SUMMARY
We determined how visuomotor tasks modulated gamma-oscillations on electrocorticography in epileptic patients who underwent epilepsy surgery. Each visual-cue consisted of either a sentence or hand gesture instructing the subject to press or not to press the button. Regardless of the recorded hemisphere, viewing sentence and gesture cues elicited gamma-augmentation sequentially in the lateral-polar occipital and inferior occipital-temporal areas; subsequently, button-press movement elicited gamma-augmentation in the Rolandic area. The magnitudes of gamma-augmentation in the Rolandic and inferior occipital-temporal areas were larger when the hand contralateral to the recorded hemisphere was used for motor responses. A double dissociation was found in the left inferior occipital-temporal cortex in one subject; the lateral portion had greater gamma-augmentation elicited by a sentence-cue, whereas the medial portion had greater gamma-augmentation elicited by a gesture-cue. The present study has increased our understanding of the physiology of the human visuomotor system.
INTRODUCTION
Movements of humans are frequently triggered by visual cues. A typical example of visually-cued movement is a driver’s response according to a traffic signal or policeman’s gesture. Visually-cued movement tasks have been used to localize the primary motor cortex in presurgical evaluation for patients with medically-uncontrolled epilepsy. Previous studies using intracranial electrocorticography (ECoG) have shown that visually-cued motor tasks elicited augmentation of gamma-oscillations in the Rolandic area [1–5], and that the area showing such movement-related gamma-augmentation was highly concordant with the primary motor area proven by electrical neurostimulation [6]. It has been generally accepted that event-related gamma-oscillations can be treated as quantitative measures of cortical activation [7]. In short, augmentation of gamma-oscillations is considered to represent cortical activation [8–12], whereas attenuation of gamma-oscillations is considered to represent cortical deactivation [13–15]. The benefits of ECoG recording include: (i) minimal artifacts from cranial muscles [1], (ii) a better signal-to-noise ratio compared to scalp electroencephalography (EEG) and magnetoencephalography (MEG), which record cortical signals from outside of the scalp [16–18] and (iii) a better temporal resolution compared to functional MRI (fMRI) [19].
Some ECoG studies showed that centrally-presented picture stimuli elicited gamma-augmentation sequentially in the lateral-polar occipital region and the inferior occipital-temporal region [15,20], but no motor task was simultaneously employed in these studies. It still remains uncertain how gamma-oscillations are modulated in the occipital and posterior temporal regions during ‘visuomotor’ tasks. In the present study of patients whose ECoG sampling involved both the occipital, posterior temporal and Rolandic areas, we addressed three questions described below. (i) We first determined whether visuomotor tasks elicit gamma-augmentation sequentially in the lateral-polar occipital region (defined as the lateral-to-polar surface of Brodmann Area 17/18 [15]), the inferior occipital-temporal region (defined as the inferior surface of Brodmann Area 19/37) and the Rolandic area (i.e.: the pre- and post-central gyri). (ii) We subsequently determined whether sentence- and gesture-cues differentially modulate gamma-oscillations in the occipital and posterior temporal regions during visuomotor tasks, and determined whether there was a double dissociation between the type of tasks and the location of gamma-modulation. (iii) Furthermore, we determined whether the laterality of motor responses affects gamma-modulation in the occipital and posterior temporal regions during a visuomotor task. Our recent study of auditory-motor tasks [21] demonstrated that the magnitude of gamma-augmentation in the superior temporal gyrus was larger when the hand contralateral to the recorded hemisphere needs to be used for motor responses, compared to when the ipsilateral hand does, and we hypothesize that a similar phenomenon can be seen during visuomotor tasks, too.
METHODS
Patients
The inclusion criteria of the present study consisted of: (i) patients with focal epilepsy undergoing extraoperative subdural ECoG recording as a part of presurgical evaluation in Children’s Hospital of Michigan, Detroit, between April 2007 and March 2009, (ii) completion of visuomotor tasks described below, and (iii) subdural electrodes chronically implanted on both pre- and post-central gyri at least 4 cm above the Sylvian fissure as well as at least on a portion of the lateral-polar occipital region (defined as the lateral-to-polar surface of Brodmann Area 17/18) or the inferior occipital-temporal region (defined as the inferior surface of Brodmann Area 19/37) [15]. The exclusion criteria consisted of: (i) the presence of massive brain malformations (such as large porencephaly, perisylvian polymicrogyria or hemimegalencephaly) which are known to confound the anatomical landmarks for the central sulcus or calcarine sulcus, (ii) history of previous epilepsy surgery, and (iii) the presence of epilepsia partialis continua. The study has been approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from the parents or guardians of all subjects.
Subdural electrode placement and video-ECoG recording
For chronic extraoperative ECoG recording and subsequent functional cortical mapping, platinum grid electrodes (10 mm intercontact distance, 4 mm diameter; Ad-tech, Racine, WI) were surgically implanted (Figure S1 on the website) [22,23]. MRI including a T1-weighted spoiled gradient echo image as well as fluid-attenuated inversion recovery image was preoperatively obtained [24–26]. Using planar x-ray images (lateral and anteroposterior) were acquired with the subdural electrodes in place for electrode localization on the brain surface [27–31]. A three-dimensional surface image was created with the location of electrodes directly defined on the brain surface [27,29,31].
Extraoperative video-ECoG recordings were obtained for 3 to 10 days, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc, Foothill Ranch, CA, USA), as previously described [25,26]. For evaluation of interictal and ictal activities as well as event-related gamma-oscillations, the sampling rate was set at 1,000 Hz with the amplifier band pass at 0.08 – 300 Hz. Antiepileptic medications were discontinued or reduced during ECoG monitoring until a sufficient number of habitual seizures were captured.
