Abstract
Endogenous electric currents generated instantly at skin wounds direct migration of epithelial cells and are likely to be important in wound healing. Migration of fibroblasts is critical in wound healing. It remains unclear how wound electric fields guide migration of dermal fibroblasts. We report here that mouse skin wounds generated endogenous electric currents for many hours. Human dermal fibroblasts of both primary and cell-line cultures migrated directionally but slowly toward the anode in an electric field of 50–100 mV mm−1. This is different from keratinocytes, which migrate quickly to the cathode. It took more than 1 hour for dermal fibroblasts to manifest detectable directional migration. Larger field strength (400 mV mm−1) was required to induce directional migration within 1 hour after onset of the field. Phosphatidylinositol-3-OH kinase (PI3 kinase) mediates cathode-directed migration of keratinocytes. We tested the role of PI3 kinase in anode-directed migration of fibroblasts. An applied electric field activated PI3 kinase/Akt in dermal fibroblasts. Dermal fibroblasts from p110γ (a PI3 kinase catalytic subunit) null mice showed significantly decreased directional migration. These results suggest that physiological electric fields may regulate motility of dermal fibroblasts and keratinocytes differently, albeit using similar PI3 kinase-dependent mechanisms.
INTRODUCTION
Wound healing involves multiple cell types undergoing regulated sequential events: inflammation, reepithelialization, and tissue remodeling (Martin, 1997; Singer and Clark, 1999). Migration of immune cells, keratinocytes, and fibroblasts are essential elements of these events. Fibroblasts migrate into the wound, deposit extracellular matrix, organize the substratum, and contract the wound. Extracellular matrix, growth factors, cytokines, and other molecules at wounds have important chemokinetic and chemotactic effects on dermal fibroblasts (DFs; Diegelmann and Evans, 2004; Falanga, 2005; Gurtner et al., 2008).
Studies suggest the importance of biophysical factors in cell migration. Mechanical forces such as stiffness of substratum and cell mechanical stress, for example, direct migration of fibroblasts (Lo et al., 2000; Parker et al., 2002). Another biophysical factor at wounds, the naturally occurring electric field (EF), is less studied. Epithelia, typically corneal epithelium with tight junctions, actively pump ions to maintain a transepithelial electric potential difference (TEP; Klyce, 1972). The epidermis also maintains a TEP. Barker and colleagues have mapped the TEP across the human body surface and the glabrous epidermis of guinea pigs. TEPs in these studies measured 15–45 mV mm−1 with the basal side positive relative to the skin surface (Barker et al., 1982; Foulds and Barker, 1983). Wounds that break down the epithelial barrier lead to flow of positive charge (electric current) from the surrounding tissues toward the wound center, establishing EFs orientated toward the wound center. The currents return between the stratum corneum and live layers of epidermis in the opposite direction (Nuccitelli, 2003). Live tissues are electrically conductive, so EFs are generated whenever electric current flow.
Keratinocytes and many other cell types respond to applied EFs by directional cell migration, a phenomenon termed galvanotaxis or electrotaxis (Robinson, 1985; Nuccitelli, 1988; Nishimura et al., 1996; Fang et al., 1998). Because there are endogenous wound EFs, and cells important to wound healing respond to applied EFs in vitro, endogenous EFs have long been proposed to regulate wound healing (Borgens et al., 1979; Jaffe and Vanable, 1984; see also reviews by McCaig and Zhao, 1997; Nuccitelli, 2003; Robinson and Messerli, 2003; McCaig et al., 2005). Remarkably, EFs of physiological strength may override other coexisting directional cues to guide migration of the corneal epithelial cells and keratinocytes (Zhao et al., 2006; Zhao, 2009). In vivo application of EFs appears to modulate corneal epithelial wound healing (Song et al., 2002, 2004; Reid et al., 2005). Importantly, electric stimulation has been approved for the treatment of some refractory skin wounds (Olyaee Manesh et al., 2006).
The effects of physiological EFs on human DFs, however, are not well understood. Embryonic quail somitic fibroblasts migrate to the cathode in weak EFs (~ 10 mV mm−1; Erickson and Nuccitelli, 1984). Much stronger EFs (400 mV mm−1) are needed to induce cathodal migration of cell line fibroblasts (CLFBs) of murine embryonic origin (3T3 and SV101) and ligament fibroblasts (Brown and Loew, 1994; Finkelstein et al., 2004; Chao et al., 2007). Fibroblasts from bovine pulmonary artery and corneal stroma migrate to the anode in an EF of 200 mV mm−1 (Soong et al., 1990; Bai et al., 2004). Recently, Isseroff and colleagues elegantly compared electrotactic migration of human DFs with human keratinocytes. They showed that unlike keratinocytes, 1 hour exposure to a physiological EF of 100 mV mm−1 did not affect migration of human DFs (Sillman et al., 2003). Endogenous wound EFs, however, may persist for many hours, even days and weeks, until wounds heal (Illingworth and Barker, 1980). We hypothesize that longer exposure to a physiological EF affects and guides migration of human DFs. We show here that mouse skin wounds maintained endogenous electric currents for at least several hours. Prolonged exposure to a physiological EF induces directional migration of human DFs to the anode through phosphatidylinositol-3-OH kinase (PI3 kinase) signaling.
