Abstract
The mechanisms underlying the low smoking cessation rates among smokers with schizophrenia (SS) are unknown. In this laboratory study, we compared the responses of 21 SS and 21 non-psychiatric controls (CS) to manipulations of 5-hour smoking abstinence, transdermal nicotine replacement (0, 21 and 42 mg), and in vivo smoking cues. Results indicate that SS were more sensitive than CS to the effects of acute abstinence on CO boost, but not more sensitive to the effects of abstinence on urge levels or withdrawal symptoms. SS and CS did not differ in urge response to in vivo smoking cues, but SS were less consistent in their reactions. These findings suggest that heightened sensitivity to the effects of abstinence on smoke intake may partially account for the low cessation rates experienced by SS, but other potential mechanisms should be explored using behavioral laboratory models.
Keywords: schizophrenia, smoking, nicotine dependence, comorbidity, craving, cue reactivity
Introduction
People with schizophrenia are three times more likely to initiate smoking and five times less likely to quit smoking than people in the general population (de Leon and Diaz, 2005). Cessation rates for smokers with schizophrenia (SS) appear limited even among those who are motivated to quit smoking and enroll in supportive treatment programs. For example, 0–19% of SS in smoking treatments that combined access to behavioral treatment with transdermal nicotine or bupropion were abstinent at the 6- or 12-month follow-up (Addington, el-Guebaly, Campbell, Hodgins, & Addington, 1998; Evins, Cather, et al., 2005; Evins et al., 2007; George et al., 2000; George et al., 2002; Ziedonis & George, 1997), compared to 21–35% of non-psychiatric heavy smokers enrolled in comparable or less-supportive programs (Richmond & Zwar, 2003; Shiffman et al., 2005; Transdermal Nicotine Study Group, 1991).
Both biological and environmental factors could contribute to a lower likelihood of smoking cessation in SS. Inadequate support for quitting and lack of interest in other reinforcing activities may reduce motivation or undermine quit attempts in these smokers (Lucksted, Dixon, & Sembly, 2000; Spring, Pingitore, & McChargue, 2003). In addition, SS may have more trouble quitting if they experience more severe effects of abstinence on affect due to illness-related abnormalities in dopaminergic transmission (Paterson & Markou, 2007). For example, nicotine withdrawal increases intracranial self-stimulation (ICSS) reward threshold, which is attenuated by bupropion (Epping-Jordan, Watkins, Koob, & Markou, 1998; Paterson, Balfour, & Markou, 2007). Heightened sensitivity to the effects of nicotine withdrawal on negative affect may reduce the abilities of SS to maintain smoking abstinence. Like other smokers, SS report that they smoke to reduce negative affect (Esterberg & Compton, 2005; Tidey & Rohsenow, 2007). However, there has been little research on the effects of abstinence on negative affect in these smokers (Tidey & Williams, 2007).
Smoking urge severity is a predictor of smoking cessation failure in smokers without psychiatric illness (e.g., Killen & Fortmann, 1997; Shiffman et al., 1997; West, Hajek, & Belcher, 1989), and the neurobiology of schizophrenia would suggest that SS may experience higher abstinence- or cue-elicited urge levels than other smokers. Nicotine and nicotine-associated stimuli (cues), increase mesolimbic DA release (Balfour, Wright, Benwell, & Birrell, 2000; Di Chiara, 2000; Di Chiara & Imperato, 1988). Repeated exposure to nicotine results in heightened neuronal DA release, produces locomotor sensitization, and may increase the motivational and appetitive effects of nicotine-associated stimuli (Balfour 2004; DiFranza & Wellman, 2005). Because schizophrenia is associated with intrinsically-enhanced mesolimbic DA function (Knable and Weinberger, 1997), people with schizophrenia may be particularly reactive to nicotine and smoking cues (Chambers, Krystal & Self, 2001).
In testing this hypothesis, one study that found that SS and equally-dependent smokers without psychiatric illness (CS) did not differ in urge response to smoking cue images (Fonder et al., 2005). However, images are generally less effective than in vivo cues at increasing urge levels (Shadel, Niaura, & Abrams, 2001), which may have reduced effect sizes in that study. Another study found that the effects of in vivo cues on smoking urge levels in SS appeared similar to those of non-psychiatric smokers, but lacked a concurrent matched control group (Tidey, Rohsenow, Kaplan, & Swift, 2005b). Therefore, one aim of this study was to directly compare reactivity to in vivo cues in SS and CS.
Controlled comparisons of the effects of abstinence, cues and nicotine replacement in SS and CS may help to clarify the degree to which these factors contribute to ongoing smoking in SS. Thus, the aims of the current study were: (1) to compare the effects of abstinence on unelicited smoking urges, nicotine withdrawal-related negative affect, and smoking behavior in SS and CS, (2) to compare the effects of transdermal nicotine on these measures, and (3) to compare reactivity to in vivo cues under non-abstinent, abstinent and nicotine replacement conditions in SS and CS.
Methods
Participants
Participants were recruited from the community. All were required to be 18 or older, to have smoked at least 20 cigarettes per day for at least the past year and to have a Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker & Fagerstrom, 1991) score of at least 6. The FTND has good internal consistency and acceptable test-retest reliability in SS (Weinberger et al., 2006; Yang, McEvoy, Wilson, Levin, & Rose, 2003). The Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1994) was used to confirm diagnoses of schizophrenia or schizoaffective disorder in SS and to rule out current Axis I disorders in CS. Participants were excluded for severe conceptual disorganization, disorientation or uncooperativeness, lack of competence to sign the informed consent, positive urine drug or pregnancy tests, positive breath alcohol at any visit, or use of medications that might affect smoking behavior or urges. Other medications were permitted as long as doses had been stable for at least 2 weeks. Procedures were approved by the institutional review boards involved. Twenty-seven SS and 24 CS were enrolled. Six SS and 3 CS withdrew before completing the study, leaving data from 21 SS and 21 CS.