Visuomotor tasks
Sentence-cue visuomotor tasks
None of the subjects had a seizure within two hours prior to the visuomotor tasks. Each subject was awake, unsedated, and comfortably seated on the bed in a dimly lit room. Each subject was instructed to press the button using the thumb when a sentence-cue saying “Press the button” was visually presented and not to press the button when one saying “Do not press” was presented (Figure S2 on the website).
Each subject completed two sentence-cue visuomotor tasks (one for each hand); each task contained 40 trials, following a practice session prior to each task. Thereby, 20 sentence-cues saying “Press the button” and 20 saying “Do not press” were presented in a pseudorandom sequence during each task. Sentence-cues were presented using a 17-inch LCD computer monitor placed 60 cm in front of subjects. Sentence cues were binocularly presented at the center of the monitor, in grayscale, on the black background, for 1,500 msec with an inter-stimulus interval of 2,000 msec [15]. The reaction time was defined as the period between the onset of presentation of sentence-cue saying “Press the button” and the onset of button-press. We determined whether the mean or standard deviation (SD) of reaction time differed between the right and left hands or between the contralateral and ipsilateral hands (Wilcoxon-Signed Ranks Test). We also determined whether the reaction time was correlated with the age of subjects (Spearman’s Rank Test).
Gesture-cue visuomotor tasks
Each subject subsequently completed two gesture-cue visuomotor tasks (one for each hand) following a practice session prior to each task, which contained 40 trials (20 to press and 20 not to press the button). Subjects were instructed to press the button using the thumb when a gesture of pressing the button was visually presented and not to press the button otherwise (Figure S3 on the website). Otherwise, the task parameters were the same as those employed in the sentence-cue visuomotor task.
Measurement of ECoG amplitude modulations elicited by visuomotor tasks
Each ECoG trial was transformed into the time-frequency domain, and we determined ‘when’ and ‘where’ gamma-oscillations were modulated. The time-frequency analysis used in the present study was previously validated [32–35]. The measures of interest in the present study included a percent change of the amplitude of gamma-oscillations relative to that during the reference period (i.e.: the resting baseline) as well as statistical significance of task-related augmentation of gamma-oscillations. Specifically, a percent change of amplitudes averaged across 50- to 150-Hz frequency bands was defined as ‘gamma-range amplitude’ in the present study [15,21,33]. The methodological details are described in the supplementary document on the website (Supplementary Document S1).
Assessment of the effect of laterality of motor responses on event-related gamma-augmentation
We determined whether the magnitude of gamma-augmentation in the ‘visual sites’ was different between when the hand contralateral to the sampled hemisphere was used for motor-responses and when the ipsilateral hand was used; thereby, ‘visual sites’ were defined as those in the lateral-polar occipital and inferior occipital-temporal regions showing significant gamma-augmentation elicted by either sentence- or gesture-cue visual stimuli. Using ECoG traces time-locked to the onset of motor responses [21], the maximum ‘gamma-range amplitude’ in each ‘visual site’ was compared between the contralateral and ipsilateral hands as well as between the left and right hands (Wilcoxon-Signed Ranks Test)
Delineation of ECoG data on three-dimensional MRI
ECoG data for each electrode channel were exported to the given electrode site on the individual three-dimensional brain surface in two different ways. In order to delineate ‘when’, ‘where’ and ‘at what frequency band’ significant alteration of spectral amplitude occurred, time-frequency plot matrixes created above were placed onto a three-dimensional MRI at the cortical sites corresponding to their respective subdural electrode positions. In order to animate ‘when’, ‘where’ and ‘how many fold’ gamma-oscillations were increased or decreased, ‘gamma-range amplitude’ was sequentially delineated on the individual three-dimensional MRI [15,21,33]. ‘Gamma-range amplitude’ for each electrode channel at each 10-msec epoch was registered into Insight II software (Persyst, Prescott, AZ, USA) [21,36], and the interpolated topography map of ‘gamma-range amplitude’ at each 10-msec epoch was accurately superimposed to the individual three-dimensional MRI. This procedure yielded a movie file showing a sequential alteration of gamma-oscillations elicited by the visuomotor task (Videos S1 and S2 on the website). The spatial and temporal patterns of gamma-modulation can be better appreciated via animation movies [21,25].
Functional cortical mapping using neurostimulation
Functional cortical mapping by electrical neurostimulation was performed during extraoperative ECoG recording as previously described (Supplementary Document S1). We determined whether neurostimulation of the ‘visual sites’ elicited visual symptoms more frequently than did that of the others in the lateral-polar and inferior occipital-temporal areas (Fisher’s exact probability test). Previous studies including our own have already demonstrated that ‘motor sites’ showing movement-related gamma-augmentation are highly concordant with the primary motor area proven by electrical neurostimulation [6,21].
RESULTS
The present study included a total of 11 right-handed patients with focal epilepsy who satisfied both inclusion and exclusion criteria (age range: 8–18 years; 7 females) (Table 1). All patients were included in our previous study of gamma-oscillations modulated by an auditory-motor task [21].
Table 1.
