Abstract
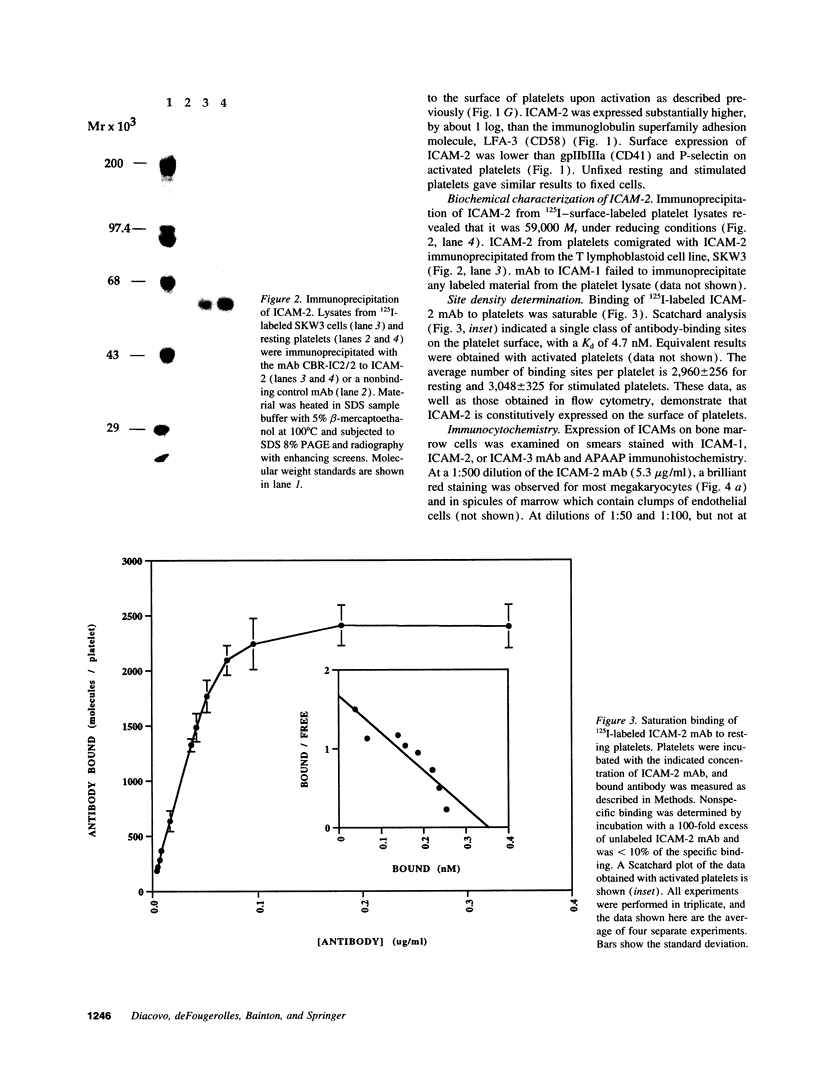
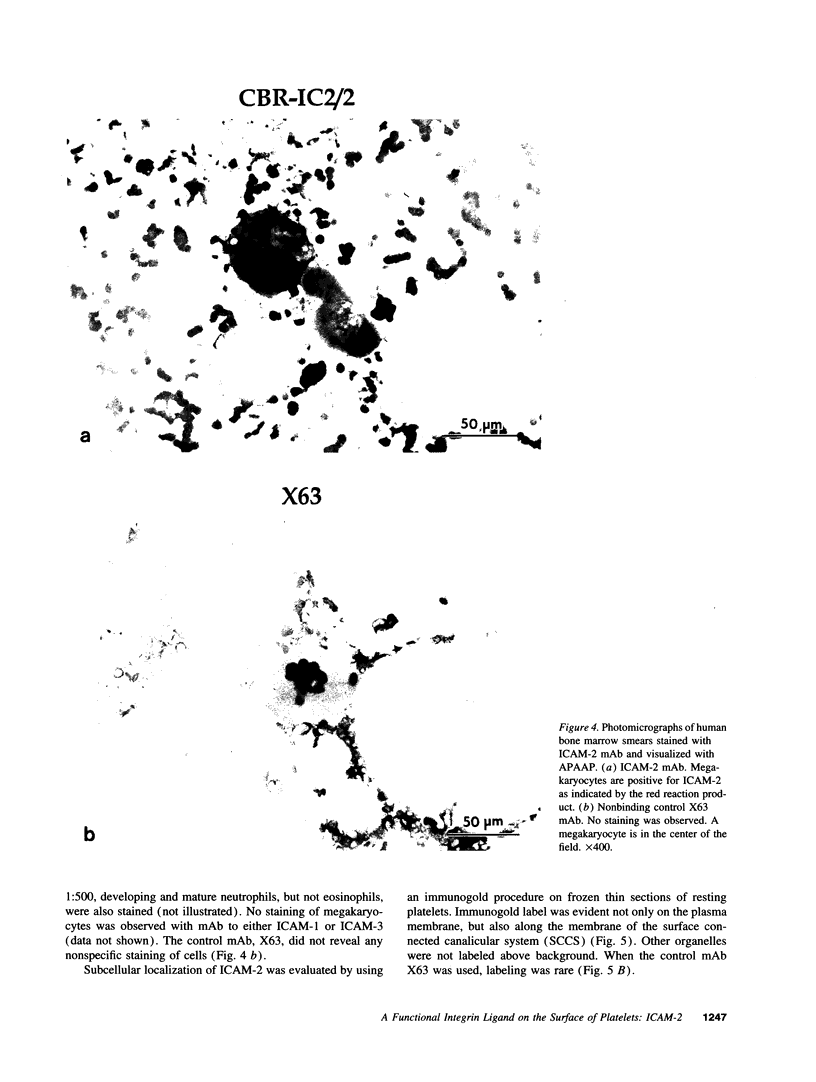
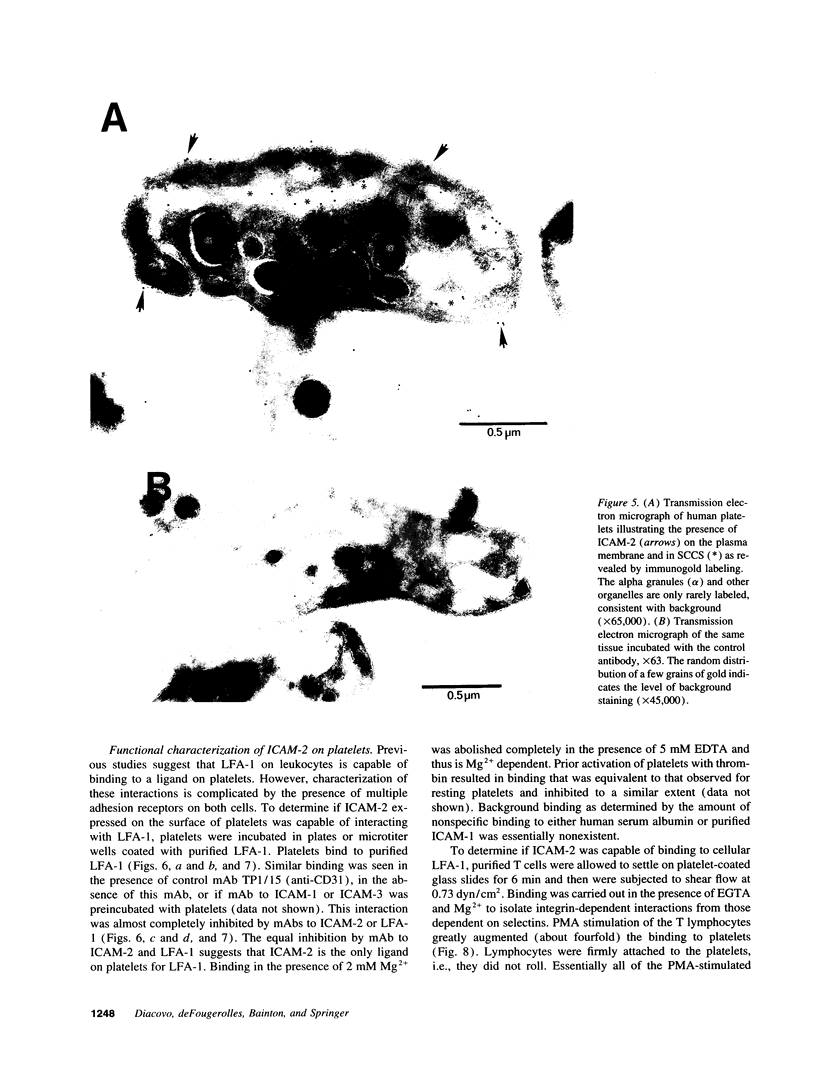
Activated platelets express P-selectin and release leukocyte chemoattractants; however, they have not been known to express integrin ligands important in the stabilization of leukocyte interactions with the vasculature. We now demonstrate the presence of intercellular adhesion molecular-2 (ICAM-2) (CD102), and lack of expression of other beta 2-integrin ligands, ICAM-1 (CD54) and ICAM-3 (CD50), on the surface of resting and stimulated platelets. ICAM-2 isolated from platelets migrates as a band of 59,000 M(r) in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Staining of bone marrow aspirates with anti-ICAM-2 mAb demonstrates strong reactivity to megakaryocytes. Using frozen thin sections and immunogold labeling, the antigen was shown to be present on the plasma membrane and surface-connected canalicular system of resting platelets. The average number of ICAM-2 molecules per platelet is 3,000 +/- 230 and does not change after activation. In adhesion assays, resting and stimulated platelets were capable of binding through ICAM-2 to purified leukocyte function-associated antigen-1. Activation of T lymphocytes with PMA stimulated binding to platelets that was Mg2+ dependent and could be specifically inhibited by mAbs to either ICAM-2 or leukocyte function-associated antigen-1. ICAM-2 is the only known beta 2-integrin ligand present on platelets, suggesting that it may play an important role in leukocyte-platelet interactions in inflammation and thrombosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abts H., Emmerich M., Miltenyi S., Radbruch A., Tesch H. CD20 positive human B lymphocytes separated with the magnetic cell sorter (MACS) can be induced to proliferation and antibody secretion in vitro. J Immunol Methods. 1989 Dec 20;125(1-2):19–28. doi: 10.1016/0022-1759(89)90073-2. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar M., Smith B., Pinto A., Mullane K. M. Neutrophil depletion suppresses 111In-labeled platelet accumulation in infarcted myocardium. J Cardiovasc Pharmacol. 1985 Sep-Oct;7(5):906–912. doi: 10.1097/00005344-198509000-00014. [DOI] [PubMed] [Google Scholar]