Design overview
Participants underwent an initial visit for the collection of individual difference measures. Then, participants completed 8 sessions in which cue reactivity and smoking behavior measures were collected under 4 conditions: non-abstinent + 2 placebo transdermal patches, 5-h abstinent + 2 placebo patches, 5-h abstinent + 2 patches that delivered a total of 21 mg nicotine, and 5-h abstinent + 2 patches that delivered a total of 42 mg nicotine. Nicotine and matching placebo patches (GlaxoSmithKline, Parsippany, NJ) were applied under double-blind conditions. The non-abstinent sessions were presented first to facilitate acclimation to study procedures. Dose order in the remaining sessions was counter-balanced across participants. Sessions were separated by at least 48 hours. Two replications of each condition were performed and averaged to reduce variability. Participants received $400 for completing the study.
Individual Difference Measures
Information was collected on demographic variables, smoking histories, and current psychiatric symptoms (SS only). Readiness to change smoking was measured using the stages-of-change algorithm (DiClemente et al., 1991), with the modification suggested by Etter and Sutton (2002), to classify participants into the precontemplation stage, contemplation stage, or preparation stage. Psychiatric symptoms (SS only) were evaluated using the Positive and Negative Syndrome Scale (PANSS; Kay, Fizbein, & Opler, 1987). The FTND was used to assess nicotine dependence. During this visit, participants practiced completing urge and nicotine withdrawal symptom measures on the computer and practiced smoking using the topography equipment.
Laboratory Procedures
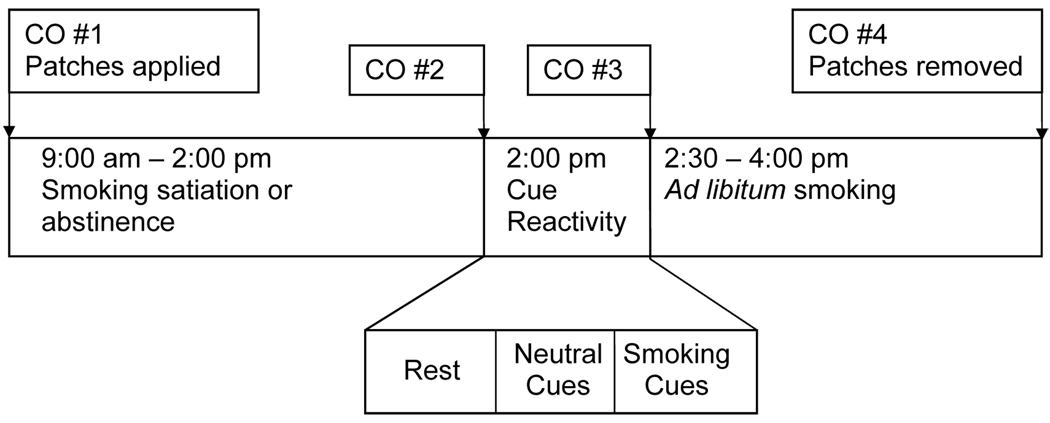
A schema depicting the laboratory sessions is shown in Figure 1. Participants provided breath samples for the assessment of carbon monoxide (CO) levels upon arrival for each session (Smokerlyzer, Bedfont Scientific Ltd., Kent, UK). At the first session, participants also provided saliva samples for cotinine analysis (Salimetrics, LLC, State College, PA). A trained interviewer used the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962) to assess psychiatric symptoms in SS before each session. Next, transdermal patches were applied to participants’ upper arms (one per arm). Over the next 5 hours, participants were either permitted to smoke freely (non-abstinent + placebo condition) or were not permitted to smoke (abstinent + placebo, abstinent + 21 mg NRT, and abstinent + 42 mg NRT conditions). Participants were under continuous observation except for bathroom and lunch breaks, with CO monitoring to assure compliance with abstinence. Breath CO levels were measured at the end of this period.
Figure 1.
Timeline of study sessions.
Participants then underwent a smoking cue reactivity assessment as described previously (Tidey et al., 2005b). After a 10-min relaxation period, participants completed measures of smoking urge and nicotine withdrawal symptoms. Urge to smoke was assessed using the Questionnaire on Smoking Urges-brief form (QSU-brief; Cox, Tiffany, & Christen, 2001) and the item “How much is your urge to smoke right now?”, rated on a 100 mm visual analogue scale (VAS), with the anchors 0 = “no urge at all” and 100 = “strongest urge you’ve ever had”. Nicotine withdrawal symptoms were measured using the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986; 1998). The items included were depression, anxiety, anger/irritability/frustration, difficulty concentrating, restlessness, and increased appetite; the item “insomnia” was omitted as participants did not undergo overnight abstinence. Symptom were rated on 100-mm VAS with the anchors 0 = “not present” and 100 = “severe”. The score provided is the mean of ratings on the six items. Participants then underwent a neutral cues trial and a smoking cues trial similar to that described in detail previously (Tidey et al., 2005b). Neutral cues were a pencil, 25 mm × 65 mm eraser and a small pad of paper, which participants looked at and handled for 4 min. Smoking cues were a lit cigarette of the participant’s preferred brand, a lighter and an ashtray, which participants looked at and handled for 4 min. Participants completed the urge measures after each trial.
