Abstract
Bone marrow mesenchymal stem cells (BMSCs) are capable of differentiating into multiple cell types, providing an alternative cell source for cell-based therapy and tissue engineering. Simultaneous differentiation of human BMSCs into smooth muscle cells (SMCs) and urothelium would be beneficial for clinical applications in bladder regeneration for patients with bladder exstrophy or cancer who need cystoplasty. We investigated the ability of human BMSCs to differentiate toward both SMCs and urothelium with cocultured or conditioned media and analyzed growth factors from a coculture system. After being cocultured with urothelium or cultured using urothelium-derived conditioned medium, human BMSCs expressed urothelium-specific genes and proteins: uroplakin-Ia, cytokeratin-7, and cytokeratin-13. When cocultured with SMCs or cultured in SMC-conditioned medium, human BMSCs expressed SMC-specific genes and proteins: desmin and myosin. Several growth factors (hepatocyte growth factor, platelet-derived growth factor-homodimer polypeptide of B chain (BB), transforming growth factor-β1, and vascular endothelial growth factor) were detected in the SMC cocultured media and in the urothelium cocultured media (epidermal growth factor, platelet-derived growth factor-BB, transforming growth factor-β1, and vascular endothelial growth factor). BMSC–scaffold constructs significantly improved cell contractility after myogenic differentiation. In vivo-grafted cells displayed significant matrix infiltration and expressed SMC-specific markers in the nanofibrous poly-l-lactic acid scaffolds. In conclusion, smooth muscle- and urothelium-like cells derived from human BMSCs provide an alternative cell source for potential use in bladder tissue engineering.
Introduction
Urological cell-based tissue engineering is one of the most promising areas in biotechnology for restoring tissues and organ functions of the urinary tract system. Current methods require a competent biological scaffold that is seeded in vitro with the patient's own bladder cells. Suitable bladder cells from the patient for this purpose are sometimes limited or unobtainable. When suitable cells are unavailable for seeding because of bladder exstrophy, malignancy, or other reasons, use of other cell types originating from the patient holds promise. This strategy avoids graft rejection and long-term use of medications usually needed after allogeneic transplantation. A suitable alternative to bladder cells could be mesenchymal stem cells (MSCs). MSCs reside primarily in the bone marrow, although they exist in other sites such as adipose tissue, peripheral and cord blood, liver, and fetal tissues. Bone marrow-derived stromal cells contain a few MSCs (1 MSC in 104 to 5 × 107 marrow cells), depending on the age of the individual.1 Despite their limited numbers, MSCs possess a high ability to both self-renew for extended periods of time and differentiate into several different specialized cell types under appropriate conditions.
It was once thought that MSCs differentiated only into a mesodermal lineage (i.e., bone, adipose, cartilage, myocytes, cardiomyocytes, fibroblasts, and myofibroblasts). However, this notion has been challenged by recent investigations showing that bone marrow MSCs (BMSCs) also differentiate into endodermal (epithelial cells of liver, lung, and pancreas) and ectodermal lineages (skin epidermal, pigment epithelial cells, neurons, astrocytes, and oligodendrocytes). In addition, MSCs display immunosuppressive,2 angiogenic,3 antifibrotic, and antiapoptotic4 effects.
BMSCs are capable of expansion and tissue-specific differentiation in vitro based on the external signals and/or the environment. There are different methodologies for induction and maintenance of a differentiated cell phenotype from BMSCs. In urological tissue engineering and regeneration, two methods that are commonly employed when using stem cells are (1) implantation of stem cells in vivo directly without any induction, and (2) induction of stem cell differentiation toward the specific target cells in vitro followed by in vivo implantation for tissue remolding. In the first approach, the host organ or tissue environment directs the fate of the stem cells to bladder cells.5 However, when the native cells or tissues are unhealthy, the implanted noninduced stem cells may fail to differentiate to the functional target cells. On the other hand, the main advantage of the second approach is that obtaining a particular cell phenotype can be controlled and achieved by the inducing agents prior to implantation, whereby the induced cells can fully differentiate into the specific cell type for tissue regeneration.
MSCs can differentiate into a smooth muscle phenotype in vitro with differentiation agents such as conditioned medium (CM) derived from smooth muscle cell (SMC) culture5 or myogenic growth factors (PDFF-BB, hepatocyte growth factor [HGF], and transforming growth factor-β [TGF-β])5–8 and migrate to a scaffold for differentiation into smooth muscle-like cells in vivo.9 Further, stem cell-seeded scaffolds that are implanted into the bladders repopulate and reorganize the bladder more rapidly, reducing fibrosis and exhibiting appropriate neural functionality.9,10 However, most of these studies focused on the vessel SMCs. Even in the case of bladder, nonhuman marrow stem cells are commonly used in differentiating into SMCs and urothelium.5,11 The optimal approaches to induce human BMSCs to differentiate into bladder SMCs, and to a more urothelial differentiation, are still under investigation. It is known that cells release cytokines to neighboring tissues, which can modulate the fate of adjacent cells via paracrine signaling. In vitro, a coculturing method is usually used in guiding stem cells to differentiate toward fully functional cells.12 This method is efficiently applied in the situation when the induced growth factors are unknown. The aims of this study were as follows: (1) to evaluate the possibility of differentiating human BMSCs into SMC- and urothelium-like cells by using CM derived from bladder cells (SMCs and urothelium, respectively) or by indirect coculturing with bladder cells; (2) to assess key cytokine growth factors existing in a coculture system or in CM derived from bladder cell culture for myogenic and uroepithelial differentiation of human BMSCs; and (3) to evaluate a BMSC-seeded nanofibrous poly-l-lactic acid (PLLA) polymer scaffold for cell–matrix infiltration, cell differentiation, and smooth muscle tissue formation in vivo, for potential use in bladder exstrophy or cancer patients needing cystoplasty.