RESULTS
Skin wounds maintained EFs for many hours
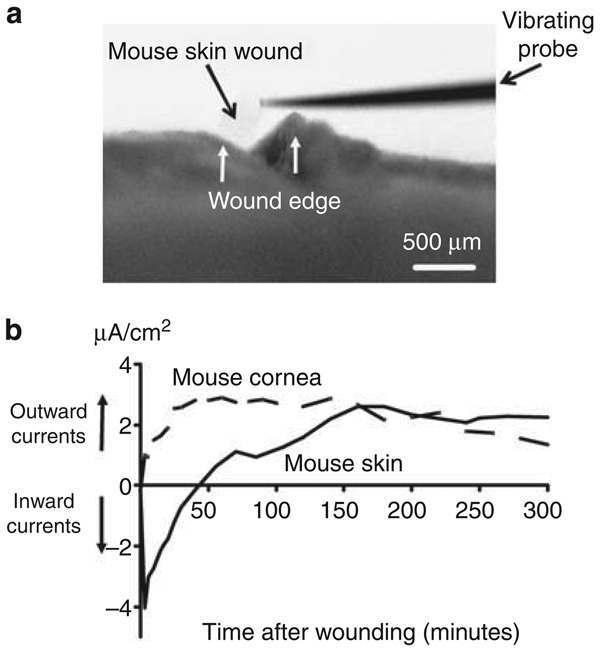
To characterize the endogenous EFs at skin wounds, we measured the endogenous electric currents at the surface of lancet wounds on the skin of recently killed mice using a vibrating probe noninvasively (Figure 1a). In moist tissues with certain resistance and conductance, the EFs are directly proportional to the electric currents as per Ohm’s law. Initially, all wounds showed transient inward currents (flow of positive charge into the wound) afterwards the current direction quickly reversed to an outward current (Figure 1b). Compared with endogenous currents at corneal wounds, the skin wound currents rose slowly, reaching a peak about 150 minutes after wounding. The electric currents were maintained at this level for up to 6 hours (the longest time point we carried out the measurements), at which time the wounds remained open with currents at ~ 3 µA cm−2.
Figure 1. Endogenous electric currents at skin wounds.
(a) View down dissecting microscope showing a vibrating probe in measuring position at a shallow lancet wound on the skin of a mouse. Bar = 500 µm. (b) Time courses of endogenous electric currents. Note the slow rise and long-lasting nature of the skin wound electric currents. Measurements were carried out immediately after cervical dislocation and wounding. Positive value: outward current; negative value: inward current. The data are grouped from three independent experiments with three individual mice.
Delayed directional migration of human dermal fibroblasts in physiological EFs
We next tested the effects of EFs on the migration of human DFs. In an EF of 100 mV mm−1 for 1 hour, cells from primary cultures, as well as a cell line of human DFs, did not show directional migration. This is consistent with a previous report (Sillman et al., 2003). Exposing the cells to the same field for longer periods, however, induced the cells to migrate directionally toward the anode. Shortly after 1 hour in an EF of 100 mV mm−1, cells extended filopodia and lamellipodia and migrated toward the anode (Figure 2; see Supplementary Movies online).
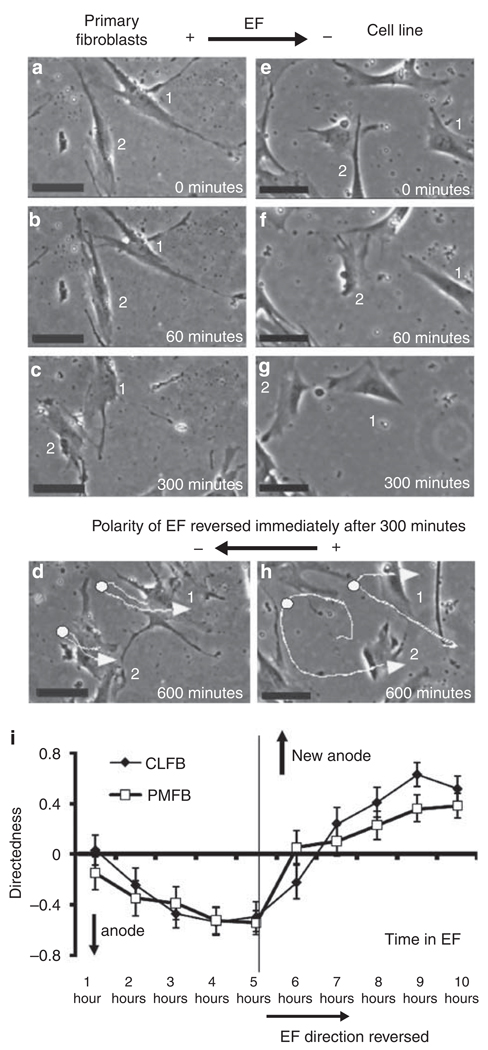
Figure 2. Human dermal fibroblasts migrate directionally toward the anode in an electric field (EF) of 100 mV mm−1 after prolonged exposure.
(a–d) Primary DFs, (e–h) fibroblast cell line. Exposure to an EF of 100 mV mm−1 for more than 60minutes started to induce cell migration to the anode. When the field polarity was reversed, the cells reversed the migration direction. Trajectories of cells are highlighted with white lines in d and h. Arrows indicate end points of the cells, and the white dots on the trajectories the point the field polarity was reversed at 5 hours (immediately after c and g). (i) Quantification of migration direction shows the reversal of cell migration following reversal of the field polarity. Data are from four independent experiments (86 cells from primary cultures and 55 cells from cell line cells). Bar = 50 µm. See Supplementary Movies online.
To ensure that the cells continued to respond to the applied EFs, we reversed the field polarity after 5 hours. This caused both types of DFs to reverse their direction of migration so that they continued to migrate toward the anode (Figure 2; Supplementary Movies). Reversal of direction of migration took place 1–2 hours after EF reverses. These events were quantified as described previously using the value of directedness (see Materials and Methods). Quantitative analyses confirmed directional migration toward the anode and the latency in response in a large number of cells from several independent experiments (Figure 2i). If cells migrated toward the left (the anode side in the first 5-hour period; Figure 2), the directedness value would be negative. If cells migrated toward the right (the anode side after reversal of the polarity in the following 5-hour period; Figure 2), the directedness value would be positive. The more directional the cells migrated to the left, the closer the directedness value is to −1. The more directional the cells migrated to the right, the closer the directedness value is to 1. Quantitative analysis confirmed the directional migration of human DFs in an applied EF and reversal of migration direction following reversal of the field polarity (Figure 2i).