Patient Profile
| Patient | Gender | Age (years) |
Antiepileptic medications |
Electrode placement |
Seizure onset zones on ECoG |
Histology | FS IQ |
VCI | PRI | WMI | PSI | Wada |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 8 | LEV, OXC | Rt FPTO | Not captured* | Gliosis | 90 | 93 | 90 | 94 | 97 | N/A |
| 2 | F | 10 | OXC, TPM | Lt FPTO | Lt TP | Tumor | 102 | 106 | 102 | 97 | 97 | Bilateral |
| 3 | M | 10 | LEV, OXC, TPM | Rt FPTO | Rt O | Dysplasia | 75 | 87 | 82 | 80 | 73 | N/A |
| 4 | M | 14 | LEV, OXC, TPM | Rt FPTO | Rt PT | Dysplasia | 64 | 77 | 82 | 62 | 56 | Left |
| 5 | F | 16 | CBZ | Rt FPTO | Not captured** | Not Available | N/A*** | N/A | N/A | N/A | N/A | N/A |
| 6 | F | 16 | CZP, PHT, TPM | Rt FPTO Lt FP |
Rt FPT | Gliosis | 80 | 93 | 88 | 80 | 73 | N/A |
| 7 | M | 16 | OXC, TPM | Rt FPTO | Rt T | Dysplasia; Hippocampal Sclerosis |
48 | 53 | 73 | 50 | 50 | Left |
| 8 | F | 17 | LTG | Lt FPTO | Lt F | Gliosis | 95 | 96 | 89 | 94 | 99 | N/A |
| 9 | F | 17 | LEV, OXC | Rt FPTO | Rt O | Gliosis | 95 | 100 | 97 | 86 | 114 | Left |
| 10 | M | 17 | OXC | Lt FPTO | Lt T | Tumor | 105 | 110 | 103 | 86 | 114 | N/A |
| 11 | F | 18 | LTG, OXC, ZNS | Rt FPTO | Rt PTO | Ulegyria | 87 | 96 | 86 | 75 | 81 | N/A |
Frequent interictal spikes were noted in the right inferior frontal-parietal regions.
Habitual seizures failed to be captured during extraoperative ECoG recording; no resective surgery was performed. Habitual seizures characterized by forced head-deviation toward the left side were captured during preoperative scalp EEG recording; thereby, delayed ictal discharges were noted over the right hemisphere but further localization of the presumed epileptogenic zone was not tenable in Patient 5.
It has been documented that her school performance was above average.
F: female. M: male. Lt: left. Rt: right. CBZ: carbamazepine. CZP: clonazepam. LEV: levetiracetam. LTG: lamotrigine. OXC: oxcarbazepine. PHT: phenytoin. TPM: topiramate. ZNS: zonisamide. F: frontal. P: parietal. T: temporal. O: occipital.
FSIQ: full scale IQ. VCI: verbal comprehension index. PRI: perceptual reasoning index. WMI: working memory index. PSI: processing speed index. N/A: not applicable. Wada test was employed in four patients. The results suggested that three patients were left-hemisphere dominant for language and that patient 2 had bilateral language representation.
Behavioral data
Regardless of sentence- or gesture-cues, there was no difference in the mean number of included trials between the right and left hands or between the contra- and ipsilateral hands, according to the group analysis across the 11 patients (Table S1 on the website; p>0.3 on the Wilcoxon-Signed Ranks Test). Regardless of sentence- or gesture-cues, there was no difference in the mean or SD of reaction time between the right and left hands or between the contralateral and ipsilateral hands (Table S1 on the website; p>0.1 on the Wilcoxon-Signed Ranks Test). The Spearman’s Rank Test revealed a significant negative correlation between ‘the age of subjects’ and ‘the reaction time averaged across the whole trials’ (rho = −0.70; p=0.02), suggesting that older children may have been more motivated, more attentive or less exhausted during the visuomotor tasks compared to younger ones.
Sequential gamma-augmentation in the lateral-polar occipital and inferior occipital-temporal areas
Intracranial electrodes involved the lateral-polar occipital area in 10 patients (all but patient 8) and the inferior occipital-temporal area in 11 patients (Table S2 on the website). The total number of electrode sites in the lateral-polar occipital area was 39 (mean: 3.5 per patient; 7 on the left and 32 on the right hemisphere), and that in the inferior occipital-temporal area was 81 (mean: 7.4 per patient; 30 on the left and 51 on the right hemisphere). Time-frequency ECoG analysis relative to the onset of visual-cue presentation revealed significant visually-elicited gamma-augmentation as follows. In the sentence-cue visuomotor task, significant gamma-augmentation was noted at 31 lateral-polar occipital sites (7 in the left and 24 in the right side) and 37 inferior occipital-temporal sites (15 in the left and 22 in the right side). In the gesture-cue visuomotor task, significant gamma-augmentation was noted at 22 lateral-polar occipital sites (7 in the left and 15 in the right side) and 32 inferior occipital-temporal regions (11 in the left and 21 in the right side). Thereby, a total of 31 lateral-polar occipital sites (7 in the left and 24 in the right side) and 44 inferior occipital-temporal sites (16 in the left and 28 in the right side) were treated as ‘visual sites’, since these 75 sites showed significant gamma-augmentation elicited by either sentence- or gesture-cues. No significant gamma-augmentation was noted in the Rolandic area immediately following presentation of visual-cues.
The earliest onset of sentence-cue visual-related gamma-augmentation was +114 msec on average (SD: 73 msec; 10 subjects) in the lateral-polar occipital area; patient 8 had no subdural electrodes involving the lateral-polar occipital area (Table S2 on the website). The earliest onset of sentence-cue visual-related gamma-augmentation was +185 msec on average (SD: 117 msec; 10 subjects) in the inferior occipital-temporal area; no significant gamma-augmentation was noted in the inferior occipital-temporal area in patient 3, whose inferior occipital-temporal area was involved by cortical dysplasia associated with the seizure onset on ECoG.
The earliest onset of gesture-cue visual-related gamma-augmentation in the lateral-polar occipital area was +125 msec on average across 8 subjects (SD: 68 msec); no significant gamma-augmentation was noted in patients 9 and 11, whose lateral-polar occipital areas were involved by the seizure onset zone. The earliest onset of gesture-cue visual-related gamma-augmentation in the inferior occipital-temporal area was +170 msec on average across 8 subjects (SD: 58 msec); no significant gamma-augmentation was noted in patients 3, 6 and 9, whose seizure onset zones involved a portion of the inferior occipital-temporal area.
The Wilcoxon-Signed Ranks test revealed that the lateral-polar occipital area exhibited significant visual-related gamma-augmentation earlier than the inferior occipital-temporal area in the sentence-cue (p=0.02) as well as gesture-cue tasks (p=0.03).
