Abstract
Purpose
Acute lung injury is characterized by an exaggerated inflammatory response and a high metabolic demand. Mechanical ventilation can contribute to lung injury, resulting in ventilator-induced lung injury (VILI). A suspended-animation-like state induced by hydrogen sulfide (H2S) protects against hypoxia-induced organ injury. We hypothesized that suspended animation is protective in VILI by reducing metabolism and thereby CO2 production, allowing for a lower respiratory rate while maintaining adequate gas exchange. Alternatively, H2S may reduce inflammation in VILI.
Methods
In mechanically ventilated rats, VILI was created by application of 25 cmH2O positive inspiratory pressure (PIP) and zero positive end-expiratory pressure (PEEP). Controls were lung-protective mechanically ventilated (13 cmH2O PIP, 5 cmH2O PEEP). H2S donor NaHS was infused continuously; controls received saline. In separate control groups, hypothermia was induced to reproduce the H2S-induced fall in temperature. In VILI groups, respiratory rate was adjusted to maintain normo-pH.
Results
NaHS dose-dependently and reversibly reduced body temperature, heart rate, and exhaled amount of CO2. In VILI, NaHS reduced markers of pulmonary inflammation and improved oxygenation, an effect which was not observed after induction of deep hypothermia that paralleled the NaHS-induced fall in temperature. Both NaHS and hypothermia allowed for lower respiratory rates while maintaining gas exchange.
Conclusions
NaHS reversibly induced a hypometabolic state in anesthetized rats and protected from VILI by reducing pulmonary inflammation, an effect that was in part independent of body temperature.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-010-2022-2) contains supplementary material, which is available to authorized users.
Keywords: Hydrogen sulfide, Suspended-animation-like state, Critical illness, Acute lung injury, Ventilator-induced lung injury, Inflammation
Introduction
Acute lung injury (ALI) is a common complication in critically ill patients [1], characterized by an exaggerated inflammatory response, often requiring mechanical ventilation. Mechanical ventilation itself, however, can contribute to lung injury, called ventilator-induced lung injury (VILI) [2]. Mechanisms of VILI include overstretching and repetitive opening and closing of the alveoli, leading to a pro-inflammatory state [3]. Mechanical and inflammatory processes probably interact: a mechanically stressed lung may produce an inflammatory reaction [4]. Conversely, inflammation renders the lung susceptible to mechanical stress [5].
Reducing mechanical stress by applying low tidal volumes reduces mortality in ALI patients [6]. Besides restrictive volume ventilation, lowering of respiratory frequency attenuated ALI in experimental models [7]. Although (mild) respiratory acidosis has been shown to decrease mortality in ALI [8], severe acidosis is usually avoided. Respiratory acidosis can compromise immune function [9] and right ventricular function [10] and decrease oxygenation [11]. Also, use of tidal volumes lower then 6 ml/kg was found to enhance lung protection [12], calling for new interventions that allow for low minute ventilation while maintaining adequate gas exchange. A hypometabolic state, with decreased CO2 production, was induced in mice using hydrogen sulfide (H2S) gas [13]. H2S inhibits cytochrome c oxidase, thereby blocking oxidative phosphorylation, leading to decreased oxygen consumption. The mice experienced a drop in body temperature, heart rate, and CO2 production, resembling a state akin to hibernation. This was termed a “suspended-animation-like state.” H2S has also anti-inflammatory effects, including inhibition of cytokine production and neutrophil function and influx [14–17].
Herein, we describe the induction of a suspended-animation-like state in a physiological in vivo VILI model, using an intravenous H2S donor. We hypothesized that H2S-induced hypometabolism protects from VILI by reducing inflammation. Alternatively, H2S-induced hypometabolism may lower CO2 production, thereby allowing for a lower respiratory rate and hence less VILI. Distinguishing between these different effects may influence future studies on VILI, redirecting efforts on reducing minute ventilation to interventions targeting inflammation, or vice versa.
