant with IFO completely prevented the increase in LDH and CK-MB induced by IFO, suggesting that PLC may have potential protective effect against IFO-induced cardiac damage. This effect could be due to cardiac membrane stabilization by the L-carnitine portion of PLC which interacts with sarcolemmal phospholipids and mitochondrial membranes42 with subsequent decrease in the release of cardiac enzymes. Moreover, PLC through its reported antioxidant defence in the heart and other tissues could protect myocytes against IFO-induced lipid peroxidation.43 The antioxidant effect of PLC has been confirmed by our results which have reported that PLC induced complete reversal of IFO-induced increase in TBARS and decrease in GSH in cardiac tissues to the control values. More recently, the antioxidant and anti-inflammatory effects of L-carnitine and PLC have been reported.35,44 In contrast, administration of IFO to carnitine-depleted rats produced a progressive increase in the activities of LDH and CK-MB. It is reported that IFO treatment is associated with nephrotoxicity in the form of Fanconi Syndrome.7,9,10 The IFO regimen used in current study (50 mg/kg/day) for 5 successive days proves to be nephrotoxic as evidenced by the increase in serum creatinine and BUN in rats. Quezado, et al. reported that development of congestive heart failure in patients with lymphoma was significantly correlated with the doubling in serum creatinine after IFO treatment.31 Our results are unique since no available experimental or clinical data about the role of endogenous carnitine system during development of IFO-induced multiple organ toxicity.
In the current study, the observed decrease in cardiac carnitine content could be a secondary event following IFO-induced inhibition of endogenous synthesis and/or inhibition of tubular reabsorption of carnitine. Since kidney is the major site for endogenous carnitine biosynthesis and 95% of filtered carnitine is reabsorbed by the proximal tubules of the nephron, therefore, IFO-induced Fanconi Syndrome may lead to inhibition of endogenous carnitine biosynthesis and increases its urinary losses with the consequent secondary deficiency of the molecule. This is in line with current data which showed that urinary carnitine excretion and carnitine clearance were increased by administration of IFO. The present results are in line with the only clinical study performed on five patients and reported that IFO increased urinary carnitine excretion.13 These results are in good agreement with earlier and recent studies which have reported that increased urinary excretion of carnitine is an early marker in chemotherapy-induced nephrotoxicity.25,45,46 Moreover, the role of IFO metabolites in kidney and heart damage cannot be ruled out. Inhibition of the oxidation of long-chain fatty acids by CAA and TDGA has been previously reported.6,19
Data reported here demonstrate that IFO administration significantly decreased intramitochondrial free CoA-SH, an indispensable activator in Kreb's cycle and β-oxidation. This effect could be explained on the basis of the high reactivity of IFO metabolites including CAA, TDGA, IPM and acrolin, with SH-containg molecules. Earlier studies have documented that the metabolic pathway of IFO leads to formation of chloroacetyl-CoA with the consequent depletion of free CoA-SH.47 L-carnitine is known to detoxify acyl-CoA moieties with the formation of acyl-carnitine and subsequent release of free CoA-SH, thus preserving substrates utilization and ATP production in mitochondria. This detoxification mechanism leads to an increased secretion of carnitine derivatives in urine with subsequent secondary deficiency of the molecule.
