Abstract
Selective loss of neurons, abnormal protein deposition and neuroinflammation are the common pathological features of neurodegenerative diseases, and these features are closely related to one another. In Parkinson's disease, abnormal aggregation and deposition of α-synuclein is known as a critical event in pathogenesis of the disease, as well as in other related neurodegenerative disorders, such as dementia with Lewy bodies and multiple system atrophy. Increasing evidence suggests that α-synuclein aggregates can activate glial cells to induce neuroinflammation. However, how an inflammatory microenvironment is established and maintained by this protein remains unknown. Findings from our recent study suggest that neuronal α-synuclein can be directly transferred to astrocytes through sequential exocytosis and endocytosis and induce inflammatory responses from astrocytes. Here we discuss potential roles of astrocytes in a cascade of events leading to α-synuclein-induced neuroinflammation.
Key words: Parkinson's disease, neuroinflammation, alpha-synuclein, amyloid, neurodegeneration
Astrocytes, which are the most abundant cells in the brain, are responsible for a wide variety of important functions, including regulation of blood flow, maintenance of the blood-brain barrier (BBB), synaptic function and plasticity and maintenance of the extracellular environment of ions, fluids and neurotransmitters.1,2 Insults to the central nervous system (CNS) from injury and disease could result in molecular, cellular and functional changes in astrocytes, leading to what is known as ‘reactive astrogliosis’, which is epitomized by an alteration in gene expression, hypertrophy and proliferation of astrocytes. Known molecular triggers of reactive astrogliosis include peptide growth factors and cytokines, such as FGF2, IL-6, TNFα and IL-1, neurotransmitters such as glutamate and noradrenalin and disease-associated products, such as β-amyloid and these factors could be released by all cell types of CNS, including neurons, microglia, oligodendrocytes, endothelia and astrocytes.
The role of reactive astrogliosis has been controversial. Neurotoxicity, inflammation and inhibition of axon regeneration are some of the negative impacts of reactive astrogliosis that have been depicted for many years. However, findings from recent studies also support the role of reactive astrogliosis in protection of brain cells. The protective roles of reactive astrocytes are implicated by uptake of glutamate, glutathione production, protection from NH4+ toxicity, degradation of neurotoxic amyloid β-peptides, blood brain barrier repair and limiting inflammatory cells and infectious agents to injury sites.
Among the functions of reactive astrocytes, their roles in regulating brain inflammation have gained attention in recent years. Various stimuli present in the brain parenchymal microenvironment could promote astrocyte production of either pro- or anti-inflammatory molecules that could exert effects on neighboring cells.3 Production of factors, such as B cell survival factor BAFF, CCL2, CCL5, CXCL10 and CXCL12, triggers adaptive immunity in the CNS. Astrocyte-derived GM-CSF, IL-6, CCL2 and CCL5 regulate migration, activation and proliferation of microglia. The inflammatory state with elevated levels of cytokines and chemokines also affects neuronal survival and many neuronal functions, such as synaptic transmission and plasticity.
Neuroinflammation in neurodegenerative diseases, such as Alzheimer's disease (AD) and Parkinson's disease (PD), has been well-documented. In particular, an increased amount of proinflammatory cytokines, proliferation of glial cells and a reduction of neurotrophic factors derived from glial cells have been associated with PD.4 However, it is not known how neuroinflammatory responses are triggered and progress in neurodegenerative diseases. A series of recent works by our group and others suggests a novel mechanism of neuroinflammation in PD and the role of astrocytes in the inflammatory process. In this Extra View, we would like to discuss how astrocytes modulate the inflammatory microenvironment in brain parenchyma in response to extracellular α-synuclein derived from neurons.
