Abstract
Human rhinovirus (RV) infection is responsible for the majority of virus-induced asthma exacerbations. Using a mouse model of human RV infection, we sought to determine the requirement of CXCR2, the receptor for ELR-positive CXC chemokines, for RV-induced airway neutrophilia and hyperresponsiveness. Wild-type and CXCR2 −/− mice were inoculated intranasally with RV1B or sham HeLa cell supernatant. Following RV1B infection, CXCR2 −/− mice showed reduced airway and lung neutrophils and cholinergic responsiveness compared to wild-type mice. Similar results were obtained in mice treated with neutralizing antibody to Ly6G, a neutrophil-depleting antibody. Lungs from RV-infected, CXCR2 −/− mice showed significantly reduced production of tumor necrosis factor (TNF)-α, MIP-2/CXCL2 and KC/CXCL1, and lower expression of MUC5B, compared to RV-treated wild-type mice. The requirement of TNF-α for RV1B-induced airways responses was tested using TNF receptor (TNFR)-1 −/− mice. TNFR1 −/− animals displayed reduced airways responsiveness to RV1B, even when exogenous MIP-2 was added to the airways. We conclude that CXCR2 is required for RV-induced neutrophilic airway inflammation, and that neutrophil TNF-α release is required for airways hyperresponsiveness.
INTRODUCTION
Viral infections trigger 80% of asthma exacerbations in children and nearly 50% in adults, with human rhinovirus (RV) being the most common virus identified. In addition, a large number of patients with chronic obstructive pulmonary disease (COPD) experience RV-induced exacerbations (1). Consistent with the notion that RV causes exacerbations of asthma, experimental RV infection has been shown to increase airway hyperreactivity in asthmatic subjects (2-4). RV has also been shown to increase maximal responses to methacholine in normal subjects (5, 6).
Human RV is a positive-stranded RNA virus of the Picornaviridae family. The major group serotypes, for example RV14, 16 and 39, bind to intercellular adhesion molecule (ICAM)-1 (7). Minor group viruses, such as RV1B, bind to low density lipoprotein family receptors (8). Recently, a third group of previously unrecognized RVs were shown to cause respiratory illness in infants (9, 10). Binding of RV to airway epithelial cell ICAM-1 triggers the induction of C-X-C chemokines with a Glu-Leu-Arg (ELR) motif including IL-8/CXCL8, epithelial-neutrophil activating peptide (ENA)-78/CXCL5 and growth related oncogene (GRO)-α/CXCL1 (11-14). Minor group serotypes such as RV1B produce a similar pattern of inflammatory cytokines upon receptor engagement (15, 16). ELR (+) C-X-C chemokines, which cause migration of neutrophils to the site of infection, bind to the G protein-coupled seven transmembrane receptor CXCR2.
IL-8 and neutrophils are found in the nasal secretions, sputum or bronchoalveolar lavage fluid of allergic subjects undergoing experimental RV infection (6, 17-20). Further, the number of neutrophils correlates with the level of IL-8 (18, 19). After RV16 infection, asthmatic patients show increased levels of IL-8 in their nasal lavage which correlates with the level of airways responsiveness (3), in contrast to unaffected individuals in whom IL-8 does not increase (21). Together, these data suggest that RV infection of airway epithelial cells may potentiate pre-existing inflammation by enhancing the production of neutrophil chemoattractants and neutrophilic airway inflammation. Upon stimulation, activated neutrophils release a variety of pro-inflammatory mediators including cytokines such as TNF-α and IL-1β, superoxide, myeloperoxidase and various proteases which could promote airway inflammation and responsiveness (22-26). However, the requirement of IL-8 and other CXCR2 ligands, or of airway neutrophils, for RV-induced airway responses has not been established.
RV1B, a minor group virus, binds to mouse airway epithelial cells (16, 27). Accordingly, a mouse model of human RV1B infection has recently been developed. We have shown in C57BL/6 mice that intranasal inoculation of high-dose RV1B, but not sham HeLa cell supernatant or UV-irradiated virus, induces migration of neutrophils and lymphocytes to the airways, as well as robust lung cytokine, chemokine and interferon production (16). The influx of inflammatory cells is also accompanied by moderate airways hyperresponsiveness to methacholine, which is present both 24 and 96 h post-infection. Inoculation with high-dose RV1B but not UV-irradiated virus also induces airway inflammation and interferon production in BALB/c mice (28). In the present study, we sought to determine the requirement of CXCR2 ligands for RV-induced airway responses, employing a CXCR2 −/− mouse strain that is impaired in neutrophil recruitment. We found that CXCR2 is required for neutrophilic airway inflammation following RV infection. Further, the reduction in airway neutrophils was accompanied by a reduction in airway responsiveness 24 h post infection. Finally, airways responsiveness was also decreased in TNFR −/− mice, suggesting that neutrophil TNF-α release is required for RV-induced airways hyperresponsiveness.
METHODS
Animals
Wild type and CXCR2−/− BALB/c mice, and TNFR1 −/− C57BL/6 mice, were purchased from Jackson Laboratories (Bar Harbor, MA). Mice were housed in a specific pathogen-free area within the animal care facility at the University of Michigan. All mice were 8 wk old females. This study was approved by the Institutional Animal Care and Use Committee.
Generation of RV stocks
RV1B was generated from an infectious cDNA clone, as described (27). Viral stocks were generated as previously described (14). Briefly, HeLa cells were infected with RV until 80% of the cells were cytopathic. HeLa cell lysates were harvested and cellular debris pelleted by centrifugation (10, 000 × g for 30 min at 4°C). RV in HeLa cell lysates was concentrated and partially purified by centrifugation with a 100,000 MW cutoff Centricon filter (2,000 rpm at 4°C for 8 h; Millipore, Billerica, MA) (29). Virus was titered by infecting confluent HeLa monolayers with serially diluted RV (range: undiluted to 10−9) and assessing cytopathic effect five days after infection. Fifty percent tissue culture infectivity doses (TCID50) values were determined by the Spearman-Karber method.
RV1B exposure
Mice were anesthetized by intraperitoneal injection with ketamine (40 mg/kg) and xylazine (5 mg/kg) and intranasally inoculated with 45 μl of 1×108 TCID50/ml RV1B or equal volume sham HeLa cell lysate, as previously described (16). Mice were euthanized 1 or 4 days post infection.