The effect of laterality of motor responses on visual-related gamma-augmentation in the inferior occipital-temporal areas
Time-frequency ECoG analysis relative to the onset of sentence-cue presentation also revealed that the maximum ‘gamma-range amplitude’ in the inferior occipital-temporal ‘visual sites’ was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean: 54% [SD: 33%] vs 44% [SD: 28%]; p<0.001 on the Wilcoxon-Signed Ranks Test); there was no difference in the maximum ‘gamma-range amplitude’ in the inferior occipital-temporal ‘visual sites’ between the left and right hands (mean: 49% [SD: 30%] vs 49% [SD: 32%]; p=0.7).
Similarly, time-frequency ECoG analysis relative to the onset of gesture-cue presentation also revealed that the maximum ‘gamma-range amplitude’ in the inferior occipital-temporal ‘visual sites’ was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean: 47% [SD: 29%] vs 41% [SD: 23%]; p=0.02); there was no difference in the maximum ‘gamma-range amplitude’ in the inferior occipital-temporal ‘visual sites’ between the left and right hands (mean: 45% [SD: 27%] vs 42% [SD: 15%]; p=0.2).
The effect of laterality of motor responses on visual-related gamma-augmentation in the lateral-polar occipital areas
Time-frequency ECoG analysis relative to the onset of sentence-cue presentation revealed that the maximum ‘gamma-range amplitude’ in the lateral-polar occipital ‘visual sites’ was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean: 106% [SD: 56%] vs 87% [SD: 42%]; p=0.003) also somewhat larger when the left hand was used for motor responses, compared to when the right hand was used (mean: 102% [SD: 46%] vs 92% [SD: 54%]; p=0.06).
Time-frequency ECoG analysis relative to the onset of gesture-cue presentation revealed that the maximum ‘gamma-range amplitude’ in the lateral-polar occipital ‘visual sites’ was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean: 106% [SD: 65%] vs 89% [SD: 63%]; p<0.001) and also larger when the left hand was used for motor responses, compared to when the right hand was used (mean: 106% [SD: 66%] vs 88% [SD: 62%]; p<0.001).
It was difficult to determine whether the usage of ‘contralateral hand’ or ‘left hand’ had a dominant effect on ‘gamma-range amplitudes’ measured in the lateral-polar occipital area, due to the collinearity problem. The sample size in the lateral-polar occipital area was small; only 7 electrodes were placed on the left lateral-polar occipital sites, whereas 30 electrodes were placed on the left inferior occipital-temporal area.
Gamma-augmentation in the Rolandic area
Intracranial electrodes involved the Rolandic area at least 4 cm above the Sylvian fissure in 12 hemispheres of all 11 patients (Table S2 on the website). The number of electrode sites in the Rolandic area at least 4 cm above the Sylvian fissure was 9.1 per hemisphere on average. Time-frequency ECoG analysis relative to the onset of motor-responses revealed movement-related gamma-augmentation in the Rolandic area as follows. The earliest onset of movement-related gamma-augmentation elicited by the contralateral hand responses was −165 msec on average (SD: 169 msec) in the sentence-cue visuomotor task and −145 msec on average (SD: 137 msec) in the gesture-cue visuomotor task. Taking into account the reaction time of about 1,000 msec in either visuomotor task, we can conclude that significant gamma-augmentation in the Rolandic areas was preceded by gamma-augmentation in the inferior occipital-temporal areas. No significant gamma-augmentation was noted in the lateral-polar or inferior occipital-temporal area during motor responses.
Time-frequency ECoG analysis for the sentence-cue visuomotor task revealed that the maximum ‘gamma-range amplitude’ in the Rolandic area was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean: 174% [SD: 55%] vs 41% [SD: 29%]; p=0.002 on the Wilcoxon-Signed Ranks Test); difference in the maximum ‘gamma-range amplitude’ between the left and right hands failed to reach significance (mean: 139% [SD: 77%] vs 77% [SD: 74%]; p=0.1). Similarly, time-frequency ECoG analysis for the gesture-cue visuomotor task revealed that the maximum ‘gamma-range amplitude’ in the Rolandic area was larger when the contralateral hand was used for motor responses, compared to when the ipsilateral hand was used (mean: 160% [SD: 54%] vs 39% [SD: 34%]; p=0.003 on the Wilcoxon-Signed Ranks Test); difference in the maximum ‘gamma-range amplitude’ between the left and right hands failed to reach significance (mean: 121% [SD: 61%] vs 78% [SD: 86%]; p=0.2).
Differential gamma-augmentation elicited by sentence- and gesture-cues
Time-frequency ECoG analysis relative to the onset of visual-cues revealed a functional double dissociation in patient 10; the relatively lateral portion of inferior occipital-temporal area had greater gamma-augmentation elicited by a sentence-cue, whereas the relatively medial portion had greater gamma-augmentation elicited by a gesture-cue (Figure 1). The spatial and temporal patterns of differential gamma-augmentation can be best appreciated via animation movies (Videos S1 and S2 on the website). Group analysis of seven subjects (excluding patients 3, 6, 9 and 11 whose seizure onset zones involved a portion of the lateral-polar or inferior occipital-temporal areas) revealed a double dissociation between the type of visuomotor tasks and the location of gamma-modulation (Figure 2). Five sites in the left and none in the right lateral-polar occipital areas showed gamma-augmentation preferably elicited by sentence-cues more than gesture-cues, whereas 2 sites in the left and 9 in the right lateral-polar occipital areas showed gamma-augmentation preferably elicited by gesture-cues more than sentence-cues (p=0.007 on the Fisher’s exact probability test). Similarly, 3 sites in the left and none in the right inferior occipital-temporal areas showed gamma-augmentation elicited by sentence-cues more than gesture-cues, whereas 2 sites in the left and 11 in the right inferior occipital-temporal areas showed gamma-augmentation elicited by gesture-cues more than sentence-cues (p=0.02). Such double dissociation at the group level failed to reach significance when all 11 subjects were included (p>0.05; Figure 2). These findings suggest that sentence-cues preferably elicited gamma-augmentation in the left and gesture-cues preferably in the right ventral visual cortices among subjects only whose seizure onset zones did not involve the ventral visual cortices.