Parkinson's Disease and α-synuclein
PD is the second most prevalent neurodegenerative disease after AD, and is characterized primarily by movement abnormalities, such as resting tremor, bradykinesia and muscle tone rigidity, and also by various psychiatric, autonomic and sensory dysfunctions.5 Pathologically, motor symptoms are attributed to selective loss of dopamine neurons in the substantia nigra pars compacta region of the midbrain.5 In the same region, some surviving neurons contain proteinacious inclusion bodies known as Lewy bodies. Although much attention has been concentrated on the pathology of the midbrain, diverse clinical symptoms suggest affliction of multiple brain systems, and, indeed, Lewy body lesions are found in multiple areas of the cerebrum and brain stem. By analysis of the patterns of Lewy body lesions in postmortem brains of PD patients, Braak and colleagues proposed a hypothesis that as the disease progresses, Lewy bodies propagate in a highly predictable manner, initiating in the lower brain stem and olfactory bulb, spreading through the midbrain and mesocortex, and finally affecting wide areas of the neocortex.6
The mechanism underlying disease initiation and progression is still unknown. However, accumulating evidence supports critical involvement of α-synuclein in the pathogenesis of the disease. Aggregated forms of this protein are the major component of Lewy bodies.7 Some rare familial forms of PD are linked to either missense or gene multiplication mutations in the α-synuclein gene.8 The α-synuclein gene locus has recently been identified as a major genetic risk factor for PD in genome-wide association studies of two large populations, one European and the other Japanese.9,10
α-Synuclein is a neuronal cytosolic protein. However, a small amount of this protein is released from neurons by unconventional exocytosis.11,12 Under various stress conditions, release of α-synuclein aggregates from neuronal cells was significantly increased.11 Extracellular α-synuclein aggregates can be taken up by neurons and glial cells by endocytosis.13,14 Inspired by these properties of α-synuclein, we have recently demonstrated that aggregates of this protein can be transferred from one neuron to another through sequential exocytosis and endocytosis, forming Lewy body-like inclusions and leading to neuronal death.15 This cell-to-cell transmission might be the mechanism underlying the propagation of Lewy body lesions during progression of PD.
In a recent study, we have shown that α-synuclein released from neuronal cells could also be transferred to and accumulate in astrocytes and induce expression of genes that are associated with immune functions.16 Conditioned medium of α-synuclein expressing SH-SY5Y neuronal cells was treated to primary astrocyte cultures and the expression changes were monitored by microarray analysis. Of those genes that changed expression in response to extracellular α-synuclein, prominent changes were observed in genes for proinflammatory cytokines and chemokines, as shown in Tables 1 and 2.
Table 1.
Changes in the cytokine and cytokine receptor mRNA expression in astrocytes by extracellular α-synuclein
| Entrez ID | Gene symbol | Gene title | Fold changes | p-value | ||||
| 6 hr | 24 hr | 6 hr | 24 hr | |||||
| pro-inflammatory | cytokines | 24493 | Il1α | interleukin 1alpha | 11.28 | 33.54 | 2E-07 | 2E-05 |
| 24494 | Il1β | interleukin 1beta | 23.03 | 76.53 | 2E-07 | 1E-05 | ||
| 24498 | Il6 | interleukin 6 | 3.488 | 3.927 | 1E-05 | 3E-04 | ||
| 116472 | Il17b | PREDICTED: interleukin 17B | 0.88 | 0.487 | 0.153 | 0.002 | ||
| 29197 | Il18 | interleukin 18 | 1.006 | 2.062 | 0.946 | 0.002 | ||
| 78965 | Csf1 | colony stimulating factor1 | 5.064 | 3.17 | 2E-06 | 3E-04 | ||
| 116630 | Csf2 | PREDICTED: colony stimulating factor2 | 2.85 | 6.194 | 7E-06 | 2E-04 | ||
| 25610 | Csf3 | colony stimulating factor3 | 1.256 | 3.8 | 0.001 | 3E-04 | ||
| cytokine receptors | 24499 | Il6rα | interleukin 6 receptor, alpha | 1.588 | 2.371 | 1E-04 | 0.002 | |
| anti-inflammatory | cytokines | 25325 | Il10 | interleukin 10 | 0.982 | 2.22 | 0.994 | 8E-04 |
| 84388 | Il18bp | interleukin 18 binding protein | 1.057 | 2.092 | 0.121 | 0.002 | ||
| 25717 | Tgfβ3 | transforming growth factor, beta3 | 0.595 | 0.192 | 3E-04 | 4E-04 | ||
| cytokine receptors | 117022 | Il1r2 | interleukin 1 receptor, type II | 1.016 | 1.665 | 0.932 | 0.005 | |
Table 2.