Bronchoalveolar lavage (BAL) and tissue inflammation
BAL was performed by exposing and intubating the trachea using a 1.7-mm OD polyethylene catheter, and instilling PBS containing 5 mM EDTA in 1-ml aliquots. Cytospins prepared from BAL cells and stained with Diff-Quick (Dade Behring, Newark, DE) and differential counts were determined by counting 200 cells. To quantify the number of inflammatory cells in the tissues, lung digests were performed by mincing the lungs with scissors and suspending the tissue in 30 mg collagenase type IV (Gibco Invitrogen, Carlsbad, CA) in 5 ml serum free RPMI for one h. Cells were isolated by straining through a 70 μm nylon mesh (BD Falcon, San Jose, CA), spun at 1500 g, and the resultant pellet treated with red blood cell lysis buffer (BD Pharmingen, San Diego, CA). Finally, leukocytes were enriched by spinning the cells through 40% Percoll (Sigma-Aldrich, St. Louis, MO), decanting the supernatant and resuspending the pellet in PBS (30). The total cell count was determined on a hemocytometer. Cytospins were examined for differential cell counts after staining with Diff-Quick.
Flow cytometry
In selected experiments, BAL fluid was examined for the number of TNF-α-expressing neutrophils. 1× 106 cells were blocked with brefeldin A (3 μg/ml) and incubated in low attachment polystyrene plates for 5-6 hours. Cells were then stained with Pacific Blue-conjugated antibody against the neutrophil cell surface marker LY6G (BD Pharmingen) and FITC-labeled anti-TNF-α (E-Bioscience, San Diego, CA). IgG antibodies were used as isotype controls. Finally, cells were fixed in 1% formaldehyde, covered with foil and refrigerated until flow cytometry was performed the following day.
Histology
Lungs were fixed in 10% formalin overnight, and then transferred to 70% ethanol and paraffin embedded. H&E staining was performed on a 5 μm section of each lung.
Cytokine/chemokine expression
Lung RNA was extracted using Trizol reagent (Sigma-Aldrich) and analyzed for the presence of MIP-2, TNF-α, GM-CSF, MUC5AC and MUC5B by quantitative two-step real time PCR using specific primers and probes. Primer probe mixes for TNF-α, GM-CSF and MIP-2 expression were purchased from Applied Biosystems (Foster City, CA). MUC5AC and MUC5B primers were from IDT (Coralville, IA) and employed FAM as the fluorescent tag and TAMRA as a quencher. For MUC5AC, the sequences were: forward primer, 5′ AAA GAC ACC AGT AGT CAC TCA GCA A 3′; reverse primer, 5′ CTG GGA AGT CAG TGT CAA ACC A/ 3BHQ_1/-3′; and probe, 5′- /56-FAM/5′ TCA CAC ACA ACC ACT CAA CCA GTG ACC A /36-TAMSp/ -3.′ For MUC5B, the sequences were: forward primer, 5′ GAG CAG TGG CTA TGT GAA AAT CAG 3′; reverse primer, 5′ CAG GGC GCT GTC TTC TTC AT-3′; and Taqman probe, 5′-/56-FAM/ ATC CGC, CTA GTC CTC ACC TTC CTG TGG/ 3 BHQ_1/3.′ The signal was normalized to GAPDH and expressed as fold-increase over sham.
Cytokine production
Lungs were homogenized in 1 ml PBS , spun for 15 minutes at 1500 g, and the supernatant assayed for murine homologs of IL-8 including MIP-2/CXCL2, KC/CXCL1, and the proinflammatory cytokines TNF-α and GM-CSF by ELISA (R&D systems, Minneapolis, MN).
Neutralizing antibody preparation
In some experiments, mice were injected intraperitoneally with 75 μg of neutralizing antibody to Ly6G (clone mAb RB6-8C5) or an equivalent dose of rat anti-mouse IgG, inoculated simultaneously with RV1B, and euthanized 24 h post infection. Ascites for anti-Ly6G, an anti-mouse granulocyte neutralizing antibody (31), was obtained from the University of Michigan Vector Core and stored at −20°C. A 20 mL volume was then thawed overnight and centrifuged at 3000 rpm for 15 min. Debris was removed and the clear suspension transferred to a fresh tube. The ascites fluid was then clarified by ultracentrifugation at 40,000 rpm for 1 h, followed by removal of any lipid masses. Five mL clear ascites was then purified on a Protein G bead column (Millipore) at 4°C. The ascites was diluted in a 1:2 ratio with binding buffer containing 0.01M sodium phosphate and 0.15 M sodium chloride adjusted to pH 7.0, and loaded onto the Protein G column. The eluate was reapplied to the column and washed 3 times with 50 mL binding buffer to remove any non specific proteins. Bound IgG was then eluted with 20 mL 0.1 M glycine hydrochloride, pH 2.6. The antibody was then stored in 10 tubes containing 0.5 ml 1.0 M Tris-HCL, pH 9.0. Each of the 10 fractions of eluate was measured at A280, and the peak fractions were pooled and dialyzed overnight with 3 changes of PBS. The final concentration of the RB6-8C5 antibody was 1.75 mg/mL.
MIP-2 administration
In some experiments, wild type C57BL/6 and TNFR1−/− mice were administered MIP-2 (1 μg/ml intranasally, R&D Systems) immediately following sham or RV1B infection. Mice were harvested for BAL fluid and airway resistance measured 24 h post-treatment.
Presence of viral RNA
RNA was extracted from lungs of mice using Trizol reagent (Sigma-Aldrich, St. Louis, MO) and analyzed for the presence of viral RNA by reverse transcriptase-PCR. Quantitative one-step real time PCR for positive-strand viral RNA was conducted using RV-specific primers and probes for RV (forward primer: 5′-GTG AAG AGC CSC RTG TGC T-3′; reverse primer: 5′-GCT SCA GGG TTA AGG TTA GCC-3′; probe: 5′-FAM-TGA GTC CTC CGG CCC CTG AAT G-TAMRA-3′ (32). Copy numbers of positive strand viral RNA were normalized to 18S RNA, which was similarly amplified using gene-specific primers and probes.
Gene arrays
Lung RNA from sham and RV-treated wild-type and CXCR2 −/− mice was subjected to a targeted PCR array examining mouse inflammatory cytokines (SA Biosciences, Frederick, MD).
Measurement of respiratory system resistance
Mice were anesthetized with sodium pentobarbital (50 mg/kg mouse, intraperitoneal injection) and intubated via cannulation of the trachea with a 20-gauge stub adapter cannula (Becton-Dickinson, Sparks, MD). Mechanical ventilation was performed using a FlexiVent ventilator (Scireq, Montreal, Quebec, Canada) at 150 breaths/min with a tidal volume of 10 ml/kg body weight. Airway responsiveness was assessed by measuring respiratory system resistance in response to increasing doses of nebulized methacholine, as described (16).