Materials and Methods
Cell culture
The Institutional Review Board Committee approved the study for obtaining human tissue samples used for this study. Normal bladder or ureter tissues came from patients undergoing ureter reimplantation or kidney transplantation. Bladder SMCs and urothelium, henceforth called bladder cells, were cultured as reported previously.9,13,14 Briefly, the muscle or mucosal tissues were dissected out, minced into small pieces (∼1 mm2), and incubated in 0.1% collagenase type IV solution for 30 min at 37°C. The digested muscle or urothelial tissues were plated in respective media for establishing primary culture. Human BSMCs (Lonza, Walkersville, MD) and SMCs were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and urothelium were cultured in keratinocyte serum-free medium (KSFM). Primary cultured BMSC samples at passage 2 were obtained from five individuals whose age ranged from 9 to 36 years. Flow cytometry was used to confirm that BMSCs expressed the stem cell markers CD105, CD166, CD29, but did not express hematopoietic stem cell markers, that is, CD14, CD34, and CD45. Cells were cultured under standard conditions at 37°C with 5% CO2 and media were replaced every other day.
Differentiation of human BMSCs in vitro
For differentiation, BMSCs (2500–3000 cells/cm2) were cocultured with bladder cells or cultured with bladder cell-derived CM for 14 days. CM was derived from bladder cells by collecting the media from cultured human bladder SMCs at 70–90% confluence and human urothelium at 60–70% confluence, respectively, every 12 h. The collected media were centrifuged at 500 g for 5 min to remove cells, filtered (0.2 μM), and diluted with an equal volume of DMEM with 10% FBS (SMC differentiation) or one-fifth the volume of DMEM with 10% FBS (urothelial cell [UC] differentiation) before use. Bladder cells (top chamber) were indirectly cocultured with BMSCs (bottom chamber) in a 10 cm transwell plate (Costar, Vernon Hills, IL) with a barrier membrane (0.4 μm) between the cell types.
We optimized the differentiated media for both SMC and UC differentiation. For SMC differentiation, we used myogenic medium consisting of CM-SMC and fresh DMEM medium with 10% FBS at different ratios. When the ratio of CM-SMC and fresh medium reached 1:1, this medium was better in cell differentiation and proliferation than those at other ratios (such as 1:5, 1:2.5, 2.5:1, and 5:1). For UC differentiation, urothelial differentiated media consisted of CM-UC and fresh DMEM. The optimal ratio of CM to fresh DMEM was 1:4 with serum at a final concentration of 2%. Although UC do not require serum for growth, addition of a small amount of serum was required for urothelial differentiation of BMSCs. In this study, we observed that BMSCs failed to thrive and differentiate in the earlier stage of urothelial differentiation when they were cultured in the media with absence or presence of only 1% serum. In contrast, UC differentiated poorly when cultured in the medium with 3% or higher serum.
To assess differentiation of BMSCs to bladder cells, lineage-specific gene analysis (reverse transcriptase–polymerase chain reaction [PCR]) and protein expression (immunoblotting and immunofluorescence) were carried out. Bladder cells were cultured in their respective medium for use as positive and negative controls for urothelial differentiation.
RNA isolation and reverse transcriptase-PCR
Total cellular RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA) and used for cDNA synthesis using SuperScript™ II Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. SMC-specific transcripts were detected using touchdown PCR with the following conditions: The initial denaturing step was at 94°C for 6 min, followed by 94°C for 30 s, 61°C for 30 s, and 72°C for 1 min for three cycles. Subsequent cycling steps were carried out by decreasing the annealing temperature by 2°C for every three cycles, until a final temperature of 53°C (30 cycles) was reached. The urothelium-specific transcript was analyzed using the following PCR conditions: 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s for 35 cycles. A 10-min extension step at 72°C was performed as the final step for all PCR experiments. The sequences of primers used, along with the expected product sizes, are listed in Table 1. The reaction products were analyzed by electrophoresis using a 1.5% agarose gel. Human glyceraldehyde-3-phosphate dehydrogenase was used as a housekeeping gene.
Table 1.
Sequence Information of Primers Used for Reverse Transcriptase–Polymerase Chain Reaction and Their Expected Product Size
| Target gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Size (bp) |
|---|---|---|---|
| Desmin | CCATCGCGGCTAAGAACATT | TCGGAAGTTGAGGGCAGAGTA | 440 |
| MHC | GGACGACCTGGTTGTTGATT | GTAGCTGCTTGATGGCTTCC | 656 |
| Calponin | ATGTCCTCTGCTCACTTCA | TTTCCGCTCCTGCTTCTCT | 453 |
| Up-Ia | ACGTCCTACACCCACCGTGA | ACCCCACGTGTAGCTGTCGAT | 360 |
| Cytokeratin-7 | TGGTGCTGAAGAAGGATGTG | CACGCTGGTTCTTGATGTTG | 405 |
| Cytokeratin-13 | GGCTTCCTACCTGGAGAAGG | CGACCACCTGGTTGCTAAAT | 422 |
| GAPDH | CGGATTTGGTCGTATTGG | TCAAAGGTGGAGGAGTGG | 861 |
MHC, myosin heavy chain; Up-Ia, uroplakin-Ia; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Immunoblotting
Human BMSC protein lysates were prepared from different treatments and subjected to immunoblotting for detection of SMC- and urothelium-specific protein expression. Briefly, cells were homogenized in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.5% Triton X-100 with protease inhibitors [Complete Mini; Roche, Indianapolis, IN]). Protein (25–50 μg) was resolved using a 10% sodium dodecyl sulfate–polyacrylamide gel. The protein was transferred to a poly vinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) and probed with primary antibodies (alpha-smooth muscle actin [α-SMA, 1:5000; Sigma, St. Louis, MO], calponin [1:1000; Santa Cruz, Santa Cruz, CA], desmin [1:500; Santa Cruz], smooth muscle myosin heavy chain [1:1000; Sigma], uroplakin-Ia [Up-Ia, 1:500, a cell marker for superficial urothelial cells15; Santa Cruz], cytokeratin-13 [CK13, 1:500, a cell marker for urothelial base cells16,17; Santa Cruz], cytokeratins [AE1/AE3, 1:1000; Dako, Glostrup, Denmark], and β-actin [1:5000; Sigma]) overnight at 4°C with constant shaking. The secondary antibody conjugated to horseradish peroxidase (1:2000; Cell Signaling, Danvers, MA) was incubated at room temperature for 1 h with constant shaking. Proteins were detected using the Super Signal West Femto chemiluminescence reagent (Pierce, Rockford, IL) and the images were captured using the Fujifilm LAS-3000 Imager system.