Methods
H2S donor
Preparations of a H2S donor were made freshly on the day of the experiments. NaHS (Sigma-Aldrich, Steinheim, Germany) was diluted in distilled water to a stock solution (90 mM), and pH adjusted to 7.5 using KCl. Further dilutions were made by uncapping the container and immediate loading of the syringe with NaHS, diluted with saline 0.9%.
Anesthesia and instrumentation
The study was approved by the animal care and use committee of our hospital. Male Sprague–Dawley rats (±350 g; Harlan, The Hague, The Netherlands) received an intraperitoneal injection of anesthesia mix (0.15 ml/100 g body weight) containing 90 mg/kg ketamine, 0.5 mg/kg medetomidine, and 0.05 mg/kg atropine. Anesthesia was maintained by infusion of 50 mg/kg ketamine at 0.5 ml/100 g/h. Tracheotomy was performed, after which a metal cannula was connected to a ventilator (Servo 900C; Siemens, Sweden). Hemodynamic monitoring was done by a carotid artery catheter connected to a monitor. Aortic flow was measured by insertion of a flow probe (T106; Transonic System, NY, USA) around the ascending aorta following thoracotomy. Mean stroke volume was calculated by dividing the heart rate by the aortic flow. Arterial blood gas analysis was performed hourly (alpha-stat, Rapidlab 865 blood gas analyzer; Bayern, Mijdrecht, The Netherlands). In the saline control groups, rectal temperature was maintained at 37°C.
VILI and lung-protective mechanical ventilation
Rats were ventilated for 4 h in a pressure-controlled mode with 25 cmH2O positive inspiratory pressure (PIP) and 0 cmH2O positive end-expiratory pressure (PEEP) (tidal volume ~15 ml/kg), thereby creating VILI [5]. Lung-protective (LP) mechanical ventilation was achieved by 13 cmH2O PIP and 5 cmH2O PEEP (tidal volume ~7.5 ml/kg). FiO2 was set at 60% and I:E ratio at 1:2. In VILI groups, respiratory rate was adjusted according to blood gas analysis to maintain normo-pH. End-tidal (et) CO2 was measured by carbon dioxide analyzer (CWE Inc., Ardmore, PA, USA).
Experimental protocol
A dose-finding experiment was performed with 18, 36, and 72 μmol/kg/h NaHS (n = 8 per group). For further experiments, a dose of 36 μmol/kg/h was used. Randomization to lung-injurious mechanical ventilation or to LP mechanical ventilation was done, followed by NaHS or saline infusion (n = 8 per group). As suspended animation is accompanied by hypothermia, additional control groups were used, in which animals were actively cooled to reproduce the H2S-induced fall in temperature, by placing ice bags on the belly.
Bronchoalveolar lavage, tissue sampling, and analyses
After 4 h of mechanical ventilation, the rats were bled. The right lung was ligated. Bronchoalveolar lavage fluid (BALF) was obtained by flushing the left lung (3 × 2 ml saline). Cell counts were determined using a hematocytometer (Z2 Coulter Particle Counter; Beckman Coulter, FL, USA). Differential counts were done on Giemsa-stained cytospins. Hematoxylin and eosin-stained lung sections were analyzed by a pathologist who was blinded to group identity. Edema, hemorrhage, infiltration, wall thickness, and hyperinflation were scored on a scale of 0–4: 0 for normal lungs, 1 for <25% lung involvement, 2 for 25–50% involvement, 3 for 50–75% involvement, and 4 for >75% lung involvement. Total histology score is the sum score of all parameters. The remaining right lobes were weighed to determine wet weight. Interleukin (IL)-6, cytokine-induced neutrophil chemoattractant 3 (CINC3), tumor necrosis factor (TNF), and IL-1β were measured by enzyme-linked immunosorbent assay (ELISA) according to instructions from the manufacturer (R&D Systems, Abingdon, UK) as were protein levels in BALF (Bradford; Oz Biosciences, Marseille, France).