Data analysis
SigmaStat computing software (SPSS, Chicago, IL) was used for data analysis. Data are represented as mean±SEM. Statistical significance was assessed by one- or two-way analysis of variance (ANOVA). Differences identified by ANOVA were pinpointed by the Student Newman-Keuls' multiple range test. For gene arrays, unpaired t tests were used to establish differences between groups.
RESULTS
RV infection of BALB/c mice
We recently demonstrated in C57BL/6 mice that RV1B, a minor group virus which binds to low density lipoprotein family receptors, induces airway inflammation and cholinergic hyperresponsiveness, as well as robust lung chemokine and interferon production (16). Airways responses to RV1B were significantly greater than those to sham HeLa cell lysate or replication-deficient UV-irradiated virus, and negative-strand viral RNA was detected in the lungs of RV1B-inoculated mice, evidence of replicative infection. In the present study, BALB/c mice were inoculated with 3 × 108 TCID50 RV1B intranasally or sham equivalent, and BAL performed 24 h and 96 h post-infection. Consistent with recent data from BALB/c mice (28), animals of this strain demonstrated greater levels of airway inflammation in response to RV1B compared to C57BL/6 mice. As in C57BL/6 mice, the initial (24 h) response to RV infection in BALB/c mice was primarily neutrophilic in character (Figures 1-3). By 96 h, BAL neutrophils were significantly reduced. To further characterize the time course of the neutrophilic response, we examined lung neutrophil counts 2, 8, 16 and 24 hours post-infection (Figures 3C, 3D). We observed maximum neutrophil infiltration 24 h post-exposure. Significant differences in sham- and RV1B-treated mice were noted as early as 8 h after infection.
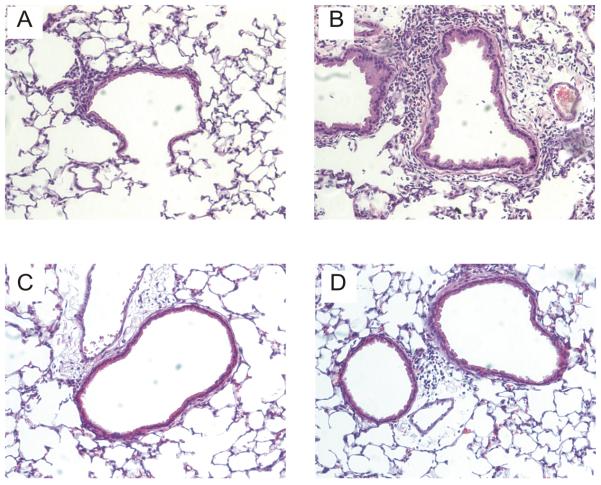
Figure 1.
Hematoxylin and eosin-stained lung sections from RV1B-infected wild-type BALB/c mice (A,B) and CXCR2 −/− mice (C,D). Airway inflammation in wild-type mice ranged from mild (left panel) to severe (right panel). Inflammation was attenuated in CXCR2 −/− mice. These results are typical of the five mice studied in each group. (Original magnification, 160X.)
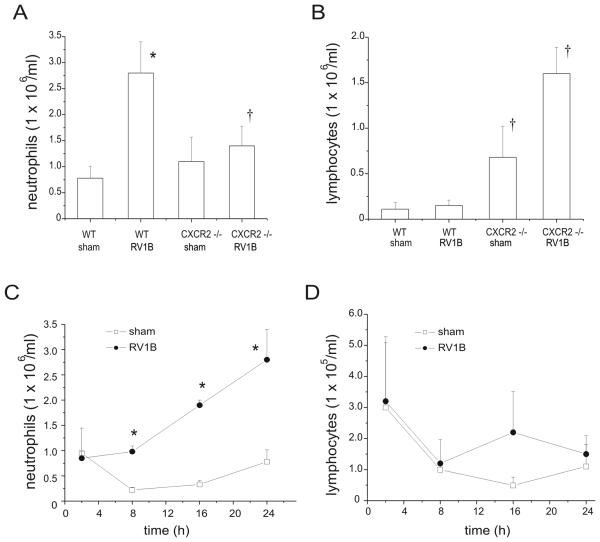
Figure 3.
Effects of CXCR2 knockout on lung inflammatory cells in sham and RV-treated mice. Mouse lungs were isolated, minced, and then digested in collagenase type IV for 1 h. Leukocytes were enriched following RBC lysis treatment, and counted for the presence of neutrophils (A, C) and lymphocytes (B, D). Panels A and B show data from 24 h after infection. Panels C and D show the early time course of neutrophil and lymphocyte influx in wild type mice. (N = 5 mice per group, bars represent mean±SEM, *different from respective sham group, p<0.05, ANOVA; †different from RV1B-treated wild- type mice, p<0.05, one-way ANOVA.)
CXCR2 −/− mice exhibit significantly reduced BAL and lung neutrophils following RV infection
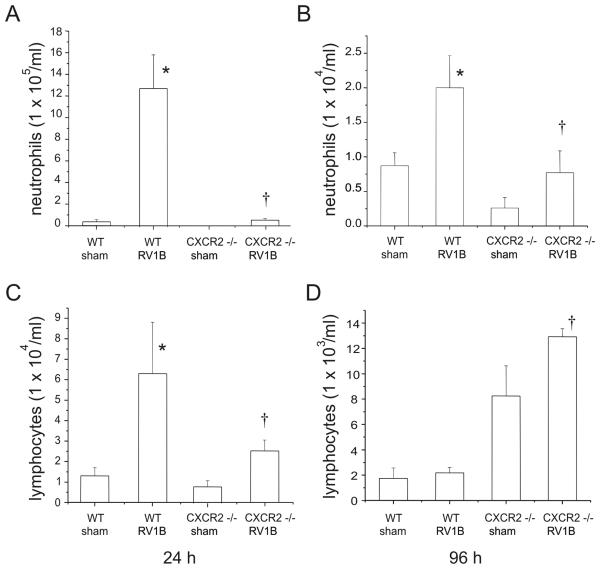
To determine the requirement of ELR (+) CXC chemokines for the observed neutrophilic inflammation. we examined the response of CXCR2 −/− mice to RV infection. CXCR2 serves as the receptor for the murine chemokines KC/CXCL1 and MIP-2/CXCL2, the homologs of human IL-8. Compared to their wild type controls, CXCR2 −/− mice showed a significant reduction in BAL neutrophils 24 h post RV1B infection (Figure 2). By 96 h, the neutrophilic response was significantly reduced compared to infiltration at 24 h, although RV1B-treated CXCR2 −/− mice still exhibited significantly lower BAL neutrophils compared to wild-type mice. Lung neutrophil influx was also significantly lower in RV1B-infected CXCR2 −/− mice (Figures 1,3). Together, these data imply that the CXCR2 ligands are the major neutrophil chemoattractants elaborated 24 h following RV infection and that this response is significantly attenuated at 96 h. Lymphocyte influx at 24 h was 30-fold higher in wild type RV treated mice than at 96 h. Interestingly, RV-infected CXCR2 −/− mice exhibited significantly more BAL lymphocytes 96 h after infection compared to wild-type RV1B-infected mice (Figure 2D). In the lungs, CXCR2−/− mice exhibited higher lymphocytes counts compared to wild type mice after both sham and RV1B infection (Figure 3B).