Immunofluorescence for BMSCs induced in vitro
Induced human BMSCs were seeded in chamber slides or cover slips and allowed to adhere overnight. Cells were fixed with 4% paraformaldehyde, permeabilized with 100% cold acetone for 1 min, and blocked. To assess BMSC differentiation to smooth muscle-like cells, α-SMA (1:100), calponin (1:200), desmin (1:50), and myosin (1:50) were used. Similarly, to assess differentiation of BMSCs to urothelial-like cells, Up-Ia (1:50) and AE1/AE3 (1:200) were used. An appropriate secondary antibody conjugated to fluorescein isothiocyanate was used. Finally, the cells were mounted in a propidium iodide-containing mount (Vector, Burlingame, CA) for staining nuclei and visualized by fluorescence microscopy using a Zeiss microscope (M1).
All stained sections were evaluated by a single experienced individual (repeated at least three times) and were graded as follows: +, 1–25% positive staining cells; ++, 25–50%; +++, 50–75%; ++++, 75–100%.
Quantitation of growth factors in CM and coculture medium
We measured the secretion of six growth factors, namely epidermal growth factor (EGF), basic fibroblast growth factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF-BB), HGF, and TGF-β1, into the culture medium, either from bladder cells or coculture systems, using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Briefly, media were collected at 12 h and 14 days after culture and centrifuged at 600 g for 10 min at 4°C. Aliquots were immediately analyzed or stored at −80°C. Fresh media were added at 12 h prior to media collection. Triplicate media samples were incubated in 96-well plates coated with an antibody specific to a particular cytokine. On completion of assays, the absorbance was measured at 450 nm (with wavelength correction at 570 nm) within 30 min. Respective media controls were used as blanks to determine the background levels of growth factors, which were assumed as 100%. In cocultures where two types of media were involved, respective medium was added to the chambers of a plate without cells and medium was harvested from the bottom chamber at indicated time points.
Contractility assessment using organ bath
Human BMSCs (2.5 × 105) were seeded on decellularized bladder submucosa extracellular matrix (ECM) strips (2 × 4 × 10 mm).9,13,18 In vitro and in vivo contractility on cell-seeded ECM were assessed by organ bath. Strips were cultured either in the presence of SMC-CM or DMEM culture medium as control for 5 days under static condition, after which four strips were loaded into a muscle bioreactor. The strips were stretched continuously (5 pulls/min) at 10% of their original length in SMC-CM for 8 days. As a control, four BMSC-seeded bladder submucosal matrix scaffold strips were cultured in static condition for the same period.
For studying the contractility response of the seeded scaffolds, electrical field stimulation at 5 V and 1 Hz was performed in an organ bath. The cell-seeded strips (n = 4) were evaluated for contractility studies as previously described.19 Briefly, the strips were suspended in 10 mL of Krebs solution and subjected to electric field stimulation (5 V, 1 Hz), with approximately 5 min between each stimulation.
Differentiation of human BMSCs in vivo
The Institutional Animal Care and Use Committee approved the animal surgery procedures for this study. A total of 10 athymic mice were used in this study. To assess the contractile function and histological structure of myogenic differentiated of BMSCs in vivo, two types of biomaterials were utilized for cells seeding, that is, (1) bladder ECM for organ bath and (2) nanofibrous PLLA polymer scaffolds for maximize muscle formation. Two grafts were subcutaneously implanted per animal. For contractility testing, BMSCs were induced to differentiate into SMCs using CM-SMCs and were seeded onto bladder ECM scaffolds (four grafts). Cell-free ECM (four grafts) and noninduced BMSC-seeded (four grafts) scaffolds were also implanted as controls. For muscle tissue formation within PLLA, BMSC-seeded (three grafts), SMC-seeded (three grafts), and cell-free PLLA scaffolds (three grafts) were implanted. All grafts were retrieved at 1 month after surgery. The implanted grafts were then fixed in 10% buffered formalin and embedded in paraffin. The consecutive cross sections of each graft were stained with hematoxylin and eosin, Masson's trichrome, and 4'6-diamidino-2-phenylindole (DAPI), and immunohistochemistry staining for smooth muscle-specific marker (desmin) was also performed.
Statistical analysis
For tensile strength analyses using organ baths, averages and standard error of the mean of replicates (n = 4 grafts for in vitro studies and n = 3 grafts for in vivo studies) were calculated. Comparisons were made between groups using a two-tailed Student's t-test with unequal variances, and differences were considered significant at p < 0.05.