Figure 1. Differential gamma-augmentation elicited by sentence- and gesture-cues in patient 10.
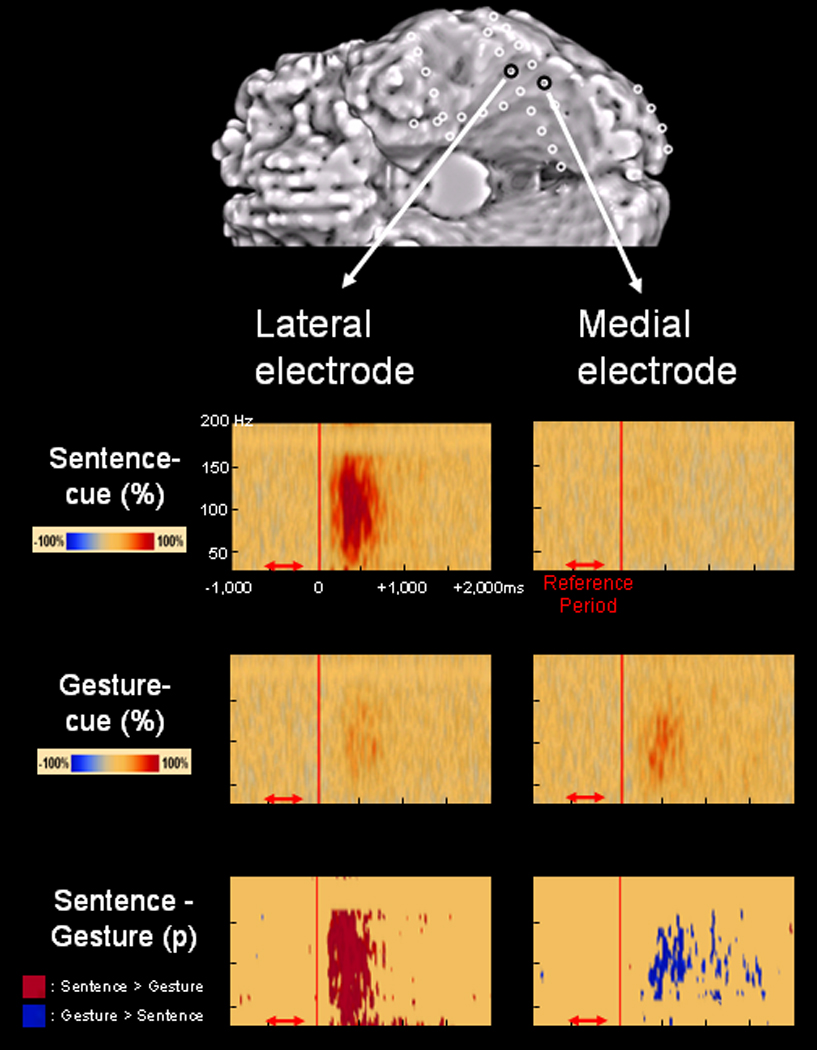
Time-frequency ECoG matrixes relative to the onset of visual-cues revealed a functional double dissociation in patient 10. The sentence-cue visuomotor task resulted in gamma-augmentation in the lateral portion but not in the medial portion of the inferior occipital-temporal area (upper row). The gesture-cue visuomotor task resulted in gamma-augmentation in the medial and lateral portions of the inferior occipital-temporal area with greater intensity in the medial portion (middle row). Comparison of amplitudes between sentence- and gesture-cues revealed that the relatively lateral portion of inferior occipital-temporal area had significantly greater gamma-augmentation elicited by a sentence-cue (denoted by red areas in the bottom row), whereas the relatively medial portion had significantly greater gamma-augmentation elicited by a gesture-cue (denoted by blue areas in the bottom row). Please also see Videos S1 and S2 on the website.
Figure 2. Differential gamma-augmentation elicited by sentence- and gesture-cues at the group level.
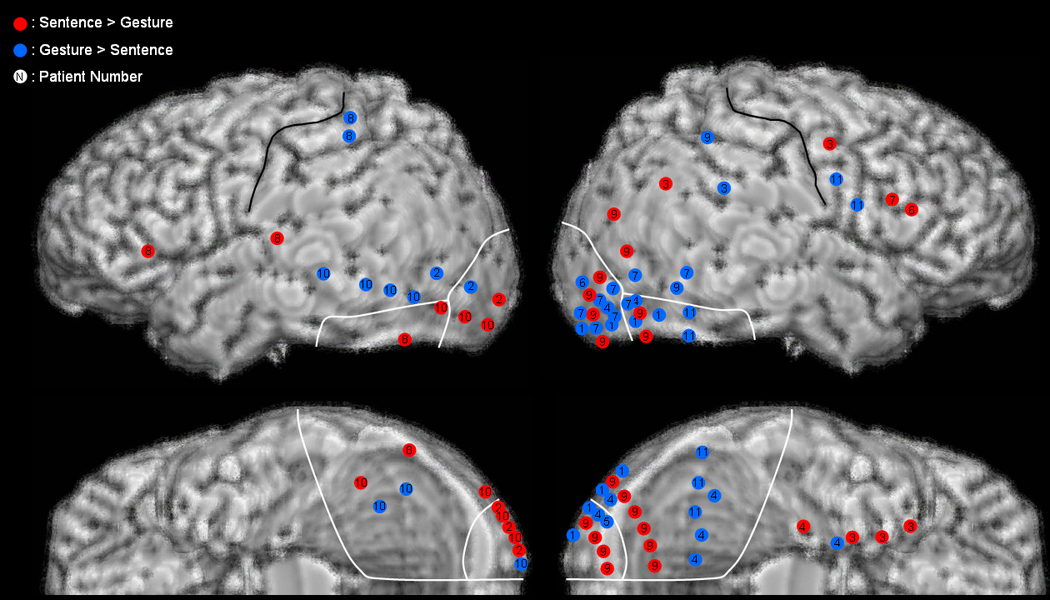
Red circles represent electrode sites showing gamma-augmentation elicited by sentence-cues significantly more than gesture-cues. Blue circles represent electrode sites showing gamma-augmentation elicited by gesture-cues significantly more than sentence-cues. The number in each circle indicates the patient number (Table 1).