Rat behavior after hydrogen sulfide infusion
Behavioral studies were performed in six additional intubated rats. NaHS was infused at 36 μmol/kg/h for 4 h. After stopping the infusion, animals were rewarmed and extubated. Behavior was monitored hourly for 8 h by observational assessment of movement, signs of stress, eating and drinking, as well as physiological parameters including breathing pattern and frequency and heart rate [18]. During 15 min of observation, the presence of anxiety (arched back, raised fur), locomotor activity (attempt to stand or any other movement), and food or water intake (each attempt to drink or eat) were scored as present (1 point) or not present (0 points).
Statistical analysis
Data are expressed as mean with standard deviation (SD), or as mean with standard error of the mean (SEM) in the figures. Intergroup differences were analyzed by analysis of variance (ANOVA) and Bonferroni’s post hoc test, or by Kruskal–Wallis test with Mann–Whitney U test according to the data distribution. A p value of <0.05 was considered significant. Statistical analyses were done using Prism (Graphpad Prism 5, CA, USA) and SPSS version 15 (SPSS Inc., IL, USA).
Results
Hydrogen sulfide dose-dependently induced physiological changes consistent with a suspended-animation-like state in anesthetized rats and reduced exhaled CO2
NaHS at 36 μmol/kg/h reduced body temperature from 36.4 ± 0.8°C to 25.7 ± 1.5°C (p < 0.05) and heart rate from 289 ± 33 to 136 ± 33 beats/min (p < 0.05). Increasing the dose to 72 μmol/kg/h showed similar effects. However, in this group, two animals died within 2 h, indicating possible toxicity. When a lower dose of 18 μmol/kg/h was used, changes in body temperature and heart rate were less profound (from 37.0 ± 1.0°C to 27.9 ± 1.3°C and from 291 ± 28 to 178 ± 61 beats/min, respectively; p < 0.05 versus 2 mg/kg/h). For all further experiments, a dose of 36 μmol/kg/h was used.
Infusion of NaHS increased stroke volume from 118 ± 14 μl at baseline to 173 ± 5 μl (p < 0.05) after 4 h. Cardiac output did not change (330 ± 170 versus 195 ± 60 μl/min, p = 0.08). Hydrogen sulfide reduced exhaled CO2 by ~33% after 4 h of infusion compared with baseline (p < 0.05), whereas hypothermia resulted in a ~16% reduction (Table 1).
Table 1.
Respiratory parameters during hydrogen sulfide donor NaHS infusion and induced hypothermia in a mechanically ventilated model of ventilator-induced lung injury (VILI), at baseline (T = 0) and after 4 h of mechanical ventilation (T = 4)
| Time (h) | LP | VILI | |||||
|---|---|---|---|---|---|---|---|
| Saline | NaHS | Hypothermia | Saline | NaHS | Hypothermia | ||