- Buttrum S. M., Hatton R., Nash G. B. Selectin-mediated rolling of neutrophils on immobilized platelets. Blood. 1993 Aug 15;82(4):1165–1174. [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., McDowall A., Back R., Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984 Oct;89(1):65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Hymes K., Nardi M., Leaf A., Karpatkin S. Role of leuCAM integrins and complement in platelet-monocyte rosette formation induced by immune complexes of human immunodeficiency virus-type 1-immune thrombocytopenic purpura patients. Blood. 1993 May 1;81(9):2375–2380. [PubMed] [Google Scholar]
- Hynes R. O. The complexity of platelet adhesion to extracellular matrices. Thromb Haemost. 1991 Jul 12;66(1):40–43. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kieffer N., Phillips D. R. Platelet membrane glycoproteins: functions in cellular interactions. Annu Rev Cell Biol. 1990;6:329–357. doi: 10.1146/annurev.cb.06.110190.001553. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Maclouf J. A., Murphy R. C. Transcellular metabolism of neutrophil-derived leukotriene A4 by human platelets. A potential cellular source of leukotriene C4. J Biol Chem. 1988 Jan 5;263(1):174–181. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- McEver R. P. GMP-140: a receptor for neutrophils and monocytes on activated platelets and endothelium. J Cell Biochem. 1991 Feb;45(2):156–161. doi: 10.1002/jcb.240450206. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Nagata K., Tsuji T., Todoroki N., Katagiri Y., Tanoue K., Yamazaki H., Hanai N., Irimura T. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62). J Immunol. 1993 Sep 15;151(6):3267–3273. [PubMed] [Google Scholar]
