Abstract
The repair of critical-sized bone defects is still challenging in the fields of implantology, maxillofacial surgery and orthopaedics. Current therapies such as autografts and allografts are associated with various limitations. Cytokine-based bone tissue engineering has been attracting increasing attention. Bone-inducing agents have been locally injected to stimulate the native bone-formation activity, but without much success. The reason is that these drugs must be delivered slowly and at a low concentration to be effective. This then mimics the natural method of cytokine release. For this purpose, a suitable vehicle was developed, the so-called biomimetic coating, which can be deposited on metal implants as well as on biomaterials. Materials that are currently used to fill bony defects cannot by themselves trigger bone formation. Therefore, biological functionalization of such materials by the biomimetic method resulted in a novel biomimetic coating onto different biomaterials. Bone morphogenetic protein 2 (BMP-2)-incorporated biomimetic coating can be a solution for a large bone defect repair in the fields of dental implantology, maxillofacial surgery and orthopaedics. Here, we review the performance of the biomimetic coating both in vitro and in vivo.
Keywords: biomimetic, biphasic calcium phosphate coating, bone morphogenetic protein-2, bone tissue engineering
1. Introduction
1.1. Critical-sized bone defect
An adequate volume of bone tissue is of paramount importance to obtain excellent restoration of maxillofacial aesthetics and musculoskeletal functions. The repair rate of a bone defect is mainly dependent on the bone wound size (Schmitz & Hollinger 1986). When the defect size is greater than the healing capacity of osteogenic tissues, the fibrous connective tissue, depending upon the faster migration mechanism of fibroblasts than that of osteoblasts, regenerates faster than bone tissue and becomes dominant in the bone defect (Schmitz & Hollinger 1986; Hollinger & Kleinschmidt 1990; Mundell et al. 1993; Stetzer et al. 2002). The critical-sized bone defect (CSBD) is defined as the smallest size intraosseous wound that will not spontaneously heal completely with bone tissue, or the defect will heal by connective tissue during the lifetime of the animal (Schmitz & Hollinger 1986; Hollinger & Kleinschmidt 1990). The CSBD may, therefore, be considered to be the prototype of discontinuity defects, as a condition of failed osteogenesis for overcoming the threshold of physiological repair processes.
The need for an appropriate bone substitute is significantly increasing: in 2004 alone, more than 1 100 000 surgical procedures involving the partial excision of bone, bone grafting and inpatient fracture repair were performed in the USA at an estimated total cost of more than $5 billion (Kretlow & Mikos 2007). And the United Nations and the World Health Organization have endorsed the years 2000–2010 as the Bone and Joint Decade (Weinstein 2000; Lidgren 2003) to recognize the global burden of musculoskeletal diseases. Although tremendous efforts have been made to solve the problem, current strategies encounter a variety of limitations and leave the effective healing of the CSBD as an unsolved problem in implantology and orthopaedics.
1.2. Autografts and allografts
Autografts are still regarded as the ‘gold standard’ of bone repair because they can provide all the necessary osteogenic elements for bone regeneration such as osteoconductive three-dimensional scaffolds, osteogenic cells and osteoinductive growth factors (Carson & Bostrom 2007). However, autografting is associated with a series of limitations such as the pain and morbidity of the donor site (Heary et al. 2002; Silber et al. 2003), prolonged surgery and limited available volume (Kretlow & Mikos 2007). Besides, when used to repair the defects on specific sites (e.g. orbital bone defects) where the restoration of the complicated anatomic structure is essential, autografts are hardly manipulated to reproduce the curvature of the local site (Borghouts & Otto 1978; Asamura et al. 2010). Furthermore, resorption rates for endochondral bone have been reported up to 75 per cent (Smith & Abramson 1974) and rates of 20–30% (Vuyk & Adamson 1998) reported for membranous bone grafts. The uncontrollable and variable spontaneous resorption of autografts may compromise the precision of the restoration of orbital volume and thus potentially lead to the asymmetry of eye globes (Potter & Ellis 2004).
The conventional alternative to an autograft is an allogeneic bone graft that is obtained either from cadaveric donors or from donors undergoing total hip arthroplasty (Ayerza et al. 2006; Muscolo et al. 2006). Although it provides a good, natural and bony scaffold, allogeneic bone carries certain risks such as disease transmission (Buck et al. 1989), toxicity associated with sterilization (Moreau et al. 2000), variable host immune response (Lewandrowski et al. 2001) and limited supplies (Carson & Bostrom 2007). Also in some areas of the world, the practice of allogeneic bone transplantation is culturally unacceptable.
1.3. Bone tissue engineering
Bone tissue engineering is an interdisciplinary field that combines the knowledge and technology of material engineering and biological factors to regenerate damaged bone tissues. It includes gene-, cell- and cytokine-based therapies and has been used by researchers as a substitute for autografts and allogeneic bone grafts (Sokolsky-Papkov et al. 2007). Although gene- and cell-based therapies are attracting increasing attention (Betz et al. 2009; Chung et al. 2009; Jiang et al. 2009; Kawai et al. 2009; Nair et al. 2009), they are still in their infancy regarding safety and efficacy for humans (Kimelman et al. 2007). In contrast, cytokine-based therapy is advantageous in terms of safety, feasibility and practical potential for nearest clinical application compared with gene- and cell-based therapies (Saito & Takaoka 2003; Habraken et al. 2007). In the field of cytokine-based bone engineering, a consensus has been achieved that an appropriate carrier to deliver cytokines sustainably and at a physiological level is of paramount importance for osteogenic efficacy of the cytokines in bone restoration (Saito & Takaoka 2003; Giannoudis et al. 2007; Cartmell 2008).
An ideal carrier should:
— not only be optimal in delivering cytokines but also biocompatible to minimize host inflammatory reactivity;
— be properly biodegradable to eliminate the need for a second operation within a certain time;
— be three-dimensionally structured and surface-osteoconductive to support vascularization and bone ingrowth (Sokolsky-Papkov et al. 2007).
Although many efforts to develop this ‘ideal scaffold’ have been made during recent decades, the criteria are far from being satisfied by the current carrier strategies.
Compared with autologous and allogeneic grafts, either a naturally derived mineral phase and organic matrix such as deproteinized bovine bone (Jung et al. 2003) and demineralized bone collagen (Milovancev et al. 2007) or synthetic polymers such as a copolymer of lactic and glycolic acid (PLGA) and Polyactive (copolymer of polyethylene oxide and polybutylene terephthalate) were not limited in available volume and have been widely accepted for the reconstruction of a bone defect. However, they are neither highly osteoconductive to facilitate the adequate migration of osteogenic cells nor are they intrinsically osteoinductive. These are the two main stumbling blocks for their applications in the CSBD.
This dilemma could be overcome by a process of consolidation, namely by coating the synthetic polymers with a layer of calcium phosphate (CaP) (Murphy et al. 2000a).