Voltage and time dependence of directional migration of dermal fibroblasts in EFs
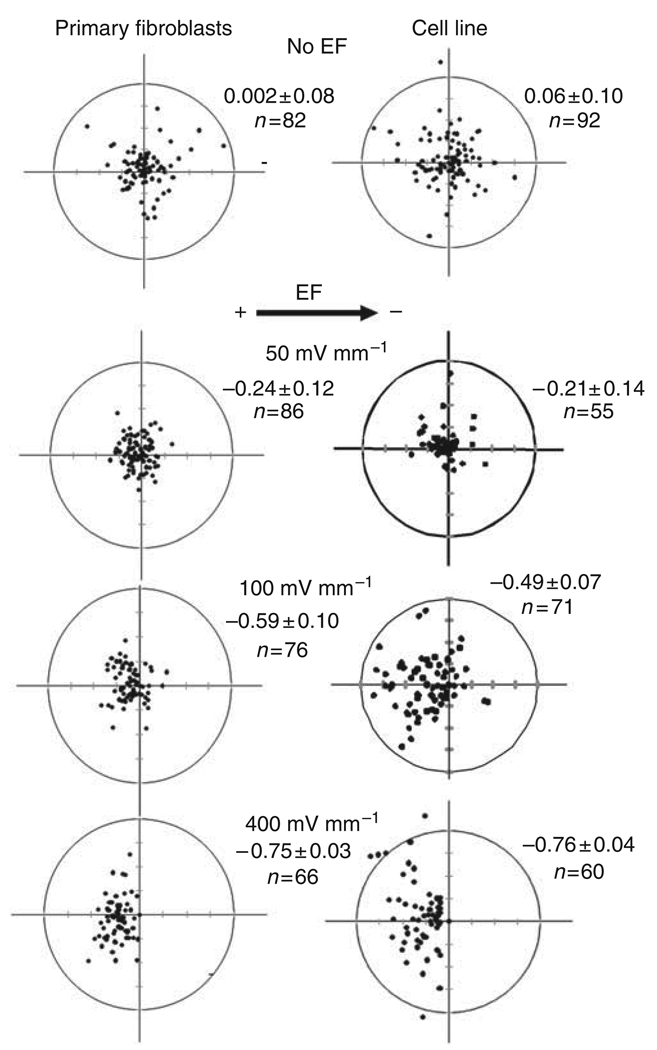
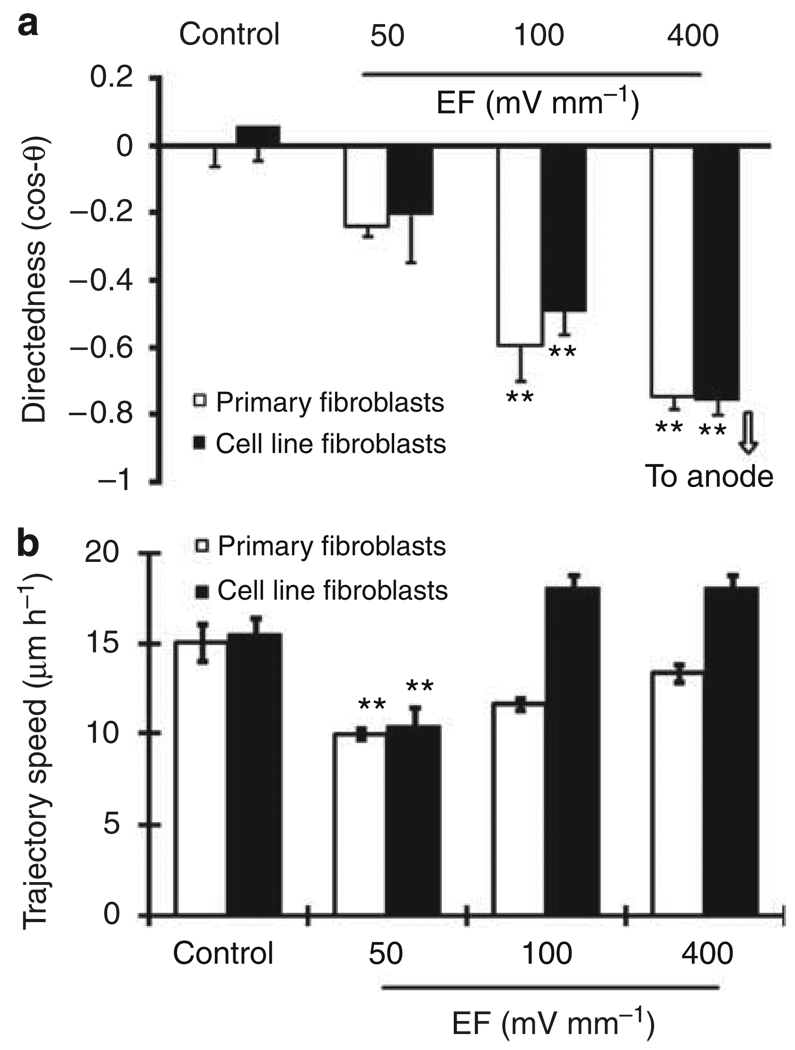
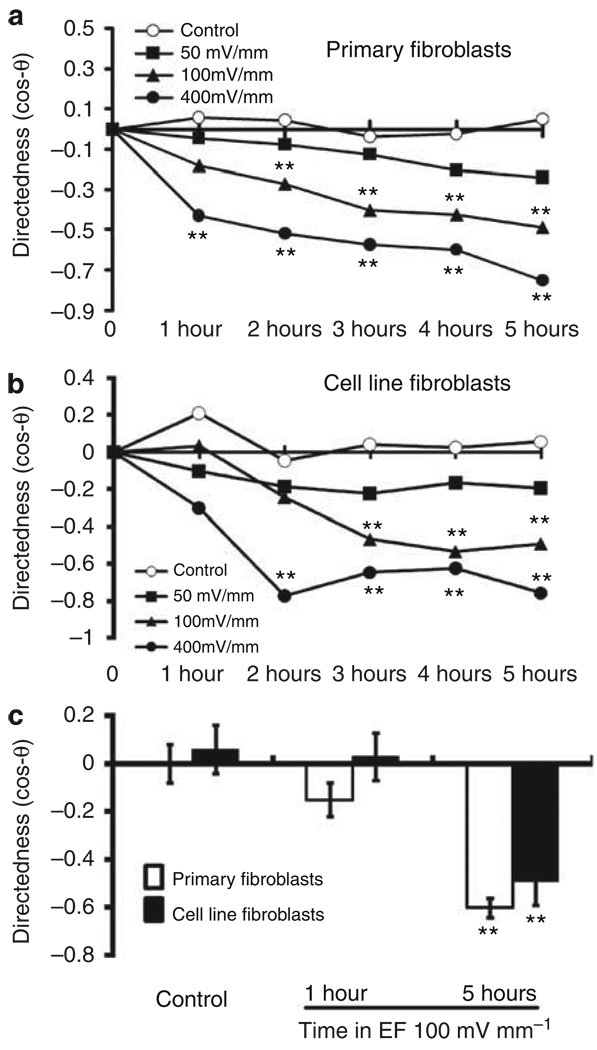
We determined the effects of different voltages on the migration of DFs. An EF of 50 mV mm−1 for 5 hours tended to induce cell migration toward the anode, but without statistical significance. A field of 100 mV mm−1 or more induced significant cell migration directed toward the anode. The higher the voltage, the more directionally the cells migrated to the anode (Figures 3 and 4a). The migration rate in cells exposed to an EF of 50 mV mm−1 was significantly reduced compared with cells not exposed to an EF (Figure 4b).
Figure 3. Scatter plots show cell migration for 5 hours in the presence or absence of an electric field (EF).
The start position of each cell was set as the origin in the center of the scatter plots (0,0). Black dots represent positions of individual cells after 5 hours. Primary culture and cell line fibroblasts migrated directionally toward the anode (left) in an EF of 100 and 400 mV mm−1. The radius of each circle represents 150 µm of translocation distance. “n” represents the total number of cells in each condition. The directedness of migration is shown on the upper right corner of each plot (mean±SEM). The cathode is to the right of each graph. The data shown are from at least four independent experiments.
Figure 4. Voltage dependence of migration directedness and migration rate of human dermal fibroblasts (DFs).
(a) Both primary cultures and a cell line of human DFs migrated directionally toward the anode. Increasing the field strength increased the migration directedness. (b) Migration rate of human DFs depended on strength of applied EF. Cell numbers 60–92 from at least three individual experiments. **P<0.01 when compared with controls with no EF.
