Abstract
Understanding and controlling chondrocyte and cartilage metabolism in osteochondral tissues may facilitate ex vivo maintenance and application, both for allografts and tissue-engineered grafts. The hypothesis of this study was that maintenance of chondrocyte viability and matrix content and release of sulfated glycosaminoglycan (sGAG) in the articular cartilage of joint-scale osteochondral fragments are temperature and metabolism dependent. The aims were to assess, for adult goat joints, the effects of incubation temperature (37°C vs. 4°C) on cartilage chondrocyte viability and tissue matrix content and mechanical function, and the effects of temperature and cellular biosynthesis on sGAG release. Chondrocyte viability was maintained with 37°C incubation for 28 days, but decreased by ∼30% with 4°C incubation. Concomitantly, with 37°C incubation, cartilage sGAG was depleted by ∼52% with the lost sGAG predominantly unable to aggregate with hyaluronan, whereas collagen content, tissue thickness, and tissue stiffness were maintained. The depletion of sGAG was diminished by slowing metabolism, with 4°C decreasing release by ∼79% compared with 37°C incubation, and cycloheximide inhibition of cell metabolism at 37°C decreasing release by ∼47%. These results indicate that the articular cartilage of joint-scale grafts have enhanced chondrocyte viability with incubation at 37°C, but may need anabolic stimuli or catabolic inhibitors to maintain sGAG content.
Introduction
The repair of articular cartilage defects using osteochondral allografts and autografts is one option for functional restoration, though the procedures are limited by a lack of donor tissue. Cartilage repair and replacement treatments are typically used for small and large cartilage defects that have not yet progressed to the point where prosthetic joint replacement is recommended.1,2 The shortage of available donor tissue3 has stimulated tissue engineering efforts to generate large, appropriately shaped, joint-scale osteochondral fragments.4–8 Allografts are currently stored at the joint scale, and the properties of cartilage in such stored allografts provide the standard for large tissue-engineered constructs.
The effects of storage conditions on cadaveric donor tissue have typically been studied using small osteochondral cores at 4°C, although studies suggest that joint-scale storage at 37°C may be beneficial. Tissue banks currently store tissue as large osteochondral fragments at 4°C in proprietary media with 10% serum. Studies of storage conditions have typically examined small osteochondral cores as a model system9–14 at 4°C in media with 10% serum, with only a few studies addressing the practical storage in joint-scale fragments.15,16 Cartilage tissue structure, cell viability, and proteoglycan (PG) metabolism can be maintained in explant culture at 37°C in certain medium formulations,17–19 but have only recently been investigated in the context of allograft storage, using small cores.20
Viable chondrocytes are necessary for long-term function of osteochondral grafts, and the viability of chondrocytes in cultured cartilage is affected by the incubation medium, temperature, and configuration (Table 1). In small osteochondral cores, chondrocyte viability is higher with the addition of serum to the culture medium at 4°C11,21 and also with 37°C compared with 4°C incubation.17,21 Chondrocyte viability at 4°C follows similar trends in joint-scale osteochondral fragments as in small cores, with significant loss of viability over several weeks,15,16 although quantitative viability results at 37°C at the joint scale have not yet been reported.
Table 1.
Osteochondral and Joint-Scale Culture: Effects of Temperature and Duration on Chondrocyte Viability
| |
|
Conditions |
|
|
||
|---|---|---|---|---|---|---|
| Tissue | Age | T (°C) | Days | FBS | Viability | Reference |
| Small osteochondral core | Mature | 4 | 28 | — | 37–32% | 11 |
| 21–44 | 10% | 50–84% | 21 | |||
| Joint-scale fragmenta | Mature | 4 | 28–60 | 10% | 50–77% | 15,16 |
| Small osteochondral core | Mature | 37 | 25 | — | 90–100% | 17 |
| Joint-scale fragment | Inmature | 37 | 1 | 5% | — | 36 |
| Mature | 37 | 10 | 10% | Viable | 31 | |
| 28 | 10% | — | ||||
Typical tissue bank incubation configuration and condition.
FBS, fetal bovine serum.
Understanding and controlling chondrocyte metabolism in different tissue configurations facilitates ex vivo maintenance of cartilage, and may be useful for storage of allografts and osteochondral tissue. Although the collagen content of cartilage explants and osteochondral cores remains relatively stable during tissue culture, sulfated glycosaminoglycan (sGAG) content can be markedly influenced by metabolic stimuli in the culture medium, such as serum, which promotes biosynthesis of aggrecan,18,22,23 the major compressive load-bearing component of cartilage.24 The first globular domain of aggrecan mediates its association with hyaluronan (HA),25 stabilizing it within the cartilage matrix. Cleavage of aggrecan can disrupt this association with HA, increasing the release of sGAG from cartilage. Aggrecan metabolism and maintenance is important for tissue function, as tissue stiffness has been correlated with sGAG content.26–28 The metabolism of sGAG in cartilage is mediated by biosynthetically active chondrocytes, as inhibition of cell biosynthesis with cycloheximide (CX) diminishes both the synthesis and the degradation of aggrecan.22,29,30 In the joint-scale configuration of intact bovine calf sesamoid bones, cartilage sGAG metabolism is affected by both static31,32 and dynamic33,34 loading. The modulation of sGAG metabolism in mature cartilage at the joint-scale with 37°C incubation remains to be investigated.
Thus, the hypothesis of this study was that maintenance of chondrocyte viability and matrix content and release of sGAG in the articular cartilage of joint-scale osteochondral fragments are temperature and metabolism dependent. The specific aims were to assess the effects of adult goat joint-scale culture temperature (37°C vs. 4°C) and duration (fresh, 12 or 28 days) on articular cartilage chondrocytes (viability and DNA content) and tissue matrix (sGAG and collagen contents, sGAG distribution, and stiffness), and the effects of temperature (37°C vs. 4°C) and cellular biosynthesis (±CX) on matrix depletion (sGAG release rate and aggregatability with HA).