After a short break, CO levels were assessed and participants were instructed that they could smoke as little or as much as they wanted for the next 90 min. Cigarettes of the participants’ usual brands were provided by the experimenters, and were smoked using a smoking topography system (CReSS, Plowshare Technologies, Baltimore, Maryland). Smoking topography measures, recorded by the CReSS, included total number of puffs smoked, number of puffs smoked per cigarette, inter-puff interval, total session smoke volume, average puff volume, average puff duration, maximum puff velocity and latency to begin smoking. At the end of the smoking period, participants completed the urge measures, provided a breath sample for CO level assessment, completed the BPRS (SS only), and had their patches removed.
Data analysis
Dependent variables were examined for distributional assumptions and collinearity. Sphericity was intact. Smoke latency and inter-puff interval data were log-transformed to normalize the data. Number of puffs per cigarette and session smoke volumes were collinear with total number of puffs smoked per session (rs ≥ 0.80), so were removed from analyses. Average puff duration was collinear with average puff volume (r = 0.84) and was removed. QSU-brief and urge VAS scores were collinear (all rs > .60), so only the single-item urge scores were retained. Analyses used SPSS for Windows 11.5.0. Differences were considered significant when p ≤ .05.
Group comparisons on demographic and smoking history measures were conducted using independent-samples t-tests for continuous variables and chi-square tests for categorical variables. Between-groups one-way analyses of variance tests (ANOVAS) were conducted to compare the groups on pre-session CO levels across sessions and on CO change during the 5-h satiation and abstinence periods (CO #2 minus CO #1 in Figure 1). A one-way ANOVA was used to analyze the effects of Condition on BPRS change scores (post-session total score minus pre-session total score) in SS only.
To compare the effects of abstinence and NRT in SS and CS, mixed-factor ANOVAs were conducted with the factors Group (SS, CS) and Condition (non-abstinent, abstinent + 0 mg NRT, abstinent + 21 mg NRT, abstinent + 42 mg NRT). Dependent variables were unelicited urge level (i.e., urge levels reported after the 10-min rest period), MNWS score, smoking topography measures and CO boost (CO #4 minus CO #3 in Figure 1). Topography data were missing for one CS participant and latency data were missing for 2 SS and 2 CS. Significant interactions were followed by simple effects tests. Effect sizes (η2) are also provided when p = .05 –.10, with η2 ≤ .05 for small, η2 = .06 – .13 for medium and η2 ≥ .14 for large effect sizes (Cohen, 1988). This study had 80% power to detect medium-sized effects.
To investigate aim 3, we first examined proportions of each group that reacted to cues. Participants were defined as “cue reactors” if their urge level during the smoking cues trial in the non-abstinent sessions exceeded their urge level during the neutral cues trial by at least one point (Fonder et al., 2005). Chi-square tests were conducted to determine whether the proportion of cue reactors differed within the SS and CS groups. T-tests and chi-square tests were used to examine differences between cue reactors and non-reactors within each group. We then conducted 3-factor ANOVAs to examine the effects of Group, Cues (Neutral, Smoke) and Condition on urge levels. These analyses were first performed within all SS and CS, consistent with analytic methods used in most smoking cue reactivity research (e.g., Hutchison et al., 1999; Sayette, Martin, Wertz, Shiffman, & Perrott, 2001; Tiffany, Cox, & Elash, 2000), and then with cue reactors only, to facilitate comparisons with Fonder et al. (2005).
Results
Individual difference measures
There were no significant differences between the groups on any demographic or smoking history measure (see Table 1). Psychiatric symptom levels for SS were similar to those reported previously by other studies in outpatients (e.g., Fonder et al., 2005; George et al., 2000). Pre-session breath CO levels, averaged across the 8 sessions, were significantly higher in SS than in CS (F (1, 40) = 10.39, p < .01; Table 1). The 5-hr satiation periods increased CO levels by 26% in SS and 20% in CS (NS). The 5-hr abstinence periods reduced CO levels by 49% in SS and 53% in CS (NS). Among SS, there was no significant effect of condition on BPRS change scores. Pre-session BPRS total scores were 28.13 ± 7.3 and post-session BPRS total scores were 27.09 ± 6.2.
Table 1.
Demographic and Baseline Smoking Characteristics of Study Participants
| SS (n = 21) | CS (n = 21) | |
|---|---|---|
| Age [M (SD)] | 44.1 (7.0) | 47.1 (10.4) |
| Male | 14 | 11 |
| Race | 12 W / 2 AA / 7 0 | 16 W / 4 AA / 1 O |
| Hispanic ethnicity | 2 | 2 |
| Employed full- or part-time | 2 | 5 |
| Annual income above US$10,000 | 7 | 13 |
| ≥ 12 years of education | 19 | 18 |
| Cigarettes per day | 32.6 (13.6) | 31.0 (12.0) |
| Nicotine dependence (FTND score) | 7.7 (1.4) | 7.5 (1.4) |
| Min to first cigarette of the day | 5.7 (7.5) | 7.8 (9.4) |
| Years of daily smoking | 26.4 (9.2) | 31.8 (11.4) |
| Salivary cotinine level (ng/ml) | 367.6 (147.4) | 331.3 (167.1) |
| Pre-session CO level (ppm) | 27.1 ± 8.6** | 19.8 ± 6.3 |
| Smoking stage of change | 12 Pre/9 Con/0 Prep | 11 Pre/7 Con/3 |
| PANSS positive scale score | 16.7 (4.4) | – |
| PANSS negative scale score | 18.3 (6.8) | – |
| PANSS general scale score | 33.6 (6.6) | – |
| PANSS total score | 68.6 (16.5) | – |
| Antipsychotic drug class | 6 Typ / 10 A / 5 O | – |
Abbreviations: SS, smokers with schizophrenia; CS, control smokers; W, white; AA, African-American; O, other; FTND, Fagerström Test of Nicotine Dependence; CO, carbon monoxide; Pre, precontemplation; Con, Contemplation; Prep, preparation; PANSS, Positive and Negative Syndrome Scale; Typ, typical, A, atypical.