To characterize the latency in directional cell migration, we analyzed the time course of migration directedness. In an EF of 100 mV mm−1 for 1 hour, neither primary DFs nor CLFBs showed directional migration (Figure 5). With continued exposure to an EF, the directionality of migration of both types of human DFs gradually increased and reached a peak by 4 hours. Therefore, EFs of a strength close to the in vivo value, if present for long enough (~ 4–5 hours), direct the migration of human DFs. The longer exposure time may compensate for the lower voltage.
Figure 5. Directional migration of human dermal fibroblasts (DFs) was time dependent.
(a, b) Time dependence of directional migration of both types of human DFs at different voltages. **P<0.01 when compared with the control values at the same time points without an electric field (EF). (c) In an EF of 100 mV mm−1 for 1 hour, cells migrated randomly, whereas in the same EF for 5 hours, cells showed significantly directional migration. **P<0.01 comparing with 1 hour groups.
PI3 kinase signaling mediates electrotaxis of dermal fibroblasts
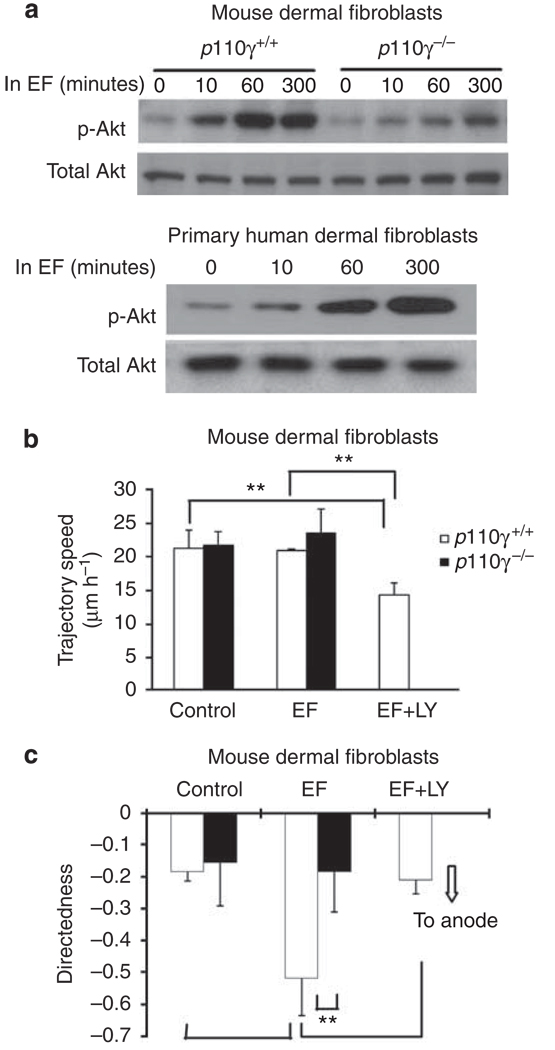
PI3 kinase mediates cathode-directed migration of corneal epithelial cells and keratinocytes (Zhao et al., 2006). We used genetic manipulation and pharmacological treatment to test if the anode-directed migration of DFs involves PI3 kinase signaling. Using western blot analysis, we found that an applied EF significantly increased the levels of phosphorylated Akt (p-Akt) in mouse and human DFs, indicating EF activation of PI3 kinase signaling (Figure 6a).