Figure 2.
Effects of CXCR2 knockout on bronchoalveolar lavage (BAL) neutrophils (A,B) and lymphocytes (C,D) in sham and RV-treated mice 24 and 96 h post infection. Wild-type BALB/c and CXCR2−/− mice were treated with 45 μl of 3 × 108 TCID50/ml RV1B or sham (HeLa cell supernatant). BAL was performed with 0.9% NaCl containing 5mM EDTA. Note the one-log difference in scale between panels A, B and C, D. (N = 5-6 mice per group, bars represent mean±SEM, *different from respective sham group, p<0.05, ANOVA; †different from RV1B-treated wild- type mice, p<0.05, one-way ANOVA.)
CXCR2 −/− display significantly reduced lung expression of pro-inflammatory cytokines
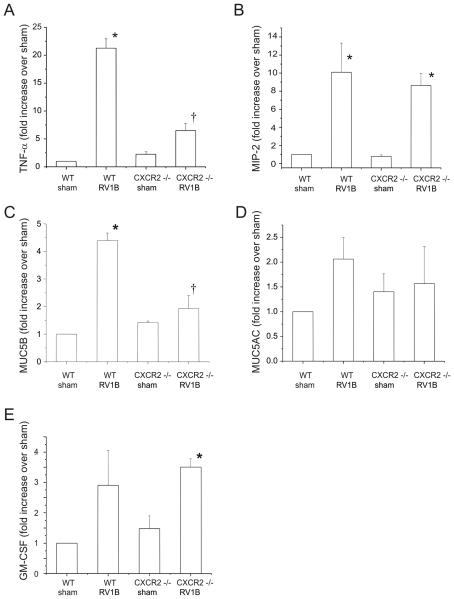
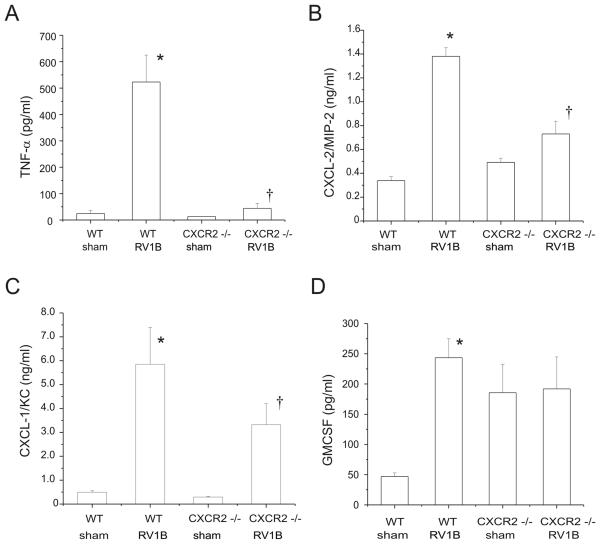
To determine the induction of pro-inflammatory cytokine and mucin gene expression, lungs were homogenized in Trizol reagent, cDNA was synthesized using reverse transcriptase, and subjected to quantitative real time PCR employing a Taqman probe. Inductions in TNF-α and MUC5B expression following RV infection were significantly higher in wild-type mice compared to CXCR2 −/− animals (Figure 4). Levels of MIP-2/CXCL2, GM-CSF and MUC5AC expression were comparable in RV-infected wild type and CXCR2 −/− mice.
Figure 4.
CXCR2−/− mice show reduced TNF-α and MUC5B mRNA expression 24 h post-RV1B exposure. A-E. Wild type and CXCR2−/− mouse lungs were homogenized in Trizol reagent. RNA was extracted and analyzed for the presence of TNF-α, MIP-2, MUC5B, MUC5AC and GM-CSF by quantitative two-step real time PCR using specific primers and probes. (N=4-5 mice per group, bars represent mean±SEM, *different from respective sham group, p < 0.05, †different from wild type-RV1B treated group, p<0.05, one-way ANOVA.)
We then subjected lung mRNA samples to gene array analysis focused on 84 inflammatory cytokines and receptors. RV infection induced a statistically significant, >2-fold increase in the expression of 26 genes, and a significant, >2-fold decrease in the expression of 6 genes (Table 1). In addition to TNF-α, genes increasing in expression included those encoding KC/CXCL1, ENA-78/CXCL5, IP-10/CXCL10, IL-1α, IL-1β, TARC/CCL17 and LARC/CCL20. We also computed the ratio of gene expression in RV1B-treated CXCR2 −/− mice compared to RV1B-treated wild-type BALB/c mice. CXCR2 knockout mice demonstrated a statistically significant, >2-fold increase in the expression of 11 genes, and a significant, >2-fold decrease in the expression of 8 genes (Table 2). In addition to TNF-α, CXCR2 −/− mice inoculated with RV1B showed significantly lower expression levels of KC/CXCL1, ENA-78/CXCL5, IL-1α, IL-1β, TARC/CCL17, LARC/CCL20 and eotaxin-2/CCL24. In contrast, RV1B-infected CXCR2 −/− mice showed an increase in the lymphocyte chemokine IP-10/CXCL10, perhaps explaining the observed increase in BAL lymphocytes 96 h after infection.
We measured lung protein levels of TNF-α, GM-CSF and the IL-8 homologs MIP-2/CXCL-2 and KC/CXCL-1 in wild type and CXCR2−/− deficient mice and found significantly lower levels of TNF-α, MIP-2/CXCL-2 and KC/CXCL-1 (Figure 5). No significant difference could be observed in the production of GM-CSF.
Figure 5.
CXCR2−/− mice show deficient cytokine and chemokine responses to RV1B infection 24 h post infection. TNF-α, MIP-2, CXCL-1, levels were significantly lower in the knockout mice as opposed to wild type RV1B infected mice. (N=5 mice per group, bars represent mean±SEM, *different from respective sham group, p<0.05, †different from wild type-RV1B treated group, p < 0.05, one-way ANOVA.)