Results
Myogenic or urothelial differentiation of BMSCs is slower than regular culture processes. BMSCs were initially plated at about 20% confluence, which proliferated for the first 2–3 days in differentiation medium. Thereafter, their growth rate appeared to slow down. It took 14 days for the induced BMSCs to reach 70–80% confluence, compared with the noninduced BMSCs, which took only 7 days to reach similar confluence.
Characterization of human BMSC differentiation
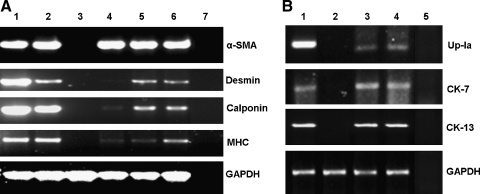
The morphology of BMSCs (Fig. 1b) started to change from spindle shapes to a polygonal epithelium-like shape as that of urothelial cells (Fig. 1a) when either cocultured with urothelium (Fig. 1c) or cultured using urothelium-CM (Fig. 1d) for differentiation to a urothelial cell lineage on day 7. About 50% of BMSCs turned over their morphology to epithelium-like cell when cultured up to 14 days. In the negative control, the number of BMSCs decreased sharply and cells shrank in morphology when cultured in KSFM. BMSCs that differentiated to SMCs still maintained a distinct spindle-shape phenotype. Semiquantitative reverse transcriptase RT-PCR was performed on RNA samples extracted from the experimented BMSCs. Amplification of smooth muscle-specific transcripts (α-SMA, calponin, desmin, and myosin) showed specific products in the positive controls and induced BMSC samples, but these were completely absent in the negative control, urothelium (Fig. 2A). The noninduced BMSCs only showed very faint amplification of most transcripts. On induction, using coculture or CM, there was a marginal increase in α-SMA transcription but a 5–18-fold induction in desmin and calponin transcription as determined by quantification of band intensity compared to the non-induced BMSC control. SMC-CM promoted expression of myosin transcripts by about eightfold, whereas coculture with SMCs only promoted expression of myosin transcripts by twofold. Specific amplification products were observed only when cells were induced for differentiation. Expression of urothelial-specific markers was carried out using CK7, CK13, and Up-Ia (Fig. 2B). Up-Ia gene was more strongly expressed when BMSCs were treated with urothelium-CM compared with coculturing with urothelium.
FIG. 1.
Urothelial differentiation of human BMSCs on day 7 after induction. (a) Human bladder urothelial cell (p4) control, (b) noninduced human BMSC (p4) control, (c) human BMSCs induced (arrows) by coculture with urothelium, and (d) human BMSCs induced (arrows) by CM from urothelium. Original magnification, × 100. BMSC, bone marrow mesenchymal stem cell; CM, conditioned medium.
FIG. 2.
Expression of muscle and urothelial lineage-specific transcripts in differentiated human BMSCs by reverse transcriptase–polymerase chain reaction on day 14. (A) Smooth muscle-specific primers (α-SMA, desmin, calponin, MHC) used on RNA extracted from human BMSCs (p4) induced with human bladder SMC-derived conditional medium for 14 days. Lane 1, fresh smooth muscle tissue; lane 2, cultured SMCs as positive controls; lane 3, cultured urothelium as negative control; lane 4, noninduced BMSCs as control; lane 5, induced BMSCs by coculture with SMCs; lane 6, induced BMSCs by SMC-CM; lane 7, no template control. (B) Urothelial-specific primers (Up-Ia, CK-7/13) used on RNA samples from human BMSCs (p4) induced by coculture with urothelium for 7 days. Lane 1, cultured urothelium as positive; lane 2, noninduced BMSCs as control; lane 3, BMSCs cocultured with urothelium; lane 4, BMSCs cultured using urothelium-CM; lane 5, no template (H2O) control. GAPDH was used as the housekeeping gene for load control. Note: Threefold excess of the reaction mixture was loaded for all the primers in (A) compared with that in (B). Moreover, the bands for all the primers in (A) are overexposed, whereas the bands in (B) are under exposured. α-SMA, alpha smooth muscle actin; MHC, myosin heavy chain; Up-Ia, uroplakin-Ia; CK-7/13, cytokeratin-7/13; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Immunoblotting was carried out after extracting total protein. Calponin, α-SMA, and desmin protein expression increased upon coculture, using SMC-CM and noninduced BMSCs (Fig. 3A). However, MHC, which was absent in the noninduced BMSC control, was specifically induced only in the SMC-CM treatment. UC-specific proteins were also expressed only when cells were induced with either coculture or CM (Fig. 3B). Uroplakin-Ia (Up-Ia) expression was stronger when BMSCs were cultured using UC-CM than coculture systems. Using AE1/AE3 antibodies produced a series of bands ranging from 40 to 70 kDa. CK13 expression was also specific to the induced cells.
FIG. 3.
Smooth muscle- and urothelial-specific protein expression on human BMSCs differentiated on day 14. (A) Lane 1, fresh smooth muscle tissue; lane 2, cultured SMCs as positive controls; lane 3, cultured urothelium as negative control; lane 4, noninduced BMSCs as control; lane 5, induced BMSCs by coculture with SMCs; lane 6, induced BMSCs by SMC-CM. The housekeeping gene, β-actin, was used as the load control. (B) Lane 1, bladder urothelium as control (p3); lane 2, noninduced BMSCs (p3); lane 3, BMSCs cocultured with urothelium; lane 4, BMSCs cultured with UC-CM. AE1/AE3, antibodies against cytokeratins; CK-13, cytokeratin-13, Up-Ia, Uroplakin-Ia.