| pH | T = 0 | 7.33 ± 0.14 | 7.38 ± 0.07 | 7.43 ± 0.08 | 7.47 ± 0.07 | 7.41 ± 0.06 | 7.47 ± 0.05 |
| T = 4 | 7.28 ± 0.14 | 7.41 ± 0.03 | 7.36 ± 0.09 | 7.46 ± 0.06 | 7.42 ± 0.10 | 7.43 ± 0.11 | |
| PaCO2 (kPa) | T = 0 | 5.3 ± 1.5 | 5.4 ± 1.0 | 4.6 ± 0.4 | 4.2 ± 0.6 | 5.0 ± 0.7 | 4.0 ± 0.8 |
| T = 4 | 6.0 ± 1.7 | 4.8 ± 1.4 | 4.9 ± 1.4 | 2.8 ± 0.5 | 3.8 ± 1.1 | 4.5 ± 1.4 | |
| PaO2 (kPa) | T = 0 | 36 ± 5 | 36 ± 3 | 37 ± 5 | 38 ± 4 | 40 ± 2 | 36 ± 4 |
| T = 4 | 40 ± 4 | 53 ± 3a | 44 ± 8 | 41 ± 2 | 48 ± 2b | 32 ± 16 | |
| HCO−3 (mmol/l) | T = 0 | 24 ± 4 | 23 ± 4 | 22 ± 3 | 23 ± 3 | 23 ± 2 | 22 ± 2 |
| T = 4 | 21 ± 4 | 21 ± 3 | 20 ± 2 | 16 ± 2 | 18 ± 3 | 22 ± 3 | |
| Respiratory rate | T = 0 | 35 ± 0 | 35 ± 0 | 35 ± 0 | 35 ± 0 | 35 ± 0 | 35 ± 0 |
| T = 4 | 36 ± 2 | 35 ± 0 | 35 ± 0 | 21 ± 2 | 16 ± 1b | 18 ± 1 | |
| End-tidal CO2 | T = 0 | 5.1 ± 0.9 | 5.2 ± 1.0 | 3.8 ± 1.2 | 4.0 ± 1.0 | 4.8 ± 1.0 | 3.1 ± 0.9 |
| T = 4 | 5.6 ± 1.2 | 3.3 ± 0.5a | 3.2 ± 1.5c | 2.6 ± 1.4 | 3.4 ± 1.0 | 2.0 ± 0.2 | |
Data are means ± SD. LP lung-protective mechanical ventilation
aLP saline versus LP + NaHS
bVILI saline versus VILI + NaHS
cLP saline versus LP + hypothermia
Effect of NaHS on body temperature and hemodynamics in a VILI model
Similar to the preliminary experiments, H2S donor NaHS induced physiologic changes akin to hibernation, reducing body temperature and heart rate compared with saline controls (Electronic Supplementary Material, Fig. 1, both p < 0.05). In the hypothermia control groups, active cooling was necessary to reach body temperatures similar to NaHS-treated animals. Induced hypothermia resulted in a decrease in heart rate (Fig. 1). During the 4 h of mechanical ventilation, blood pressure did not drop in all groups.
Effect of suspended animation on pulmonary inflammation in a VILI model
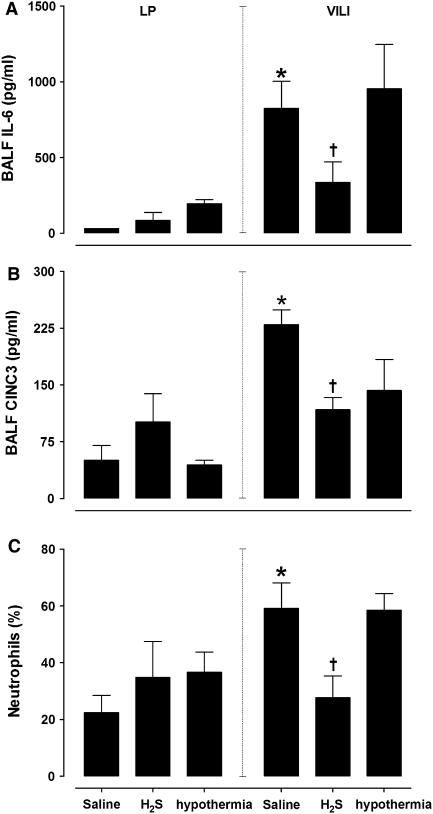
VILI was characterized by an increase in pulmonary wet weight compared with lung-protective mechanical ventilation (p < 0.05, Table 2), accompanied by an increase in BALF cell count and protein levels (p < 0.05 for all). VILI also resulted in increased BALF levels of IL-6, CINC3, and neutrophil influx compared with lung-protective mechanically ventilated controls (IL-6: 824 ± 437 versus 31 ± 0 ng/ml, CINC3: 230 ± 47 versus 51 ± 44 pg/ml, neutrophils: 59 ± 22% versus 22 ± 14%, p < 0.05, Fig. 1). Levels of TNF and IL-1β were below detection limits in all groups. Histopathology showed more neutrophil influx, alveolar edema, and cell wall thickening in VILI compared with lung-protective ventilated controls (p < 0.05, Table 2; Fig. 2, Electronic Supplementary Material).
Table 2.