CXCR2 −/− mice show reduced airway responsiveness to methacholine 24 h following RV1B infection
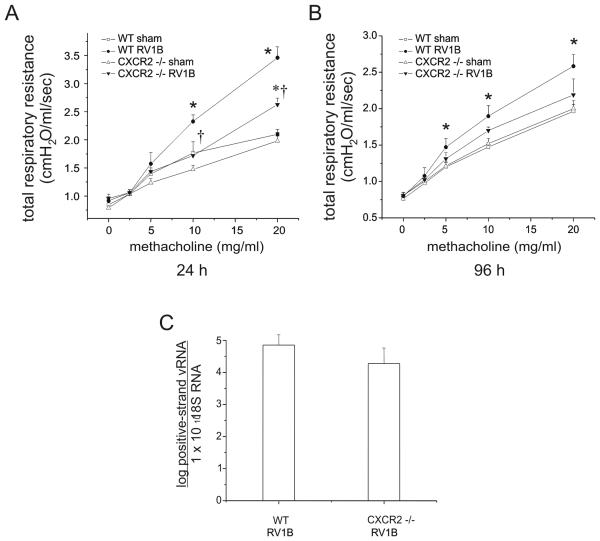
In order to determine the contribution of neutrophils to RV1B induced airway responsiveness, CXCR2 −/− and wild type BALB/c mice were tested for responsiveness to the bronchoconstrictor agonist methacholine 24 and 96 h after RV1B or sham treatment. Compared to sham-infected mice, RV1B infection was also associated with moderate but significant airways cholinergic hyperresponsiveness which persisted up to 96 h after treatment (Figure 6A). At 24 h, RV-infected CXCR2 −/− mice demonstrated significantly lower airways responses than wild-type mice (p<0.001, two-way ANOVA). However, RV1B-treated CXCR2 −/− mice still showed a significantly higher maximum methacholine response compared to sham-infected CXCR2 −/− mice. These data suggest that CXCR2 and airway neutrophils are required for maximal RV-induced methacholine responsiveness, but do not completely account for the RV response.
Figure 6.
CXCR2−/− mice show reduced airways cholinergic responsiveness 24 h post-infection. Following anesthesia and endotracheal intubation, changes in respiratory system resistance to nebulized methacholine were measured using the FlexiVent system (Scireq, Montreal, CA). Mice were studied either 24 h (A) or 96 h post-viral exposure (B). (N=5 mice per group. Open squares, sham-treated wild-type mice; closed circles, RV1B-treated wild-type mice; open triangles, sham-treated CXCR2 −/− mice; closed triangles, RV1B-treated CXCR2 −/− mice; error bars represent ±SEM, *different from respective sham group, †different from wild type RV1B-treated group, p<0.05, two-way ANOVA.) C. RV1B-infected CXCR2−/− mice show comparable viral loads compared to RV1B-treated wild type mice 24 h post infection. Wild type and CXCR2−/− mouse lungs were homogenized in Trizol reagent 24 h post exposure. RNA was extracted and analyzed for the presence of positive strand RV RNA. Viral copy number was normalized to the quantity of 18S RNA present in mouse lungs. (N=6 mice per group, bars represent geometric mean±SEM.)
At 96 h, the airways responsiveness of RV-infected CXCR2 −/− mice was no longer different than RV1B-treated wild type mice (Figure 6B). However, the response to RV infection in CXCR2 −/− mice appeared attenuated, as there was no difference in airways responsiveness between RV-infected and sham-treated CXCR2 −/− mice. These data suggest that neutrophils play a lesser role in the determination of airway responses at later time points. We therefore focused further experiments on the 24 h time point. First, we examined lung mRNA for viral load. There was no difference in RV copy number between the lungs of infected wild-type and CXCR2 −/− mice (Figure 6C).
Anti-Ly6G treated BALB/c mice exhibit reduced airway neutrophils and methacholine responsiveness 24 h after RV1B treatment
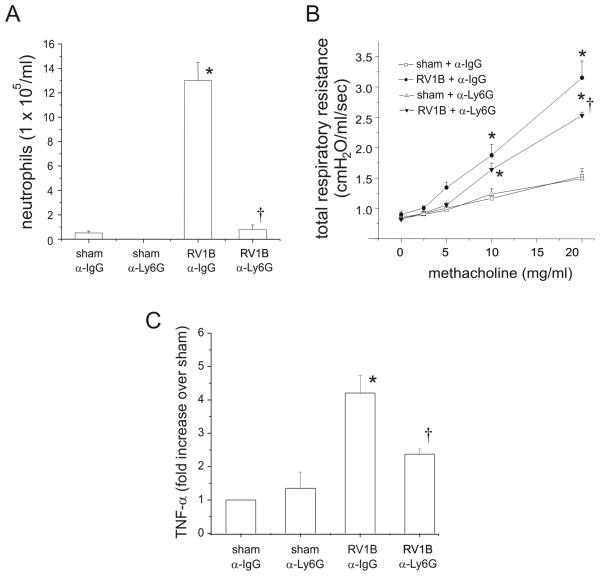
Although CXCR2 is classically expressed on neutrophils (33), it may also be expressed on monocytes, macrophages and lymphocytes (34-36). To confirm that RV-induced airways hyperresponsiveness was due to the contribution of granulocytes, we examined the effect of granulocyte depletion using the RB6-8C5 monoclonal antibody to Ly6G, an antigen expressed widely on granulocytes, including neutrophils (31). Mice were injected with 75 μg of neutralizing antibody to mouse Ly6G or the corresponding isotype IgG control, and inoculated intranasally with RV1B or sham. Treatment with anti-Ly6G significantly attenuated neutrophil numbers 24 h following RV infection compared to RV1B/IgG-treated animals (Figure 7A). Finally, depletion of neutrophils in the RV1B/anti-LY6G group was associated with a partial but statistically significant reduction in maximal methacholine response compared to RV1B/anti-IgG group (Figure 7B). Neutrophil depletion was also accompanied by a partial but significant reduction in lung TNF-α levels (Figure 7C). These data suggest that neutrophils are required for airways hyperresponsiveness 24 h post-RV1B infection.
Figure 7.
Anti-Ly6G treated BALB/c mice exhibit reduced airway granulocytes including neutrophils, methacholine responsiveness and lung TNF-α expression 24 h after RV1B treatment. Mice were injected intraperitoneally with 75 μg of anti-LY6G antibody or isotype conrol anti-IgG antibody. A. RV1B/anti-LY6G mice showed a significant reduction in BAL neutrophils 24 h post-infection. (N=5 mice per group, bars represent mean±SEM, *different from respective sham group, p<0.05, † different from wild-type-RV1B treated group, p<0.05, one-way ANOVA. B. RV1B/anti-LY6G treatment reduced maximal airway responsiveness to methacholine compared to RV1B/anti-IgG treated mice. (N=3-4 mice per group, error bars represent SEM, *different from respective sham group, †different from IgG-treated RV1B group, p<0.05, two-way ANOVA. C. RV1B/anti-LY6G treatment reduced lung TNF-α expression compared to RV1B/anti-IgG treated mice. (N=5 mice per group, error bars represent SEM, *different from respective sham group, †different from IgG-treated RV1B group, p<0.05, one-way ANOVA.