p < .01
Unelicited urge and nicotine withdrawal symptoms
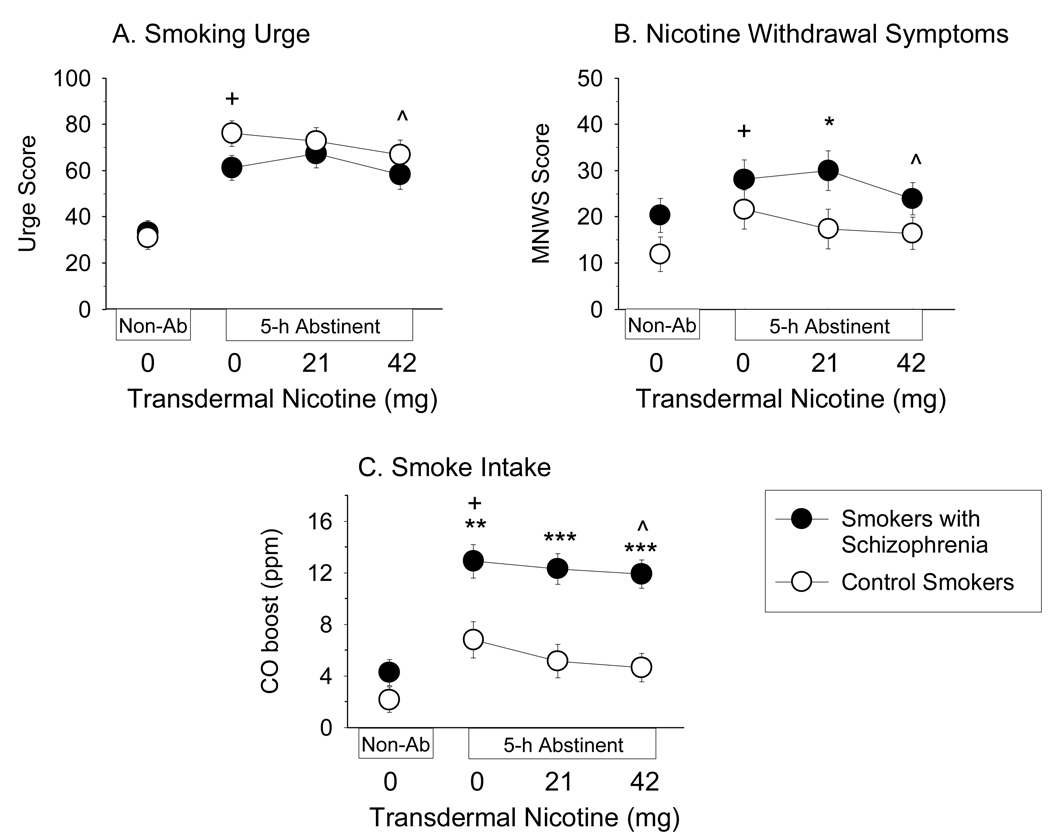
There was a significant effect of Condition on unelicited urge level (F (3, 120) = 55.14, p < .001). Post-hoc tests indicated that urge levels were higher in all abstinent conditions compared to the non-abstinent condition (ps < .05), and that urge levels in the abstinent + 42 mg NRT condition were significantly lower than urge levels in the other abstinent conditions (ps < .05; Figure 2A). The effect of Group on unelicited urge level was not significant. The Group × Condition interaction on unelicited urge level approached significance (F (3, 120) = 2.23, p = .09; η2 = .05), with abstinence tending to increase urge levels more in CS.
Figure 2.
Effects of 5-hr abstinence and 0, 21 and 42 mg transdermal nicotine (NRT) on (A) unelicited smoking urge levels, (B) nicotine withdrawal symptoms and (C) CO boost in smokers with schizophrenia (n = 21; solid symbols) and equally-heavy smokers without psychiatric illness (n = 21; open symbols). Data points represent M ± SEM. Plus signs indicate significant differences between the Non-abstinent + 0 mg NRT and Abstinent + 0 mg NRT conditions; carets indicate significant effects of NRT; asterisks indicate significant differences between groups (* p < .05; ** p < .01; *** p < .001).
There was a significant effect of Condition on MNWS score (F (3, 120) = 7.90, p < .001). MNWS scores were significantly higher in the abstinent + 0 mg NRT and abstinent + 21 mg NRT conditions compared to the non-abstinent condition (ps < .05), and 42 mg NRT reduced MNWS scores to non-abstinent levels (Figure 2B). The effect of Group on MNWS score approached significance (F (1, 40) = 3.09, p = .09, η2 = .07), with SS tending to have higher MNWS scores under all conditions. The Group × Condition interaction on MNWS score was not significant (η2 = .02).