Effect of hydrogen sulfide donor NaHS and induced hypothermia on cell influx and protein concentrations in bronchoalveolar lavage fluid, pulmonary wet weight, and lung pathology scores in ventilator-induced lung injury (VILI)
| LP | VILI | |||||
|---|---|---|---|---|---|---|
| Saline | NaHS | Hypothermia | Saline | NaHS | Hypothermia | |
| Lung wet weight (mg) | 750 ± 116 | 784 ± 111 | 669 ± 101 | 967 ± 82a | 896 ± 117 | 1,206 ± 309 |
| Cell count (×104 cells/ml) | 13.4 ± 13 | 23.3 ± 21 | 35.6 ± 10 | 83.9 ± 47.9a | 41.7 ± 40 | 129 ± 149 |
| Protein (μg/ml) | 274 ± 185 | 373 ± 88.4 | 341 ± 186 | 658 ± 26a | 448 ± 174 | 769 ± 355 |
| Pathology score | 1.3 ± 1.0 | 2.0 ± 0.9 | 1.1 ± 0.4 | 4.2 ± 1.0a | 2.8 ± 0.8b | 3.6 ± 1.1 |
Data are means ± SD. LP lung-protective mechanical ventilation
aLP saline versus VILI saline
bVILI saline versus VILI + NaHS
Fig. 1.
Interleukin-6 (a) and chemokine CINC3 (b) concentrations, and neutrophil influx (c) in bronchoalveolar lavage fluids of animals treated with hydrogen sulfide donor NaHS, saline and hypothermic controls, mechanically ventilated with either lung-protective (LP) or lung-injurious mechanical ventilation, creating ventilator-induced lung injury (VILI). Data are mean ± SEM. * LP versus VILI, p < 0.05; † VILI versus VILI + NaHS, p < 0.05 (n = 8 per group)
NaHS reduced BALF neutrophil influx in VILI (59 ± 22% versus 28 ± 20%, p < 0.05, Fig. 1), with a decrease in BALF CINC3 levels (230 ± 47.3 versus 81.6 ± 34.8 pg/ml, p < 0.05) and a tendency to decrease IL-6 levels (824 ± 437 versus 336 ± 360 ng/ml, Fig. 1, p = 0.07). NaHS improved histopathologic abnormalities (p < 0.05, Table 2; Fig. 2). Pulmonary edema, cell influx, and protein levels were nonsignificantly reduced, which may have been due to large variations within the groups.
Effect of hypothermia on pulmonary inflammation in a VILI model
To determine whether the protective effect of NaHS was mediated by a reduction in body temperature, hypothermia was induced to a temperature that paralleled the H2S effect. Pulmonary inflammation in VILI did not decrease by active cooling to a body temperature comparable to the NaHS group. None of the inflammatory parameters in VILI were reduced by hypothermia (Table 2; Fig. 1).
Effect of suspended animation on respiratory rate and gas exchange in a VILI model
As expected, the respiratory rate in animals with VILI had to be reduced during the experiment to maintain normo-pH in a physiological VILI model (Table 1). Both NaHS-treated animals and hypothermic controls allowed for a more profound reduction in respiratory rate compared with saline controls (p < 0.05 for both). Adequate oxygenation was maintained in all groups. An increase in pO2 was observed in all groups treated with H2S compared with saline controls (p < 0.05 for both), an effect that was not observed in the hypothermia groups.
Reversibility of suspended animation induced by an H2S donor
To be of potential therapeutic interest, H2S-induced effects need to be reversible. Therefore, we conducted a behavioral experiment in six intubated animals. Comparable to the previous results, NaHS reduced body temperature from 37 ± 0.1°C to 29 ± 0.5°C and heart rate from 297 ± 29 to 217 ± 28 beats/min. etCO2 decreased by 38 ± 4% compared with baseline (p < 0.05). After cessation of NaHS and active rewarming, heart rate and etCO2 values returned to baseline within 30 min. After cessation of anesthesia, animals awoke and were extubated. During the first 4 h, animals lay calmly in their cages with normal breathing pattern. Gradually, the animals started moving, drinking, and eating. After 8 h of observation, the rats showed no behavioral abnormalities (Table 3, Electronic Supplementary Material).