TNFR1 −/− mice show reduced airway neutrophils and methacholine responsiveness following RV1B infection
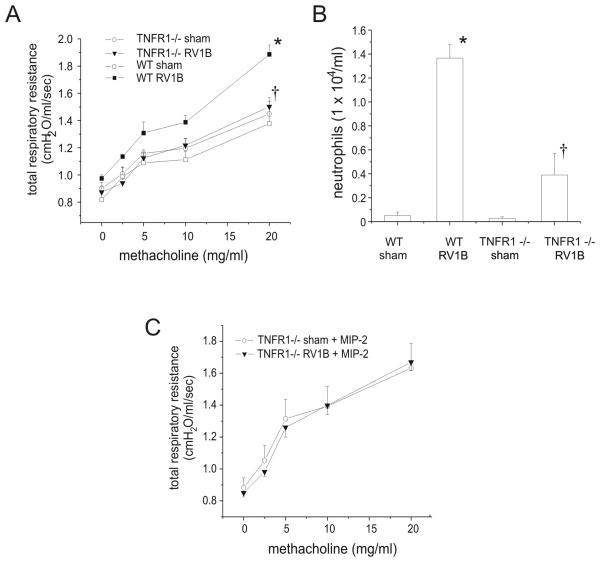
Based on the reduced TNF-α mRNA expression found in RV1B-infected CXCR2 −/− mice, we wondered whether neutrophil-derived TNF-α could be responsible for the airways cholinergic hyperresponsiveness observed 24 following RV infection. TNF-α has been demonstrated to increase the responsiveness of airway smooth muscle to contractile agonists (37-39). First, we measured the number of TNF-α-expressing neutrophils in the BAL of sham and RV1B-inoculated BALB/c mice by flow cytometry. RV-infected mice demonstrated a 16-fold increase in the number of LY6G- and TNF-α-positive cells. Next, to test the requirement for TNF-α, wild type C57BL/6 mice and TNFR1 −/− mice were inoculated with RV1B or sham, and airway inflammation and resistance measured 24 h post infection. RV-treated TNFR1 −/− mice showed a partial but significant reduction in methacholine response relative to wild-type RV treated mice (Figure 8A), consistent with the notion that TNF-α signaling is required for maximum RV-induced airway responsiveness.
Figure 8.
TNFR1−/− mice show reduced airway responsiveness and BAL neutrophils 24 h post-RV1B infection. TNFR1−/− and wild type C57BL/6 mice were inoculated with sham or RV1B and examined 24 h after infection. A. Changes in respiratory system resistance to nebulized methacholine were measured using the FlexiVent system. B. BAL was performed with 0.9% NaCl containing 5mM EDTA. C. Intranasal administration of MIP-2 fails to restore airways hyperresponsiveness in RV-infected TNFR1 −/− mice. (N=6 per group. Data represent mean±SEM, *different from respective sham group, †different from wild type-RV1B treated group, p<0.05, one- or two-way ANOVA, as appropriate.)
TNFR1 −/− mice also showed a significant reduction in BAL neutrophils after RV treatment compared to wild type mice (Figure 8B). It is therefore conceivable that the observed reduction in airways responsiveness in TNFR −/− mice is secondary to neutrophil diminution, rather than a defect in TNF-α signaling. To test this, BAL neutrophils were restored in RV-treated TNFR1−/− mice by intranasal administration of the neutrophil chemokine MIP-2. MIP-2 administration dramatically increased airway neutrophils in sham and RV1B-inoculated TNFR−/− mice (sham, 15.1±5.4 × 105 cells/ml; RV1B, 21.7±6.3 × 105 cells/ml, n=5). However, airway hyperresponsiveness in MIP-2- treated RV1B-infected TNFR1−/− mice was not restored (Figure 8C). Taken together, these data demonstrate that CXCR2, airway neutrophils, and intact TNFR1 receptor are required for RV1B-induced airways hyperresponsiveness.
DISCUSSION
RV is responsible for the majority of the common colds and approximately 50% of asthma exacerbations. We and others have shown that RV1B infects mouse airway epithelial cells (16, 27). Further, infection of C57BL/6 and BALB/c mice induces airway neutrophilia and hyperresponsiveness to methacholine challenge 24 h post infection (16, 28) We therefore sought to determine the contribution of ELR(+) CXC chemokines and neutrophils to RV1B-induced airway inflammation and hyperreactivity. Upon stimulation, activated neutrophils release a variety of pro-inflammatory mediators including cytokines such as IL-8 and TNF-α, superoxide, myeloperoxidase and various proteases which could promote airway inflammation and obstruction (22-25). Experimental RV infection has been shown to increase airway neutrophilic inflammation in asthmatic subjects (6, 17-20). Finally, RV infection has been shown to increase airway neutrophils (20) and maximal responses to methacholine (5, 6) in normal subjects.
In addition to ELR (+) CXC chemokines, other neutrophil chemoattractants include complement activation products such as C5a, lipid mediators such as leukotriene B4 and platelet activating factor, and host derived peptides such as N-acetyl-Pro-Gly-Pro, a degradation product of the extracellular matrix (40-42). To test for the contribution of ELR(+) CXC chemokines to RV-induced airways hyperresponsiveness, we employed a CXCR2 −/− mouse strain. CXCR2 serves as the receptor for the neutrophil chemoattractants and IL-8 homologs KC/CXCL1, MIP-2/CXCL2 and ENA-78/CXCL5. Twenty-four h after infection, CXCR2 −/− mice demonstrated significantly fewer airway neutrophils. CXCR2 −/− mice also showed lower lung mRNA and protein levels of the neutrophil chemokines KC/CXCL1 and MIP-2/CXCL-2, and lower expression of ENA-78/CXCL5 than RV-infected wild-type BALB/c mice. These data demonstrate that CXCR2 ligands are the main chemoattractants mediating RV-induced neutrophilic airway inflammation. In addition, they suggest that, in the context of RV infection, lung neutrophils intensify granulocyte infiltration of the airways by secreting their own chemokines. Previous studies have demonstrated the ability of neutrophils to express pro-inflammatory cytokines and chemokines, including IL-8/CXCL8, ENA-78/CXCL5 and GRO-α/CXCL1 (26). Finally, RV-infected CXCR2 −/− mice showed significantly reduced methacholine responsiveness compared to wild type RV1B-infected mice, consistent with the notion that airway neutrophils significantly contribute to RV-induced airways responses.