Immunofluorescence experiments performed on BMSCs cultured using SMC-CM showed expression of α-SMA and calponin in induced and noninduced cells (Fig. 4a, b, e, f), irrespective of their induction status. However, expression of desmin (Fig. 4c, g) and myosin (Fig. 4d, h) was specifically seen only in the induced cells, and approximately 30–50% of the cells expressed these specific markers on day 14 (Fig. 4g, h). SMCs isolated from bladder tissue were used as a positive staining control (Fig. 4i–l). The summary of expression levels of in vitro differentiated BMSCs to the SMC phenotype using SMC-CM is given in Table 2.
FIG. 4.
Myogenic differentiation of human BMSCs using SMC-derived CM. Human BMSCs (p4) were stained with α-SMA (a, e, i), calponin (b, f, j), desmin (c, g, k), and myosin (d, h, l) antibodies without induction as negative control (a–d) and with induction for 14 days (e–h). SMCs were also stained with the same antibodies as a positive control (i–l). Scale bar = 50 μM. Color images available online at www.liebertonline.com/ten.
Table 2.
Summary of In Vitro Differentiation of Human Bone Marrow Mesenchymal Stem Cells to Bladder Cell Phenotype by Coculture or Conditioned Medium
| |
Smooth muscle-specific marker expressiona |
Urothelial-specific marker expression |
|||||
|---|---|---|---|---|---|---|---|
| Culture conditions | α-SMA | Calponin | Desmin | Myosin | Cell contraction | Up-Ia | AE1/AE3 |
| BMSC: SMC or UC-CMa/coculture | +++ | ++++ | +++ | +++ | +++ | +++ | +++ |
| BMSCs nontreated (−) control | ++++ | ++ | −/+ | −/+ | −/+ | −/+ | −/+ |
| Bladder SMCs (+) control | ++++ | ++++ | ++++ | ++++ | ++++ | ND | |
| Bladder UC (+) control | ND | ++++ | ++++ | ||||
Using only smooth muscle cell (SMC)-conditioned medium (CM).
α-SMA, alpha-smooth muscle actin; BMSCs, bone marrow mesenchymal stem cells; UCs, urothelial cells; AE1/AE3, antibodies against cytokeratins; ND, not done.
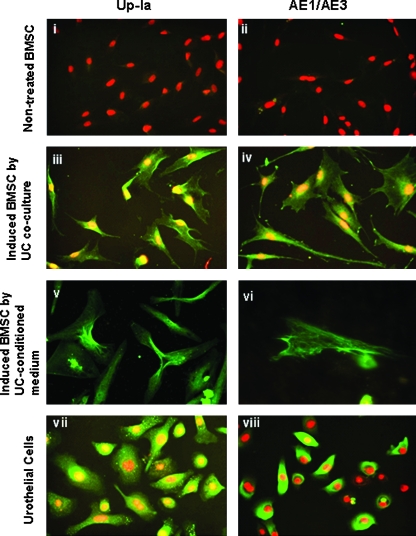
Staining for Up-Ia and pancytokeratins (AE1/AE3) on BMSCs cocultured with urothelium revealed specific expression of urothelial markers only in induced cells (Fig. 5-iii, iv). A high percentage (50–60%) of cells induced with CM also expressed Up-Ia and pancytokeratin proteins on day 14 (Fig. 5v, vi). Non-induced cells (Fig. 5i, ii) and urothelial cells (Fig. 5vii, viii) were used as controls. The overall expression levels of in vitro differentiated BMSCs to the urothelium phenotype using urothelium-CM and coculture with urothelium are given in Table 2.
FIG. 5.
Urothelial differentiation of human BMSCs using coculture approach. Top panel: (i, ii) noninduced BMSCs as negative control (iii, iv) BMSCs induced to urothelial differentiation by coculture with urothelium; (v, vi) urothelial differentiation of BMSC by UC-CM; (vii, viii) urothelial cells (p4) as positive controls. Cells were stained with urothelial-specific markers Up-Ia and AE1/AE3 (pancytokeratin). The secondary antibody was conjugated to fluorescein isothiocyanate (green) and nuclei counterstained using propidium iodide (red). Scale bar = 50 μM. Color images available online at www.liebertonline.com/ten.
Growth factor analysis of cocultured and CM
CM from bladder cell cultures and media from the bottom chamber of the cocultured dish showed levels of EGF and basic fibroblast growth factor below those of control medium. VEGF and PDGF-BB levels increased in urothelium-CM and BMSC cocultured with urothelium. By contrast, VEGF could be detected in the early and late stages of differentiation (12 h and 14 days); PDGF could be detected only at a late stage (Fig. 6i). In the SMC-CM and SMC coculture system, TGF-β1, HGF, and VEGF secretion levels were significantly higher in the early and late stages of the differentiation process (Fig. 6ii, iii, iv, respectively). Interestingly, HGF (Fig. 6iii) was abundantly secreted into the medium with time only in the presence of SMCs, whereas VEGF secretion did not show any specificity and increased in all treatments with time (Fig. 6iv).
FIG. 6.
Myogenic and urothelium-induced growth factors secreted from bladder cell culture conditions. Human BMSCs (p4) were cultured with SMCs/urothelium (coculture) or CM from SMCs/urothelium and media collected after 12 h and 14 days for growth factor estimation by ELISA. (i) PDGF and (ii) TGF-β1 levels were very low and are plotted as a percentage of control (dotted arrow). However, (iii) HGF and (iv) VEGF were significantly secreted into the media during coculture as well as with CM, represented as actual concentration (pg/mL). Epidermal growth factor and basic fibroblast growth factor levels were biologically insignificant and are hence not depicted. 1, BMSC coculture with SMCs (12 h); 2, BMSC coculture with urothelium (12 h); 3, SMC-CM (12 h); 4, urothelium-CM (12 h); 5, BMSC coculture with SMCs (14 days); 6, BMSC coculture with urothelium (14 days); 7, SMC-CM (14 days); 8, urothelium-CM (14 days). PDGF, platelet-derived growth factor; TGF-β1, transforming growth factor-β1; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor.