Materials and Methods
Experimental design
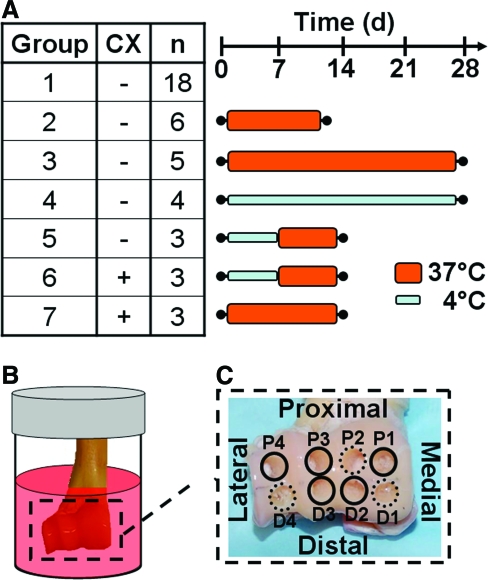
A total of 42 distal humeri, harvested bilaterally from 22 adult goats, were used. The humeri harvested bilaterally were always used for different experimental conditions. In particular, 18 humeri from different goats were used as fresh controls. The remaining 24 humeri were used for various incubation conditions. (The 4 out of the 24 humeri that were harvested bilaterally from 2 goats were used for different incubation conditions.) This allowed efficient usage of the available animal tissue. Groups included [1] fresh controls (n = 18) or joints incubated for [2] 12 days (n = 6) or [3] 28 days (n = 5) at 37°C without CX, for [4] 28 days (n = 4) at 4°C without CX, for 7 days at 4°C and then 7 days at 37°C (4/37) [5] without CX (n = 3) or [6] with CX (n = 3), or for [7] 14 days at 37°C with CX (n = 3) (Fig. 1A). Although the goats were of two types, Boer males (16) and mixed-breed females (6), they were of similar age and joint size, with average cartilage thicknesses of (mean ± standard deviation) 0.35 ± 0.05 mm and 0.34 ± 0.05 mm and average surface areas of 12.5 ± 2.5 cm2 and 11.2 ± 0.9 cm2, respectively. In addition, type had no significant effect on the outcome variables described below (p = 0.18–0.86). The left and right limbs were divided as evenly as possible into each group, with either the same number of left and right limbs in each group or at most one more left or right limb in groups with an odd number of samples.
FIG. 1.
Schematic diagrams of (A) experimental groups, (B) joint-scale bioreactor setup, and (C) core isolation locations from the distal humerus. Color images available online at www.liebertonline.com/ten.
Harvest and culture
Distal humeri were aseptically isolated, affixed to the lid of a bioreactor, and suspended in media. Thoracic limbs were received on wet ice within 24 h of animal sacrifice. Elbow joints35 were aseptically isolated essentially as described previously.36 Briefly, the mid-diaphyses of the radius and ulna were exposed and transected. The bone marrow was removed, and the cavities were rinsed with 3% hydrogen peroxide and then with phosphate-buffered saline (PBS) with 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL Fungizone (PSF). The distal humerus was exposed, separated from the radius and ulna; soft tissue, including the periosteum on the diaphysis, was removed. Each humerus was suspended in a 250 mL polycarbonate jar functioning as a bioreactor, with a stainless steel bolt affixed to the lid and attached to the diaphysis with bone cement (Osteobond; Zimmer, Warsaw, IN) by filling the diaphysis with ∼10 mL of wet cement, inserting the bolt, and allowing the cement to set for 15 min. For incubation at 37°C, the bioreactor lids were modified to allow gas exchange via 0.45 μm filters. Each bioreactor was filled with the medium, secured with a lid, and placed at 4°C or 37°C. One hundred milliliters of the medium was used per bioreactor (low-glucose Dulbecco's modified Eagle's medium, 10 mM HEPES, 0.1 mM non-essential amino acids, 0.4 mM L-proline, 2 mM L-glutamine, PSF, and 25 μg/mL ascorbic acid) (DMEM+) supplemented with 10% fetal bovine serum, and also with 0.5 mM CX to inhibit biosynthesis30 in certain studies. The lid with suspended distal humerus was screwed on to the bioreactor base (Fig. 1B), and incubated at 37°C within a humidified environment at 5% CO2 or at 4°C for the appropriate durations. For joints incubated at 4°C, the closed container effectively maintained the CO2 environment since the bicarbonate-dependent pH of the spent incubation medium was 7.40 ± 0.05. The medium was changed twice per week, and the spent medium was collected and stored at −20°C until processing.
Joint surface area
Joint surface area was estimated from geometries determined from photographs and a scaling factor determined from three-dimensional (3D) surface scanning of selected samples. Photographs of the articulating face of the distal humerus of all joints were taken before core isolation with a digital camera. The width of the humeral head, as well as the diameters of the medial and lateral edges, was measured using ImageJ (National Institutes of Health, Bethesda, MD). The shape of the humeral head was approximated as a truncated hemicone with half of the circumferential face corresponding to the surface area of cartilage, as calculated from the diameters and width. To determine a scaling factor between geometrical measures and cartilage surface area, three joint surfaces were scanned in 3D (3D Desktop Scanner; NextEngine, Santa Monica, CA), and cartilage surface areas were determined directly from 3D reconstructions (ScanStudio; NextEngine). The difference between surface area estimated from the geometrical estimate and the direct measurement was 6.6% ± 3.2%. Thus, surface area was estimated routinely by the geometrical estimate scaled up by 1.066.
Isolation of osteochondral cores for analysis
After harvest or incubation, eight 3.2-mm-diameter osteochondral cores were isolated with the articular cartilage intact, and samples were prepared for analysis of viability and histology or for analysis of stiffness and biochemistry. Cores were isolated from four proximal (P1–P4) and four distal (D1–D4) sites spanning the medial to lateral margin of the distal humerus (Fig. 1C) using a custom stainless steel coring bit under constant irrigation with PBS with PSF kept at 4°C. Cores from three positions (D1, D4, and P3) were placed in DMEM + with 10% fetal bovine serum for 1–2 h until viability staining and analysis. The cores from the remaining five positions were rinsed in PBS with protease inhibitors (1 mM phenylmethanesulfonyl fluoride, 2 mM disodium ethylenediamine tetraacetate, 5 mM benzamidine-HCl, and 10 mM N-ethylmaleimide), for 1 h at 4°C and then stored at −20°C until further processing.
Viability and histology
Cores from three positions (D1, D4, and P3) were bisected axially with a razor blade and one half was analyzed for chondrocyte viability. The half-cores were stained with Live/Dead® (Molecular Probes; Invitrogen, Carlsbad, CA) and the en face and vertical profiles were imaged at 10 × with a fluorescence microscope (Eclipse TE300; Nikon, Melville, NY). Images were analyzed using a custom image-processing routine in MATLAB® v7.5 to determine the percent of live cells. The vertical profile was divided into superficial, middle, and deep zones each comprising 15%, 30%, and 55% of the total thickness based on cellular morphological features. Since the superficial zone contained only one to three layers of cells, viability estimates from vertical profiles were variable due to the low number of cells observed in each field of view (mean ± standard deviation: 49 ± 21 cells). Instead, the viability of the chondrocytes in the superficial zone was determined from en face views, which provided a larger numbers of cells in each field of view (601 ± 156 cells).
The second half of each core was prepared for histology and stained with Toluidine blue. The half-cores were fixed in 4% paraformaldehyde in PBS for ∼1 week at 4°C and then decalcified in 1 mL of PBS with 15% ethylenediaminetetraacetic acid (EDTA) on a rocker at room temperature. The EDTA solution was changed every other day for ∼3 weeks to allow decalcification. The decalcified cores were cryostat sectioned at 10 μm, stained with 0.04% Toluidine blue, and imaged at 10 × magnification.