Smoking topography
Means and ANOVA results are shown in Table 2. Significant effects of Group were found on CO boost (Figure 2C), total puffs smoked, inter-puff interval, and puff velocity, with these variables consistently indicating that SS smoked more intensely than CS. There were effects of Condition on CO boost (Figure 2C), puff volume and latency to smoke, and a trend was found for total puffs (p = .06, η2 = .06). Post-hoc tests indicated that abstinence increased CO boost and number of puffs smoked, and reduced puff volume and latency to smoke (ps < .05). The effect of abstinence on CO boost was significantly reduced by 42 mg NRT and the effect of abstinence on latency to smoke was significantly reversed by 21 mg NRT (ps < .05). There was also a significant Condition × Group interaction on CO boost (Table 2). Post-hoc tests indicated that abstinence increased CO boost to a greater extent in SS than CS (F (1, 40) = 5.01, p < .05), but effects of NRT were not significantly different for the two groups (Figure 2C).
Table 2.
Smoking measures [M(SD)] during 90-minute smoking periods.
| Measure | Non-Abstinent | Abstinent | Group F (1, 39) |
Cond F (3, 117) |
Group × Cond F (3, 117) |
|||
|---|---|---|---|---|---|---|---|---|
| 0 mg NRT | 0 mg NRT | 21 mg NRT | 42 mg NRT | |||||
| CO Boost (ppm) |
SS | 4.3 (4.9) | 12.9 (7.5) | 12.3 (6.8) | 11.9 (5.7) | 17.64** | 27.69** | 5.02** |
| CS | 2.2 (3.8) | 6.6 (4.9) | 5.0 (4.2) | 4.7 (4.3) | ||||
| Total Puffs | SS | 59.4 (44.2) | 69.7 (58.6) | 66.9 (51.7) | 70.7 (65.1) | 9.18** | 2.46† | 1.03 |
| CS | 26.2 (22.1) | 31.7 (26.9) | 27.5 (18.6) | 26.9 (18.9) | ||||
| Latency to Smokea |
SS | 135.2 (2.5) | 100.3 (3.3) | 114.0 (3.2) | 79.6 (3.2) | 0.11 | 4.88** | 0.79 |
| CS | 158.9 (2.5) | 104.2 (3.7) | 111.8 (2.6) | 92.2 (2.7) | ||||
| Puff Volume (ml) |
SS | 51.7 (16.4) | 46.0 (14.5) | 45.4 (14.6) | 46.6 (14.1) | 1.28 | 3.27* | 2.26† |
| CS | 43.2 (14.6) | 41.8 (14.4) | 43.6 (16.3) | 41.4 (13.9) | ||||
| Inter-Puff Interval (s)a |
SS | 21.4 (1.5) | 20.4 (1.5) | 20.4 (1.5) | 20.5 (1.6) | 15.14** | 0.90 | 0.88 |
| CS | 35.0 (1.7) | 33.6 (1.5) | 35.3 (1.6) | 32.2 (1.5) | ||||
| Puff Velocity (ml/s) |
SS | 44.2 (9.0) | 42.3 (10.0) | 42.8 (11.4) | 42.5 (10.7) | 4.99* | 1.24 | 1.35 |
| CS | 36.7 (7.8) | 36.4 (7.5) | 36.5 (7.0) | 37.4 (8.5) | ||||
Abbreviations: Cond, condition; SS, smokers with schizophrenia; CS, control smokers.
Data were log-transformed prior to analysis. Geometric means and standard deviations are reported.
p < .01,
p < .05,
p < .10
Smoking cue reactivity
Twenty SS (95%) and 21 CS (100%) met the definition of “cue reactor” in at least one of the non-abstinent sessions. However, SS were less consistent than CS in their cue reactivity: 11 SS (52%) were cue reactors in both sessions, 9 (43%) were cue reactors in only one session, and one (5%) did not react to cues in either session. In contrast, 19 CS (91%) reacted in both sessions, two (10%) reacted in only one session, and there were no non-reactors. This pattern of response was significantly different for the two groups (Χ2 (2, N = 42) = 7.59, p < .05). There was no indication that the consistent cue reactors differed from non-reactors within either group on any smoking or demographic variable (ps for all t-test and Χ2 comparisons > 0.20).