Functional analysis of differentiated cells
Cultures of seeded scaffolds cultured in SMC-CM with repetitive mechanical strain significantly improved the cell contractility response to electrical field stimulation (3.7 ± 0.1mg in vitro, 7.8 ± 4.6 in vivo) compared with static culture (1.8 ± 1.5 mg in vitro, 2.3 ± 1.6 in vivo; p < 0.05) (Fig. 7).
FIG. 7.
Muscle tissue contractility following myogenic differentiation of human BMSCs. BSMs were seeded with human BMSCs and were continuously stretched (5 pulls/min) to 10% of their original length for 8 days. Contractility response of human BMSC-seeded BSM: (left) in vitro and (right) in vivo. Organ baths were used to study contractility of muscle-induced human BMSCs by subjecting them to an electrical field stimulation of 5 V, 1 Hz. Seeded BSMs were cultured in vitro using SMC-CM (stretch and static) or standard culture medium (control) for 14 days and assayed for contractility or in vivo implanted into nude mice and assayed after 4 weeks. Nude mice used for in vitro studies (n = 4) and in vivo studies (n = 3). *p < 0.05 (t-test), compared with static culture. BSMs, bladder submucosal matrixes.
In vivo differentiation studies
The bladder extracellular matrix (ECM) graft was infiltrated with numerous BMSCs as seen by DAPI staining after 1 month in vivo (Fig. 8A-a). Trichrome staining (Fig. 8A-b) showed ample collagen production and the cells also stained for desmin (Fig. 8A-c). Figure 8B (a, c, e, g) shows hematoxylin and eosin staining of the PLLA matrix when cultured with different cells. Cell–matrix infiltration was remarkable in the cell-seeded PLLA compared with the cell-free PLLA matrix. Moreover, blood vessels are also seen in the stained sections, indicating graft acceptance and successful regeneration. The PLLA matrix was shown as background light brown staining, as seen in the right panels (Fig. 8B-b, B-d, B-f, B-h). The intensity of desmin staining increased in the grafts seeded with the induced (using SMC-CM) BMSCs (Fig. 8B-h) when compared with the nontreated BMSCs (Fig. 8B-f).
FIG. 8.
Muscle tissue formation in vivo. (A) Bladder extracellular matrix and (B) PLLA. Cross sections of the implanted scaffold were retrieved from the nude mice after 1 month. (A-a) Immunofluorescence analysis for DAPI (4′,6-diamidino-2-phenylindole) and (A-b) Masson's trichrome, and (A-c) immunohistochemistry analysis for desmin staining of cell-seeded bladder extracellular matrix implants. Images are shown at 400 ×. (B) Immunohistochemistry staining of cell-free PLLA implant (B-a, B-b), SMC-seeded PLLA as a positive control (B-c, B-d), noninduced BMSC-seeded PLLA as negative control (B-e, B-f), and PLLA seeded with BMSCs cultured using SMC-CM for 14 days (B-g, B-h). Hematoxylin and eosin-stained sections (on the left) are shown at 200 × and desmin-stained sections (on the right) at 400 ×. PLLA, poly-l-lactic acid. Color images available online at www.liebertonline.com/ten.
Discussion
Urinary bladder is a hollow organ composed of tissues from the endoderm (i.e., urothelium) and mesoderm (i.e., SMCs). The interaction between urothelium and SMCs is essential for the development and maintenance of structural integrity of the bladder and its contractile function. During de novo reconstruction of functional bladder using MSCs for tissue engineering, it is necessary to induce stem cell differentiation into both urothelium and SMCs for the bladder regeneration processes. In this study, we used BMSCs as an alternative cell source for urothelium and SMC differentiation, for potential use in bladder, urethral, or other urological tissue engineering. Our data showed a promoting effect of indirect coculture of bladder cells or CM from bladder cell cultures on the expression of smooth muscle- and uroepithelial-specific genes and proteins in BMSCs in vitro. The CM are more efficient in inducing BMSC differentiation. The CM derived from bladder cells contain cytokine growth factors that can be used to guide BMSCs to differentiate into SMCs and urothelium. Further, use of a novel biodegradable material, a nanofibrous PLLA polymer scaffold, not only provided a three-dimensional structure for maximizing cell–matrix infiltration and enhancing cell differentiation but also promoted muscle tissue remolding.
In this study, human BMSCs were induced to differentiate into SMC- and urothelium-like cells by either indirect coculture of bladder cells or CM derived from bladder cells. Both approaches generated similar results in inducing bladder cell differentiation of BMSCs. The indirect coculture system is the closest possible “in vitro” setup that mimics the “in vivo” surroundings without cell types coming into direct contact, yet with all the benefits of being in each other's proximity. An indirect coculture system allows soluble regulatory proteins/growth factors secreted by the target cells to induce differentiation of the cocultured cells.
Several growth factors secreted into the CM can be quantified for use in cell-free differentiation systems. Identification of such cytokine growth factors would allow their exogenous use for improving the efficiency of myogenic and uroepithelial differentiation of BMSCs in future studies. Over the years, much research has addressed the question of true differentiation versus fusion of stem cells.20,21 This study shows that BMSC differentiation could not have taken place by cell fusion, as BMSCs and bladder cells were in two separate chambers and never made contact or were nourished using cell-free conditioned medium.