Discussion
In the present study, an intravenous H2S donor reversibly induced a suspended-animation-like state in anesthetized and mechanically ventilated rats. NaHS reduced pulmonary injury caused by the mechanical ventilator by reducing pulmonary inflammation, an effect which was independent of a mere reduction in body temperature. As both NaHS and hypothermia allowed for lower respiratory rates, reduction of respiratory rate did not contribute to the observed protective effect of NaHS in our VILI model.
H2S gas can induce a suspended-animation-like state in mammals that do not normally hibernate [13, 19, 20]. In the present study, we show that H2S donor NaHS in anesthetized rats induced comparable physiological changes. Also, NaHS reduced the amount of exhaled CO2 (etCO2) at unchanged ventilator settings. We did not measure CO2 production. However, as a fall in CO2 delivery to the lungs is unlikely to account for the observed decrease in etCO2 at unchanged cardiac output and unchanged minute ventilation, the decrease in etCO2 may indicate decreased metabolic rate in this model. Use of a parenteral solution instead of gas has practical advantages, as there is no need for an inhalation device system and less risk of exposure to the gas.
We found that NaHS attenuated lung injury in an in vivo VILI model by inhibiting inflammatory processes. NaHS decreased pulmonary neutrophil influx, with a decrease in chemokine CINC3 levels. A recent experiment using H2S gas in a mice model of VILI also found a reduction in extravasation of neutrophils and neutrophil apoptosis [21]. As influx of neutrophils is a hallmark of acute lung injury, H2S-induced suspended animation may also be beneficial in other causes of lung injury. In addition, we found that NaHS reduced levels of the pro-inflammatory cytokine IL-6 in VILI. Comparably, in models of lung injury, bolus treatment with H2S reduced levels of IL-1 and IL-8 and increased levels of IL-10 [14]. The anti-inflammatory properties of H2S have also been shown after inhibition of endogenously produced H2S [22]. However, endogenously produced H2S also mediated inflammation during experimental endotoxemia [23], suggesting a dual role of H2S in inflammation. In our study, hibernating doses of NaHS reduced inflammation, which may be mediated by a reduction in neutrophil influx at the site of injury.
As mild hypothermia has been found to reduce inflammation in VILI [24, 25], we determined whether the NaHS-induced reduction in inflammatory parameters in our study was due to a reduction in body temperature. We found that animals needed to be actively cooled to reproduce the NaHS-induced fall in temperature, which was not achieved by merely switching off the heating pad. This suggests that the profound reduction of body temperature is a specific effect of NaHS. In addition, although hypothermia reduced metabolism, it did not protect from lung injury. This accords with findings in a mice VILI model, in which H2S reduced lung injury, but mild hypothermia did not [21]. In these experiments, fixed body temperatures presumably did not allow for a reduction in metabolism, as blood gas analysis did not change during H2S inhalation and a reduction in heart rate was not mentioned. Therefore, protection by H2S occurred via reduction of inflammation. In this study, it is not clear whether the protective effect of H2S occurred mainly via reduction of metabolic rate or via reduction of inflammation. Interestingly, H2S has an effect on mitochondrial structure and function [26]. Also, H2S, but not hypothermia, changes substrate utilization [27], suggesting a distinct effect on metabolism. This issue warrants further exploration.
Besides tidal volume, the repetitive strain of respiratory cycles may contribute to lung injury. In this study, both NaHS and hypothermia allowed for lower respiratory rates compared with controls. As lowering respiratory rates did not decrease injury in the hypothermia group, reduction of tachytrauma may not have contributed to the protective effect of NaHS. In contrast, a reduction in respiratory frequency reduced injury in an isolated perfused model of VILI [7]. Differences may be related to different study designs, as compliance in ex vivo VILI models is altered. Also, importantly, the reduction in our model was only modest. Furthermore, in our physiological VILI model, study groups did not allow for differentiation between effects of hypothermia and low respiratory rate. It is possible that a potential beneficial effect of reducing respiratory rate was counteracted by the deleterious effect of profound hypothermia on lung tissue.