This progressive decrease of carnitine level in cardiac tissue by IFO in carnitine depleted rats was parallel to the marked increase in LDH and CK-MB, which may point to the possible consideration of carnitine deficiency as a risk factor in IFO-induced cardiotoxicity. This aggravated cardiomyopathy could be explained on the basis of myocardial carnitine deficiency with subsequent impairment of fatty acid oxidation and ATP production. It is well known that L-carnitine is an essential cofactor for mitochondrial transport and oxidation of long chain fatty acids which are the preferred substrates for ATP production in normal, well-oxygenated adult myocardium.48 Depletion of the heart from carnitine and CoA-SH either by IFO, DC-MD or both would impair the beta-oxidation of long chain fatty acids and pyruvate oxidation with the consequent decrease in ATP production and heart contractile function. This was supported by the marked decrease of ATP and free CoA-SH levels in heart tissues observed in carnitine-depleted rats, which renders the cardiac cells vulnerable to damage by IFO. In the current study, utilizing PLC as a source of carnitine prevented IFO and DC-MD induced decrease in cardiac carnitine content, intramitochondrial CoA-SH and ATP production by replenishing the myocardium with adequate carnitine for energy production. A possible explanation for this is that, in the mitochondria, PLC has higher affinity for CoA-SH:carnitine acetyltransferase (CAT) and being metabolized into free L-carnitine and propionyl-CoA.49,50 The L-carnitine portion of PLC will increase fatty acid oxidation by increasing its mitochondrial transport through CPT-I and/or decreasing the intramitochondrial acetyl-CoA/CoA-SH ratio. The propionyl-CoA formed in mitochondria from PLC metabolism will stimulate substrates oxidation since it can be converted into succinyl-CoA in a reaction mediated by propionyl-CoA carboxylase, thus increasing the flux of acetyl-CoA through TCA cycle. In summary, this study suggests that IFO is usually associated in the setting of nephrotoxicity and increased urinary carnitine loss, with severe cardiotoxicity.
Materials
Holoxan vials (Baxter oncology GmbH, Germany) were gifted from King Khalid University Hospital drug store, King Saud University, Kingdom of Saudi Arabia. Each holoxan vial contains 1 g IFO in a dry lyophilized powder form. The content of each vial was freshly dissolved in sterile water for injection immediately before injection. Propionyl-L-carnitine (PLC), D-carnitine (DC) and Mildronate (MD) were kindly supplied by Dr. Zaven Orfalian, Sigma-Tau Pharmaceuticals, Pomezia, Italy. It has been supplied as white powder in a non-commercial plastic bottles contains 100 g and it was freshly dissolved in normal saline prior to injection. All other chemicals used were of the highest analytical grade.
Animals.
Adult male Wistar albino rats, weighing 180–200 g, were obtained from the Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia and were housed in metabolic cages under controlled environmental conditions (25°C and a 12 h light/dark cycle). Animals had free access to pulverized standard rat pellet food and tap water. The protocol of this study has been approved by Research Ethics Committee of College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia.
Experimental design.
Experimental animal model of carnitine deficiency was developed according to Paulson and Shug,51 Whitmer,52 and Tsoko et al.53 In the current study, carnitine depletion was induced in rats by daily intraperitoneal (I.P.) injection of DC (250 mg/kg/day) combined with MD (200 mg/kg/day) for 10 successive days according to previously published studies.51–53 Depletion of L-carnitine by DC occurs via an exchange of the D-and L-isomers across the cell membrane. Moreover, DC possesses an inhibitory effect upon carnitine transferase enzymes and competitive inhibitory effect upon L-carnitine uptake.51–53 Depletion of L-carnitine by MD occurs via inhibition of gamma-butyrobetaine hydroxylase, a key enzyme in the biosynthesis of carnitine.53 Also, Fanconi Syndrome was induced in rats by administration of IFO (50 mg/kg/day, I.P.) for 5 successive days according to the previously published protocols.7,10,54 To achieve the ultimate goals of this study, a total of 60 adult male Wistar albino rats were used and divided at random into 6 groups of 10 animals each. Rats of Group 1 (control group) were received I.P. injection of normal saline (2.5 ml/kg/day) for 10 successive days. Animals in Group 2 (carnitine-depleted group) were given DC (250 mg/kg/day, I.P.) and MD (200 mg/kg/day, I.P.) for 10 successive days. Animals in Group 3 (carnitine-supplemented group) were given PLC (250 mg/kg/day, I.P.) for 10 successive days. Rats of Group 4 (IFO group) were received normal saline for 5 successive days followed by IFO (50 mg/kg/day, I.P.) for 5 successive days. Rats of Group 5 (IFO-carnitine depleted rats) were given the same doses of DC-MD as group 2 for 5 days before and 5 days concomitant with IFO as group 4. Rats of Group 6 (IFO-carnitine supplemented rats) were given the same doses of PLC as Group 3 for 5 days before and 5 days concomitant with IFO as Group 4. The following experimental table outlines the sequence of studies for each experimental animal model used.