Proper collection and storage of these CM are also vital for preserving the biological activity of the secreted factors. In our study, we used 60–70% confluent cultures with media changed at 12 h prior to collection. The filtered CM was also stored at −80°C to preserve biological activity. Using bladder cell-CM from healthy, subconfluent cultures is simple and convenient and displays more efficient stem cell differentiation. In SMCs and urothelial differentiation of BMSCs, bladder cell-CM treatment appears more efficient in inducing BMSC differentiation into both SMCs and urothelium when comparing coculturing with bladder cells. Interestingly, SMC-CM can enhance myosin transcript and protein expression in BMSCs compared with coculturing with SMCs. HGF, a myogenic growth factor, is significantly higher in SMC-CM than coculturing with SMCs. The most likely explanation is the clogging of the porous membrane in the top chamber due to cell confluence over time, leading to inefficient cytokine growth factor secretion to the bottom well, whereas all the cytokine growth factors secreted were detected in CM. We detected several cytokine growth factors in the SMC-CM (i.e., PDGF-BB, TGF-β1, HGF, and VEGF); the last two were expressed in high levels over 14 days. However, VEGF level was elevated in coculture compared with the CM. This finding is consistent with other findings with coculture model.22 Previous study with this model showed that VEGF is significantly increased in the cocultured medium compared with the medium from hepatocytes or neroblastoma grown alone.22 VEGF may be just one of many myogenic growth factors. SMC-CM both enhanced SMC marker expression and improved contractility of the induced BMSCs. Cell markers such as α-SMA, calponin, and desmin are expressed in SMCs and myofibroblasts as well, whereas myosin is a SMC-specific marker.23 Notably, induced BMSCs displayed myosin expression and contractile function in the presence of serum and calcium ionophores, similar to SMCs in myosin expression and cell contractility assays. Noninduced human BMSCs expressed α-SMA, which is similar to findings in dog BMSCs.9
We observed that myogenic or urothelial differentiation of human BMSCs was time dependent. The morphology change was observed as early as day 3. Phenotypic changes were confirmed on day 7 using RT-PCR, western blot, and immunocytochemistry. However, myogenic or urothelial differentiation of human BMSCs were significantly enhanced on day 14. For myogenic differentiation of human BMSCs, the processes took longer than that in rodent model. Generally, it takes about 8 days to induce rat BMSCs to smooth muscle-like cells.5 The urothelial differentiation of cells using EGF treatments24 was slightly faster than those observed by coculture or CM.
Additionally, in an in vivo study, induced BMSCs displayed SMC markers at 4 weeks after implantation, indicating that the BMSCs induced with SMC-CM in vitro indeed maintained the SMC phenotype and persisted with the myogenic differentiation in vivo. In uroepithelial differentiation of BMSCs, urothelium-CM could promote the RNA transcript of Up-Ia, but with coculture of urothelium, weaker expression of Up-Ia gene and protein was observed. Although urothelial markers were expressed in BMSCs following urothelial induction, only about half of BMSCs changed from a spindle shape to a polygonal shape after 14 days of differentiation.
A biodegradable PLLA polymeric scaffold with nanofibers and a porous structure has been successfully used in tissue engineering, for example, in bone,25 cartilage,26 liver,27 and nerve regeneration in vitro and in vivo. This nanostructured PLLA scaffold mimics the natural ECM and provides a highly porous and fibrous architecture for good cell–matrix penetration and maintains the physiological strength of the replaced tissue in vivo. This scaffold is free of any cytotoxic and heterogeneous proteins to prevent immunological reactions and can be biodegraded within 3–6 months. In this study, the induced BMSCs displayed abundant cell–matrix infiltration and SMC differentiation. The cell-seeded nanofibrous PLLA scaffold induced smooth muscle tissue regeneration with abundant formation of capillaries, superior to those induced by cell-seeded natural collagen matrix. The main advantage of using PLLA as a substrate is that its porous structure provides cellular interactions with the polymer, prompting cell migration, proliferation, and tissue formation in a three-dimensional construct.
In conclusion, BMSCs can be differentiated into both SMCs and urothelium by coculture of bladder cells or using CM derived from bladder cell culture, indicating that MSCs can simultaneously give rise to mesoderm and endoderm cell lineages of bladder tissue. CM from bladder cell culture shared definite cytokine growth factors (PDGF-BB, TGF-β1, and VEGF), whereas HGF was specifically involved in induction of myogenic differentiation of human BMSCs. More studies are needed to answer the question whether the cell phenotypes are temporally or permanently changed after BMSCs are induced to differentiation. As a promising scaffold, the nanofiber PLLA matrix with micropores significantly increased cell–matrix infiltration and maximized stem cell proliferation and differentiation into SMCs, thereby promoting formation of bladder cells in vivo. Establishment of MSC differentiation to bladder cell lineages (SMCs and urothelium) may have significant applications in cellular approaches to restore bladder capacity and function for the patients with bladder extrophy or cancer who need cystoplasty.