Correlation between ‘visual sites’ suggested by ECoG analysis and symptoms elicited by neurostimulation
Electrical stimuli were delivered to 17 electrode pairs including the lateral-polar occipital ‘visual sites’; a phosphene was elicited by neurostimulation of 13 out of the 17 pairs. No visual symptom was elicited when electrical stimuli were delivered to a single electrode pair in the lateral-polar occipital area not classified as ‘visual sites’. Electrical stimuli were delivered to 28 electrode pairs including the inferior occipital-temporal ‘visual sites’, and a phosphene was elicited by neurostimulation of 11 pairs and visual-language failure was elicited by neurostimulation of a pair. Electrical stimuli were delivered to seven electrode pairs in the inferior occipital-temporal area not classified as ‘visual sites’; a phosphene was elicited by neurostimulation of only 1 of the seven pairs. The chance of neurostimulation of pairs including ‘visual sites’ eliciting a visual symptom was 4.4 times higher than that of the remaining pairs in the ventral visual cortices assessed in the present study (p=0.05, Fisher’s exact probability test).
DISCUSSION
(i) Both sentence- and gesture-cue visuomotor tasks elicited sequential gamma-augmentation in the lateral-polar occipital, inferior occipital-temporal and Rolandic areas. (ii) A double dissociation was found in the left inferior occipital-temporal area in one subject; the lateral portion had greater gamma-augmentation elicited by a sentence-cue, whereas the medial portion had greater gamma-augmentation elicited by a gesture-cue. (iii) The magnitudes of gamma-augmentation in the inferior occipital-temporal and the Rolandic areas in the recorded hemisphere were larger when the hand contralateral to the recorded hemisphere was used for motor responses, compared to when the ipsilateral hand was. (iv) Neurostimulation of the sites showing significant visually-elicited gamma-augmentation frequently resulted in congruent clinical symptoms.
Significance of gamma-augmentation in the lateral-polar occipital and inferior occipital-temporal areas
Our observation of visually-elicited gamma-augmentation is consistent with previous ECoG studies. It was previously demonstrated that centrally-presented objects such as abstract shapes, faces, and houses elicited gamma-augmentation at 30 to 200 Hz [15,20] and large evoked potential peaks [37,38] initially in the lateral-polar occipital area and subsequently in the inferior occipital-temporal area in either hemisphere. Such a well-replicated observation supports the notions that early gamma-augmentation in the lateral-polar occipital area represents the early cortical processing for external visual stimuli and that subsequent gamma-augmentation in the inferior occipital-temporal area may represent further cognitive processing associated with the nature of visual stimuli.
Double dissociation of the left inferior occipital-temporal sites showing gamma-augmentation elicited by sentence- and gesture-cue visual stimuli is a novel finding in the present study, although this is a finding in a single subject whose ECoG was sampled from the left hemisphere. This observation supports the notions that the lateral portion of the inferior occipital-temporal region (i.e.: posterior inferior temporal gyrus) on the left side plays a crucial role in word recognition, whereas the medial portion of inferior occipital-temporal region (i.e.: the fusiform gyrus) plays a role in body part recognition. Previous ECoG studies have shown that visually-presented language stimuli elicited gamma-augmentation [39–42] in the posterior inferior temporal gyrus on the left side more intensely than on the right side [39,42]. It was also reported that focal resection of the left posterior inferior temporal region resulted in a marked reading deficit, while recognition of other visual categories remained intact [43]. Previous studies of healthy subjects using fMRI also showed that viewing words was associated with increased blood oxygen level-dependent (BOLD) responses in the posterior inferior temporal gyrus on the left side more intensely than on the right side [44,45]. On the other hand, other fMRI studies showed that imitation of a body gesture resulted in increased BOLD responses in the fusiform gyri bilaterally [46,47].
Group analysis of seven subjects whose seizure onset zones failed to involve the ventral visual pathway revealed a double dissociation between the type of visuomotor tasks and the location of gamma-modulation. The left ventral visual pathway was activated by sentence-cues more than gesture-cues whereas the right ventral visual pathway was activated by gesture-cues more than sentence-cues. This novel observation seems to be consistent with the generally-accepted notion that language information is processed preferably in the left hemisphere [39,42,44,45].
The effect of the laterality of movements on activation in the inferior occipital-temporal areas
The magnitude of gamma-augmentation in the inferior occipital-temporal ‘visual sites’ was larger by 15 – 23% when the hand contralateral to the recorded hemisphere needed to be used for motor responses, compared to when the ipsilateral hand was. A similar observation was noted in our recent ECoG study of auditory-motor tasks, which demonstrated that the magnitude of gamma-augmentation in the superior temporal gyrus was larger when the contralateral hand was used, compared to when the ipsilateral hand was [21]. Increased attention or movement preparation given to the assigned hand might have resulted in increased functional connectivity between the contralateral Rolandic and inferior occipital-temporal areas via back-projections in an intra-hemispheric pathway. A previous fMRI study showed that tactile stimulation to a hand enhanced visually-induced BOLD responses in the inferior occipital-temporal area on the side contralateral to the hand [48].