Core diameter, thickness, and indentation stiffness
Cores from the remaining five positions (D2, D3, P1, P2, and P4) were analyzed for diameter and thickness. The diameter of each core was measured using calipers (Mitutoyo, Aurora, IL). For cartilage thickness, images were captured at 0°, 60°, and 120° around the perimeter of the core, thickness was measured from the images (ImageJ) at three locations per angle, and the average of the nine measurements was used.
The short-time indentation stiffness was determined. Samples were compressed at the articular surface by 20% of cartilage thickness at a rate of 0.1 mm/s (over <1 s) with a plane-ended, impermeable, 400-μm-diameter indenter tip, and the load was measured using a mechanical testing apparatus (v500cs; BioSyntech Canada, Laval, Canada). The tip was held at maximum displacement for 0.5 s, the load recorded, and test repeated at three sites 400 μm apart on each core. The average load for each core was divided by the indentation depth to determine the indentation stiffness.
DNA, collagen, and sGAG contents
After indentation testing, the cartilage was analyzed for content of DNA, collagen, and sGAG. For each core, cartilage was removed from the bone and solubilized with proteinase K at 60°C for 16 h, and portions of the digests were analyzed for DNA by the PicoGreen dye binding assay,37 sulfated sGAG by the dimethylmethylene blue dye binding assay,38 and collagen by p-dimethylaminobenzaldehyde detection of hydroxyproline.39 The hydroxyproline content was converted to collagen content using a mass ratio of 7.25 collagen to hydroxyproline.40,41 Biochemical measures were normalized to core surface area to allow comparison between sGAG in the tissue and released to the medium. Portions of the spent medium were also analyzed for sGAG content and normalized to joint surface area per day, as passive loss of cleaved sGAG molecules from cartilage matrix in explants is a surface-area-dependent phenomenon.42 Total sGAG in the culture system was calculated by summing the mass of sGAG in the spent medium over the culture duration normalized to joint surface area, and adding that to the average sGAG per surface area in the cores, scaled up to the joint surface area.
Aggregation of sGAG in conditioned medium with HA
The size and aggregatability with HA of sGAG in the conditioned medium and from freshly extracted controls were analyzed using gel electrophoresis. Pooled samples of the conditioned medium from the initial days in culture of joints incubated at 37°C were concentrated to ∼1 μg/μL sGAG with a 100 kDa filter. As a control sample, cartilage was harvested from one adult goat distal humerus and sGAG was extracted43 (with an extraction efficiency of 97%). The ability of aggrecan in the spent medium and in the cartilage extract control to aggregate with HA was analyzed by the addition of 5% (w/w) Healon and 5% (w/w) link protein (LP), isolated from bovine calf cartilage.44 Samples were dissociated by dialyzing against 4 M GuHCl and 0.1 M NaAc (pH 6.8) overnight at 4°C. Then, samples were allowed to associate by dialysis against 0.1 M NaAc (pH 6.8) for 24 h at 4°C. Subsequently, as a control for association mediated by HA, portions of each sample were incubated with Streptomyces hyaluronidase (10 units/mL in 0.1 M NaH2PO4 and 0.15 M NaCl, pH 6) overnight at 37°C to digest HA. Sample volumes with an equivalent of 10 μg of sGAG were electrophoresed in tris-acetate-EDTA buffer (pH 8) in a 1% agarose gel; fixed; stained in 0.2% (w/v) Alcian blue 8GX, 3% acetic acid, and 0.05 M MgCl2, pH 245; destained; and imaged.
Statistics
Data are presented as mean ± standard error of the mean. The effects of incubation condition (fresh control, 12 days at 37°C, 28 days at 37°C, or 28 days at 4°C) and position (P3, D1, and D4) on en face, middle, and deep zone viabilities, and remaining positions (P1, P2, P4, D2, and D3) on cartilage thickness and stiffness, and DNA, sGAG, and COL contents were determined by two-way analysis of variance with condition as a fixed effect and position as a repeated measure. Because humeri were distributed among the various experimental conditions, with no two humeri from the same animal being used for the same condition, the statistical model did not attempt to account for animal donor effects. Viability data were arcsine transformed to improve normality.46 The effects of incubation condition (4°C–CX, 4/37°C–CX, 4/37°C + CX, 37°C–CX, or 37°C + CX) and day (0:3.5:28) on sGAG in the medium samples were determined by two-way analysis of variance with condition as a fixed effect and day as a repeated measure on log10 transformed data to improve homoscedasticity.46 Tukey post hoc comparisons were performed to compare groups when significant differences (p < 0.05) were detected between conditions. Statistical analyses were performed using Systat 10.2 (Systat Software, Chicago, IL).
Results
Viability
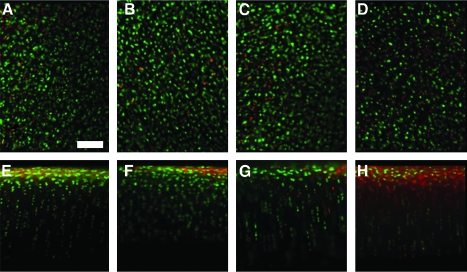
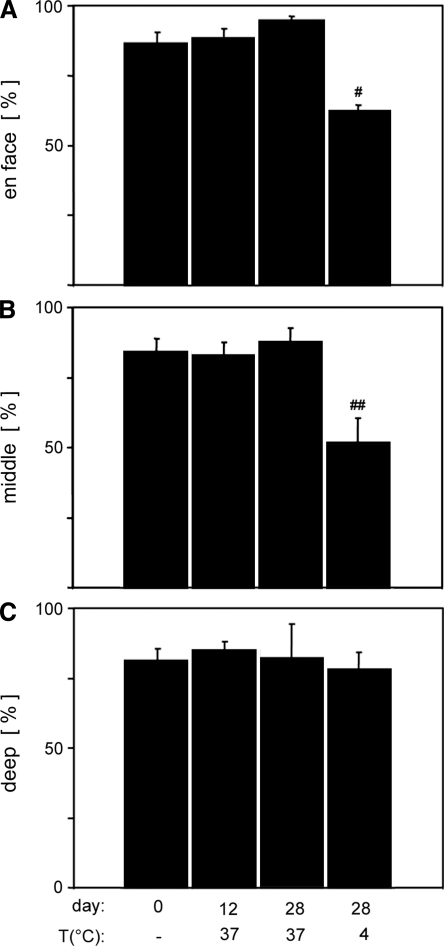
Chondrocyte viability was high in the en face view and middle zone of fresh controls and joints incubated at 37°C, but lower in joints incubated at 4°C, whereas no effect of condition was observed in the deep zone (Fig. 2). Quantitative analysis was consistent with these observations, with a significant effect of incubation condition on viability in the en face (p < 0.05) and middle zone (p < 0.05), though not in the deep zone (p = 0.98). A significantly lower percentage of viable cells were present in joints incubated at 4°C in the en face profile (62%) compared with all other groups (86–95%, Fig. 3A, p < 0.05) and in the middle zone (52%) compared with fresh controls (84%) and joints incubated for 28 days at 37°C (88%, Fig. 3B, p < 0.05).