Acknowledgments
This work was supported by R21 DK071791-01. The authors thank Ms. Karen Klein (Research Support Core, WFUHS Office of Research) for her editorial assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 2.Gnecchi M. He H. Noiseux N. Liang O.D. Zhang L. Morello F. Mu H. Melo L.G. Pratt R.E. Ingwall J.S. Dzau V.J. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto R. Omura T. Yoshiyama M. Hayashi T. Inamoto S. Koh K.R. Ohta K. Izumi Y. Nakamura Y. Akioka K. Kitaura Y. Takeuchi K. Yoshikawa J. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1168. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 4.Lim S.Y. Kim Y.S. Ahn Y. Jeong M.H. Hong M.H. Joo S.Y. Nam K.I. Cho J.G. Kang P.M. Park J.C. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Kanematsu A. Yamamoto S. Iwai-Kanai E. Kanatani I. Imamura M. Adam R.M. Tabata Y. Ogawa O. Induction of smooth muscle cell-like phenotype in marrow-derived cells among regenerating urinary bladder smooth muscle cells. Am J Pathol. 2005;166:565. doi: 10.1016/S0002-9440(10)62278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamama K. Sen C.K. Wells A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008;17:897. doi: 10.1089/scd.2007.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narita Y. Yamawaki A. Kagami H. Ueda M. Ueda Y. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008;333:449. doi: 10.1007/s00441-008-0654-0. [DOI] [PubMed] [Google Scholar]
- 8.Ross J.J. Hong Z. Willenbring B. Zeng L. Isenberg B. Lee E.H. Reyes M. Keirstead S.A. Weir E.K. Tranquillo R.T. Verfaillie C.M. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y. Lin H.K. Frimberger D. Epstein R.B. Kropp B.P. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005;96:1120. doi: 10.1111/j.1464-410X.2005.05741.x. [DOI] [PubMed] [Google Scholar]
- 10.Frimberger D. Morales N. Shamblott M. Gearhart J.D. Gearhart J.P. Lakshmanan Y. Human embryoid body-derived stem cells in bladder regeneration using rodent model. Urology. 2005;65:827. doi: 10.1016/j.urology.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Anumanthan G. Makari J.H. Honea L. Thomas J.C. Wills M.L. Bhowmick N.A. Adams M.C. Hayward S.W. Matusik R.J. Brock J.W., 3rd Pope J.C.t. Directed differentiation of bone marrow derived mesenchymal stem cells into bladder urothelium. J Urol. 2008;180:1778. doi: 10.1016/j.juro.2008.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuhara S. Tomita S. Yamashiro S. Morisaki T. Yutani C. Kitamura S. Nakatani T. Direct cell-cell interaction of cardiomyocytes is key for bone marrow stromal cells to go into cardiac lineage in vitro. J Thorac Cardiovasc Surg. 2003;125:1470. doi: 10.1016/s0022-5223(02)73610-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y. Kropp B.P. Lin H.K. Cowan R. Cheng E.Y. Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng. 2004;10:181. doi: 10.1089/107632704322791835. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y. Kropp B.P. Moore P. Cowan R. Furness P.D., 3rd Kolligian M.E. Frey P. Cheng E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: potential applications for tissue engineering technology. J Urol. 2000;164:928. doi: 10.1097/00005392-200009020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lobban E.D. Smith B.A. Hall G.D. Harnden P. Roberts P. Selby P.J. Trejdosiewicz L.K. Southgate J. Uroplakin gene expression by normal and neoplastic human urothelium. Am J Pathol. 1998;153:1957. doi: 10.1016/S0002-9440(10)65709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser M. Thomas D.F. Pitt E. Harnden P. Trejdosiewicz L.K. Southgate J. A surgical model of composite cystoplasty with cultured urothelial cells: a controlled study of gross outcome and urothelial phenotype. BJU Int. 2004;93:609. doi: 10.1111/j.1464-410x.2003.04675.x. [DOI] [PubMed] [Google Scholar]
- 17.Cross W.R. Eardley I. Leese H.J. Southgate J. A biomimetic tissue from cultured normal human urothelial cells: analysis of physiological function. Am J Physiol Renal Physiol. 2005;289:F459. doi: 10.1152/ajprenal.00040.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y. Frimberger D. Cheng E.Y. Lin H.K. Kropp B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98:1100. doi: 10.1111/j.1464-410X.2006.06447.x. [DOI] [PubMed] [Google Scholar]
- 19.Vaught J.D. Kropp B.P. Sawyer B.D. Rippy M.K. Badylak S.F. Shannon H.E. Thor K.B. Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: functional innervation and receptor expression. J Urol. 1996;155:374. [PubMed] [Google Scholar]
- 20.Ying Q.L. Nichols J. Evans E.P. Smith A.G. Changing potency by spontaneous fusion. Nature. 2002;416:545. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 21.Terada N. Hamazaki T. Oka M. Hoki M. Mastalerz D.M. Nakano Y. Meyer E.M. Morel L. Petersen B.E. Scott E.W. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 22.Beierle E.A. Strande L.F. Berger A.C. Chen M.K. VEGF is upregulated in a neuroblastoma and hepatocyte coculture model. J Surg Res. 2001;97:34. doi: 10.1006/jsre.2001.6097. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. Autologous cell sources for urological applications. In: Atala A., editor; Denstedt J., editor. Biomaterials and Tissue Engineering in Urology. Cambridge, England: Woodhead Publishing Limited; 2008. [Google Scholar]
- 24.Tian H. Bharadwaj S. Liu Y. Ma H. Ma P.X. Atala A. Zhang Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. 870;31 doi: 10.1016/j.biomaterials.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu J. Liu X. Ma P.X. Induction of osteoblast differentiation phenotype on poly(l-lactic acid) nanofibrous matrix. Biomaterials. 2008;29:3815. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Z. Gao C. Gong Y. Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26:1253. doi: 10.1016/j.biomaterials.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Torok E. Vogel C. Lutgehetmann M. Ma P.X. Dandri M. Petersen J. Burda M.R. Siebert K. Dullmann J. Rogiers X. Pollok J.M. Morphological and functional analysis of rat hepatocyte spheroids generated on poly(l-lactic acid) polymer in a pulsatile flow bioreactor. Tissue Eng. 2006;12:1881. doi: 10.1089/ten.2006.12.1881. [DOI] [PubMed] [Google Scholar]