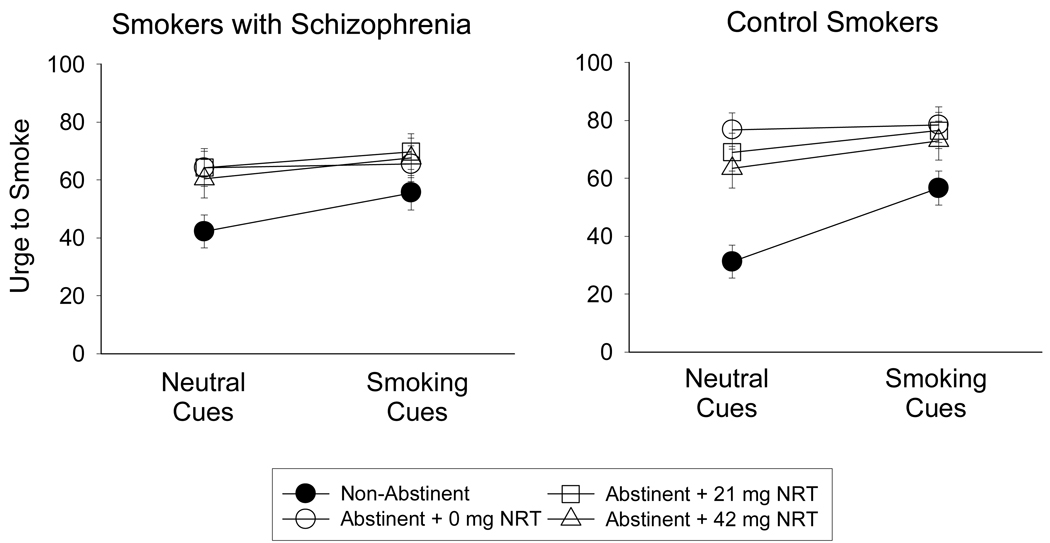
In analyses that included all participants, there was a significant effect of Cues on urge levels (F (1, 40) = 23.53, p < .001), with means indicating that urge scores were higher during the smoking cues trial than during the neutral cues trial. There was a significant effect of Condition on urge (F (3, 120) = 19.61, p < .001). Post-hoc tests indicated that abstinence increased smoking urge levels and 42 mg NRT reduced urge levels relative to the abstinent + 0 mg NRT condition (ps < .05; Figure 3). There was also a significant Condition × Cues interaction on urge (F (3, 120) = 16.08; p < .001). Post-hoc simple effects tests indicated that effects of cues on urge were significant in the non-abstinent condition (F (1, 40) = 41.40, p < .001), were not significant in the abstinent + 0 mg NRT condition, and became significant again in the abstinent + 21 mg NRT and abstinent + 42 mg NRT conditions (F (1, 40) = 6.64, p < .05; F (1, 40) = 17.40, p <.001, respectively). As shown in Figure 3, NRT reduced urge levels in the neutral cues trial but not in the smoking cues trial.
Figure 3.
Effects of 5-hr abstinence and transdermal nicotine (NRT) on urge levels following exposure to neutral cues and smoking cues in (left) smokers with schizophrenia (n = 21) and (right) equally-heavy smokers without psychiatric illness (n = 21). Data points represent M ± SEM.
The main effect of Group on urge level was not significant (η2 < .01). The Condition × Group and Condition × Cues × Group interactions approached significance (F (3, 120) = 1.94, p = .12, η2 = .05; F (3, 120) = 2.11, p = .10, η2 = .05, respectively), with means indicating that NRT tended to reduce urge levels during neutral cues trials in CS but not in SS (Figure 3).
Analyses were repeated with the cue reactors only (11 SS, 19 CS). These analyses did not alter the outcomes of the ANOVAs. There were significant main effects of Cues and Condition on urge level (F (1, 28) = 17.95, p < .001, F (3, 84) = 34.25, p < .001, respectively). There was a significant Condition × Cues interaction (F (3, 84) = 29.26; p < .001) with effects of cues significant only in the non-abstinent and abstinent + 42 mg NRT conditions. There was no significant main effect of Group or significant interaction between Group and any other variable on urge level.
Discussion
The results of this study indicate that abstinence increased smoking urge, nicotine withdrawal symptoms and several smoking variables in SS and CS. NRT reduced nicotine withdrawal symptoms and smoking urges and increased latency to smoke in both groups. SS were more sensitive to the effects of abstinence on CO boost but not other variables, and SS were not less sensitive than CS to the effects of NRT. There are a number of points on which we would like to comment.
First, urge levels reported by SS and CS were strikingly similar under the various study conditions. Although few studies have systematically compared urge levels under non-abstinent and abstinent conditions in these groups, Weinberger et al. (2007) recently published a comparison under non-abstinent and overnight-abstinent conditions, with similar findings. In contrast to their similar urge levels, MNWS scores tended to be higher in SS across conditions. This is not surprising as most of the items included in this measure are associated with schizophrenia (American Psychiatric Association [APA], 1994). However, the fact that abstinence increased, and NRT decreased, MNWS scores in both groups supports the validity of MNWS as a measure of nicotine withdrawal in SS. Group differences on smoking topography indicated that SS smoke more intensely according to several topography measures, consistent with findings that we and others have reported previously (Tidey, Rohsenow, Kaplan, & Swift, 2005a; Williams, Gandhi, Karavidas, & Foulds, 2006).
Abstinence increased urge levels, nicotine withdrawal symptoms and CO boost, and reduced smoking latency and puff volumes in both groups, with SS being particularly sensitive to the effects of abstinence on CO boost. The heightened sensitivity of the smokers with schizophrenia to the effects of abstinence on smoke intake is particularly interesting and it is noteworthy that this effect is greater than we would have predicted from looking at effects of abstinence on urge and MNWS scores in these smokers. Although this dissociation might suggest that the urge and MNWS measures are less valid for SS, the systematic manner in which SS altered their urge and MNWS reports in response to abstinence, cues and NRT argues against this interpretation. Another interpretation is that SS may experience exacerbations in another subjective state, not measured in this study, which in turn leads to increased smoking. For example, abstinence disrupts visuospatial working memory task performance in SS and resumption of smoking reverses this deficit (George et al., 2002; Sacco et al., 2005; but see Evins, Deckersbach, et al., 2005). Another possibility is that SS may be more sensitive to the effects of abstinence on sensorimotor factors associated with smoking (e.g., Brauer et al., 2001). Blood nicotine levels were not collected in this study; their inclusion would have indicated the proportion of nicotine replaced by NRT in these participants and helped to clarify this finding. It should also be noted that the effects of NRT were small in this study, which was powered to detect medium-sized effects. As recently noted by Perkins, Stitzer, & Lerman (2006), the fact that NRT often fails to reduce smoking cue-elicited urge levels in laboratory analogue studies, despite its proven efficacy on smoking cessation, may be related to the fact that many laboratory studies such as this one enroll participants who are not actively attempting to quit smoking during the study.