Changes in the chemokine and chemokine receptor mRNA expression in astrocytes by extracellular α-synuclein
| Entrez ID | Gene symbol | Gene title | Fold changes | p-value | |||
| 6 h | 24 h | 6 h | 24 h | ||||
| Chemokines | 24770 | Ccl2 | chemokine (C-C motif) ligand 2 | 1.267 | 5.213 | 0.0012 | 0.0003 |
| 25542 | Ccl3 | chemokine (C-C motif) ligand 3 | 18.01 | 40.43 | 3E-07 | 1E-05 | |
| 116637 | Ccl4 | chemokine (C-C motif) ligand 4 | 5.591 | 34.61 | 2E-06 | 2E-05 | |
| 81780 | Ccl5 | chemokine (C-C motif) ligand 5 | 13.56 | 154.1 | 4E-07 | 4E-06 | |
| 287910 | Ccl6 | chemokine (C-C motif) ligand 6 | 1.028 | 1.685 | 0.5854 | 0.0051 | |
| 287561 | Ccl7 | chemokine (C-C motif) ligand 7 | 3.013 | 11.54 | 4E-06 | 3E-05 | |
| 287562 | Ccl12 | PREDICTED: chemokine (C-C motif) ligand 12 | 1.455 | 78.03 | 0.0012 | 1E-05 | |
| 362506 | Ccl19 | PREDICTED: chemokine (C-C motif) ligand 19 | 1.087 | 3.956 | 0.0193 | 0.0003 | |
| 29538 | Ccl20 | chemokine (C-C motif) ligand 20 | 120.2 | 349.2 | 2E-08 | 2E-05 | |
| 81503 | Cxcl 1 | chemokine (C-x-C motif) ligand 1 | 19.6 | 39.27 | 4E-08 | 0.0002 | |
| 114105 | Cxcl 2 | chemokine (C-x-C motif) ligand 2 | 32.85 | 18.17 | 5E-08 | 5E-05 | |
| 360918 | Cxcl 4 | chemokine (C-x-C motif) ligand 4 | 1.352 | 4.232 | 0.0302 | 0.0003 | |
| 60665 | Cxcl 5 | PREDICTED: chemokine (C-x-C motif) ligand 5 | 52.5 | 249.5 | 1E-08 | 2E-06 | |
| 246759 | Cxcl 9 | chemokine (C-x-C motif) ligand 9 | 1.212 | 3.885 | 0.0012 | 0.0005 | |
| 245920 | Cxcl 10 | chemokine (C-x-C motif) ligand 10 | 10.36 | 12.47 | 4E-06 | 6E-05 | |
| 305236 | Cxcl 11 | chemokine (C-x-C motif) ligand 11 | 4.762 | 33.66 | 2E-05 | 3E-05 | |
| 24772 | Cxcl 12 | chemokine (C-x-C motif) ligand 12 | 1.249 | 4.094 | 0.0289 | 0.0003 | |
| 497942 | Cxcl 16 | similar to chemokine (C-x-C motif) ligand 16 | 4.033 | 13.27 | 9E-06 | E-05 | |
| 89808 | Cx3cl1 | chemokine (C-x3-C motif) ligand 1 | 9.005 | 24.16 | 2E-07 | 5E-05 | |
| - | Xcl1 | small inducible cytokine subfamily C, member 1 | 2.646 | 2.698 | 3E-05 | 0.0009 | |
| Chemokine Receptors | 57301 | Ccr1 | chemokine (C-C motif) receptor 1 | 0.969 | 0.928 | 0.9487 | 0.2708 |
| 60463 | Ccr2 | chemokine (C-C motif) receptor 2 | 1.09 | 0.978 | 0.1826 | 0.7404 | |
| 117027 | Ccr3 | chemokine (C-C motif) receptor 3 | 0.976 | 0.834 | 0.6092 | 0.2104 | |
| 171054 | Ccr4 | chemokine (C-C motif) receptor 4 | 0.995 | 1.026 | 0.9553 | 0.9216 | |
| 117029 | Ccr5 | chemokine (C-C motif) receptor 5 | 1.048 | 1.007 | 0.2268 | 0.7863 | |
| 308163 | Ccr6 | chemokine (C-C motif) receptor 6 | 0.91 | 1.098 | 0.0109 | 0.1073 | |
| 287673 | Ccr7 | chemokine (C-C motif) receptor 7 | 0.987 | 0.829 | 0.965 | 0.5081 | |
| 282832 | Ccr9 | chemokine (C-C motif) receptor 9 | 0.951 | 0.926 | 0.6239 | 0.0994 | |
| 60628 | Cxcr4 | chemokine (C-x-C motif) receptor 4 | 0.58 | 0.383 | 0.0003 | 0.0007 | |
| 84475 | Cxcr3 | chemokine (C-x-C motif) receptor 3 | 1.115 | 1.037 | 0.0893 | 0.7357 | |
| 171056 | Cx3cr1 | chemokine (C-x3-C) receptor 1 | 1.036 | 0.958 | 0.3237 | 0.993 | |
| - | Xcr1 | PREDICTED: chemokine (C motif) receptor 1 | 1.034 | 0.959 | 0.2971 | 0.8277 | |
Expression of Inflammatory Factors in Astrocytes Exposed to Neuron-derived α-Synuclein
Cytokines that are differentially expressed in astrocytes in response to extracellular α-synuclein are shown in Table 1. Many proinflammatory cytokines, such as IL-1α, IL-1β, IL-6, IL-18 and colony-stimulating factors (CSF-1, -2, -3), have shown increased expression as early as 6 hours post-treatment. The most dramatic changes have been observed for IL-1α and IL-1β. In 6 hours, about 11-fold and 23-fold changes were detected for IL-1α and IL-1β, respectively. The increase in expression continues until 24 hours, resulting in 33-fold and 76-fold changes, respectively. IL-1α and IL-1β are well-characterized proinflammatory cytokines, and, thus, the drastic increase in expression of these cytokines suggests a strong inflammatory response from astrocytes upon exposure to neuronderived α-synuclein. Other proinflammatory cytokines induced in astrocytes by neuron-derived α-synuclein include IL-6, IL-18 and CSFs.