1.4. Biomimetic coatings
The technology whereby calcium phosphate layers are deposited upon a substratum has been so greatly improved during recent years that it renders the process possible at physiological (Kokubo et al. 1990; Kokubo 1991) rather than at grossly unphysiological temperatures (greater than 1000°C; Klein et al. 1994; Berkhout et al. 1997; Takahashi et al. 2008). Moreover, the structure of the crystals formed (carbonated apatite) is more akin to that of bone mineral than are those of hydroxyapatite and tri- or tetracalcium phosphate (Klein et al. 1994), which are produced at exceedingly high temperatures.
1.5. Functionalization of biomimetic coatings by bioactive agents
The beauty of the so-called biomimetic coating technique is that it can be rendered osteoinductive through the coprecipitation of an osteogenic agent and, consequently, it is incorporated into the crystalline latticework of a biomimetic coating (Wen et al. 1999; Liu et al. 2003). Because such agents are proteinaceous and so inactivated at high temperatures, formerly they could be only superficially adsorbed onto a preformed biomimetic calcium phosphate coating (Murphy et al. 2000a). When exposed to a physiological milieu, such a superficially adsorbed depot of an osteogenic agent is released so rapidly as to be exhausted within a few hours, and the transient high local concentration of the drug that is thereby generated may compromise osteogenic activity (Liu et al. 2005, 2007a). In contrast, a coating-incorporated depot of such an agent is liberated gradually (Liu et al. 2005) and in a cell-mediated manner over a period of several weeks (Wernike et al. 2009). Under these conditions, osteogenic activity can be efficaciously induced and sustained at both ectopic and orthotopic sites in animal models (Liu et al. 2005, 2007a).
2. Deposition of biomimetic coating on bone substitutes
The concept of biomimetic mineralization was introduced by Kokubo and his colleagues in 1990 (Kokubo et al. 1990). The layer of calcium phosphate that is produced can reduce fibrous encapsulation (Nagano et al. 1996) and promote the differentiation of bone marrow stromal cells into osteoblasts (Ohgushi & Caplan 1999), as well as enhance the ingrowth of osseous tissue and its direct contact with the implanted materials (Yan et al. 1997; Barrere et al. 2003; Li 2003). This coating technique can be applied to various types of materials, including bioceramics (Vallet-Regi et al. 1999), metals (Campbell et al. 1996) and organic polymers (Tanahashi et al. 1995). However, using the original biomimetic technique, successful coating of the materials can be achieved only on those surfaces with active chemical groups (Stancu et al. 2004; Yin et al. 2004; Song et al. 2005), which serve as nucleation sites for mineralization (Tanahashi & Matsuda 1997; Filmon et al. 2002; Yin et al. 2004). Active chemical groups such as dihydrogen phosphate (H2PO4) or carboxylic acid (COOH) have been shown to be highly conducive to biomimetic mineralization, and those composed of methyl groups (CH3) to be unpropitious for the process (Tanahashi & Matsuda 1997). But since many of the bone substitutes available on the market, especially the polymeric materials, lack active chemical groups on their surfaces, attempts have been made to attach functional anionic ones covalently (Filmon et al. 2002). However, most of the bone substitutes that are in clinical use are either not amenable to such manipulation or, if they are, the modifications carry the risk of compromising the physico-chemical and biological properties of the material. Moreover, not only the surface chemistry but also the surface geometry and the three-dimensional structure of a polymer can influence its biomimetic mineralization (Oliveira et al. 2007). Different kinds of bone defects also resulted in the requirement for bone substitutes with different physico-chemical properties: voluminous bone defects need block- or granule-structured bone substitutes, whereas laminar bone defects need membrane-structured ones. The variety of bone substitutes makes it very difficult to modify their surface by mineralizing them.
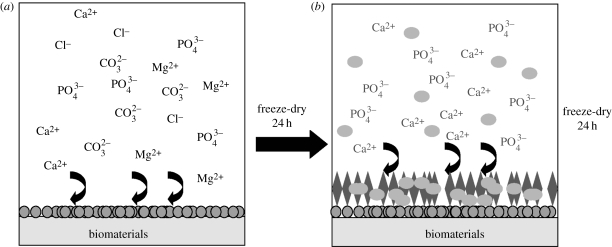
To overcome this dilemma, we refined a biomimetic coating technique that comprises two basic steps (figure 1; Liu et al. 2004a, 2006). (The coating procedure together with the in vitro characterization and in vivo evaluation of the coated biomaterials can be seen in the materials and methods section of the electronic supplementary material.)
Figure 1.
The procedure of the biphasic biomimetic calcium phosphate coating. (a) Step 1: the biomaterial is immersed in a fivefold concentrated simulated body fluid for 24 h at 37°C, 150 r.p.m. with stirring. A fine, dense layer of amorphous calcium phosphate (ACaP) thereby formed serves as a seeding substratum for the deposition of a more substantial crystalline layer. (b) Step 2: after freeze-drying, the crystalline layer will be produced by immersing the ACaP-coated biomaterials in a supersaturated calcium phosphate solution for 48 h at 37°C, 60 r.p.m. with shaking. The samples are then freeze-dried. If the crystalline layer is to be functionalized by the incorporation of bioactive agents such as bone morphogenetic protein-2 (BMP-2), then these agents are introduced into the latter medium at a certain concentration. Circles, amorphous seedling layer; ellipsoids, BMP-2; diamonds, crystalline protein-carrying layer.
Using this biphasic biomimetic coating technique, not only titanium implants (Liu et al. 2001, 2003, 2007b) but also deproteinized bovine bone (Wu et al. 2009) and four polymeric bone substitutes (Wu et al. 2008, in press; see table 1) with different physico-chemical properties can be biomimetically coated with a layer of calcium phosphate without any additional surface modification (figure 2). The morphological and physico-chemical properties of the coatings were independent of either the surface chemistry, or the surface geometry or the three-dimensional structures of the underlying materials (Wu et al. in press). The consistency of the result is due to the amorphous seeding layer. Previously, when this biphasic biomimetic coating technique was applied to titanium alloy implants (Liu et al. 2005), the ‘adhesion’ thereto of the amorphous layer of calcium phosphate was attributed to the affinity of Ca2+ and HPO42− ions for titanium (Healy & Ducheyne 1992; Barrere et al. 2002a,b). But this chemical affinity may not be involved here since such an amorphous layer of calcium phosphate can also be precipitated on polymers with the diverse surface chemistries used in our studies. A more likely explanation is that the tiny particles of calcium phosphate—which are formed under the nucleation-inhibiting influence of Mg2+ (Barrere et al. 2002a,b) and HCO32− (Barrere et al. 2002a,b)—can be captured and immobilized on the substratum by a process of mechanical gomphosis. These particles then serve as seeding centres for the subsequent growth of a crystalline latticework of calcium phosphate under conditions that are conducive to nucleation (Barrere et al. 2001). A series of findings indicated that our biphasic biomimetic CaP-coating technique has the potential to mineralize a broader spectrum of bone substitutes without the need for any additional surface modification.
Table 1.