Figure 6. Role of phosphatidylinositol-3-OH kinase (PI3 kinase) signaling in electric field (EF)-directed migration of dermal fibroblast (DF) cells.
(a) Western blot analysis shows that exposure to an EF increased cell levels of phosphorylated Akt (p-Akt), indicating activation of PI3 kinase signaling. Knockout of PI3 kinase catalytic subunit (p110γ) significantly reduced PI3 kinase signaling. (b, c) Knockout of p110γ abolished directional migration, whereas the migration speed did not change significantly. Pharmacological inhibition of PI3 kinase also resulted in loss of directional migration in an EF. Cell numbers were 59–69, and 60–92 for p110γ+/+ and p110γ−/− cells, respectively, from three independent experiments. EF = 100 mV mm−1. **P<0.01.
We then used a p110γ knockout mouse to confirm the role of PI3 kinases. p110γ is the catalytic subunit of PI3 kinase-γ, a major isoform of PI3 kinases. Absence of p110γ significantly decreased EF-induced PI3 kinase signaling, as indicated by significantly reduced p-Akt levels (Figure 6a). DFs from p110γ knockout mice also showed significantly reduced EF-directed cell migration, whereas the motility (trajectory speed) was largely unaffected (Figure 6b and c). To exclude developmental defects of p110γ knockout on EF-directed migration of DFs, we further confirmed the role for PI3 kinase signaling with pharmacological inhibition of PI3 kinases. Treatment of mouse DFs with LY294002 (a PI3 kinase inhibitor) caused significant decrease in EF-directed anodal migration (Figure 6b and c).
DISCUSSION
Our study results show that skin wounds generated endogenous electric currents for at least 5 hours, probably much longer, until the epithelial wound closes and the TEP is restored (Illingworth and Barker, 1980). The outward electric currents rise to a peak slower than that at corneal wounds (Figure 1b; Reid et al., 2005). The ionic currents leave the wound and generate lateral fields on either side of the wound. DFs at wounds are therefore exposed to long-lasting endogenous currents and EFs. We mimicked the long-lasting endogenous EFs and exposed human DFs to physiological EFs for several hours. After a latent period > 1 hour, human DFs started migrating toward the anode, in a direction opposite to that of keratinocytes, which migrate toward the cathode.
Physiological EFs have different effects on migration of human DFs than they do on non-DFs and keratinocytes. In strong EFs (200–400 mV cm−1), non-DFs derived from murine embryos (NIH3T3 and SV101), fibroblasts from bovine ligament, and embryonic quail somites migrate toward the cathode. In contrast, corneal stroma fibroblasts and pulmonary artery fibroblasts migrate toward the anode (Nuccitelli and Erickson, 1983; Soong et al., 1990; Brown and Loew, 1994; Bai et al., 2004; Finkelstein et al., 2004; Chao et al., 2007). The EFs inducing these responses are larger than endogenous EFs, while the strongest endogenous EFs and currents are measured at the wound edge (Barker et al., 1982; Chiang et al., 1992; Reid et al., 2005; Mukerjee et al., 2006). EFs of approximately 150 mV mm−1 are typical of the cornified layer of the epidermis (Nuccitelli et al., 2008). In the dermis, where fibroblasts reside, the EFs are likely to be smaller because of relatively lower resistance and voltage dissipation in three-dimensional tissues. Human skin fibroblasts do not respond within 1 hour to an EF of 100 mV mm−1 (Sillman et al., 2003). However after a latent period of 1–2 hours, the cells migrate directionally to the anode (Figure 2). In an EF of 50 mV mm−1, primary cultured DFs migrated at lower rates compared with that of control cells cultured without EF (Figure 4b). The effects of physiological EFs on the direction of migration and rate are different from those on keratinocytes, which migrate to the cathode with increased speed (Nishimura et al., 1996; Farboud et al., 2000; Pullar et al., 2006; Zhao et al., 2006).
Sequential migration of different cell types into the wound has to be well choreographed to achieve proper wound healing. Physiological EFs have different effects on different cell types (for example, the effect on migration speed and direction of DFs and keratinocytes). This observation supports a recent intriguing theory of “traffic control” of sequential cell migration in wound healing (Henry et al., 2003; Bandyopadhyay et al., 2006). The authors propose that a “plasma-to-serum-to-plasma” transition during wound healing orchestrates the orderly migration of epidermal and dermal cells. Transforming growth factor-β3 (TGF-β3) binds to the type II TGFβ receptor and inhibits cell motility. Wounding exposes skin cells to serum, which contains high level of TGF-β3. Bandyopadhyay et al. (2006) showed that DFs expressed higher levels of type II TGFβ receptor than keratinocytes. Addition of TGF-β3 in a medium with plasma significantly decreased migration of DFs in a dose-dependent manner. Neutralizing TGF-β3 with antibodies abolished this effect of TGF-β3. This inhibitory effect was not present when neutralizing antibodies against TGF-β1 or TGF-β2 were added. The authors therefore suggest that high TGF-β3 level in serum may selectively inhibit motility of DFs and favor reepithelialization at an early stage after wounding (Bandyopadhyay et al., 2006). As the wound heals, the cells experience a transition of serum back to plasma with less inhibitory effects on the migration of DFs, which then migrate into the wound. Differential effects of wound EFs on the motility of keratinocytes and DFs may represent a similar regulatory mechanism that participates in the “traffic control” for the orderly and organized migration of keratinocytes and DFs during wound healing. The in vivo control of the “traffic” is likely to involve multiple factors. For example, serum and wound fluid contain many mitogens and motogens. A major one of these is platelet-derived growth factor (Werner and Grose, 2003; Barrientos et al. 2008). Platelet-derived growth factor significantly stimulates both growth and migration of DFs (Katz et al., 1991; Li et al., 2004). Biochemical factors thus may collectively regulate cell motility and migration direction, and control the traffic with biophysical cues. Coordination of these controllers will be an important topic for future research.