Another interesting observation was a significant decrease of TGFβ3 (by 80% after 24 hours) (Table 1). TGFβ is a well-known anti-inflammatory molecule that acts to reduce synthesis and release of proinflammatory cytokines and chemokines. TGFβ1 has been shown to reduce amyloid plaque load in mouse models of AD, possibly through increase of Aβ phagocytosis by microglia.
As shown in Table 1, mild increases in anti-inflammatory cytokines were also observed. IL-10 is a potent inhibitor of cytokines IL-1, -2, -6, -8, -12 and CSF1 and chemokines, such as CCL3. IL-18 binding protein (IL-18BP) is a soluble extracellular domain of the IL-18 receptor, which acts as a decoy receptor and binds circulating IL-18.17 Increased expression of IL-1 receptor type II (Il1r2) may counteract the action of IL-1β by prevention of binding to IL-1 receptor type I. However, these anti-inflammatory responses are late events and are much milder than the proinflammatory responses.
Exposure to neuron-derived α-synuclein also caused dramatic changes in chemokine expression in astrocytes (Table 2). CC-type (CCL-3, -4, -5, -12, -20), CXC-type (CXCL-1, -2, -5, -10, -11, -12, -16) and CX3C-type (CX3CL1) chemokines showed increased response to neuron-derived extracellular α-synuclein. These chemokines are involved in a variety of functions, such as recruitment of monocytes and macrophages, migration of microglia and neural progenitors, regulation of myelination, regulation of microglial activity, proliferation and survival of astrocytes and synaptic plasticity and transmission.3
Increased expression of cytokines and chemokines has also been detected in several neurodegenerative diseases. Higher levels of TNFα, IL-1β, IL-2, IL-4, IL-6, TGFα, TGFβ1 and TGFβ2 have been shown in the brain parenchyma and CSF of human PD patients.18 Chemokines CCL2, CCL3, CCL5 and CCL8 and cytokines IL-1β, IFNγ and TNFα were increased in peripheral blood monocytes of PD patients.19 CXCL12/CXCR4 expression was increased in the nigrostriatal system of PD, as well as in brain tumors, ischemia and HIV encephalitis.20
Effects on Microglia of Inflammatory Cytokines and Chemokines Released by Astrocytes
Microglia are the main immune responsive cells in the CNS. These cells produce a large variety of inflammatory cytokines and chemokines, which could affect the parenchymal microenvironment in both auto- and paracrine fashion. Microglia could also receive inflammatory signals from neighboring astrocytes, neurons, endothelium and leukocyte infiltrates, as they express receptors for inflammatory modulators on the cell surface. Astrocytes could be the major source of these inflammatory modulators, as recent advances have shown that astrocytes could produce many inflammatory cytokines and chemokines upon various stimuli. As we have described above, astrocytes exposed to neuron-derived α-synuclein induce production of proinflammatory cytokines and chemokines that can activate microglia. Interestingly, in response to release of extracellular α-synuclein from neuronal cells, microglia increased expression of receptors for cytokines and chemokines (unpublished data). These results indicate that neuron-derived α-synuclein not only stimulates astrocytes for production of microglia-activating soluble factors, but also prepares microglia to respond to these factors. Inflammatory modulators upregulated in astrocytes by extracellular α-synuclein regulate microglial chemotaxis, activation and proliferation.3 Therefore, by release of factors that recruit and activate microglia, astrocytes might act as an intermediary signal ampli- fier, sensing neuron-derived extracellular α-synuclein and relaying this signal to microglia for a potentially explosive inflammatory response.