As noted above, stimulated neutrophils produce a large number of pro-inflammatory substances which could promote airway inflammation and obstruction, including TNF-α, IL-1β and neutrophil elastase (22-25). In human airway smooth muscle cells loaded with fura 2, TNF-α enhances thrombin- and bradykinin-evoked elevations of intracellular Ca2+ (37). TNF-α increases the Ca2+ sensitivity of myofilaments by activating the RhoA signaling pathway, which in turn leads to an inhibition of myosin light chain phosphatase and an increase in myosin light chain phosphorylation (38). Recently, TNF-α-enhanced contractile responses in cultured airway smooth muscle cells were found to depend on activation of CD38, a multifunctional ectoenzyme involved in cell adhesion, signal transduction and calcium signaling (39).
In the present study, we observed a consistent reduction in TNF-α expression in CXCR2-deficent mice, as well as mice treated with a granulocyte-depleting antibody, anti-LY6G. On this basis, we employed TNFR1−/− mice to determine the contribution of TNF-α signaling to RV1B-induced airway inflammation and hyperresponsiveness. TNFR1−/− mice exhibited a significant reduction in the maximal response to methacholine 24 h after RV1B exposure, consistent with the notion that TNF-α is required for airways hyperresponsiveness following RV infection. However, our analysis was complicated by a significant reduction in airway neutrophils in TNFR1 −/− mice; hence, we could not distinguish the contribution of TNF-α signaling from the previously uncovered neutrophil requirement described above. TNF-α has been shown to mediate recruitment of neutrophils to the airways following allergen sensitization and challenge (43). Despite reconstitution of the neutrophil response with exogenous MIP-2, RV-infected TNFR1−/− mice remained less responsive to methacholine than wild type mice, indicating that a functional TNFR1 receptor is required for RV-induced airways hyperresponsiveness. Taken together with our previous results, these data suggest that neutrophils, attracted to the airways by CXCR2 ligands, induce a state of hyperresponsiveness by elaboration of TNF-α.
It is possible that other mechanisms play a role in RV-induced airways hyperresponsiveness. For example, neutrophil elastase induces airway constriction and hyperresponsiveness, as well as airway mucus production (23, 25). In our study, MUC5B gene expression was highly induced in the wild-type mice 24 h after RV1B infection, but not in the CXCR2−/− mice, suggesting that neutrophils play a role in mucin gene expression following RV infection. We also observed a significant reduction in airway lymphocytes and the lymphocyte chemotactic factors TARC/CCL17 and LARC/CCL20.in CXCR2−/− mice 24 h after RV1B infection. It is therefore conceivable that lymphocytes also play a role in the observed RV-induced airway hyperresponsiveness. Consistent with this, airway neutrophils are reduced 10-fold at 96 h following RV infection, yet airways hyperresponsiveness persists (16). However, CXCR2 −/− RV-infected mice with attenuated airway cholinergic responses exhibited significantly higher airway lymphocytes compared to the wild type mice. Since phagocytic neutrophils are ordinarily briskly recruited to the lung upon infection, the heightened lymphocytic infiltration of the airways in CXCR2 −/− animals may represent a compensatory response to the functional immunodeficiency of these mice.
In this study, we did not examine the effect of RV on mice with pre-existing airways inflammation. These initial studies in control animals are necessary to assess the disease-specific mechanisms of virus-induced asthma exacerbations. For example, our unpublished data indicate that airway inflammatory and constrictor responses in RV-infected ovalbumin sensitized- and -challenged mice are qualitatively and quantitatively different than those in normal mice. Also, we did not rule out the possibility that RV infection of airway smooth muscle cells directly influences contractile responses. However, as far as we are aware, there is no evidence that RV infects airway smooth muscle cells in vivo, and immunohistochemical stains of RV1B-infected mice have shown infection to be limited to the airway epithelium and perhaps airway inflammatory cells (16). Finally, we did not examine the requirement of CXCR2 for major group RV responses. Recent studies suggest that minor group viruses are more cytotoxic (44) and stimulate higher IFN-β production (45) in bronchial epithelial cells. On the other hand, we have shown that major and minor groups viruses stimulate similar levels of IL-8 and Akt phosphorylation (14), and major and minor groups viruses have been shown to stimulate nearly identical patterns of mRNA expression in primary human bronchial epithelial cells (46). Also, we (16) and others (28) have found similar effects of RV1B and RV16 infection in wild-type and human ICAM-1 transgenic mice, respectively.
In summary, we have demonstrated that, in naïve mice, CXCR2, neutrophils and TNF-α play a causal role in RV-induced airways hyperresponsiveness. Following RV infection, airway neutrophils release factors that regulate neutrophil chemotaxis, mucin expression and airway smooth muscle responses. Further studies using mouse models of RV1B infection may elucidate mechanisms underlying exacerbations of asthma, COPD and other chronic airways diseases.