Information on the biomaterials that were functionalized by the BMP-2-incorporated biomimetic calcium phosphate coating.
| brand name | manufacturer | composition | three-dimensional structure | status |
|---|---|---|---|---|
| Helistat | Integra, USA | collagen bovine deep flexor tendon | porous | on the market |
| Polyactive | IsoTis B.V., Bilthoven, The Netherlands | 70% polyethylene oxide (PEO) and polybutylene terephthalate (PBT) | porous | on the market |
| Ethisorb | Johnson & Johnson Medical Ltd, USA | glactin 910 and P-dioxanon | fibrous | on the market |
| PLGA | courtesy of Smith and Nephew, UK | poly(lactic-co-glycolic acid) | fibrous | clinical trial |
| Bio-Oss | Geistlich Biomaterials, Inc., Switzerland | deproteinized bovine bone (DBB) | porous | on the market |
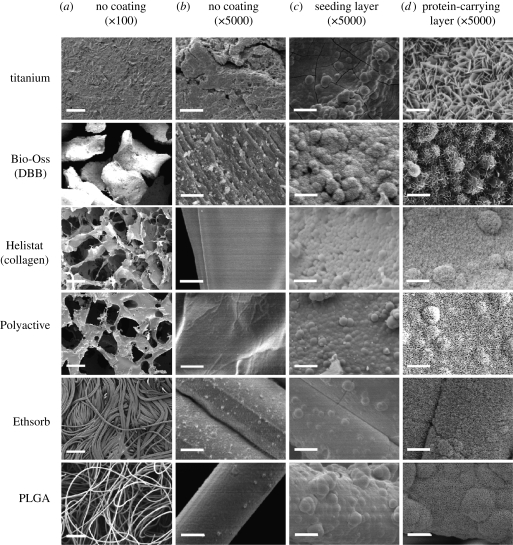
Figure 2.
(a) Scanning electron micrographs of the geometry at low magnifications and (b) the topography at high magnifications of six biomaterials in the native condition, and (c) after coating first with an amorphous seeding layer of calcium phosphate and (d) then with a crystalline one, which was coprecipitated with bovine serum albumin (BSA). The six materials include: one with metallic material titanium plates; one with bovine-derived mineral material Bio-Oss granules (deproteinized bovine bone, DBB); one with bovine-derived organic sponge Helistat (collagen); and three with synthetic polymeric membranes (Polyactive, Ethisorb and PLGA) (from top to bottom). Scale bars, (a) 200 µm and (b–d) 5 µm. (Part of this figure was reproduced with kind permission from the Journal of Tissue Engineering and Mary Ann Liebert, Inc., New Rochelle, NY).
In bone tissue engineering, a three-dimensional scaffold needs interconnective porosity to ensure the ingrowth of osteogenic cells and vascular cells. However, current direct coating techniques, either biomimetic or unphysiological, failed to mineralize on the deep surfaces of three-dimensional bone substitutes with complicated structures. This can be a major concern for scaffolds with small pores, and the apatite on the pore surface can decrease the original scaffold pore size and even block some of the tiny pores. Li et al. (2007) have reported an indirect coating preparation. Apatite was first coated on the surface of paraffin spheres of the desired sizes. The coated paraffin spheres were then moulded into foam. PLGA/pyridine solution was cast into the interspaces among the coated paraffin spheres. After the paraffin spheres were dissolved and removed by solvent, apatite was left on the pore surfaces inside the scaffold. However, the risk of some residual solvent, which is harmful to transplanted cells or host tissues (Yang et al. 2001; Lee et al. 2004), may increase dramatically because critical measures could not be taken to remove this in the presence of cytokines. Another possibility is to apply a dynamic perfusion technique to prepare coatings (Oliveira et al. 2007). Dynamic conditions allowed for the production of thicker apatite layers as a consequence of higher mineralizing rates when compared with static conditions. Micro-CT analysis clearly demonstrated that flow perfusion was the most effective condition in order to obtain well-defined apatite layers in the inner parts of the scaffolds.
3. Functionalization, coating thickness and incorporation rate of proteins
Although biomimetic calcium phosphate coatings improve the osteoconductivity of metal implants, they do not confer osteoinductivity on the implants. This is a feature of promoting the differentiation of immature progenitor cells down an osteoblastic lineage. To overcome this problem, one possible approach is to integrate functional biological agents, such as growth factors, into the biomimetic coating (Krout et al. 2005; Schliephake et al. 2005a,b). Growth factors are proteins that serve as signalling agents for cells and stimulate cellular differentiation, proliferation, migration, adhesion and gene expression (Solheim 1998a,b; Lieberman et al. 2002; Oreffo 2004). Bone tissue regeneration is an orchestrated process including angiogenesis, osteogenesis and remodelling, which can be the targets of various growth factors.
Among the most important growth factors are the sulphate-linked dimeric bone morphogenetic proteins (BMPs) that belong to the transforming growth factor-β (TGF-β) superfamily and that can specifically induce ectopic bone formation. BMP-2 has been shown to be one of the most potent members of the BMP family for the induction of bone formation in vivo (Murakami et al. 2003; Liu et al. 2005, 2007a; Takahashi et al. 2005; Jeon et al. 2007; Liu et al. 2007b). Other osteogenic BMPs, such as BMP-4 (Sun et al. 2003; Wang et al. 2003) and BMP-7 (Haidar et al. 2009c), were also shown to be potent in inducing bone regeneration. Very recently, we also described the functional characteristics of the BMP-2/7 heterodimer (more potent BMP forms) during osteoblastogenesis in comparison with BMP-2 and BMP-7 homodimers. BMP-2/7 showed a significantly lower effective concentration range than the respective homodimers and thus it facilitated a promising application potential in tissue engineering (Zheng et al. 2010). Apart from the osteoinductive growth factors, angiogenic growth factors such as basic fibroblast growth factor (β-FGF) (Park et al. 2006), vascular endothelial growth factor (VEGF) (Murphy et al. 2000b) and platelet-derived growth factor (Arm et al. 1996; Hafeman et al. 2008; Moore et al. 2009) were also shown to promote significantly bone regeneration in vivo since angiogenesis is an essential prerequisite for bone survival and regeneration (Kanczler & Oreffo 2008). Furthermore, bone regeneration can also be promoted by other bioactive factors such as osteoclast-suppressing bisphosphonate (Cartmell 2008) and cathepsin inhibitors.