Interestingly, some of the inflammatory modulators expressed in astrocytes may have opposite effects on microglia. IL-10 may act on microglia to suppress inflammatory responses. Chemokines CX3CL1 and CCL5 are known to inhibit microglia toxicity and suppress production of pro-inflammatory cytokines from activated microglia.3 Production of these modulators with a precise spatiotemporal regulation may play an important role in fine-tuning of microglia activation.
Effects of Inflammatory Cytokines and Chemokines on Astrocytes
Cytokines and chemokines induced by extracellular α-synuclein may directly target astrocytes. TNFα, IFNγ and IL-1 are the main activators of astrocytes. Chemokines, such as CCL2, CCL5, CCL20, CXCL1, CXCL2 and CX3CL1, promote chemotaxis, cell proliferation and survival of astrocytes. CXCL12 and CCL5 induce glutamate release and synthesis of cytokines and chemokines in astrocytes, implying a role in glia-glia and glia-neuron communications. An interesting observation was that unlike in microglia, changes in expression of receptors for these factors in response to extracellular α-synuclein were minimal in astrocytes (Tables 1 and 2). The reason for this may be that the main target for these inflammatory molecules produced by astrocytes is not astrocytes themselves, but microglia. Furthermore, astrocytes seem to downregulate some chemokine receptors, such as CXCR4 and increase expression of anti-inflammatory cytokine receptor IL-1 receptor type II (IL1r2), perhaps for negative feedback regulation. Other reports have also shown that astrocytes could survive inflammatory insults and death-receptor-induced apoptosis.3,21 Therefore, astrocytes produce cytokines and chemokines that may act on astrocytes themselves; however, astrocytes may also activate the mechanism that restricts their own inflammatory responses.
Effects of Inflammatory Cytokines and Chemokines on Neurons
Inflammatory cytokines and chemokines are known to regulate many neuronal functions, including neurogenesis, neurotoxicity and synaptic transmission and plasticity. For example, pro-inflammatory cytokines, such as IL-1 and TNFα, induce neurotransmitter release in neurons. TGFβ3 is known to play a neuroprotective role in animal models of neurodegeneration and also to induce dopaminergic neurogenesis.22,23 Therefore, reduction of TGFβ3 in response to extracellular α-synuclein (Table 1) may not only compromise anti-inflammatory function of astrocytes, but also leave neurons more vulnerable to toxic insults. On the other hand, chemokines, such as CXCL12 and CCL2, have a protective role by promoting migration and survival of neural precursors. As CX3CL1 is a potent inhibitor of microglial neurotoxicity, upregulation of constitutively expressed CX3CL1 in astrocytes may protect neurons. It is also known to modulate evoked excitatory synaptic transmission.24 Therefore, changes in expression and secretion of cytokines and chemokines by extracellular α-synuclein in astrocytes may have a significant effect on neuronal activity and survival in multifaceted ways.