In contrast to the effect of H2S gas in a mice model of VILI [21], NaHS improved oxygenation in our VILI model. These contrasting results may relate to the different compound used. Notably, we analyzed blood gases without temperature correction, which presumably results in preservation of intracellular enzymes and other protein structures. However, as the increase in oxygenation was not found in hypothermic controls, it seems unlikely that a shift in oxyhemoglobin dissociation curve caused by hypothermia during suspended animation contributed to the observed increased oxygenation [28].
The present study does not address several important issues related to H2S-induced hypometabolism. Reducing metabolism in small animals has important limitations, because body temperature is reduced much faster [29]. Of note, in larger animals including piglets and sheep, H2S gas failed to induce a suspended-animation-like state [30, 31]. However, H2S donor NaHS reduced body temperature, O2 uptake, and CO2 production in a pig model of ischemia–reperfusion injury, indicative of a reduction of metabolism [32]. Whether reducing metabolism with the appropriate compound is feasible in naturally nonhibernating mammals remains to be determined in dose-finding studies that induce hibernation-like states without inducing toxicity in appropriately sized animal models.
As discussed, H2S and H2S donors may exert inflammatory and toxic effects, limiting applicability. There was a nonsignificant increase in pulmonary cell influx, wet weight, protein levels, and cytokine levels in the lung-protective control group compared with saline controls, which may indicate a possible toxic effect. Obviously, this issue warrants further investigation. A final limitation of the study is that we did not measure H2S content, rendering the exact dose of H2S given unknown.
Conclusions
An intravenous H2S donor reversibly induced physiologic changes consistent with a suspended-animation-like state in anesthetized and mechanically ventilated rats. NaHS protected from VILI by reducing inflammation, an effect that was, at least in part, independent of body temperature. Reducing metabolism may be a new therapeutic approach to protect the lungs from ventilator-associated lung injury.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Mean arterial pressure (MAP) (a), heart rate (HR) (b), and body temperature (c) during hydrogen sulfide (NaHS) infusion in a model of ventilator-induced lung injury (VILI). LP lung-protective mechanical ventilation, treated with saline. Data are means ± SD (JPEG 536 kB)
Lung histopathology slides (H&E stained): left panel lung-protective (LP) mechanical ventilation, and right panel lung-injurious mechanical ventilation creating ventilator-induced lung injury (VILI), in rats infused with either saline or NaHS or actively cooled to a body temperature paralleling the NaHS-induced fall in body temperature (JPEG 3,143 kB)
Behavioral study following NaHS infusion and in nontreated animals (doc 34 kB)
Acknowledgments
This work was supported by a grant from the European Society of Intensive Care Medicine (ECCRN Basic Sciences Award 2007). We would like to thank Gezina T.M.L. Oei, M.Sc., Department of Anesthesiology, Academic Medical Center, Amsterdam, The Netherlands, for her contribution in the rat behavior experiment.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care. 2005;11:43–49. doi: 10.1097/00075198-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 3.Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care. 2003;7:233–241. doi: 10.1186/cc1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.CCM.0000149854.61192.DC. [DOI] [PubMed] [Google Scholar]
- 5.Haitsma JJ, Schultz MJ, Hofstra JJ, Kuiper JW, Juco J, Vaschetto R, Levi M, Zhang H, Slutsky AS. Ventilator-induced coagulopathy in experimental Streptococcus pneumoniae pneumonia. Eur Respir J. 2008;32:1599–1606. doi: 10.1183/09031936.00045908. [DOI] [PubMed] [Google Scholar]
- 6.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss JR, Jr, Blanch L, Murias G, Adams AB, Olson DA, Wangensteen OD, Leo PH, Marini JJ. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med. 2000;161(2 Pt 1):463–468. doi: 10.1164/ajrccm.161.2.9811008. [DOI] [PubMed] [Google Scholar]