There is a general consensus that, to maximize their osteoinductive efficacy, BMPs need to be carried and released in a controlled and sustained way rather than in a burst (Lutolf et al. 2003; Sokolsky-Papkov et al. 2007). Many control release systems including polymers, ceramics and hybrid materials have been developed and they have been reviewed elsewhere (Lee & Shin 2007; Mourino & Boccaccini 2009). In this review, we focus mainly on the materials functionalized by BMP-2-incorporated biomimetic calcium phosphate coatings. Using conventional coating methods, osteogenic agents can be deposited only superficially upon preformed coatings, either by adsorption (Ono et al. 1995a,b; Esenwein et al. 2001) or by binding to biofunctional proteins (Endo 1995) or by chemical treatment (Kim et al. 1996). Although high protein-incorporating efficiency (nearly 100%) is obtained with this method, the disadvantage of such attachment is that the biologically active molecules are released too rapidly to be effective upon exposure to a physiological environment (Liu et al. 2005, 2007a). Using the biomimetic technique, osteogenic agents can be incorporated into the crystal latticework of the coatings (Liu et al. 2004b). Evidence for the phenomenon is provided elsewhere (Liu et al. 2001, 2004a; 2005) and was confirmed in our recent study by Fourier-transform infrared spectroscopy (Wu et al. in press). The incorporated proteins can be released gradually and steadily at a low pharmacological level; not rapidly as in a single high-dose burst (Liu et al. 2005).
During the biomimetic approach, the osteoinductive protein is added to the modified simulated body fluid (m-SBF) solution and coprecipitated with the CaP coating onto the substrate material (Wen et al. 1999; Du et al. 2005; Liu et al. 2007a). One disadvantage of this method is that the protein-incorporating rate reported is relatively low, with approximately 3–15% of the proteins in the SBF being incorporated into the CaP coating (Liu et al. 2004b, 2007b; Luong et al. 2006). This results in a waste of the extremely expensive osteoinductive proteins, which can be a substantial burden for patients. Improvement in the biomimetic coating technique to enhance the protein-incorporating rate is therefore needed to broaden their clinical applications. Yu et al. (2009) attempted to improve the protein-incorporating rate by carefully adjusting the substrate surface area to SBF volume ratio (SSA/SV ratio). A low volume of SBF was employed to increase the SSA/SV ratio to ensure that most of the calcium and phosphate ions in the SBF solution contribute to the coating formation. They also tried to optimize the protein-loading efficiency by varying the specific configurations of the coating system, such as solution height and container selection for the SBF. The loading efficiency of a model protein, bovine serum albumin (BSA), was as high as 90 per cent, which was achieved when the ratio of the SSA to m-SBF volume was as high as 0.072.
The achievement of such a high incorporation rate is very important for decreasing the cost of growth factors that are used in biomimetic coatings. However, in this work, the final coating thickness was not clarified, because a certain coating thickness is important for restoring an adequate amount of bioactive agents and ensuring an adequate length of time for the drug delivery to support bone formation (Liu et al. 2005). Decreasing the volume of the coating solution will decrease the total content of calcium and phosphate and thus will definitely decrease the coating yield and thickness. So whether the decreased coating thickness could survive long enough to sustain the new bone formation is largely unknown. Moreover, when this method is applied to mineralize porous bone substitutes whose surface area could be a dozen times larger than a plain titanium strip, the coating thickness could be further decreased.
The essential reason for increasing the incorporation rate of protein is to decrease the volume of the coating solutions and thus the total dose of proteins. In our recent study, another way was tried to decrease the solution volume without compromising the coating thickness. We significantly increased the concentration of calcium and phosphate in the coating solutions to five times higher than in the conventional solutions we used. The preliminary results showed that a coating of a certain thickness can be achieved by using a dramatically smaller volume of the highly concentrated coating solution than that in a conventional one (G. Wu et al. 2010, unpublished data). However, the reaction condition still needs to be optimized since a slight change both in the pH value of the coating solution and in the chemical components will result in either the failure of the coating or changes in the coating properties (Chou et al. 2004; Qu & Wei 2008).
4. Release mechanisms of coating-incorporated BMP-2
The slow release kinetics of proteins can be obtained by many delivery modes; for example, entrapping agents into the carrier materials, such as polymers and compounds of calcium phosphates and gelatin, or binding proteins to titanium implants following chemical modification of the metal surface (Chen et al. 2005; Takahashi et al. 2005; Prabu et al. 2006). The mechanisms of slow delivery of growth factors by carriers can be (i) diffusion controlled, (ii) chemical and/or enzymatic reaction controlled, (iii) solvent controlled, or (iv) controlled by combinations of these mechanisms (Luginbuehl et al. 2004; Haidar et al. 2009c). The slow release of incorporated proteins into biomimetic coatings was also orchestrated by the combination of several mechanisms. Initially, a burst release (higher release rate) of incorporated proteins can be detected when exposed to an in vitro release system—phosphate buffer saline with a pH value of 7.4 (Wu et al. in press). This release lasts 3–5 days at a very high release rate and it can be attributed to the diffusion-controlled release that is governed by the solubility and diffusion coefficient of the protein, protein partitioning between the aqueous medium and the material of the delivery system as well as the protein loading and the diffusional distance (Li & Wozney 2001). This release pattern always occurs in the adsorbed proteins and is influenced by the three-dimensional structures for steric hindrance. A typical example of such a release is the release of BMP-2 from porous scaffolds that was regulated via adjustment of pore size (Whang et al. 1998) or particle size (Demers et al. 2002). In accordance with this research in our study, the influence of three-dimensional structures on the burst release rate was also shown by the initial burst release rates of coating-incorporated proteins from the two porous polymers (11% d−1) that were significantly higher than those from the two fibrous polymers (6–8% d−1). The existence of a larger depot of entrapped and incidentally adsorbed proteins in association with the porous structure than with the fibrous structures would account for the phenomenon (Wu et al. in press). Burst release of BMP-2 may cause a transient local high concentration of BMPs that may potentially over-stimulate the osteoclastic activity (Toth et al. 2009) and compromise early osteogenic activities (Zheng et al. 2010), etc. Therefore, further efforts should be made to reduce the ‘burst release’.
Subsequent to the diffusion-controlled release, a slow release profile of incorporated proteins was exhibited over a monitoring span of five weeks, after which around 65–75% for the two porous carriers and 30–40% for the two fibrous carriers of the total protein loading remained (Wu et al. in press). The protein release rates for the four carriers were similar during this slow release period and the release could be attributed to a chemical reaction-controlled process, namely coating dissolution (Wu et al. in press). In contrast, the adsorbed protein exhibited a burst release for only 3–5 days and then a complete clearance (Wu et al. in press).
Until now, investigations of release kinetics were performed by incubating protein-containing biomaterials in physiological solutions such as cell culture media or simulated body fluid, thus addressing the passive, non-cell-mediated release of the proteins from the carrier only. In vivo, however, inflammatory cells such as macrophages/foreign-body giant cells (FBGCs; multi-nucleated giant cells that mediate foreign-body reactions and eliminate foreign bodies) and osteoclasts may interact with the biomaterial. Therefore, apart from the aforementioned mechanisms, the release of incorporated proteins can also result from the interaction of host tissues. When coatings were implanted in vivo, multi-nucleated cells such as FBGCs and osteoclasts could resorb the coatings and form a resorption lacuna on the lattice work of coatings (Liu et al. 2005). It is reasonable to assume that cell-mediated protein liberation significantly influences the temporal bioavailability of growth factors within a biomaterial implantation site. This suggestion is supported by a recent in vitro study by Lee et al. (2007), who found a significant increase in the release of BSA from polymer scaffolds in the presence of rat vascular smooth muscle cells when compared with the passive release of the protein into DMEM culture media.