Methodological limitations
Inevitable limitations of ECoG recording include: sampling limitation, antiepileptic drugs, and inability to study healthy volunteers. In our study, most of patients had subdural electrodes placed only on the cortical surface of the presumed epileptogenic hemisphere; we were not able to evaluate the other hemisphere or subcortical structures. Since large bridging veins were present, we were not able to place large grid subdural electrodes but strip electrodes in the lateral-polar occipital and inferior occipital-temporal areas in the majority of our patients. It has been reported that interictal epileptiform activities in animals and patients with focal epilepsy include paroxysmal gamma-band oscillations (also known as ‘ripples’) [49], which may potentially influence the measurements of event-related gamma-oscillations. Such ripples are observed most frequently during non-REM sleep and rather rarely during wakefulness [50]. In order to reduce the effects of ‘ripples’ on the results of time-frequency analysis, trials affected by runs of interictal epileptiform discharges were excluded from the analysis. All subjects included in the present study had a diagnosis of focal epilepsy and the sampled hemispheres were suspected to be dysfunctional. It is possible that the effects of interictal spikes might be lingering and we still don’t know the duration of their effects on cortical function in the areas showing interictal spikes or the remote effects of spikes occurring elsewhere. Thus, interpretation and generalization of our results must be made cautiously along with the observations in studies of healthy humans using noninvasive diagnostic modalities such as fMRI [48]. Our observation of the magnitudes of visually-elicited gamma-augmentation being larger when the contralateral hand was used would be difficult to explain by the effect of underlying disease process alone. Antiepileptic drugs may affect the findings of neurostimulation and time-frequency ECoG analysis. Here, phenytoin was loaded intravenously prior to neurostimulation to minimize the risk of stimulation-related seizures. It was reported that phenytoin elevated motor thresholds to TMS but had no effect on motor-evoked potential amplitudes [51]. We recognize that failure to elicit clinical symptoms by neurostimulation could be partially attributed to the acute effect of phenytoin given prior to neurostimulation. The sentence-cue task was given prior to the gesture-cue task in all patients. Although all patients had a practice session prior to each task, we cannot rule out the effects of order of tasks on the results of time-frequency analysis.
Supplementary Material
Four examples of sentence-cues are shown. Each sentence in each trial was written in a different font. Visual-cues instructed the subject to press or not to press the button in a pseudorandom order. The size of sentences was 2.5 cm in height and ranged from 13 to 19 cm in width.
Four examples of gesture-cues are shown. Gestures in the upper and lower rows instruct subjects to press and not to press the button, respectively (presented in a pseudorandom order). The size of gesture-cues was 10 – 12 cm in height and width.
Gamma-augmentation involved the lateral-polar occipital region 500 msec prior to the onset of button-press, the inferior occipital-temporal region, and the Rolandic area immediately prior to the onset of button-press. The magnitude of gamma-augmentation was greater when the right hand (i.e.: contralateral hand) was used for motor responses, compared to when the left hand (i.e.: ipsilateral hand) was used. The lateral portion of the inferior occipital-temporal region had greater gamma-augmentation elicited by a sentence-cue, whereas the medial portion had greater gamma-augmentation elicited by a gesture-cue.
Gamma-augmentation involved the lateral-polar occipital region 500 msec prior to the onset of button-press, the inferior occipital-temporal region, and the Rolandic area immediately prior to the onset of button-press. The magnitude of gamma-augmentation was greater when the right hand (i.e.: contralateral hand) was used for motor responses, compared to when the left hand (i.e.: ipsilateral hand) was used. The medial portion of the inferior occipital-temporal region had greater gamma-augmentation elicited by a gesture-cue, whereas the lateral portion had greater gamma-augmentation elicited by a sentence-cue.
Acknowledgement
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano). We are grateful to Harry T. Chugani, M.D., Lunliya Thampratankul, M.D., Carol Pawlak, R.EEG/EP.T, Ruth Roeder, R.N., M.S. and the staff of the Division of Electroneurodiagnostics at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 2.Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA. Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor tasks. Clin Neurophysiol. 1999;110:524–537. doi: 10.1016/s1388-2457(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez SL, Grave de Peralta R, Thut G, Millán Jdel R, Morier P, Landis T. Very high frequency oscillations (VHFO) as a predictor of movement intentions. Neuroimage. 2006;32:170–179. doi: 10.1016/j.neuroimage.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schalk G, Leuthardt EC, Brunner P, Ojemann JG, Gerhardt LA, Wolpaw JR. Real-time detection of event-related brain activity. Neuroimage. 2008;43:245–249. doi: 10.1016/j.neuroimage.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner P, Ritaccio AL, Lynch TM, Emrich JF, Wilson JA, Williams JC, Aarnoutse EJ, Ramsey NF, Leuthardt EC, Bischoi H, Schalk G. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–286. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 8.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 9.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 10.Nishida M, Juhász C, Sood S, Chugani HT, Asano E. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. Neuroimage. 2008;42:1275–1284. doi: 10.1016/j.neuroimage.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band electrocorticographic activity. J Neurosci. 2009;29:10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lachaux JP, Jung J, Mainy N, Dreher JC, Bertrand O, Baciu M, Minotti L, Hoffmann D, Kahane P. Silence is golden: transient neural deactivation in the prefrontal cortex during attentive reading. Cereb Cortex. 2008;18:443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- 14.Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–2027. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009;45:477–489. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaetz W, Otsubo H, Pang EW. Magnetoencephalography for clinical pediatrics: the effect of head positioning on measurement of somatosensory-evoked fields. Clin Neurophysiol. 2008;119:1923–1933. doi: 10.1016/j.clinph.2008.04.291. [DOI] [PubMed] [Google Scholar]