In addition to the endogenous wound EFs, exogenously applied EFs of higher voltage have been used in the treatment of some chronic and refractive wounds and have shown some beneficial effects (reviewed by Weiss et al., 1990; Davis and Ovington, 1993; Lee et al., 1993; Kloth and McCulloch, 1996; Poltawski and Watson, 2009). For example, a frequency of 105 Hz, an intraphase interval of 50 µ seconds, and voltages of 100–175 V over 15cm significantly accelerated healing of stage IV decubitis ulcers in humans. The percentage of healed wound increased several-fold with this scheme of electric stimulation (Kloth and Feedar, 1989). These EFs are relatively large, about 10 times the physiological field strength (~ 100 mV mm−1). In addition to the possible effects on cell migration, such EFs might have other effects, such as activating intracellular signaling pathways or stimulating blood circulation (Zhao, 2009). We showed in this study that strong EFs (400 mV mm−1) induced responses of DFs within 1 hour (Figure 5a and b), supporting the effectiveness of high voltage stimulation in regulating migration of human DFs. We speculate that exposure duration and voltage strength may compensate each other in regulating cell migration, suggesting that lower voltages for more prolonged periods of time might have an equally beneficial effect (Figure 4b).
Genetic disruption of the p110γ gene, the catalytic subunit of an isoform of PI3 kinase, significantly reduced EF-induced PI3 kinase signaling and inhibited electrotaxis of mouse DFs. This was corroborated with a PI3 kinase inhibitor (Figure 6). PI3 kinase, the same molecule that regulates cathode-directed migration in keratinocytes, also mediates anode-directed migration in fibroblasts. Considerable amount of information is also available regarding ligand–receptor interactions at the cell surface in epithelial cells in an EF, but little is presently known about fibroblasts (Forrester et al., 2007). The activation of PI3 kinase may not define the directionality; some upstream molecules are likely to do this. Further investigation to identify molecules upstream of PI3 kinase may yield direction “sensor(s).”
In conclusion, physiological EF stimulation lasting for more than 1 hour induces human DFs to migrate toward the anode, mediated by the PI3 kinase signaling pathway. Thus, endogenous wound EFs may contribute to cell “traffic control” during wound healing through differentially regulated motility of different cell types. Long exposure (> 1 hour) to physiological EFs, or short exposure (< 1 hour) to strong EFs, may modulate wound healing by regulating migration of DFs.
MATERIALS AND METHODS
Materials
Falcon tissue culture dishes (100 mm) were from BD Bioscience (Franklin Lakes, NJ). Eagle’s minimum essential medium and PI3 kinase inhibitor (LY294002) were from Sigma-Aldrich (St Louis, MO). CO2-independent medium, penicillin, and streptomycin were from Gibco Life Technology (Paisley, Scotland, UK). Antibodies against total and phosphorylated forms of Akt (Ser473) were from Cell Signaling (Beverly, MA).
Measurement of EFs at skin wounds
C57BL/6 mice were killed by cervical dislocation, which was approved by the Ethical Committee of University of Aberdeen and the Home Office. Hair was removed from the back and a lancet wound (~ 3 mm in length) was made by cutting into the full thickness skin, one wound on each of the three mice. Endogenous wound electric currents were measured using a vibrating probe (Vibrating Probe Company, Davis, CA) as described earlier (Reid et al., 2007). Briefly, an insulated stainless steel electrode was electroplated with gold and platinum. When the probe is vibrated in a fluid through which a steady current flows, it detects a voltage gradient. Measurements were carried out at room temperature with the probe approximately 50 µm away from the wound edge surface (see Figure 1a). Measurements were carried out in phosphate-buffered saline solution (pH 7.4; Sigma-Aldrich, catalog no. P4417).
Cultures of human and mouse dermal fibroblasts
A human DF cell line (161BR, ECACC No. 90011809; CLFB) and primary cultures of human DFs (PMFB) were maintained in Eagle’s minimum essential medium (10% fetal bovine serum, 100 U ml−1 penicillin, and 100 U ml−1 streptomycin), at 37 °C in a 5% CO2 incubator, and the medium was changed every 2–3 days.
Primary cultured human DFs were derived from the eyelids of adult donors (59–66 years old) who underwent surgical blepharoplasty and did not have preexisting pathological conditions. The dermal tissue was washed with Ca+ +-Mg+ +-free PBS, minced with sterile scissors, and allowed to adhere to tissue culture flasks for 30 minutes in a CO2 incubator at 37 °C. Eagle’s minimum essential medium was then added to the flasks. Fibroblasts were allowed to grow out of the tissue pieces attached to the tissue culture flask. Cells were subcultured when they reached 50–70% confluence. The fourth passage of cells from four donors was used for all experiments. Use of human donor tissues was approved by the National Health Service Grampian Ethical Committee and adhered to the Declaration of Helsinki Principles. Informed consent was obtained from each donor.