4.1. Osteoclast-mediated release of growth factors from natural bone matrix
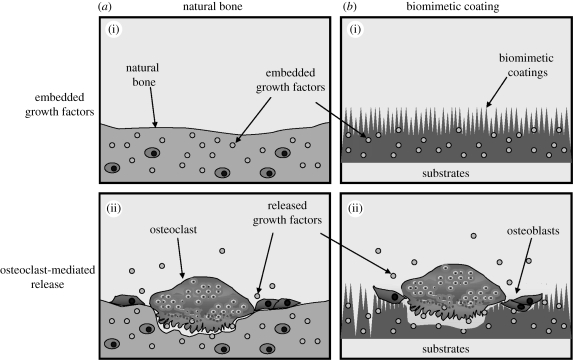
The BMP-2-incorporated CaP coating is a biomimetic because the coating degradation and the release of the growth factor partially follow the principles of natural bone remodelling (figure 3). With natural bone formation, a large amount of growth factors such as BMPs, insulin-like growth factor I and II and transforming growth factor (TGF)-β are pre-embedded in the bone inorganic mineral phase. Osteoclasts resorb bone by secreting hydrions and cathepsin K into a specialized extracellular hemivacuole formed at the interface between the cell and the bone (Chambers 2000). While hydrions dissolve bone mineral, cathepsin K can degrade native collagen and dissolve the organic matrix (Delaisse et al. 1984). When the matrix is resorbed by osteoclasts, the pre-embedded growth factors can be excavated with matrix dissolution, and activated by the prevailing acid pH in the resorption lacuna of osteoclasts. Finally, the growth factors are released and are thus available to act upon osteoblast precursors to promote bone formation. This theory was proposed by Baylink and colleagues (Howard et al. 1981) as the pathway of a local ‘coupling factor’ linking bone resorption to subsequent formation.
Figure 3.
(a)(i,ii) Graph depicting both osteoclastic release of pre-embedded growth factors in bone matrix during bone remodelling and (b)(i,ii) the osteoclast-mediated release of growth factors that were incorporated into the calcium phosphate coatings of biomaterials.
4.2. Cell-mediated release of the incorporated proteins from the biomimetic coating
Interestingly, in our technique, growth factors such as BMP-2 were also pre-embedded in the mineral phase CaP coating. When the titanium implants with BMP-2-incorporated coatings were implanted subcutaneously in rats, multi-nucleated cells such as FBGCs and osteoclasts were found on the surface of coatings and formed a resorptive lacuna on the coatings (Liu et al. 2005). By the fifth week, the mild acute inflammatory response was almost completely quelled, but the resorption of coatings by FBGCs and osteoclasts continued.
The release of coating-incorporated proteins mediated by osteoclasts was corroborated by our previous study (Wernike et al. 2009). In biphasic calcium phosphate (BCP) ceramics adsorbed with [3H]-labelled BSA ([3H]-BSA) the release kinetics were not modified by the presence of monocytes/macrophages or osteoclasts, showing that the release of the protein was induced exclusively by passive mechanisms. Superficial adsorption does not provide the possibility of modulating the liberation of proteins by means of cell-mediated release. When [3H]-BSA was coprecipitated to BCP ceramics, bone marrow cell (BMC)-derived osteoclasts markedly influenced the liberation of [3H]-BSA, inducing a sustained, cell-mediated protein release. The cell-mediated release was found exclusively with BMCs differentiated towards osteoclasts. In contrast, BMCs differentiated towards monocytes/macrophages did not modify the release kinetics of [3H]-BSA.
Although the cell-mediated release of coating-incorporated proteins by osteoclasts was proved by others' and our studies, whether such a release mode can also be mediated by FBGCs remains unclear. Although FBGCs are considered to originate from the fusion of monocyte–macrophage lineage cells and have been induced in vitro from human blood monocytes as Langhans giant cells and osteoclasts, molecular and cell biology studies have shown that the FBGC has distinctly different functional and phenotypic characteristics (Anderson 2000). In vivo, FBGCs often occupied portions of the coatings that were not covered with bone (Liu et al. 2005; Wu et al. 2009), which did not show a coupling manner.
In summary, BMP-2 incorporated into biomimetic coatings exhibited three release mechanisms: initial diffusion-controlled burst release, release chemically controlled by coating dissolution and cell (osteoclast)-mediated release.
5. in vivo degradation of biomimetically coated substrate
The basic function of bone substitutes is to provide a three-dimensional scaffold for the migration and proliferation of osteogenic and angiogenic cells. After the establishment of a bone and vascular system, the substitute should be completely degraded and eventually replaced by natural bone (Sokolsky-Papkov et al. 2007). Therefore, a certain degradation rate of a bone substitute is important to fulfil its function: a too rapid degradation of bone substitutes will result in the disorder of osteogenic activities and the dominance of connective tissue healing; on the other hand, a too slow degradation will hinder the replacement by natural bone. Many efforts have been made to modulate the degradation rate of bone substitutes (Sokolsky-Papkov et al. 2007; Mistry et al. 2009, 2010). As a methodology to improve the osteoconductivity of and to confer the osteoinductivity to bone substitutes, BMP-2-incorporated CaP coating furnishes the substitutes with a layer of calcium phosphate of a certain thickness and induces a series of biological activities, which can also modulate the degradation rate of biodegradable bone substitutes. The modulating effect of the BMP-2-incorporated CaP coating on the degradation rate of the bone substitutes is complicated by many factors such as biocompatibility and biodegradability of bone substitutes, protein incorporation and the subsequent osteogenic activities.
The biomimetic coating prepared by our technique is consistent in chemical composition and physical morphology with the substitutes of different physico-chemical properties (Wu et al. in press). After implantation, it is the coating instead of the bone substitutes themselves that interacts directly with the host tissues and determines the formation of FBGCs. The intrinsic biodegradability of the five kinds of biomaterials selected in our experiments was examined in subcutaneous sites after a five-week implantation. The highest degradation rate occurred in collagen, which was completed degraded within the monitoring span. The lowest degradation occurred in Bio-Oss and Polyactive, in which no significant degradation could be detected. Degradation rates of PLGA and Ethisorb fell in between. The degradation rates of each kind of bone substitute that bore an adsorbed depot of BMP-2 were similar to the respective untreated ones. This suggested that the adsorbed BMP-2 of the amount used in our study did not drastically affect the degradation rate of bone substitutes. Accordingly, the volume densities of the FBGCs within the bone substitutes with adsorbed BMP-2 were also similar to those of the respective untreated bone substitutes. The finding was contradictory to previous studies in that adsorbed BMP-2 was shown to increase the degradation of neutralized glass ceramic (Kessler et al. 2003). This inconsistency may result from the relatively lower amount of BMP-2 and thus the subsequent smaller bone volume induced by the adsorbed BMP-2 in our experiment.