Immediately after the last dose of the treatment protocol, 24-hour urine was collected for monitoring urinary carnitine excretion and carnitine clearance. Animals were then sacrificed by decapitation after exposure to ether in a dessicator kept in a well-functioning hood and blood samples were obtained. Serum was separated for measurement of serum creatinine, blood urea nitrogen (BUN) lactate dehydrogenase (LDH), creatine phosphokinase iso-enzyme (CK-MB) and total carnitine. Hearts were quickly excised, washed with saline, blotted with a piece of filter paper and homogenized, in normal saline or 6% perchloric acid as indicated in the procedures of measurement of each parameter, using a Branson sonifier (250, VWR Scientific, Danbury, CT).
Methods
Assessment of serum creatinine and blood urea nitrogen (BUN).
Serum creatinine and BUN concentrations were measured spectrophotometrically according to the methods of Fabiny and Ertingshausen55 and Tabacco et al.56 respectively.
Assessment of serum creatine kinase (CK-MB) and lactate dehydogenase (LDH) activity.
Serum activities of LDH and CK-MB were determined according to the methods of Buhl and Jackson57 and Wu and Bowers,58 respectively.
Determination of total carnitine in serum, urine and cardiac tissues.
Total carnitine concentrations were determined in serum, urine and cardiac tissues according to the method reported by Prieto et al.59 In brief, carnitine reacts with acetyl-CoA forming acetylcarnitine in areaction mediated by carnitine acetyltransferase enzyme. The liberated CoA-SH reacts with 5,5-dithiobis-(2-nitrobenzoic acid) and forming thiophenolate ion, whose generation is proportional to the amount of carnitine and can be measured spectrophotometrically at 412 nm. Serum, urine and heart tissues were deproteinized with equal volume of ice-cold 0.6 M perchloric acid and allowed to stand in an ice bath for 10 min. The mixture was centrifuged at 1,000× g at 4°C for 5 min. The supernatant was used directly for measuring free carnitine after neutralization with 1.2 M potassium carbonate. For the assay of total carnitine, a part of supernatant was mixed with 1 M KOH and incubated at 37°C for 20 min for the hydrolysis of acylcarnitines. Carnitine level was computed from a calibration curve for carnitine hydrochloride.
Determination of CoA-SH and acetyl-CoA in isolated rat heart mitochondria.
Rat heart mitochondria were isolated according to the procedure of Chappel and Hansford.60 The isolation buffer contained 0.21 M mannitol, 0.07 M sucrose, 5 mM Tris-HCl (pH 7.4) and 1 mM EGTA. In brief, heart tissues were homogenized in mitochondrial isolation buffer and centrifuged at 1,000× g for 10 min at 4°C. The resulting supernatant was decanted and further centrifuged at 1,000× g for 10 min and the resulting pellet (mitochondria) was resuspended in the isolation buffer. Protein concentration of the mitochondrial was determined by BioRad protein assay according to the method of Bradford.61 Free CoA-SH and acetyl-CoA were determined in isolated heart mitochondria using HPLC (Jasco Corporation, Ishikawa-Cho, Hachioji, Tokyo, Japan) according to Lysiak, et al.62 In brief, mitochondria were mixed with ice-cold 6% perchloric acid, centrifuged at 300x g for 5 min at 0.5°C, and the resulting supernatant fluid was neutralized to pH 6–7, and then injected into HPLC. Chromatographic separation was performed using ODS-Hypersil, 150 × 4.6 mm I.D., 5 µm column (Supelco SA, Gland, Switzerland). The UV detector was operated at 254 nm and set at 0.005. A mobile phase of 220 mM potassium phosphate containing 0.05% dithioglycol (A) and 98% methanol, 2% chloroform (B) was used. The flow rate was 0.6 ml/min and the gradient was as follows: at zero time, 94% A and 6% B; at 8 min, 92% A and 8% B; at 14 min, 87% A and 13% B; at 25 min, 80% A and 20% B; at 40 min, 55% A and 45% B; at 45 min, 55% A and 45% B; and at 60 min, 94% A and 6% B.