Wild-type C57BL/B6 mice and p110γ null mice were as described previously (Zhao et al., 2006). Genotypes were confirmed with PCR. All mice were kept according to institutional guidelines. Killed mice were shaved and their back skin was excised. After trimming off the interstitial tissues, DF cells were cultured using procedures as described earlier.
EF exposure and quantification of cell responses
The electrotaxis experiments were carried out as described (Zhao et al., 1996). The electrotaxis chamber was coated with calf collagen I (Sigma-Aldrich, catalog no. C 8919) at 10 mg cm−2 and incubated at 37 °C for 24 hours. Excess solution was then removed by washing with PBS and dishes were air-dried before experiments. Cells were seeded in the chamber and incubated up to 24 hours (37 °C; 5% CO2), allowing them to settle and adhere to the dish, before a roof of no. 1 cover glass was applied as a lid and sealed with silicone grease to create a thin, wafer-like chamber of ~ 200 µm deep. The small cross-sectional area provided a high resistance to the current flow and minimized Joule heating. CO2-independent culture medium was used in electrotaxis experiments to maintain stable pH.
The cells were exposed to an EF (at 50, 100, and 400 mV mm−1) for 5 hours at 37 °C. In some experiments, the field polarity was reversed for another 5 hours. To inhibit PI3 kinases, we pretreated cells with 30 µm LY294002 (a PI3 kinase inhibitor) for 2 hours before EF application. Time-lapse images of cell migration were acquired at 10 minute intervals and analyzed using the MetaMorph 6.0 imaging system (Universal Imaging Corporation, Downingtown, PA). To exclude the possible influence of cell–cell interactions on cell behavior, we analyzed only isolated cells that were not in contact with other cells.
Two components of cell migration were quantified: (1) trajectory speed, which is used to define the migration rate, is the distance (in µm) traveled by the cell divided by time (5 hours); and (2) directedness, as a parameter to define how directionally cells migrated in response to EFs (Pu and Zhao, 2005). The angle that each cell moved with respect to the EF vector was measured and its cosine value was calculated as directedness (Gruler and Nuccitelli, 1991). The cosine of this angle would be equal to 1 if the cell moved perfectly along the field vector toward the right, 0 if the cell moved perpendicularly to the field vector, and −1 if the cell moved directly toward the left. The average of all the cosine values (Σicosθ/n) gives the mean migration directedness of a population of cells, where θ is the angle between the field vector and the cellular translocation direction and n the total number of cells analyzed in each experimental condition. In the case of experiments where cells were treated with an EF and then the polarity reversed, the second half of the EF with the polarity reversed was treated as a new experiment for the calculation. Unpaired, two-tailed Student’s t-test was used for statistical analysis.
Western blotting
Primary cultured DFs from humans or mice were starved in serum-free culture medium for 8 hours before EF stimulation at 100 mV mm−1. After application of the EF for different time periods, cells (~ 5 × 106) were lysed in 500 µl SDS sample buffer. Cell extracts were resolved on 4–12% Bis-Tris gels and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk in Tris-buffered saline, and incubated with specific antibodies. Antibodies against total and phosphorylated forms of Akt (Ser473) were used at 1:1,000 dilution.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Wellcome Trust for continuous support. This work was partially supported by the UC Davis dermatology development fund. Dr Min Zhao was supported by grants from the National Eye Institute of the National Institutes of Health (R01EY019101), California Institute of Regenerative Medicine (RB1-01417), and National Science Foundation (MCB-0951199).
Abbreviations
- CLFB
cell line fibroblast
- DF
dermal fibroblast
- EF
electric field
- PI3 kinases
phosphatidylinositol-3-OH kinase
- TEP
transepithelial electric potential difference
- TGF-β3
transforming growth factor-β3
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Bai H, McCaig CD, Forrester JV, et al. DC electric fields induce distinct preangiogenic responses in microvascular and macrovascular cells. Arterioscler Thromb Vasc Biol. 2004;24:1234–1239. doi: 10.1161/01.ATV.0000131265.76828.8a. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay B, Fan J, Guan S, et al. A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093–1105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT, Jaffe LF, Vanable JW., Jr The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–R366. doi: 10.1152/ajpregu.1982.242.3.R358. [DOI] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Role of subdermal current shunts in the failure of frogs to regenerate. J Exp Zool. 1979;209:49–56. doi: 10.1002/jez.1402090106. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Loew LM. Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J Cell Biol. 1994;127:117–128. doi: 10.1083/jcb.127.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PH, Lu HH, Hung CT, et al. Effects of applied DC electric field on ligament fibroblast migration and wound healing. Connect Tissue Res. 2007;48:188–197. doi: 10.1080/03008200701424451. [DOI] [PubMed] [Google Scholar]
- Chiang M, Robinson KR, Vanable JW., Jr Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye. Exp Eye Res. 1992;54:999–1003. doi: 10.1016/0014-4835(92)90164-n. [DOI] [PubMed] [Google Scholar]
- Davis SC, Ovington LG. Electrical stimulation and ultrasound in wound healing. Dermatol Clin. 1993;11:775–781. [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Nuccitelli R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol. 1984;98:296–307. doi: 10.1083/jcb.98.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- Fang KS, Farboud B, Nuccitelli R, et al. Migration of human keratinocytes in electric fields requires growth factors and extracellular calcium. J Invest Dermatol. 1998;111:751–756. doi: 10.1046/j.1523-1747.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- Farboud B, Nuccitelli R, Schwab IR, et al. DC electric fields induce rapid directional migration in cultured human corneal epithelial cells. Exp Eye Res. 2000;70:667–673. doi: 10.1006/exer.2000.0830. [DOI] [PubMed] [Google Scholar]