The volume of the remaining Helistat (collagen) biomimetically coated was significantly higher than the untreated Helistat. However, for the other three polymers and the deproteinized bovine bone, we did not find such a significant difference. The results indicated that the protraction effect of coating on the degradation rate of bone substitutes can only be apparent for relatively highly degradable materials such as Helistat. It is of great interest that the volume of remaining materials such as Helistat, PLGA and Ethisorb that were functionalized with BMP-2-incorpororated CaP coating were significantly lower than respective ones that were coated with CaP coating without incorporation of BMP-2. For PLGA and Ethisorb, the volume of the remaining material was also significantly lower than with the other three groups respectively. Since the BMP-2 alone (adsorbed) did not alter the degradation of bone substitutes, it must be the BMP-2-induced osteogenic activities that contribute to such an increase in the degradation rate of Helistat, PLGA and Ethisorb. What is even more interesting is that the volume density of FBGCs was found to be lower surrounding the bone substitutes with coating-incorporated BMP-2. The mechanism accounting for the relatively higher degradation rate of the bone substitutes with coating-incorporated BMP-2 remains a mystery to be solved.
6. Osteogenic activities induced by coating-incorporated BMP-2
BMP-2 has already been intensively investigated in recent years to enhance bone formation. Hitherto, there is a general consensus that, to maximize their osteoinductive efficacies, growth factors such as BMPs need to be released in a controlled and sustained manner rather than in a burst manner (Lutolf et al. 2003; Sokolsky-Papkov et al. 2007; Haidar et al. 2009a,b). Using our technique of biomimetic production of BMP-2-incorporated CaP coatings, BMP-2 of a pharmacologically low dose is thereby incorporated into the inorganic crystal latticework and released gradually in a cell-mediated, physiological-like manner. This mode of BMP-2 delivery is more conducive to sustaining osteogenic activity than the adsorbed BMP-2. Significantly higher volume densities of newly formed bone tissue were consistently induced by coating-incorporated BMP-2 than by the adsorbed BMP-2 of similar amounts for metallic implants (Liu et al. 2005, 2007b), deproteinized bovine bone (Wu et al. 2009) and four kinds of polymers (Wu et al. 2008; figure 4).
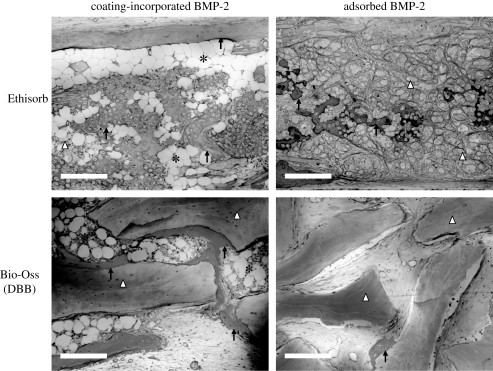
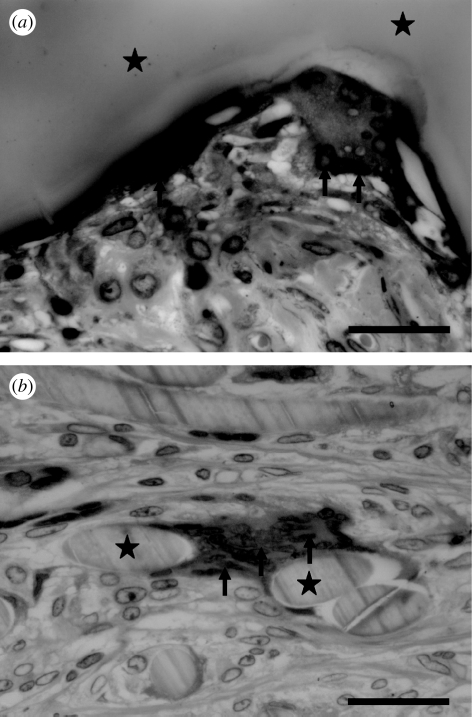
Figure 4.
Light micrographs of cross sections through the samples of Ethisorb (as a sample of a polymeric material) and Bio-Oss (deproteinized bovine bone, DBB) in the two groups (coated polymer bearing an incorporated depot of bone morphogenetic protein-2 (coating-incorporated BMP-2); uncoated polymer bearing an adsorbed depot of BMP-2 (adsorbed BMP-2)), five weeks after implantation in rats and stained with McNeil's tetrachrome, basic fuchsin and toluidine blue O. Scale bars, 200 µm. Newly regenerated bone tissue (indicated by black arrows) was extensively deposited along the surface of the two materials when they were functionalized by coating-incorporated BMP-2; a tremendous amount of bone marrow tissue (black asterisks) filled in the interstitial space. In contrast, bone tissue was sporadically deposited along the surface of the two materials that had an adsorbed depot of BMP-2; bone marrow tissue was rarely found in this group.
The three-dimensional structure of bone substitutes is also a determining factor in bone regeneration. In the current concept of bone tissue engineering, the three-dimensional geometry of the bone substitutes can be optimized to enhance their osteoconductivity for rapid bone ingrowth. A design standard such as an interconnective porous structure with a pore size greater than 300 µm and porosity less than 90 per cent was recommended owing to enhanced new bone formation and the formation of capillaries (Karageorgiou & Kaplan 2005). However, when functionalized by our technique, bone substitutes such as Polyactive, which has a similar typical structure to that which was recommended, resulted in a significantly lower volume density of bone tissue than the other three polymers (Wu et al. 2008). Furthermore, for the bone substitutes that are functionalized by the technique of BMP-2-incorporated biomimetic coating, the volume density of newly regenerated bone after a five-week implantation correlated proportionally to the surface area density of polymers at time 0 (Wu et al. 2008). Hence, for polymers that bore a BMP-2-functionalized calcium phosphate coating, the surface area density of the polymer itself overrode the influence of the other characteristics on bone formation, such as chemical composition or macroscopic form (fibrillar or sponge-like). The mechanism accounting for this phenomenon is not yet clarified. One explanation is that the higher surface area density can provide an even slower release profile of BMP-2 in vivo and the relatively lower tissue permeation also extended the release of BMP-2. Another possibility is that the compacted fibrous structures could provide a relatively stable three-dimensional environment and a good support for newly generated bone (Nie et al. 2008). This hypothesis can be supported by our data, which showed that, among the four polymers with adsorbed BMP-2, the volume density of bone tissue was also the highest within Ethisorb, which had a compacted fibrous structure and the highest surface area density (Wu et al. 2008). However, these mechanisms are still insufficient to explain the proportional correlation between the surface area density and volume density of newly formed bone tissue. In a very recent study, we mapped the spatial characteristics of osteogenic activities within the functionalized Ethisorb patch. These findings gave us some clues for the correlation. When BMP-2 was adsorbed to the Ethisorb, newly formed bone occurred in the interval connective tissue (connective tissue-located intramembraneous ossification) and did not directly contact with Ethisorb fibres. In contrast, when BMP-2 was delivered by coating incorporation, apart from the connective tissue-located intramembraneous ossification, a characteristic ossification type—BMP-2-incorporated coating-originated intramembraneous ossification—could be found within the functionalized Ethisorb (G. Wu et al. 2010, unpublished data). This type of ossification originated directly on the surface of the BMP-2-incorporated coating that was deposited on the Ethisorb fibres and formed an ‘ossification ring’. In the primary stage of this type of ossification, no ossification could be found in the surrounding connective tissue. This suggested that the coating-immobilized BMP-2 could also exert an osteoinductive effect and enrich osteoblasts directly on the interface between the coating and surrounding tissues. After the formation of ossification rings, osteotoid formed centring on the ossification rings with scattered calcified points. More calcified points could be found in the area closer to the original ossification rings. The calcified points eventually joined and formed a calcified woven bone surrounding the original ossification rings. These specific spatial characteristics suggested that this type of ossification was initiated and motivated by the coating-incorporated BMP-2. Within the inner space of BMP-2-incorporated coating-functionalized Ethisorb discs, the BMP-2-incorporated coating-originated intramembraneous ossification was the main type of bone formation and the unique mechanism for the advantages of functionalized Ethisorb discs in bone regeneration over the Ethisorb discs with adsorbed BMP-2.