With regard to smoking cue reactivity, we found that SS were significantly less consistent than CS in their reactivity to cues, but there was no indication that the groups differed in the extent to which cues increased urge levels. Fonder et al. (2005) have also reported that baseline and image cue-elicited urge levels were closely similar in SS and CS. Together, the results of these studies do not support the idea that cues provoke higher urge levels in SS. Both SS and CS in this study were less sensitive to cues during abstinence. Similar findings have been reported in smokers with schizophrenia by Fonder et al. (2005) and in non-psychiatric smokers by others (e.g., Drobes and Tiffany, 1997; Maude-Griffin and Tiffany, 1996). It has been suggested that the endogenous effects of abstinence are more salient than the exogenous effects of cues in heavy smokers (Herman, 1974). Interestingly, NRT reduced urge levels during the neutral cue trials, but not the smoking cue trials. This is consistent with previous findings that NRT appears relatively ineffective against smoking cue-elicited urge levels in non-psychiatric smokers (Tiffany et al., 2000; Waters et al., 2004; Rohsenow et al., 2007).
An unexpected finding was that morning CO levels were higher in SS than CS, despite the groups’ similarities in numbers of cigarettes smoked per day, salivary cotinine levels, FTND scores and minutes to their first cigarette after awakening. This difference could result from different diurnal patterning of smoking in these groups, i.e., the SS may have smoked more intensely in the early morning. The fact that group means on minutes to first cigarette of the day were highly similar in this study suggests that the SS were not restricted from smoking in their residences (Steinberg, Williams, Steinberg, Krejci, & Ziedonis, 2005).
Limitations of this study include its focus on acute effects of abstinence and NRT and its use of self-report measures of urge and withdrawal. Future studies could include a broader range of measures, including measures that do not rely on self report (e.g., Waters, Heishman, Lerman, & Pickworth, 2007). The generalizability concern raised by Perkins et al. (2006) could be addressed by incorporating a brief motivational intervention at study onset (e.g., Steinberg, Ziedonis, Krejci, & Brandon, 2004) or by offering modest monetary reinforcement for smoking reductions (e.g., Tidey, Higgins, Bickel & Steingard, 1999; Tidey, O’Neill, & Higgins, 2002). Nevertheless, this study contributes to the literature by demonstrating that SS are more sensitive than CS to the effects of acute abstinence on smoke intake, while not being more sensitive to effects of abstinence on urges and nicotine withdrawal symptoms. Future studies should compare other effects of abstinence in smokers with schizophrenia and controls to clarify this finding.
Acknowledgments
This research was supported by NIDA grant R01-DA-14002 to the first author and a Research Career Scientist Award from the Department of Veterans Affairs to the second author. GlaxoSmithKline provided the nicotine and placebo patches for this study but did not influence its design, conduct or results. Portions of these data were presented at annual meetings of the College on Problems of Drug Dependence and the Society for Research on Nicotine and Tobacco. We thank Laura Dionne for her assistance with data management, and the individuals who participated in this study for their contributions.
Footnotes
This work was conducted at the Providence Veterans Affairs Medical Center and the Brown University Center for Alcohol and Addiction Studies, Providence, RI, USA.
Contributor Information
Jennifer W. Tidey, Brown University Center for Alcohol and Addiction Studies and Providence VA Medical Center
Damaris J. Rohsenow, Providence VA Medical Center and Brown University Center for Alcohol and Addiction Studies
Gary B. Kaplan, VA Boston Healthcare System and Boston University School of Medicine
Robert M. Swift, Providence VA Medical Center and Brown University Center for Alcohol and Addiction Studies
Amy B. Adolfo, Brown University Center for Alcohol and Addiction Studies
References
- Addington J, el-Guebaly N, Campbell W, Hodgins DC, Addington D. Smoking cessation treatment for patients with schizophrenia. American Journal of Psychiatry. 1998;155:974–976. doi: 10.1176/ajp.155.7.974. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; 1983. [PubMed] [Google Scholar]
- Balfour DJK. The neurobiology of tobacco dependence: A preclinical perspective on the role of the dopamine projections to the nucleus accumbens. Nicotine & Tobacco Research. 2004;6:899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- Balfour DJK, Wright AE, Benwell MEM, Birrell CE. The putative role of extra-symaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behavioral and Brain Research. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE. Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine & Tobacco Research. 2001;3:101–109. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-Brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. European Journal of Pharmacology. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats; Proceedings of the National Academy of Sciences; 1988. pp. 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation and preparation stages of change. Journal of Consulting and Clinical Psychology. 1991;59:295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal and tolerance: Integrating the clinical and basic science literature. Nicotine & Tobacco Research. 2005;7:9–26. doi: 10.1080/14622200412331328538. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. Journal of Abnormal Psychology. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Esterberg ML, Compton MT. Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Social Science and Medicine. 2005;61:293–303. doi: 10.1016/j.socscimed.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Etter J-F, Sutton S. Assessing ‘stage of change’ in current and former smokers. Addiction. 2002;97:1171–1182. doi: 10.1046/j.1360-0443.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Deckersback T, Freudenreich O, Culhane MA, Olm-Shipman C, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. Journal of Clinical Psychopharmacology. 2005;25:218–225. doi: 10.1097/01.jcp.0000162802.54076.18. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion SR added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Psychopharmacology. 2007;27:380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- Evins AE, Deckersbach T, Cather C, Freudenreich O, Culhane MA, Henderson DC, et al. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. Journal of Clinical Psychiatry. 2005;66:1184–1190. doi: 10.4088/jcp.v66n0915. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV axis-I Disorders – patient edition (SCID -I / P, Version 2.0) New York: Biometric Research Department; 1994. [Google Scholar]