Determination of adenosine triphosphate in cardiac tissues.
Adenosine triphosphate was determined in heart tissues using HPLC system (Jasco Corporation, Ishikawa-Cho, Hachioji, Tokyo, Japan) according to Botker, et al.63 In brief, heart tissue was homogenized in ice-cold 6% perchloric acid, centrifuged at 1,000 rpm for 15 min at 0.5°C and the supernatant fluid was injected into HPLC after neutralization to pH 6–7. Chromatographic separation was performed at a flow rate of 1.2 ml/min, using ODS-Hypersil, 150 x 4.6 mm I.D., 5 µm column (Supelco SA, Gland, Switzerland) and 75 mM ammonium dihydrogen phosphate as mobile phase. The ATP peaks were eluted at 3.2 min and the UV detector was operated at 254 nm.
Determination of reduced glutathione and lipid peroxidation in cardiac tissues.
The tissue levels of the acid soluble thiols, mainly GSH, were assayed spectrophotometrically at 412 nm, according to the method of Ellman,64 using a Shimadzu (Tokyo, Japan) spectrophotometer. The contents of GSH were expressed as mmol/g wet tissue. The degree of lipid peroxidation in cardiac tissues was determined by measuring thiobarbituric acid reactive substances (TBARS) in the supernatant tissue from homogenate.65 The homogenates were centrifuged at 3,500 rpm and supernatant was collected and used for the estimation of TBARS. The absorbance was measured spectrophotometrically at 532 nm and the concentrations were expressed as nmol TBARS/g wet tissue.
Statistical analysis.
Differences between obtained values (mean ± SEM, n = 10) were carried out by one way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test. p ≤ 0.05 was taken as a criterion for a statistically significant difference.
Conclusions
Data from this study suggest that: (1) serum and urine carnitine levels should be viewed as markers that indicate early toxicity and should be monitored during IFO-therapy. (2) oxidative stress and carnitine deficiency provoke IFO-induced cardiotoxicity. (3) carnitine supplementation, using PLC prevents the development of IFO-induced cardiotoxicity through improving mitochondrial function and antioxidant signalling. It would be worthwhile considering the addition of PLC as adjunctive therapy to reduce cancer chemotherapy related complications.
| Group number | Types and duration of treatments (days) | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1 | Normal saline | |||||||||
| 2 | D-carnitine combined with mildronate | |||||||||
| 3 | Propionyl-L-carnitine | |||||||||
| 4 | Normal saline | Ifosfamide | ||||||||
| 5 | D-carnitine combined with mildronate | Ifosfamide D-carnitine combined with mildronate | ||||||||
| 6 | Propionyl-L-carnitine | Ifosfamide Propionyl-L-carnitine | ||||||||
Acknowledgements
The present work was supported by operating grant from King Abdulaziz City for Science and Technology (AT-16-94).