This ossification may provide an explanation for the dependence of bone formation on the surface area density of the functionalized materials. The technique thus changed the current concept in tissue engineering in which the pore size and porosity are heavily emphasized (Karageorgiou & Kaplan 2005).
With the process of bone regeneration, bone marrow tissues can also be induced by both coating-incorporated BMP-2 and adsorbed BMP-2. The interstitial space in Bio-Oss/polymers with BMP-2-incorporated CaP coating and newly formed bone was filled with a significantly higher amount of bone marrow than in Bio-Oss/polymers with adsorbed BMP-2 (Wu et al. 2008, 2009). This phenomenon can be observed within all the selected substrates after a five-week implantation. The regeneration of bone marrow tissue is of great importance because it plays critical roles in many biological functions such as nutrition resources. Marrow stromal cells (MSCs) comprise a heterogeneous population of cells, including reticular endothelial cells, fibroblasts, adipocytes and osteogenic precursor cells, that play a role in the regulation of haematopoiesis (Lichtman 1981; Tavassoli & Friedenstein 1983; Allen et al. 1990). The occurrence of large amounts of bone marrow tissue indicated that the coating-incorporated BMP-2 not only induced massive bone regeneration but also conferred complete biological functions on the tissue.
7. Inflammation-modulating factors
Host reactions following the implantation of biomaterials include injury, blood–material interactions, provisional matrix formation, acute inflammation, chronic inflammation, granulation tissue development, foreign-body reaction and fibrosis/fibrous capsule development (Anderson 2000; Gretzer et al. 2006; Luttikhuizen et al. 2006). All foreign materials used for bone substitutes, such as polymers, deproteinized bovine bone or ceramic, trigger an inflammatory response in which macrophages and FBGCs participate (figure 5) (Ratner & Bryant 2004; Rodriguez et al. 2008). The pro-inflammatory cytokines that are released by migrant T-lymphocytes can suppress bone formation (Lacey et al. 2008). This osteoinhibitory cytokine signal far outweighs the potentially osteoinductive one that emanates from macrophages in the form of BMP-2 release. As a result of this inflammatory reactivity, the polymeric scaffold becomes ensheathed by a capsule of dense, fibrous connective tissue. The walling-off of the implanted material impedes its osseointegration into the surrounding tissue. This situation can be changed from pro-fibrogenic to pro-osteogenic by using an osteogenic agent such as BMP-2.
Figure 5.
Light micrographs of cross sections through discs of the two polymer types ((a) polyactive; (b) PLGA in the group of unfunctionalized uncoated polymer only), five weeks after subcutaneous implantation in rats and stained with McNeil's tetrachrome, basic fuchsin and toluidine blue O. Scale bars, 30 µm. (a) The surface of Polyactive (indicated by black asterisks) was tightly covered by a spindle-like FBGC with several nuclei (indicated by black arrows) and ruffled edges. (b) Elliptic or round cross-sectioned PLGA fibres (indicated by black asterisks) sporadically distributed in connective tissues. In the middle of the image, a PLGA fibre was embraced and tightly covered by a FBGC with more than 10 nuclei (indicated by black arrows).
In our studies, owing to the highly aseptic operation, there was no persistent acute inflammation induced by the materials and thus we focused mainly on the long-term host foreign-body reaction to implants, including FBGCs and dense fibrous capsulation, which may lead to the failure of implants. To mimic the pro-fibrogenic environment in a large volume or large area CSBD, a subcutaneous ectopic bone induction model was adopted.
After a five-week implantation, significantly lower volume densities of FBGCs were found within the polymers with coating-incorporated BMP-2 than with the controls, such as the polymers with adsorbed BMP-2, polymers with coating or polymer alone. This phenomenon was found for three polymers, Polyactive, PLGA and Ethisorb (Wu et al. 2008), but was not found for collagen, which degraded so fast that no collagen remained after a five-week implantation and thus no FBGC reactions could be detected in the two groups without coatings. A consistent result was also found for metallic materials (Liu et al. 2005) and deproteinized bovine bone (Wu et al. 2009). The findings indicated that the BMP-2-incorporated biomimetic coating not only induced and sustained bone formation with a higher efficiency, but also reduced host inflammation such as FBGC formation. The mechanisms accounting for the phenomenon remain unclear. However, past research provides some clues for the possible pathways.
Firstly, FBGC formation is dependent on the surface properties of implants. When cells approach an implant material, it is unlikely that they will make direct contact with its surface. Rather, the rapid adsorption of proteins from blood (or serum) effectively translates the structure and composition of the foreign surface into a biological language (Wilson et al. 2005). The apatite coating can reduce fibrous encapsulation (Nagano et al. 1996), promote bone ingrowth, enhance direct bone contact (Yan et al. 1997) and has also been shown to promote differentiation of bone marrow stroma cells along the osteogenic lineage (Ohgushi & Caplan 1999). This may be partially due to the enhanced adsorption of both fibronectin and vitronectin on calcium phosphate, compared with titanium, corresponding to a significant increase in osteoblast precursor attachment (Kilpadi et al. 2001). In our very recent study, after a 14 day implantation when the coating still remained, the volume densities of FBGCs in the groups of Ethisorb coated with or without incorporated BMP-2 were significantly lower than those in the groups of Ethisorb uncoated with or without adsorbed BMP-2 (G. Wu et al. 2010, unpublished data). However, the influence of coatings on the foreign-body reaction can only be apparent before complete degradation, after which the biocompatibility of underlying substrates becomes dominant in determining the foreign-body reaction. This could account for the phenomenon that volume densities of FBGCs in the groups of coated polymers or deproteinized bovine bone were similar to those in the groups of polymers or deproteinized bovine bone either alone or with adsorbed BMP-2 after a five-week implantation (Wu et al. 2008).