FIG. 2.
Osteochondral cores were analyzed for chondrocyte viability by Live/Dead® fluorescence staining in the en face (A–D) and vertical (E–H) profiles. Representative images of cores from fresh control (A, E), 12 days at 37°C (B, F), 28 days at 37°C (C, G), and 28 days at 4°C (D, H) are shown. Scale bar: 100 μm. Color images available online at www.liebertonline.com/ten.
FIG. 3.
Fluorescent Live/Dead images were analyzed for percentage viable chondrocytes in the en face profile (A) and the middle (B) and deep (C) zones of the vertical profile from fresh control, 37°C at 12 days, 37°C at 28 days, and 4°C at 28 days. #Significantly less than all other bars (p < 0.05); ##significantly less than fresh control and 37°C at 28 days groups (p < 0.05).
Thickness and stiffness
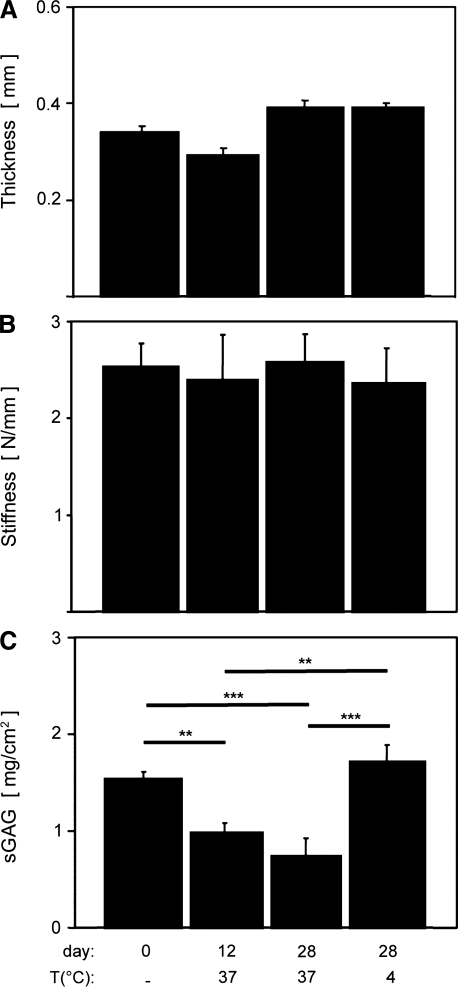
Cartilage thickness varied slightly with incubation condition, though stiffness was not detectably affected. Average distal humerus cartilage thickness ranged from approximately 0.3 to 0.4 mm, and varied slightly, but significantly, with incubation condition (p < 0.01). There were no differences between the thickness of fresh controls and any incubation condition (Fig. 4A, p = 0.14–0.24), but slight differences between the 12 days at 37°C group and 28 days at 37°C (p < 0.01) and 28 days at 4°C (p < 0.05) groups. Average cartilage indentation stiffness ranged from approximately 2.4 to 2.8 N/mm and did not vary with incubation condition (Fig. 4B, p = 0.98).
FIG. 4.
Osteochondral cores from fresh control, 37°C at 12 days, 37°C at 28 days, and 4°C at 28 days were analyzed for thickness (A), indentation stiffness (B), and sGAG content (C). **p < 0.01 and ***p < 0.001. sGAG, sulfated glycosaminoglycan.
DNA and collagen content
DNA and collagen contents of the cartilage digests were not affected by incubation condition. DNA content averaged 18.8 ± 1.2 μg/cm2 and was not affected by incubation condition (p = 0.12). Collagen content averaged 5.3 ± 0.4 mg/cm2 and was not affected by incubation condition (p = 0.75). The trends for DNA and collagen contents normalized to volume, 603 ± 38 μg/cm3 and 169 ± 13 mg/cm3, respectively, were generally similar (p = 0.06, 0.30).
sGAG content and distribution
sGAG content was decreased in joints incubated at 37°C compared with fresh controls. sGAG content was significantly affected by incubation condition (p < 0.001). sGAG content was significantly lower (∼35%) in joints incubated for 12 days at 37°C compared with fresh controls (p < 0.01) and compared with 4°C incubated joints (Fig. 4C, p < 0.01), and also lower (∼52%) in joints incubated for 28 days at 37°C compared with fresh control (Fig. 4C, p < 0.001) and compared with 4°C incubated joints (p < 0.001). The same trends were observed when normalizing core sGAG mass to tissue volume instead of area, with a fresh control average of 49.1 ± 2.4 mg/cm3.
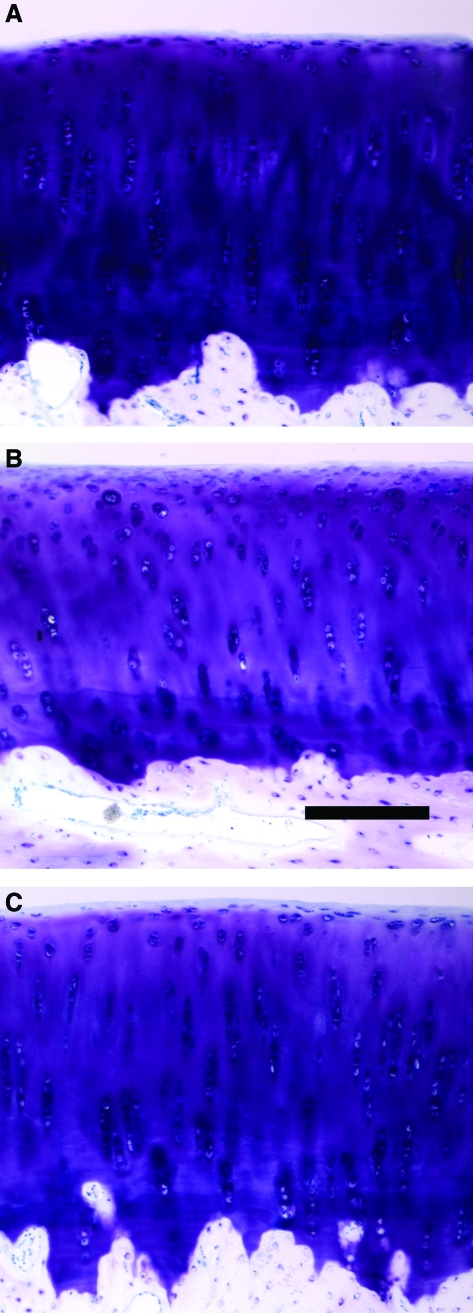
Representative histological sections of cartilage from cores isolated for fresh control, 28 days at 37°C, and 28 days at 4°C showed typical cartilage morphology and an intact surface (Fig. 5). Qualitatively, the staining intensity overall was similar in fresh controls and joints incubated at 4°C, though slightly less intense at 4°C (Fig. 5A vs. 5C). However, the staining intensity was decreased overall, and particularly in the deep zone of sections of joints incubated for 28 days at 37°C (Fig. 5B).