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/12859
References
- 1.Preiss R, Baumann F. Cyclophosphamide and related anticancer drugs. J Chromatogr B Biomed Sci Appl. 2001;764:173–192. doi: 10.1016/s0378-4347(01)00279-1. [DOI] [PubMed] [Google Scholar]
- 2.Skinner R, Sharkey I, Pearson A, Croft A. Ifosfamide, mesna and nephrotoxicity in children. J Clin Oncol. 1993;11:173–190. doi: 10.1200/JCO.1993.11.1.173. [DOI] [PubMed] [Google Scholar]
- 3.Ho P, Zimmerman K, Wexler L, Blaney S, Jarosinski P, Weaver-McClure L, et al. A prospective evaluation of ifosfamide-related nephrotoxicity in children and young adults. Cancer. 1995;76:2557–2564. doi: 10.1002/1097-0142(19951215)76:12<2557::aid-cncr2820761223>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Sangster G, Kaye SB, Calman KC, Dalton JF. Failure of 2-mercaptoethane sulphonate sodium (mesna) to protect against ifosfamide nephrotoxicity. Eur J Cancer Clin Oncol. 1984;20:435–436. doi: 10.1016/0277-5379(84)90093-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Lu H. Ifosfamide induces acute renal failure via inhibition of the thioredoxin reductase activity. Free Radic Biol Med. 2007;43:1574–1583. doi: 10.1016/j.freeradbiomed.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Visarius TM, Bähler H, Küpfer A, Cerny T, Lauterburg HB. Thiodiglycolic acid is excreted by humans receiving ifosfamide and inhibits mitochondrial function in rats. The Am Soc Pharmacol Exp Therap. 1998;26:193–196. [PubMed] [Google Scholar]
- 7.Nissim I, Horyn O, Daikhin Y, Nissim I, Luhovyy B, Phillips BC, et al. Ifosfamide-induced nephrotoxicity: mechanism and prevention. Cancer Res. 2006;66:7824–7831. doi: 10.1158/0008-5472.CAN-06-1043. [DOI] [PubMed] [Google Scholar]
- 8.Woodland C, Ito S, Granvil CP, Wainer IW, Klein J, Koren G. Evidence of renal metabolism of ifosfamide to nephrotoxic metabolites. Life Sci. 2000;68:109–117. doi: 10.1016/s0024-3205(00)00915-2. [DOI] [PubMed] [Google Scholar]
- 9.Goren MP, Wright RK, Horowitz ME, Pratt CB. Ifosfamide-induced subclinical tubular nephrotoxicity despite MESNA. Cancer Treat Rep. 1987;7:127–130. [PubMed] [Google Scholar]
- 10.Nissim I, Weinberg JM. Glycine attenuates maleate or ifosfamide induced Fanconi syndrome in rats. Kidney Int. 1996;49:684–695. doi: 10.1038/ki.1996.97. [DOI] [PubMed] [Google Scholar]
- 11.Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486:1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 12.Mancinelli A, Longo A, Shanahan K, Evans AM. Disposition of L-carnitine and acetyl-L-carnitine in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1995;274:1122–1128. [PubMed] [Google Scholar]
- 13.Marthaler NP, Visarius T, Küpfer A, Lauterburg BH. Increased urinary losses of carnitine during ifosfamide chemotherapy. Cancer Chemother Pharmacol. 1999;44:170–172. doi: 10.1007/s002800050963. [DOI] [PubMed] [Google Scholar]
- 14.Dodson WL, Sachan DS, Krauss S, Hanna W. Alterations of serum and urinary carnitine profiles in cancer patients: hypothesis of possible significance. J Am Coll Nutr. 1989;8:133–142. doi: 10.1080/07315724.1989.10720288. [DOI] [PubMed] [Google Scholar]
- 15.Cruciani RA, Dvorkin E, Homel P, Malamud S, Culliney B, Lapin J, et al. Safety, tolerability and symptom outcomes associated with l-Carnitine supplementation in patients with cancer, fatigue and carnitine deficiency: A Phase I/II study. J Pain Symptom Manage. 2006;32:551–559. doi: 10.1016/j.jpainsymman.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Karlic H, Lohninger S, Koeck T, Lohninger A. Dietary L-carnitine stimulates carnitine acyltransferases in the liver of old rats. J Histochem Cytochem. 2002;50:205–212. doi: 10.1177/002215540205000208. [DOI] [PubMed] [Google Scholar]