REFERENCES
- 1.Hershenson MB, Johnston SL. Rhinovirus infections: More than a common cold. Am. J. Respir. Crit. Care Med. 2006;174:1284–1285. doi: 10.1164/rccm.200609-1387ED. [DOI] [PubMed] [Google Scholar]
- 2.Lemanske RF, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunberg K, Timmers MC, Smits HH, de Klerk EP, Dick EC, Spaan WJ, Hiemstra PS, Sterk PJ. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grünberg K, Kuijpers EA, de Klerk EP, de Gouw HW, Kroes AC, Dick EC, Sterk PJ. Effects of experimental rhinovirus 16 infection on airway hyperresponsiveness to bradykinin in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1997;155:833–838. doi: 10.1164/ajrccm.155.3.9117013. [DOI] [PubMed] [Google Scholar]
- 5.de Kluijver J, Grunberg K, Sont JK, Hoogeveen M, van Schadewijk WAAM, de Klerk EPA, Dick CR, van Krieken JHJM, Sterk PJ. Rhinovirus infection in nonasthmatic subjects: effects on intrapulmonary airways. Eur Respir J. 2002;20:274–279. doi: 10.1183/09031936.02.00247202. [DOI] [PubMed] [Google Scholar]
- 6.Fleming H, Little F, Schnurr D, Avila P, Wong H, Liu J, Yagi S, Boushey H. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar chages in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 7.Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, Kamarck ME, McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 8.Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, Kuechler E, Blass D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci USA. 1994;91:1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, Dean A, George K, Briese T, Lipkin WI. Mass tag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee W-M, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Jr., Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J. Clin. Invest. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griego SD, Weston CB, Adams JL, Tal-Singer R, Dillon SB. Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J Immunol. 2000;165:5211–5220. doi: 10.4049/jimmunol.165.9.5211. [DOI] [PubMed] [Google Scholar]
- 13.Donninger H, Glashoff R, Haitchi HM, Syce JA, Ghildyal R, van Rensburg E, Bardin PG. Rhinovirus induction of the CXC chemokine epithelial-neutrophil activating peptide-78 in bronchial epithelium. J. Infect. Dis. 2003;187:1809–1817. doi: 10.1086/375246. [DOI] [PubMed] [Google Scholar]
- 14.Newcomb DC, Sajjan U, Nanua S, Jia Y, Goldsmith AM, Bentley JK, Hershenson MB. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J. Biol. Chem. 2005;280:36952–36961. doi: 10.1074/jbc.M502449200. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MR, Johnson MW, Johnston SL. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- 16.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, Hershenson MB. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunberg K, Smits HH, Timmers MC, De Klerk EPA, Dolhain RJEM, Dick EC, Hiemstra PS, SteRK PJ. Experimental rhinovirus 16 infection . effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 18.Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 19.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 20.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kluijver J, Grunberg K, Pons D, de Klerk EP, Dick CR, Sterk PJ, Hiemstra PS. Interleukin-1beta and interleukin-1ra levels in nasal lavages during experimental rhinovirus infection in asthmatic and non-asthmatic subjects. Clin Exp Allergy. 2003;33:1415–1418. doi: 10.1046/j.1365-2222.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 22.Busse WW, Vrtis RF, Steiner R, Dick EC. In vitro incubation with influenza virus primes human polymorphonuclear leukocyte generation of superoxide. Am J Respir Cell Mol Biol. 1991;4:347–354. doi: 10.1165/ajrcmb/4.4.347. [DOI] [PubMed] [Google Scholar]
- 23.Fahy JV, Schuster A, Ueki I, Boushey HA, Nadel JA. Mucus hypersecretion in bronchiectasis. The role of neutrophil proteases. Am Rev Respir Dis. 1992;146:1430–1433. doi: 10.1164/ajrccm/146.6.1430. [DOI] [PubMed] [Google Scholar]
- 24.Cassatella MA, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, Wang W, Lin JT, Shirato K, Mitsuhashi H, Inoue H. Aerosolized human neutrophil elastase induces airway constriction and hyperresponsiveness with protection by intravenous pretreatment with half-length secretory leukoprotease inhibitor. Am J Respir Crit Care Med. 1996;153:1405–1411. doi: 10.1164/ajrccm.153.4.8616573. [DOI] [PubMed] [Google Scholar]
- 26.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunological Reviews. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 27.Tuthill TJ, Papadopoulos NG, Jourdan P, Challinor LJ, Sharp NA, Plumpton C, Shah K, Barnard S, Dash L, Burnet J, Killington RA, Rowlands DJ, Clarke NJ, Blair ED, Johnston SL. Mouse respiratory epithelial cells support efficient replication of human rhinovirus. J Gen Virol. 2003;84:2829–2836. doi: 10.1099/vir.0.19109-0. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffery PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blair ED, Clarke NJ, Johnston SL. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J. Biol. Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 30.Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R, III, Moore BB. Defective Phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol. 2003;171:4416–4424. doi: 10.4049/jimmunol.171.8.4416. [DOI] [PubMed] [Google Scholar]
- 31.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 32.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 33.Thomas KM, Taylor L, Navarro J. The interleukin-8 receptor is encoded by a neutrophil-specific cDNA clone, F3R. J. Biol. Chem. 1991;266:14839–14841. [PubMed] [Google Scholar]
- 34.Bonecchi R, Facchetti F, Dusi S, Luini W, Lissandrini D, Simmelink M, Locati M, Bernasconi S, Allavena P, Brandt E, Rossi F, Mantovani A, Sozzani S. Induction of functional IL-8 receptors by IL-4 and IL-13 in human monocytes. J Immunol. 2000;164:3862–3869. doi: 10.4049/jimmunol.164.7.3862. [DOI] [PubMed] [Google Scholar]
- 35.Patel L, Charlton SJ, Chambers JK, Macphee CH. Expression and functional analysis of chemokine receptors in human peripheral blood leukocyte populations. Cytokine. 2001;14:27–36. doi: 10.1006/cyto.2000.0851. [DOI] [PubMed] [Google Scholar]
- 36.Tani K, Su SB, Utsunomiya I, Oppenheim JJ, Wang JM. Interferon-gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998;28:502–507. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Amrani Y, Krymskaya V, Maki C, Panettieri RA., Jr Mechanisms underlying TNF-alpha effects on agonist-mediated calcium homeostasis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 1997;273:L1020–1028. doi: 10.1152/ajplung.1997.273.5.L1020. [DOI] [PubMed] [Google Scholar]
- 38.Hunter I, Cobban HJ, Vandenabeele P, MacEwan DJ, Nixon GF. Tumor necrosis factor-alpha-induced activation of RhoA in airway smooth muscle cells: role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol Pharmacol. 2003;63:714–721. doi: 10.1124/mol.63.3.714. [DOI] [PubMed] [Google Scholar]
- 39.Guedes AGP, Jude JA, Paulin J, Kita H, Lund FE, Kannan MS. Role of CD38 in TNF-{alpha}-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2008;294:L290–299. doi: 10.1152/ajplung.00367.2007. [DOI] [PubMed] [Google Scholar]
- 40.Czermak BJ, Lentsch AB, Bless NM, Schmal H, Friedl HP, Ward PA. Role of complement in in vitro and in vivo lung inflammatory reactions. J Leukoc Biol. 1998;64:40–48. doi: 10.1002/jlb.64.1.40. [DOI] [PubMed] [Google Scholar]
- 41.Medoff BD, Tager AM, Jackobek R, Means TK, Wang L, Luster AD. Antibody-antigen interaction in the airway drives early granulocyte recruitment through BLT1. Am J Physiol Lung Cell Mol Physiol. 2006;290:L170–178. doi: 10.1152/ajplung.00212.2005. [DOI] [PubMed] [Google Scholar]
- 42.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 43.Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995;154:5411–5417. [PubMed] [Google Scholar]
- 44.Bossios A, Psarras S, Gourgiotis D, Skevaki CL, Constantopoulos AG, Saxoni-Papageorgiou P, Papadopoulos NG. Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir Res. 2005;6:114. doi: 10.1186/1465-9921-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wark PA, Grissell T, Davies B, See H, Gibson PG. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology. 2009;14:180–186. doi: 10.1111/j.1440-1843.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, Yagi S, Dolganov G, Boushey H, Avila P, Wu R. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]