FIG. 5.
Sections of osteochondral cores from fresh control (A), 28 days 37°C (B), and 28 days 4°C (C) were prepared and stained with Toluidine blue. Scale bar: 200 μm. Color images available online at www.liebertonline.com/ten.
Quantity and aggregatability of sGAG released into the medium
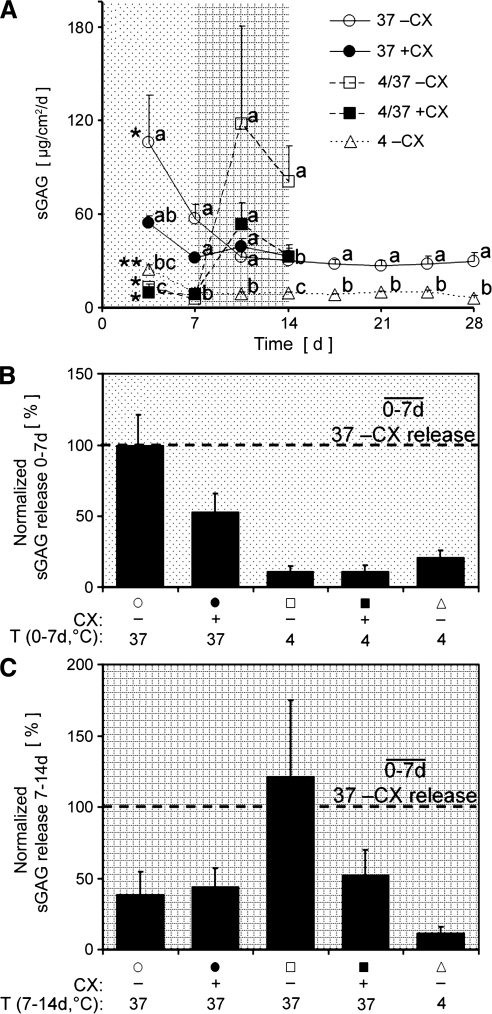
The amount of sGAG released into the medium varied with incubation condition (p < 0.01) and by day (p < 0.001). sGAG released during incubation without CX at 4°C was significantly lower at all time points than at 37°C (Fig. 6A, open triangles vs. open circles, p < 0.001–0.05). sGAG released during incubation for 7 days at 4°C and then 7 days at 37°C without CX was low and no different than other 4°C data at 3.5 days (p = 0.42) and 7 days (p = 0.46), but there was a trend for it to be higher than other 37°C data at 10.5 days (p = 0.07) and it was significantly higher at 14 days (p < 0.05, Fig. 6A, open squares). The addition of CX to the 4/37°C condition resulted in a decrease in the amount of sGAG released compared with without CX, starting with a tendency for a 55% decrease at 10.5 days (p = 0.61) that became a significant 60% decrease at 14 days (p < 0.05, Fig. 6A, filled vs. open squares). Within each incubation condition, the amount of sGAG released varied by day (Fig. 6A, p < 0.001–0.01), except for 37°C with CX (p = 0.11). The sGAG released during 7 days for each group, over 0–7 days (Fig. 6B) and over 7–14 days (Fig. 6C), normalized to the sGAG release from the 37°C without CX group from 0 to 7 days, illustrated the effects of CX and temperature.
FIG. 6.
Conditioned medium samples collected every 3.5 days were analyzed for sGAG content and normalized to joint surface area. Joints were incubated with (filled) or without (open) 0.5 mM cycloheximide (CX) at 4°C, 37°C, or for 7 days at 4°C and then 7 days at 37°C (4/37°C) (A). Within each time point, letters (a, b, and c) designate significantly different groups (p < 0.05), with a shared letter indicating no difference. *Initial time point not different from second point, but different from the remaining time points (p < 0.05), **Initial time point different from the remaining time points (p < 0.05). Average sGAG release for each group over 0–7 days (B) and 7–14 days (C) was normalized to the sGAG release from the 37–CX group from 0 to 7 days to emphasize differences due to CX and temperature.
Total sGAG in the culture system, defined as sGAG in the tissue plus that in the medium, summed over the incubation duration, did not vary with incubation condition (p = 0.16), whereas the relative percentage remaining in the tissue did vary with incubation condition (Fig. 7, p < 0.001). The percentage left in the tissue was significantly lower for joints incubated 28 days at 37°C than all other groups (34% vs. 60–83%, p < 0.001), and significantly higher for joints incubated 28 days at 4°C than all other groups (p < 0.001–0.05), except for joints incubated 14 days at 4/37°C + CX (p = 0.58). The addition of CX to the medium of joints incubated for 12–14 days at 37°C resulted in an increased fraction (73 vs. 59%) of sGAG remaining in the tissue (p < 0.001), just as it resulted in an increased fraction of sGAG (78 vs. 64%) for joints incubated for 14 days at 4/37°C (p < 0.01).
FIG. 7.
Percentage of total sGAG content (sGAGtissue + sGAGmedia) detected in the core digests (dark gray) and cumulative amount in the medium summed over the incubation period (light gray). Incubation time, temperature (4/37 indicates 1 week at 4°C and then 1 week at 37°C), and the presence or absence of 0.5 mM CX are indicated. **p < 0.01; ***p < 0.001; #different than all other groups, p < 0.001; ##different than all other groups (p < 0.001–0.05) except 14 days at 4/37 with CX (p = 0.58).
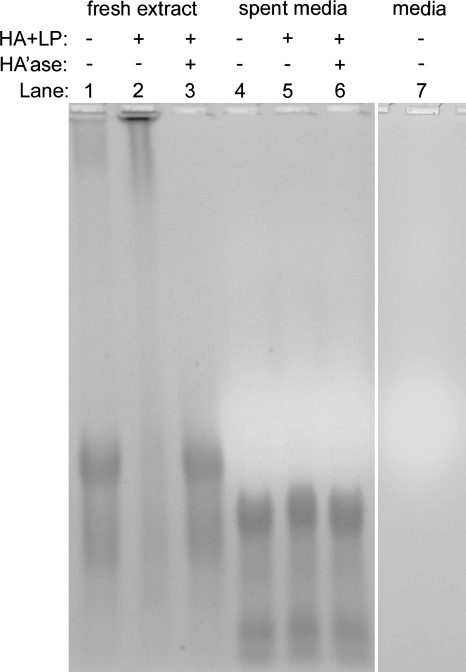
Differences in PG size and aggregatability between sGAG in the 37°C conditioned medium and that from freshly extracted cartilage were apparent from gel electrophoretic analysis. Compared with the sGAG of freshly extracted cartilage (Fig. 8, lane 1), the major band of sGAG from the 37°C conditioned medium (Fig. 8, lane 4) had a higher electrophoretic mobility and an additional, highly mobile band was visible, indicative of lower-molecular-weight sGAG molecules. sGAG in the control extract was able to aggregate with HA and LP, as indicated by the shift of the major sGAG band to a lower mobility position (Fig. 8, lane 2). This shift depended on intact HA, as digestion of the extract + HA + LP sample with hyaluronidase resulted in a band distribution (Fig. 8, lane 3) similar to control extract alone (Fig. 8, lane 1). In contrast, the mobility of the sGAG in the 37°C conditioned medium was not affected by the addition of HA and LP or digestion of HA (Fig. 8, lanes 4–6).