- 17.Dalal SS, Baillet S, Adam C, Ducorps A, Schwartz D, Jerbi K, Bertrand O, Garnero L, Martinerie J, Lachaux JP. Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage. 2009;45:1289–1304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Zaveri HP, Duckrow RB, Spencer SS. Concerning the observation of an electrical potential at a distance from an intracranial electrode contact. Clin Neurophysiol. 2009;120:1873–1875. doi: 10.1016/j.clinph.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Menon RS, Kim SG. Spatial and temporal limits in cognitive neuroimaging with fMRI. Trends Cogn Sci. 1999;3:207–216. doi: 10.1016/s1364-6613(99)01329-7. [DOI] [PubMed] [Google Scholar]
- 20.Tallon-Baudry C, Bertrand O, Hénaff MA, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- 21.Nagasawa T, Rothermel R, Juhasz C, Fukuda M, Nishida M, Akiyama T, Sood S, Asano E. Cortical gamma-oscillations modulated by auditory-motor tasks. -Intracranial recording in patients with epilepsy- Hum Brain Mapp. 2010 doi: 10.1002/hbm.20963. doi: 10.1002/hbm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, Sood S, Chugani HT. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46:1086–1097. doi: 10.1111/j.1528-1167.2005.05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–1047. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida M, Asano E, Juhász C, Muzik O, Sood S, Chugani HT. Cortical glucose metabolism correlates negatively with delta-slowing and spike-frequency in epilepsy associated with tuberous sclerosis. Hum Brain Mapp. 2008;29:1255–1264. doi: 10.1002/hbm.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida M, Sood S, Asano E. In-vivo animation of midazolam-induced electrocorticographic changes in humans. J Neurol Sci. 2009;287:151–158. doi: 10.1016/j.jns.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda M, Juhász C, Hoechstetter K, Sood S, Asano E. Somatosensory-related gamma-, beta- and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clin Neurophysiol. 2010;121:366–375. doi: 10.1016/j.clinph.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U. A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage. 1997;5:S514. [Google Scholar]
- 28.Miller KJ, Makeig S, Hebb AO, Rao RP, denNijs M, Ojemann JG. Cortical electrode localization from X-rays and simple mapping for electrocorticographic research: The "Location on Cortex" (LOC) package for MATLAB. J Neurosci Methods. 2007;162:303–308. doi: 10.1016/j.jneumeth.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT. Multimodality Data Integration in Epilepsy. Int J Biomed Imaging. 2007 doi: 10.1155/2007/13963. doi:10.1155/2007/13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS. Localization of neurosurgically implanted electrodes via photograph-MRI-radiograph coregistration. J Neurosci Methods. 2008;174:106–115. doi: 10.1016/j.jneumeth.2008.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkonyi B, Juhasz C, Muzik O, Asano E, Saporta A, Shah A, Chugani HT. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- 33.Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–1131. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, Asano E. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–1805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010;49:2735–2745. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiyama T, Otsubo H, Ochi A, Galicia EZ, Weiss SK, Donner EJ, Rutka JT, Snead OC., 3rd Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 37.Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- 38.Yoshor D, Bosking WH, Ghose GM, Maunsell JH. Receptive fields in human visual cortex mapped with surface electrodes. Cereb Cortex. 2007;17:2293–2302. doi: 10.1093/cercor/bhl138. [DOI] [PubMed] [Google Scholar]
- 39.Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–3293. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, Gordon B. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 41.Mainy N, Jung J, Baciu M, Kahane P, Schoendorff B, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Cortical dynamics of word recognition. Hum Brain Mapp. 2008;29:1215–1230. doi: 10.1002/hbm.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trébuchon-Da Fonseca A, Bénar CG, Bartoloméi F, Régis J, Démonet JF, Chauvel P, Liégeois-Chauvel C. Electrophysiological study of the basal temporal language area: a convergence zone between language perception and production networks. Clin Neurophysiol. 2009;120:539–550. doi: 10.1016/j.clinph.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 43.Gaillard R, Naccache L, Pinel P, Clémenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, Cohen L. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 44.Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 45.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- 46.Grèzes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions? Neuroimage. 2001;13:775–785. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- 47.Chaminade T, Meltzoff AN, Decety J. An fMRI study of imitation: action representation and body schema. Neuropsychologia. 2005;43:115–127. doi: 10.1016/j.neuropsychologia.2004.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science. 2000;289:1206–1208. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- 49.Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 50.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen R, Samii A, Caños M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four examples of sentence-cues are shown. Each sentence in each trial was written in a different font. Visual-cues instructed the subject to press or not to press the button in a pseudorandom order. The size of sentences was 2.5 cm in height and ranged from 13 to 19 cm in width.
Four examples of gesture-cues are shown. Gestures in the upper and lower rows instruct subjects to press and not to press the button, respectively (presented in a pseudorandom order). The size of gesture-cues was 10 – 12 cm in height and width.
Gamma-augmentation involved the lateral-polar occipital region 500 msec prior to the onset of button-press, the inferior occipital-temporal region, and the Rolandic area immediately prior to the onset of button-press. The magnitude of gamma-augmentation was greater when the right hand (i.e.: contralateral hand) was used for motor responses, compared to when the left hand (i.e.: ipsilateral hand) was used. The lateral portion of the inferior occipital-temporal region had greater gamma-augmentation elicited by a sentence-cue, whereas the medial portion had greater gamma-augmentation elicited by a gesture-cue.
Gamma-augmentation involved the lateral-polar occipital region 500 msec prior to the onset of button-press, the inferior occipital-temporal region, and the Rolandic area immediately prior to the onset of button-press. The magnitude of gamma-augmentation was greater when the right hand (i.e.: contralateral hand) was used for motor responses, compared to when the left hand (i.e.: ipsilateral hand) was used. The medial portion of the inferior occipital-temporal region had greater gamma-augmentation elicited by a gesture-cue, whereas the lateral portion had greater gamma-augmentation elicited by a sentence-cue.