- 8.Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34:1–7. doi: 10.1097/01.CCM.0000194533.75481.03. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Chacko BK, Ricksecker A, Shingarev R, Andrews E, Patel RP, Lang JD., Jr Modulatory effects of hypercapnia on in vitro and in vivo pulmonary endothelial-neutrophil adhesive responses during inflammation. Cytokine. 2008;44:108–117. doi: 10.1016/j.cyto.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekontso DA, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, Vieillard-Baron A. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–1858. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang JD, Figueroa M, Sanders KD, Aslan M, Liu Y, Chumley P, Freeman BA. Hypercapnia via reduced rate and tidal volume contributes to lipopolysaccharide-induced lung injury. Am J Respir Crit Care Med. 2005;171:147–157. doi: 10.1164/rccm.200302-305OC. [DOI] [PubMed] [Google Scholar]
- 12.Terragni PP, Del SL, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 13.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 14.Esechie A, Kiss L, Olah G, Horvath EM, Hawkins H, Szabo C, Traber DL. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci (Lond) 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- 15.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di SM, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Persson S, Claesson R, Carlsson J. Chemotaxis and degranulation of polymorphonuclear leukocytes in the presence of sulfide. Oral Microbiol Immunol. 1993;8:46–49. doi: 10.1111/j.1399-302X.1993.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood) 2008;233:1081–1087. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 18.Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- 19.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 20.Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, Zapol WM. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 22.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 24.Akinci OI, Celik M, Mutlu GM, Martino JM, Tugrul S, Ozcan PE, Yilmazbayhan D, Yeldandi AV, Turkoz KH, Kiran B, Telci L, Cakar N. Effects of body temperature on ventilator-induced lung injury. J Crit Care. 2005;20:66–73. doi: 10.1016/j.jcrc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Hotchkiss JR, Takahashi T, Olson D, Adams AB, Marini JJ. Effect of core body temperature on ventilator-induced lung injury. Crit Care Med. 2004;32:144–149. doi: 10.1097/01.CCM.0000098857.14923.44. [DOI] [PubMed] [Google Scholar]
- 26.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgart K, Wagner F, Groger M, Weber S, Barth E, Vogt JA, Wachter U, Huber-Lang M, Knoferl MW, Albuszies G, Georgieff M, Asfar P, Szabo C, Calzia E, Radermacher P, Simkova V. Cardiac and metabolic effects of hypothermia and inhaled hydrogen sulfide in anesthetized and ventilated mice. Crit Care Med. 2010;38:588–595. doi: 10.1097/CCM.0b013e3181b9ed2e. [DOI] [PubMed] [Google Scholar]
- 28.Bacher A. Effects of body temperature on blood gases. Intensive Care Med. 2005;31:24–27. doi: 10.1007/s00134-004-2369-3. [DOI] [PubMed] [Google Scholar]
- 29.Singer D. Metabolic adaptation to hypoxia: cost and benefit of being small. Respir Physiol Neurobiol. 2004;141:215–228. doi: 10.1016/j.resp.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Haouzi P, Notet V, Chenuel B, Chalon B, Sponne I, Ogier V, Bihain B. H2S induced hypometabolism in mice is missing in sedated sheep. Respir Physiol Neurobiol. 2008;160:109–115. doi: 10.1016/j.resp.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhang G, Cai S, Redington AN. Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr Crit Care Med. 2008;9:110–112. doi: 10.1097/01.PCC.0000298639.08519.0C. [DOI] [PubMed] [Google Scholar]
- 32.Simon F, Giudici R, Duy CN, Schelzig H, Oter S, Groger M, Wachter U, Vogt J, Speit G, Szabo C, Radermacher P, Calzia E. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean arterial pressure (MAP) (a), heart rate (HR) (b), and body temperature (c) during hydrogen sulfide (NaHS) infusion in a model of ventilator-induced lung injury (VILI). LP lung-protective mechanical ventilation, treated with saline. Data are means ± SD (JPEG 536 kB)
Lung histopathology slides (H&E stained): left panel lung-protective (LP) mechanical ventilation, and right panel lung-injurious mechanical ventilation creating ventilator-induced lung injury (VILI), in rats infused with either saline or NaHS or actively cooled to a body temperature paralleling the NaHS-induced fall in body temperature (JPEG 3,143 kB)
Behavioral study following NaHS infusion and in nontreated animals (doc 34 kB)