FIG. 8.
Fresh extract controls (lanes 1–3) and spent medium (day 3.5 at 37°C, lanes 4–6) samples were separated on a 1% agarose gel and stained with Alcian blue.
Discussion
The results of this study demonstrate that many properties of articular cartilage can be maintained during incubation at 37°C in a joint-scale configuration, although sGAG content is affected in a metabolism-dependent manner. The viability of chondrocytes was maintained during incubation at 37°C, whereas 4°C incubation led to a loss in viability (Figs. 2 and 3A, B), while still maintaining tissue thickness and stiffness (Fig. 4A, B), and DNA and collagen contents, suggesting that osteochondral tissue stored for use in allograft procedures may benefit in terms of viability from 37°C storage. More sGAG was depleted from the tissue and released during incubation at 37°C compared with 4°C (Figs. 4C, 5, 6, and 7), and the release was partially cell mediated (Fig. 6). The released sGAG macromolecules were smaller than controls, and their inability to aggregate with HA (Fig. 8) indicates that the sGAG lost was likely actively cleaved from the tissue matrix. For allograft storage and tissue-engineered grafts at the joint-scale in the conditions used here, there may be a trade-off between higher cell viability with 37°C incubation, but loss of matrix sGAG content.
A limitation of working at the joint-scale is that each sample requires intensive preparation, limiting the number of samples that can be obtained in a given amount of time. In this study, multiple small samples were taken across the joint surface to account for regional variation. In addition, analyzing the entire joint surface for biochemical contents was impractical as it would require accurately removing a thin layer of cartilage from the entire irregularly shaped joint surface. Thus, sample values were scaled to joint area to estimate properties of the full joint surface, assuming that the values for individual cores were collectively representative of the entire joint. Experiments at the joint-scale may be best suited for translational studies after preliminary studies at a smaller (e.g., tissue) scale. Still, despite the variability between animals and limited sample numbers, there were clear differences in viability, tissue sGAG content, and sGAG released to the medium between incubation conditions.
The increased viability in 37°C versus 4°C storage suggests that allograft tissue stored at 37°C may have improved long-term function. Graft retrieval studies after recipient death have identified donor tissue cells or their progeny remaining in the repaired cartilage defect 29 years after the surgical procedure.47 Although preservation of the tissue structure is important to the allograft procedure as it allows immediate restoration of the weight-bearing function of the defected region, the tissue matrix will eventually degrade without the presence of living cells synthesizing matrix constituents.48,49 Thus, maintaining the viability of allograft tissue is likely critically important for the long-term success of a tissue grafting procedure. Although the release of sGAG from the tissue at 37°C incubation reported here is not ideal for the goal of tissue preservation, the increased viability of chondrocytes in the tissue stored at 37°C versus 4°C is likely more important to long-term graft success.
The initial bolus release of sGAG from cartilage during the first few days in 37°C and experimental modulation of that release (Fig. 6) suggest that a cell-mediated process is responsible for aggrecan cleavage in and release from the cartilage of such cultures. A product of chondrocytes may cleave aggrecan directly or activate proteinases already present in the matrix. Likely candidates mediating such aggrecan cleavage include the matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase with thrombospondin motif (ADAMTS) families50,51 and reactive oxygen species,52,53 such as hydrogen peroxide.54,55 MMP and ADAMTS enzymes cleave the interglobular domain of aggrecan at various locations,25 resulting in large PG fragments unable to aggregate with HA. The inability of the sGAG fragments lost to the medium to aggregate with HA (Fig. 8) suggests that these fragments lack this first globular domain, consistent with the likely cleavage sites, though exposure of PG aggregates to hydrogen peroxide also generates large sGAG fragments that are unable to interact with HA.54 The difference in magnitude of sGAG release at 37°C versus 4°C and the delay of the large bolus release by 7 days in the joints incubated for 7 days at 4°C and then moved to 37°C indicate that the molecules participating in sGAG cleavage do not simply diffuse away while they are inactive. Further analysis is needed to clarify the mechanism of aggrecan degradation and release from cartilage in the joint-scale culture configuration so that appropriate catabolic inhibitors could be added to the culture medium to reduce sGAG matrix depletion.
Adaptation of joint-scale culture techniques to other tissues may require consideration of several factors that are likely to affect the response seen here, including the maturity of the tissue, tissue configuration, and the presence of serum in the medium. Chondrocyte metabolism decreases with age, making the use of adult tissue necessary to represent most cadaveric allograft storage tissue. The anabolic stimulus in this study was the addition of 10% serum to the incubation medium. Assuming that the tissue had achieved a balanced synthesis and release rate by the end of the 28-day culture duration, the turnover rate or half-life of sGAG with 37°C incubation, in the order of 20 days, was comparable to reports in the literature for cartilage explants,22,42 indicating that after the initial bolus sGAG loss, the tissue achieved a similar synthesis and degradation rate as in explant culture. Preservation of sGAG content during incubation in a joint-scale configuration might be achieved by including anabolic stimuli in the culture medium, such as serum, insulin-like growth factor-1,56,57 or transforming growth factor-β1,58 or by providing mechanical stimuli, such as those that stimulate chondrocyte sGAG biosynthesis.30,59
The lack of change in tissue stiffness despite the release of sGAG was somewhat surprising, as matrix sGAG content is generally correlated to tissue stiffness.26,27 The content of sGAG in cartilage tissue provides the resistance to compression due to its high negative charge density, which creates a swelling pressure that resists compression.60 One likely explanation for this lack of stiffness change with sGAG release is that the rapid indentation test is most sensitive to the region of tissue immediately adjacent to the indenter, rather than the deep zone where sGAG appears to be predominantly lost (Fig. 4B). Also, since the deep zone is relatively stiff compared with the overlying zones of cartilage,61 changes in deep zone sGAG and stiffness properties may not be reflected by a surface indentation stiffness measurement. More detailed characterization of the cartilage portion of the osteochondral cores, including the deep zone, could be attempted using compression testing, although determination of material properties is complicated by depth-associated variations and would be difficult for such thin cartilage. The thin cartilage also would have made it difficult to remove the tissue from the underlying bone for traditional isolated tests.