Conclusions
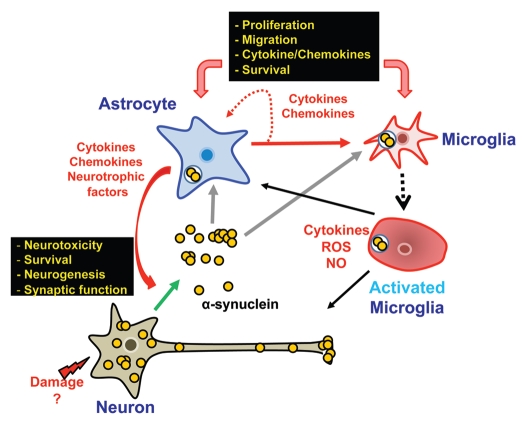
Based on our gene expression profiling study16 and other recent studies, we propose a working model for the α-synuclein-mediated neuroinflammation process (Fig. 1). Neurons under stress release increased amounts of α-synuclein into the extracellular space. Released α-synuclein proteins then induce inflammatory responses from neighboring glia. As many recent studies have shown, microglia, the major immune cells in the CNS, can be directly activated by α-synuclein. Microglia activation can also be achieved indirectly by activation of astrocytes. Astrocytes, upon stimulation by neuron-derived α-synuclein, synthesize and release a number of proinflammatory cytokines and chemokines that can in turn recruit and activate microglia. Therefore, through triggering the production of inflammatory factors in astrocytes, the effects of small amounts of α-synuclein protein released from neurons can be amplified and sustained, thereby establishing an inflammatory microenvironment and further damaging neurons. On the other hand, some factors released from α-synuclein-stimulated astrocytes have neuroprotective functions. Thus, it is tempting to speculate that astrocytes may act as a key modulator, sensing the levels of α-synuclein proteins released from neurons; in some conditions, establishing a neuroprotective environment, but in other conditions, causing fullblown inflammation. Investigation of the role of astrocytes in α-synuclein-mediated neuroinflammation would likely provide critical insight into the mechanisms of neuron-glia and glia-glia interactions in a parenchymal inflammatory microenvironment in brains of PD and other related neurodegenerative diseases.
Figure 1.
Role of astrocytes in modulation of a brain inflammatory microenvironment induced by neuron-derived α-synuclein. Under stress conditions, neurons release α-synuclein into the extracellular space. Neighboring astrocytes internalize the extracellular α-synuclein. when α-synuclein proteins accumulate in the cytoplasm, astrocytes promote the production of cytokines and chemokines, which in turn recruit and activate microglia, the major immune cells in the brain. Changes in cytokine/chemokine expression in astrocytes could also affect neuronal viability and function.
Acknowledgements
This work was supported by the faculty research fund of Konkuk University in 2007.
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/12809
References
- 1.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanagida T, Tsushima J, Kitamura Y, Yanagisawa D, Takata K, Shibaike T, et al. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid Med Cell Longev. 2009;2:36–42. doi: 10.4161/oxim.2.1.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat Disord. 2005;11:9–15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 9.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 10.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 18.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 19.Reale M, Greig NH, Kamal MA. Peripheral chemocytokine profiles in Alzheimer's and Parkinson's diseases. Mini Rev Med Chem. 2009;9:1229–1241. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain or reduce? Neurotherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JH, Bellail A, Tse MC, Yong VW, Hao C. Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. J Neurosci. 2006;26:3299–3308. doi: 10.1523/JNEUROSCI.5572-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HC, Bing G, Kim SJ, Jhoo WK, Shin EJ, Bok Wie M, et al. Kainate treatment alters TGF-beta3 gene expression in the rat hippocampus. Brain Res Mol Brain Res. 2002;108:60–70. doi: 10.1016/s0169-328x(02)00514-4. [DOI] [PubMed] [Google Scholar]
- 23.Li K, Xue B, Wang Y, Wang X, Wang H. Ventral mesencephalon astrocytes are more efficient than those of other regions in inducing dopaminergic neurons through higher expression level of TGF-beta3. J Mol Neurosci. 2009;37:288–300. doi: 10.1007/s12031-008-9146-7. [DOI] [PubMed] [Google Scholar]
- 24.Bertollini C, Ragozzino D, Gross C, Limatola C, Eusebi F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology. 2006;51:816–821. doi: 10.1016/j.neuropharm.2006.05.027. [DOI] [PubMed] [Google Scholar]