- 17.Karlic H, Lohninger A, Laschan C, Lapin A, Böhmer F, Huemer M, et al. Downregulation of carnitine acyltransferases and organic cation transporter OCTN2 in mononuclear cells in healthy elderly and patients with myelodysplastic syndromes. J Mol Med. 2003;81:435–442. doi: 10.1007/s00109-003-0447-6. [DOI] [PubMed] [Google Scholar]
- 18.Waldner R, Laschan C, Lohninger A, Gessner M, Tüchler H, Huemer M, et al. Effects of doxorubicin-containing chemotherapy and a combination with L-carnitine on oxidative metabolism in patients with non Hodgkin lymphoma. J Cancer Res Clin Oncol. 2006;132:121–128. doi: 10.1007/s00432-005-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visarius TM, Stucki JW, Lauterburg BH. Inhibition and stimulation of long-chain fatty acid oxidation by chloroacetaldehyde and methylene blue in rats. J Pharmacol. Exp Ther. 1999;289:820–824. [PubMed] [Google Scholar]
- 20.Al-Shabanah OA, Aleisa AM, Al-Yahya AA, Al-Rejaie SS, Bakheet SA, Fatani AG, et al. Increased urinary losses of carnitine and decreased intramitochondrial coenzyme a in gentamicin-induced acute renal failure in rats. Nephrol Dial Transplant. 2010;25:69–76. doi: 10.1093/ndt/gfp457. [DOI] [PubMed] [Google Scholar]
- 21.Aleisa AM, Al-Majed AA, Al-Yahya AA, Al-Rejaie SS, Bakheet SA, et al. Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin Exp Pharmacol Physiol. 2007;34:1252–1259. doi: 10.1111/j.1440-1681.2007.04714.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Majed AA. Carnitine deficiency provokes cisplatininduced hepatotoxicity in rats. Basic Clin Pharmacol Toxicol. 2007;100:145–150. doi: 10.1111/j.1742-7843.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, et al. Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol Res. 2006;53:278–286. doi: 10.1016/j.phrs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Sayed-Ahmed MM, Eissa MA, Kenawy SA, Mostafa N, Calvani M, Osman AM. Progression of cisplatin-induced nephrotoxicity in a carnitine-depleted rat model. Chemotherapy. 2004;50:162–170. doi: 10.1159/000080689. [DOI] [PubMed] [Google Scholar]
- 25.Heuberger W, Berardi S, Jacky E, Pey P, Krahenbuhl S. Increased urinary excretion of carnitine in patients treated with cisplatin. Eur J Clin Pharmacol. 1998;54:503–508. doi: 10.1007/s002280050504. [DOI] [PubMed] [Google Scholar]
- 26.Arafa HM. Carnitine deficiency aggravates carboplatin nephropathy through deterioration of energy status, oxidant/anti-oxidant balance and inflammatory endocoids. Toxicol. 2008;254:51–60. doi: 10.1016/j.tox.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad S, Robertson HT, Golper TA, et al. Multicenter trial of l-carnitine in maintenance hemodialysis patients II. Clinical and biochemical effects. Kid Intern. 2001;38:912–918. doi: 10.1038/ki.1990.290. [DOI] [PubMed] [Google Scholar]
- 28.Meinardi MT, Gietema JA, van Veldhuisen DJ, et al. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev. 2000;26:429–447. doi: 10.1053/ctrv.2000.0175. [DOI] [PubMed] [Google Scholar]
- 29.Kandylis K, Vassilomanolakis M, Tsoussis S, Efremidis AP. Ifosfamide cardiotoxicity in humans. Cancer Chemother Pharmacol. 1989;24:395–396. doi: 10.1007/BF00257451. [DOI] [PubMed] [Google Scholar]
- 30.Franzen D, Pöhler E, Hilger HH. Pneumo- and cardiotoxic side effects following combination chemotherapy with epirubicin and ifosfamide. Med Klin (Munich) 1990:133–136. [PubMed] [Google Scholar]