- Finkelstein E, Chang W, Chao PH, et al. Roles of microtubules, cell polarity and adhesion in electric-field-mediated motility of 3T3 fibroblasts. J Cell Sci. 2004;117:1533–1545. doi: 10.1242/jcs.00986. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Lois N, Zhao M, et al. The spark of life: the role of electric fields in regulating cell behaviour using the eye as a model system. Ophthalmic Res. 2007;39:4–16. doi: 10.1159/000097901. [DOI] [PubMed] [Google Scholar]
- Foulds IS, Barker AT. Human skin battery potentials and their possible role in wound healing. Br J Dermatol. 1983;109:515–522. doi: 10.1111/j.1365-2133.1983.tb07673.x. [DOI] [PubMed] [Google Scholar]
- Gruler H, Nuccitelli R. Neural crest cell galvanotaxis: new data and a novel approach to the analysis of both galvanotaxis and chemotaxis. Cell Motil Cytoskeleton. 1991;19:121–133. doi: 10.1002/cm.970190207. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Henry G, Li W, Garner W, et al. Migration of human keratinocytes in plasma and serum and wound re-epithelialisation. Lancet. 2003;361:574–576. doi: 10.1016/S0140-6736(03)12510-X. [DOI] [PubMed] [Google Scholar]
- Illingworth CM, Barker AT. Measurement of electrical currents emerging during the regeneration of amputated finger tips in children. Clin Phys Physiol Meas. 1980;1:87–89. [Google Scholar]
- Jaffe LF, Vanable JW., Jr Electric fields and wound healing. Clin Dermatol. 1984;2:34–44. doi: 10.1016/0738-081x(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Katz MH, Alvarez AF, Kirsner RS, et al. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol. 1991;25:1054–1058. doi: 10.1016/0190-9622(91)70306-m. [DOI] [PubMed] [Google Scholar]
- Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther. 1989;69:702. doi: 10.1093/ptj/68.4.503. [DOI] [PubMed] [Google Scholar]
- Kloth LC, McCulloch JM. Promotion of wound healing with electrical stimulation. Adv Wound Care. 1996;9:42–45. [PubMed] [Google Scholar]
- Klyce SD. Electrical profiles in the corneal epithelium. J Physiol. 1972;226:407–429. doi: 10.1113/jphysiol.1972.sp009991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Canaday DJ, Doong H. A review of the biophysical basis for the clinical application of electric fields in soft-tissue repair. J Burn Care Rehabil. 1993;14:319–335. doi: 10.1097/00004630-199305000-00003. [DOI] [PubMed] [Google Scholar]
- Li W, Fan J, Chen M, et al. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309. doi: 10.1091/mbc.E03-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, et al. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, et al. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. Bioessays. 1997;19:819–826. doi: 10.1002/bies.950190912. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Mukerjee EV, Isseroff RR, Nuccitelli R, et al. Microneedle array for measuring wound generated electric fields; Conf Proc IEEE Eng Med Biol Soc; 2006. pp. 4326–4328. [DOI] [PubMed] [Google Scholar]
- Nishimura KY, Isseroff RR, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci. 1996;109:199–207. doi: 10.1242/jcs.109.1.199. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Physiological electric fields can influence cell motility, growth and polarity. Adv Cell Biol. 1988;2:213–234. [Google Scholar]
- Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R, Erickson CA. Embryonic cell motility can be guided by physiological electric fields. Exp Cell Res. 1983;147:195–201. doi: 10.1016/0014-4827(83)90284-7. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R, Nuccitelli P, Ramlatchan S, et al. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 2008;16:432–441. doi: 10.1111/j.1524-475X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olyaee Manesh A, Flemming K, Cullum NA, et al. Electromagnetic therapy for treating pressure ulcers. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD002930.pub3. CD002930. [DOI] [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16:1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- Poltawski L, Watson T. Bioelectricity and microcurrent therapy for tissue healing—a narrative review. Phys Ther Rev. 2009;14:104–114. [Google Scholar]
- Pu J, Zhao M. Golgi polarization in a strong electric field. J Cell Sci. 2005;118:1117–1128. doi: 10.1242/jcs.01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar CE, Baier BS, Kariya Y, et al. Beta4 Integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell. 2006;17:4925–4935. doi: 10.1091/mbc.E06-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc. 2007;2:661–669. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- Reid B, Song B, McCaig CD, et al. Wound healing in rat cornea: the role of electric currents. FASEB J. 2005;19:379–386. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol. 1985;101:2023. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA. Left/right, up/down: the role of endogenous electrical fields as directional signals in development, repair and invasion. Bioessays. 2003;25:759–766. doi: 10.1002/bies.10307. [DOI] [PubMed] [Google Scholar]
- Sillman AL, Quang DM, Farboud B, et al. Human dermal fibroblasts do not exhibit directional migration on collagen I in direct-current electric fields of physiological strength. Exp Dermatol. 2003;12:396–402. doi: 10.1034/j.1600-0625.2002.120406.x. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Song B, Zhao M, Forrester J, et al. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J Cell Sci. 2004;117:4681–4690. doi: 10.1242/jcs.01341. [DOI] [PubMed] [Google Scholar]
- Song B, Zhao M, Forrester JV, et al. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci USA. 2002;99:13577–13582. doi: 10.1073/pnas.202235299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong HK, Parkinson WC, Bafna S, et al. Movements of cultured corneal epithelial cells and stromal fibroblasts in electric fields. Invest Ophthalmol Vis Sci. 1990;31:2278–2282. [PubMed] [Google Scholar]
- Weiss DS, Kirsner R, Eaglstein WH. Electrical stimulation and wound healing. Arch Dermatol. 1990;126:222–225. [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and ytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Zhao M. Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Zhao M, Agius-Fernandez A, Forrester JV, et al. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J Cell Sci. 1996;109:1405–1414. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.