The results of this study may have practical implications for storage of osteochondral grafts and also provide directions for future investigations. Incubation of joints at 37°C may provide an alternative to the standard cartilage tissue culture configurations especially to maintain chondrocyte viability in osteochondral grafts. Delineation of the turnover of cartilage PG in terms of the temperature and metabolism dependence of matrix release and retention in a joint-scale configuration provides a foundation for future investigations of cartilage at the joint-scale. The techniques for joint-scale culture developed here could also be useful in the scale-up of tissue engineering for the creation of joint surfaces, as well as for the storage of joints before use. The storage conditions examined in this study identify the importance of temperature and cell-mediated metabolism for the maintenance of extracellular matrix at the joint scale. The same principles may apply to tissue-engineered cartilage in various shapes and sizes.
Acknowledgments
This work was supported by grants from the National Institutes of Health (ROI AR0044058 and R0I AR055637) and an award to the University of California–San Diego from the Howard Hughes Medical Institute through the HHMI Professors Program (for R.L.S). We thank Harold M. Aberman, D.V.M., M.S.E., for his donation of goat tissue; Won C. Bae, Ph.D., for his work on the image processing script; and EunHee Han, M.S., for her donation of LP.
Disclosure Statement
The authors have no commercial interests relating to this work to disclose.
References
- 1.Gortz S. Bugbee W.D. Fresh osteochondral allografts: graft processing and clinical applications. J Knee Surg. 2006;19:231. doi: 10.1055/s-0030-1248112. [DOI] [PubMed] [Google Scholar]
- 2.McNickle A.G. Provencher M.T. Cole B.J. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 3.Alford J.W. Cole B.J. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 4.Hung C.T. Lima E.G. Mauck R.L. Taki E. LeRoux M.A. Lu H.H. Stark R.G. Guo X.E. Ateshian G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 5.Isogai N. Landis W. Kim T.H. Gerstenfeld L.C. Upton J. Vacanti J.P. Formation of phalanges and small joints by tissue-engineering. J Bone Joint Surg Am. 1999;81-A:306. doi: 10.2106/00004623-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Williams G.M. Lin J.W. Sah R.L. Cartilage reshaping via in vitro mechanical loading. Tissue Eng. 2007;13:2903. doi: 10.1089/ten.2007.0053. [DOI] [PubMed] [Google Scholar]
- 7.Han E.H. Bae W.C. Hsieh-Bonassera N.D. Wong V.W. Schumacher B.L. Gortz S. Masuda K. Bugbee W.D. Sah R.L. Shaped, stratified, scaffold-free grafts for articular cartilage defects. Clin Orthop Relat Res. 2008;466:1912. doi: 10.1007/s11999-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams G.M. Chan E.F. Temple-Wong M.M. Bae W.C. Masuda K. Bugbee W.D. Sah R.L. Shape, loading, and motion in the bioengineering design, fabrication, and testing of personalized synovial joints. J Biomech. 2010;43:156. doi: 10.1016/j.jbiomech.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball S.T. Amiel D. Williams S.K. Tontz W. Chen A.C. Sah R.L. Bugbee W.D. The effects of storage media on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 10.Malinin T. Temple H.T. Buck B.E. Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg Am. 2006;88:762. doi: 10.2106/JBJS.D.02991. [DOI] [PubMed] [Google Scholar]
- 11.Pennock A.T. Robertson C.M. Emmerson B.C. Wagner F. Bugbee W.D. Harwood F.L. Amiel D. The effects of varying storage conditions on fresh human osteochondral allografts during prolonged storage. Int Cart Repair Soc. 2006;6:12. [Google Scholar]
- 12.Rohde R.S. Studer R.K. Chu C.R. Mini-pig fresh osteochondral allografts deteriorate after 1 week of cold storage. Clin Orthop Relat Res. 2004;427:226. doi: 10.1097/01.blo.0000138955.27186.8e. [DOI] [PubMed] [Google Scholar]
- 13.Pennock A.T. Robertson C.M. Wagner F. Harwood F.L. Bugbee W.D. Amiel D. Does subchondral bone affect the fate of osteochondral allografts during storage? Am J Sports Med. 2006;34:586. doi: 10.1177/0363546505281815. [DOI] [PubMed] [Google Scholar]
- 14.Teng M.S. Yuen A.S. Kim H.T. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466:1804. doi: 10.1007/s11999-008-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams R.J., 3rd Dreese J.C. Chen C.T. Chondrocyte survival and material properties of hypothermically stored cartilage: an evaluation of tissue used for osteochondral allograft transplantation. Am J Sports Med. 2004;32:132. doi: 10.1177/0095399703258733. [DOI] [PubMed] [Google Scholar]
- 16.Williams J.M. Virdi A.S. Pylawka T.K. Edwards R.B., 3rd Markel M.D. Cole B.J. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23:831. doi: 10.1016/j.orthres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Dumont J. Ionescu M. Reiner A. Poole A.R. Tran-Khanh N. Hoemann C.D. McKee M.D. Buschmann M.D. Mature full-thickness articular cartilage explants attached to bone are physiologically stable over long-term culture in serum-free media. Connect Tissue Res. 1999;40:259. doi: 10.3109/03008209909000704. [DOI] [PubMed] [Google Scholar]
- 18.Hascall V.C. Handley C.J. McQuillan D.J. Hascall G.K. Robinson H.C. Lowther D.A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983;224:206. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- 19.Hascall V.C. Morales T.I. Hascall G.K. Handley C.J. McQuillan D.J. Biosynthesis and turnover of proteoglycans in organ culture of bovine articular cartilage. J Rheumatol. 1983;10S:45. [PubMed] [Google Scholar]
- 20.Pallante A.L. Bae W.C. Chen A.C. Gortz S. Bugbee W.D. Sah R.L. Chondrocyte viability is higher after prolonged storage at 37C than at 4C for osteochondral grafts. Am J Sports Med. 2009 doi: 10.1177/0363546509351496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearsall A.W., IV Tucker J.A. Hester R.B. Heitman R.J. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125. doi: 10.1177/0095399703258614. [DOI] [PubMed] [Google Scholar]
- 22.Campbell M.A. Handley C.J. Hascall V.C. Campbell R.A. Lowther D.A. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch Biochem Biophys. 1984;234:275. doi: 10.1016/0003-9861(84)90350-3. [DOI] [PubMed] [Google Scholar]
- 23.Benya P.D. Jaffe S. Raffo A. The capacity of chondrocytes to respond to serum is enhanced by organ culture in the absence of serum, stimulated by serum, and modified by ascorbate. Arch Biochem Biophys. 1984;232:323. doi: 10.1016/0003-9861(84)90548-4. [DOI] [PubMed] [Google Scholar]