- Fonder MA, Sacco KA, Termine A, Boland BS, Seyal AA, Dudas MM, et al. Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biological Psychiatry. 2005;57:802–808. doi: 10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry. 2002;52:53–61. doi: 10.1016/s0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. American Journal of Psychiatry. 2000;157:1835–1842. doi: 10.1176/appi.ajp.157.11.1835. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herman CP. External and internal cues as determinants of the smoking behavior of light and heavy smokers. Journal of Personality and Social Psychology. 1974;30:664–672. doi: 10.1037/h0037440. [DOI] [PubMed] [Google Scholar]
- Holmberg KS, Kane C. Health and self-care practices of persons with schizophrenia. Psychiatric Services. 1999;50:827–829. doi: 10.1176/ps.50.6.827. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, Niaura RS, Sirota AD. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology. 1999;142:139–143. doi: 10.1007/s002130050872. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. Journal of Psychopharmacology. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- Lucksted A, Dixon LB, Sembly JB. A focus group pilot study of tobacco smoking among psychosocial rehabilitation clients. Psychiatric Services. 2000;51:1544–1548. doi: 10.1176/appi.ps.51.12.1544. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin PM, Tiffany ST. The production of smoking urges through imagery: the impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4 198-108. [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. European Journal of Neuroscience. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotoxicity Research. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug and Alcohol Reviews. 2003;22:203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, et al. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Experimental and Clinical Psychopharmacology. 2007;15:81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas M, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia. Archives of General Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB. Effect of different cue stimulus delivery channels on craving reactivity: comparing in vivo and video cues in regular cigarette smokers. Journal of Behavior Therapy and Experimental Psychiatry. 2001;32:203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology. 2006;184:608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg J, Paty J, Perz WG, Gnys M, Kassel JD, et al. A day at a time: predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Di Marino ME, Pillitteri JL. The effectiveness of nicotine patch and nicotine lozenge in very heavy smokers. Journal of Substance Abuse Treatment. 2005;28:49–55. doi: 10.1016/j.jsat.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed and nonpatient smokers. American Journal of Psychiatry. 2003;160:316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Williams JM, Steinberg HR, Krejci JA, Ziedonis DM. Applicability of the Fagerstrom Test for Nicotine Dependence in smokers with schizophrenia. Addictive Behaviors. 2005;30:49–59. doi: 10.1016/j.addbeh.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Ziedonis DM, Krejci JA, Brandon TH. Motivational interviewing with personalized feedback: a brief intervention for motivating smokers with schizophrenia to seek treatment for tobacco dependence. Journal of Consulting and Clinical Psychology. 2004;72:723–728. doi: 10.1037/0022-006X.72.4.723. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Higgins ST, Bickel WK, Steingard S. Effects of response requirement and the availability of an alternative reinforcer on cigarette smoking by schizophrenics. Psychopharmacology. 1999;145:52–60. doi: 10.1007/s002130051031. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug and Alcohol Dependence. 2005a;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine & Tobacco Research. 2005b;7:421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Tidey J, Rohsenow D. Smoking outcome expectancies in smokers with schizophrenia and controls. Poster session presented at the annual meeting of the Society for Research on Nicotine and Tobacco; Austin, TX. 2007. Feb, [Google Scholar]
- Tidey JW, Williams JM. Clinical indices of tobacco use among people with schizophrenia. In: Ziedonis D, Montoya I, editors. Smoking and Schizophrenia. Journal of Dual Diagnosis, Supplement. (in press) [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation: six-month results from two multicenter controlled clinical trials. Journal of the American Medical Association. 1991;266:3133–3138. [PubMed] [Google Scholar]
- Waters AJ, Heishman SJ, Lerman C, Pickworth W. Enhanced identification of smoking-related words during the attentional blink in smokers. Addictive Behaviors. 2007 doi: 10.1016/j.addbeh.2007.05.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Reutenauer EL, Allen TM, Termine A, Vessicchio J, Sacco KA, et al. Reliability of the Fagerström Test for Nicotine Dependence, Minnesota Withdrawal Scale, and Tiffany Questionnaire for Smoking Urges in Smokers with and without Schizoprhenia. Drug and Alcohol Dependence. 2006;86:278–282. doi: 10.1016/j.drugalcdep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sacco KA, Creeden CL, Vessicchio JC, Jatlow PI, George TP. Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral measures of cigarette smoking in schizophrenia. Schizophrenia Research. 2007;91:217–225. doi: 10.1016/j.schres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Karavidas M, Foulds J. More puffs and shorter interpuff interval in smokers with schizophrenia using CReSSmicro topography device. Poster presented at the annual meeting of the Society for Research on Nicotine and Tobacco Dependence; Orlando, FL. 2006. Feb, [Google Scholar]
- Yang YK, McEvoy JP, Wilson WH, Levin ED, Rose JE. Reliabilities and intercorrelations of reported and objective measures of smoking in patients with schizophrenia. Schizophrenia Research. 2003;60:9–12. doi: 10.1016/s0920-9964(02)00208-6. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, George TP. Schizophrenia and nicotine use: Report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophrenia Bulletin. 1997;23:247–254. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]