- 31.Quezado ZM, Wilson WH, Cunnion RE, Marker MM, Reda D, Bryant G, et al. High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann Intern Med. 1993;118:31–36. doi: 10.7326/0003-4819-118-1-199301010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi J, Kawashima R, Abe Y, Fukuda H, Kubota K, Yamada K, et al. Ventricular premature contraction observed after anti-cancer chemotherapy with ifosfamide. Gan To Kagaku Ryoho. 1994;21:1681–1684. [PubMed] [Google Scholar]
- 33.Rossi R, Kleta R, Ehrich JHH. Renal involvement in children with malignancies. Pediatr Nephrol. 1999;13:153–162. doi: 10.1007/s004670050585. [DOI] [PubMed] [Google Scholar]
- 34.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.03.003. In press. [DOI] [PubMed] [Google Scholar]
- 35.Fatani AG, Darweesh AQ, Rizwan L, Aleisa AM, Al-Shabanah OA, Sayed-Ahmed MM. Carnitine deficiency aggravates cyclophosphamide-induced cardiotoxicity in rats. Chemotherapy. 2010;56:71–81. doi: 10.1159/000298822. [DOI] [PubMed] [Google Scholar]
- 36.Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, et al. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009;25:812–817. doi: 10.1016/j.nut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Abraham P, Rabi S. Nitrosative stress, protein tyrosine nitration, PARP activation and NAD depletion in the kidneys of rats after single dose of cyclophosphamide. Clin Exp Nephrol. 2009;13:281–287. doi: 10.1007/s10157-009-0160-z. [DOI] [PubMed] [Google Scholar]
- 38.Hanly L, Chen N, Rieder M, Koren G. Ifosfamide nephrotoxicity in children: a mechanistic base for pharmacological prevention. Expert Opin Drug Saf. 2009;8:155–168. doi: 10.1517/14740330902808169. [DOI] [PubMed] [Google Scholar]
- 39.Kolli WK, Abraham P, Isaac B, Selvakumar D. Neutrophil Infiltration and Oxidative Stress May Play a Critical Role in Methotrexate-Induced Renal Damage. Chemotherapy. 2009;55:83–90. doi: 10.1159/000192391. [DOI] [PubMed] [Google Scholar]
- 40.Yin HY, Ma XF, Liu F, Xia M, Xu AT. Protective Effect of Geranylgeranylacetone on Cisplatin Ototoxicity. Chemotherapy. 2009;55:1–5. doi: 10.1159/000166382. [DOI] [PubMed] [Google Scholar]
- 41.Fisher-Wellman K, Bell HK, Bloomer RJ. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid Med Cell Longev. 2009;1:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battelli D, Bellei M, Arrigoni-Martelli E, Muscatello U, Bobyleva V. Interaction of carnitine with mitochondrial cardiolipin. Biochem Biophys Acta. 1992;1117:33–36. doi: 10.1016/0304-4165(92)90158-q. [DOI] [PubMed] [Google Scholar]
- 43.Fritz IB, Arrigoni-Martelli E. Sites of action of carnitine and its derivatives on the cardiovascular system: interactions with membranes. Trends Pharmacol Sci. 1993;14:355–360. doi: 10.1016/0165-6147(93)90093-y. [DOI] [PubMed] [Google Scholar]
- 44.Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Bakheet SA. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid Med Cell Longev. 2009;2:73–81. doi: 10.4161/oxim.2.2.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mancinelli A, D'Iddio S, Bisonni R, Graziano F, Lippe P, Calvani M. Urinary excretion of L-carnitine and its short-chain acetyl-L-carnitine in patients undergoing carboplatin treatment. Cancer Chemother Pharmacol. 2007;60:19–26. doi: 10.1007/s00280-006-0341-3. [DOI] [PubMed] [Google Scholar]
- 46.Haschke M, Vitins T, Lüde S, Todesco L, Novakova K, Herrmann R, et al. Urinary excretion of carnitine as a marker of proximal tubular damage associated with platin-based antineoplastic drugs. Nephrol Dial Transplant. 2010;25:426–433. doi: 10.1093/ndt/gfp456. [DOI] [PubMed] [Google Scholar]
- 47.Dubourg L, Michoudet C, Cochat P, Baverel G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J Am Soc Nephrol. 2001;12:1615–1623. doi: 10.1681/ASN.V1281615. [DOI] [PubMed] [Google Scholar]