- Nesheim M. E., Pittman D. D., Wang J. H., Slonosky D., Giles A. R., Kaufman R. J. The binding of 35S-labeled recombinant factor VIII to activated and unactivated human platelets. J Biol Chem. 1988 Nov 5;263(31):16467–16470. [PubMed] [Google Scholar]
- Palabrica T., Lobb R., Furie B. C., Aronovitz M., Benjamin C., Hsu Y. M., Sajer S. A., Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992 Oct 29;359(6398):848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth S. S., Joneckis C. C., Parise L. V. Regulation of vascular integrins. Blood. 1993 Jun 1;81(11):2827–2843. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Optimal techniques for the immunocytochemical demonstration of beta-thromboglobulin, platelet factor 4, and fibrinogen in the alpha granules of unstimulated platelets. Histochem J. 1984 Sep;16(9):983–1001. doi: 10.1007/BF01003853. [DOI] [PubMed] [Google Scholar]
- Timmons S., Hawiger J. Separation of human platelets from plasma proteins including factor VIII VWF by a combined albumin gradient-gel filtration method using HEPES buffer. Thromb Res. 1978 Feb;12(2):297–306. doi: 10.1016/0049-3848(78)90300-6. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Present state of immunocryoultramicrotomy. J Histochem Cytochem. 1983 Jan;31(1A):164–167. [PubMed] [Google Scholar]
- Tuszynski G. P., Knight L. C., Kornecki E., Srivastava S. Labeling of platelet surface proteins with 125I by the iodogen method. Anal Biochem. 1983 Apr 1;130(1):166–170. doi: 10.1016/0003-2697(83)90664-4. [DOI] [PubMed] [Google Scholar]
- Vonderheide R. H., Tedder T. F., Springer T. A., Staunton D. E. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J Cell Biol. 1994 Apr;125(1):215–222. doi: 10.1083/jcb.125.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Springer T. A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992 Jan 1;175(1):185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]