- 24.Buckwalter J.A. Mankin H.J. Articular cartilage. Part I: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg Am. 1997;79-A:600. [PubMed] [Google Scholar]
- 25.Sandy J.D. A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis Cartilage. 2006;14:95. doi: 10.1016/j.joca.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Treppo S. Koepp H. Quan E.C. Cole A.A. Kuettner K.E. Grodzinsky A.J. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 27.Nugent G.E. Law A.W. Wong E.G. Temple M.M. Bae W.C. Chen A.C. Kawcak C.E. Sah R.L. Site- and exercise-related variation in structure and function of cartilage from equine distal metacarpal condyle. Osteoarthritis Cartilage. 2004;12:826. doi: 10.1016/j.joca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Bae W.C. Temple M.M. Amiel D. Coutts R.D. Niederauer G.G. Sah R.L. Indentation testing of human cartilage: sensitivity to articular surface degeneration. Arthritis Rheum. 2003;48:3382. doi: 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- 29.Kimura J.H. Caputo C.B. Hascall V.C. The effect of cycloheximide on synthesis of proteoglycans by cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981;256:4368. [PubMed] [Google Scholar]
- 30.Sah R.L. Kim Y.J. Doong J.H. Grodzinsky A.J. Plaas A.H.K. Sandy J.D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 31.Korver G.H.V. van de Stadt R.J. van Kampen G.P.J. Kiljan E. van der Korst J.K. Bovine sesamoid bones: a culture system for anatomically intact articular cartilage. In Vitro Cell Dev Biol. 1989;25:1099. doi: 10.1007/BF02621260. [DOI] [PubMed] [Google Scholar]
- 32.Korver G.H.V. van de Stadt R.J. van Kampen G.P.J. van der Korst J.K. Composition of proteoglycans synthesized in different layers of cultured anatomically intact articular cartilage. Matrix. 1990;10:394. doi: 10.1016/s0934-8832(11)80147-2. [DOI] [PubMed] [Google Scholar]
- 33.Korver G.H.V. van de Stadt R.J. van Kampen G.P.J. van der Korst J.K. Amsterdam, The Netherlands: 1991. Effects of culture and loading on the turnover of proteoglycans in anatomically intact articular cartilage [Ch 6, pp. 105–124, Ph.D. Thesis] [Google Scholar]
- 34.Korver G.H.V. van de Stadt R.J. van Kampen G.P.J. van der Korst J.K. The effects of loading on the synthesis of proteoglycans in different layers of anatomically intact articular cartilage in vitro. J Rheumatol. 1992;19:905. [PubMed] [Google Scholar]
- 35.Getty R. Sisson and Grossman's the Anatomy of the Domestic Animals. fifth. Philadelphia, London, Toronto: W.B. Saunders Co.; 1975. [Google Scholar]
- 36.Nugent-Derfus G.E. Takara T. O'Neill J.K. Cahill S.B. Gortz S. Pong T. Inoue H. Aneloski N.M. Wang W.W. Vega K.I. Klein T.J. Hsieh-Bonassera N.D. Bae W.C. Burke J.D. Bugbee W.D. Sah R.L. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGowan K.B. Kurtis M.S. Lottman L.M. Watson D. Sah R.L. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 38.Farndale R.W. Sayers C.A. Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 39.Woessner J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 40.Herbage D. Bouillet J. Bernengo J.-C. Biochemical and physicochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161:303. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal S. Tang L.-H. Choi H. Habermann E. Rosenberg L. Roughley P. Poole A.R. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- 42.Bolis S. Handley C.J. Comper W.D. Passive loss of proteoglycan from articular cartilage explants. Biochim Biophys Acta. 1989;993:157. doi: 10.1016/0304-4165(89)90158-x. [DOI] [PubMed] [Google Scholar]
- 43.Hascall V.C. Sajdera S.W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969;244:2384. [PubMed] [Google Scholar]
- 44.Tang L.-H. Rosenberg L. Reeiner A. Poole A.R. Proteoglycans from bovine nasal and articular cartilage: properties of a soluble form of link protein. J Biol Chem. 1979;254:10523. [PubMed] [Google Scholar]
- 45.Wall R.S. Gyi T.J. Alcian blue staining of proteoglycans in polyacrylamide gels using the “critical electrolyte concentration” approach. Anal Biochem. 1988;175:298. doi: 10.1016/0003-2697(88)90392-2. [DOI] [PubMed] [Google Scholar]
- 46.Sokal R.R. Rohlf F.J. Biometry. third. New York: WH Freeman and Co.; 1995. [Google Scholar]
- 47.Jamali A.A. Hatcher S.L. You Z. Donor cell survival in a fresh osteochondral allograft at twenty-nine years. A case report. J Bone Joint Surg Am. 2007;89:166. doi: 10.2106/JBJS.F.00618. [DOI] [PubMed] [Google Scholar]
- 48.Enneking W.F. Mindell E.R. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73-A:1123. [PubMed] [Google Scholar]
- 49.Enneking W.F. Campanacci D.A. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83-A:971. [PubMed] [Google Scholar]
- 50.Patel K.P. Sandy J.D. Akeda K. Miyamoto K. Chujo T. An H.S. Masuda K. Aggrecanases and aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine. 2007;32:2596. doi: 10.1097/BRS.0b013e318158cb85. [DOI] [PubMed] [Google Scholar]
- 51.Sandy J.D. Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiku M.L. Gupta S. Deshmukh D.R. Aggrecan degradation in chondrocytes is mediated by reactive oxygen species and protected by antioxidants. Free Radic Res. 1999;30:395. doi: 10.1080/10715769900300431. [DOI] [PubMed] [Google Scholar]
- 53.Pylawka T.K. Virdi A.S. Cole B.J. Williams J.M. Reversal of suppressed metabolism in prolonged cold preserved cartilage. J Orthop Res. 2008;26:247. doi: 10.1002/jor.20487. [DOI] [PubMed] [Google Scholar]
- 54.Roberts C.R. Mort J.S. Roughley P.J. Treatment of cartilage proteoglycan aggregate with hydrogen peroxide. Relationship between observed degradation products and those that occur naturally during aging. Biochem J. 1987;247:349. doi: 10.1042/bj2470349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts C.R. Roughley P.J. Mort J.S. Degradation of human proteoglycan aggregate induced by hydrogen peroxide. Protein fragmentation, amino acid modification and hyaluronic acid cleavage. Biochem J. 1989;259:805. doi: 10.1042/bj2590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sah R.L. Chen A.C. Grodzinsky A.J. Trippel S.B. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 57.Sah R.L. Trippel S.B. Grodzinsky A.J. Differential effects of serum, insulin-like growth factor-I, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- 58.Morales T.I. Roberts A.B. Transforming growth factor-β regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828. [PubMed] [Google Scholar]
- 59.Grodzinsky A.J. Levenston M.E. Jin M. Frank E.H. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 60.Maroudas A. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- 61.Schinagl R.M. Gurskis D. Chen A.C. Sah R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]