- 48.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 49.Paulson DJ, Traxler J, Schmidt M, Noonan J, Shug AL. Protection of the ischaemic myocardium by l-propionylcarnitine: Effects on the recovery of cardiac output after ischaemia and reperfusion, carnitine transport and fatty acid oxidation. Cardiovasc Res. 1986;20:536–541. doi: 10.1093/cvr/20.7.536. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C. Therapeutic effects of l-carnitine and propionyl-l-carnitine on cardiovascular diseases: A review. Ann NY Acad Sci. 2004;1033:79–91. doi: 10.1196/annals.1320.007. [DOI] [PubMed] [Google Scholar]
- 51.Paulson DJ, Shug AL. Tissue specific depletion of L-carnitine in rat heart and skeletal muscle by D-carnitine. Life Sci. 1981;28:2931–2938. doi: 10.1016/0024-3205(81)90269-1. [DOI] [PubMed] [Google Scholar]
- 52.Whitmer JT. L-carnitine treatment improves cardiac performance and restores high energy phosphate pools in cardiomyopathic syrian hamster. Circ Res. 1987;61:396–408. doi: 10.1161/01.res.61.3.396. [DOI] [PubMed] [Google Scholar]
- 53.Tsoko M, Beau-Seigneur F, Greste J, Niot I, Demarquoy J, Biochot J, et al. Enhancement of activities relative to fatty acid oxidation in the liver of rats depleted of L-carnitine by D-carnitine and a γ-Butyrobetaine hydroxylase inhibitor. Biochem Pharmacol. 1995;49:1403–1410. doi: 10.1016/0006-2952(95)00019-v. [DOI] [PubMed] [Google Scholar]
- 54.Sehirli O, Sakarcan A, Velioglu-Ogünç A, Cetinel S, Gedik N, Yegen BC, et al. Resveratrol improves ifosfamide-induced Fanconi syndrome in rats. Toxicol Appl Pharmacol. 2007;222:33–41. doi: 10.1016/j.taap.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Fabiny DL, Ertingshausen G. Automated reaction-rate method fordetermination of serum creatinine with the Centrifi Chem. Clin Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- 56.Tabacco A, Meiattini F, Moda E, Tarli P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin Chem. 1979;25:336–337. [PubMed] [Google Scholar]
- 57.Buhl SN, Jackson KY. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactateto-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30 and 37 degrees C. Clin Chem. 1978;24:828–831. [PubMed] [Google Scholar]
- 58.Wu AHB, Bowers CN. Evaluation and comparison of immunoinhibition and immunopreceptation methods for differentiating MB and BB from macro forms of creatine kinase isoenzymes in patients and healthy individuals. Clin Chem. 1982;28:2017–2021. [PubMed] [Google Scholar]
- 59.Prieto JA, Andrade F, Aldámiz-Echevarría L, Sanjurjo P. Determination of free and total carnitine in plasma by an enzymatic reaction and spectrophotometric quantitation spectrophotometric determination of carnitine. Clin Biochem. 2006;39:1022–1027. doi: 10.1016/j.clinbiochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Chappel JB, Hansford RG. In: Subcellullar components. 2nd ed. Birnie GD, editor. London: Butterworths; 1969. pp. 77–91. [Google Scholar]
- 61.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 62.Lysiak W, Lilly K, DiLisa F, Toth PP, Bieber LL. Quantitation of the effect of L-Carnitine on the levels of acid-soluble short-chain acyl-CoA and CoASH in rat heart and liver mitochondria. J Biol Chem. 1988;263:1151–1156. [PubMed] [Google Scholar]
- 63.Botker HE, Kimose HH, Helligso P, Nielsen TT. Analytical evaluation of high energy phosphate determination by high performance liquid chromatography in myocardial tissue. J Mol Cell Cardiol. 1994;26:41–48. doi: 10.1006/jmcc.1994.1006. [DOI] [PubMed] [Google Scholar]
- 64.Ellman GL. Tissue sulfahydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 65.Ohkawa H, Ohish N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]