The foreign-body reaction was found to be significantly reduced in the presence of coating-incorporated BMP-2. In contrast, the BMP-2 adsorbed onto the substrates, even though it had the same or a similar amount of BMP-2 as the coating-incorporated one, failed to do so (Wu et al. 2008, 2009). The findings suggested that the biomimetic coating and its incorporated BMP-2-induced osteogenic activities instead of BMP-2 itself accounted for the significantly reduced inflammatory reaction. In our very recent study, the advantage of coating-incorporated BMP-2 in suppressing the FBGC reaction was not apparent before the vigorous regeneration of bone tissue. Therefore, the BMP-2-induced osteogenic activities should account for the phenomenon (G. Wu et al. 2010, unpublished data). The mechanism may lie in both the blocking of the antigen presentation and the biochemical depression of the inflammatory reaction by the newly formed bone tissue. The coating can significantly improve the osteogenic cell adhesion and thus expedited the deposition of bone tissue that can shield the antigen presentation of the overlying substrates. On the other hand, osteopontin (CD44 ligands) regenerated during BMP-2-induced bone formation may combine competitively with the CD44 surface receptors on macrophages, and thus inhibit their multi-nucleation process (Sterling et al. 1998), which is indispensable for the formation of FBGCs (Tsai et al. 2005). In our study of Bio-Oss, the volume densities of the dense fibrous capsule surrounding the Bio-Oss with coating were significantly lower than those surrounding the Bio-Oss with adsorbed BMP-2 or that was untreated. Also, the dense fibrous capsule could be further significantly reduced when BMP-2 was incorporated. Enhanced vascularization during BMP-2-induced osteoinductive activities (Hausman et al. 2001) was shown to reduce fibrotic activity (Kyriakides et al. 1999). The effect may be mediated partially by the increased access of plasma fibronectin, which could significantly reduce the thickness of fibrous capsules (Keselowsky et al. 2007). Moreover, newly formed bone marrow tissue (figure 4) may also suppress the inflammation. In bone marrow, a population of non-phagocytic and fibroblast-like adherent cells, termed the mesenchymal stem cell (Prockop 1997; Gerson 1999; Koc et al. 1999; Reese et al. 1999), can suppress the activation of T cells and modulate the immune function of the major cell populations involved in alloantigen recognition and elimination, including antigen-presenting cells and natural killer cells (Rasmusson 2006). MSCs are capable of mediating the immunosuppressive effect and decreasing the production of inflammatory cytokines (Aggarwal & Pittenger 2005; Corcione et al. 2006), which may partially account for the reduced inflammatory reaction, such as FBGCs and fibrous capsules in our experiment.
8. Conclusions
The repair of CSBDs remains a formidable challenge in the fields of implantology, maxillofacial surgery and orthopaedics. The traditional bone fillers such as autografts and allografts are associated with various disadvantages. As alternatives, both synthetic and xenograft materials have been developed. However, most of these materials are neither intrinsically osteoinductive nor highly osteoconductive. Cytokine-based tissue engineering is a promising strategy to solve the problem. Various slow control release systems of bioactive cytokines have been developed to promote bone regeneration based on different scaffolds. Compared with some other techniques, the biphasic biomimetic calcium phosphate coating exhibited a very broad applicability to the biomaterials that were of different three-dimensional geometries, surface topographies and surface chemistries. The biomimetic coating enabled a controlled, local and slow release of BMP-2 by means of three mechanisms: initial diffusion-controlled burst release, release chemically controlled by coating dissolution and cell (osteoclast)-mediated release. The biomimetic coating can protract the in vivo degradation of biomaterials of a high but not low degradability. The coating-incorporated BMP-2 induced the vigorous regeneration of bone and bone marrow tissues with significantly higher efficiency than the adsorbed BMP-2. Meanwhile, the host foreign-body reaction to the implanted biomaterials could be significantly reduced through many pathways. Further studies of this technique can focus on several aspects such as the coating preparation on voluminous three-dimensional scaffolds, the mechanisms of immunosuppression and the adoption of more potent BMP forms like BMP-2/7 heterodimers, etc.
The biomaterials that are functionalized using the biomimetic calcium phosphate coating technique are a promising therapy for the bone engineering of CSBDs.
Footnotes
One contribution to a Theme Supplement ‘Scaling the heights—challenges in medical materials: an issue in honour of William Bonfield, Part II. Bone and tissue engineering’.
References
- Aggarwal S., Pittenger M. F. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. ( 10.1182/blood-2004-04-1559) [DOI] [PubMed] [Google Scholar]
- Allen T. D., Dexter T. M., Simmons P. J. 1990. Marrow biology and stem cells. Immunol. Ser. 49, 1–38. [PubMed] [Google Scholar]
- Anderson J. M. 2000. Multinucleated giant cells. Curr. Opin. Hematol. 7, 40–47. ( 10.1097/00062752-200001000-00008) [DOI] [PubMed] [Google Scholar]
- Arm D. M., Tencer A. F., Bain S. D., Celino D. 1996. Effect of controlled release of platelet-derived growth factor from a porous hydroxyapatite implant on bone ingrowth. Biomaterials 17, 703–709. ( 10.1016/0142-9612(96)86740-8) [DOI] [PubMed] [Google Scholar]
- Asamura S., Ikada Y., Matsunaga K., Wada M., Isogai N. 2010. Treatment of orbital floor fracture using a periosteum–polymer complex. J. Craniomaxillofac. Surg. 38, 197–203. ( 10.1016/j.jcms.2009.06.011) [DOI] [PubMed] [Google Scholar]
- Ayerza M. A., Aponte-Tinao L. A., Abalo E., Muscolo D. L. 2006. Continuity and function of patellar tendon host-donor suture in tibial allograft. Clin. Orthop. Relat. Res. 450, 33–38. ( 10.1097/01.blo.0000229291.21722.b5) [DOI] [PubMed] [Google Scholar]
- Barrere F., Layrolle P., Van Blitterswijk C. A., De Groot K. 2001. Biomimetic coatings on titanium: a crystal growth study of octacalcium phosphate. J. Mater. Sci. Mater. Med. 12, 529–534. ( 10.1023/A:1011271713758) [DOI] [PubMed] [Google Scholar]
- Barrere F., van Blitterswijk C. A., de Groot K., Layrolle P. 2002a Nucleation of biomimetic Ca–P coatings on ti6A14V from a SBF ×5 solution: influence of magnesium. Biomaterials 23, 2211–2220. ( 10.1016/S0142-9612(01)00354-4) [DOI] [PubMed] [Google Scholar]
- Barrere F., van Blitterswijk C. A., de Groot K., Layrolle P. 2002b Influence of ionic strength and carbonate on the Ca-P coating formation from SBFx5 solution. Biomaterials 23, 1921–1930. ( 10.1016/S0142-9612(01)00318-0) [DOI] [PubMed] [Google Scholar]
- Barrere F., van der Valk C. M., Meijer G., Dalmeijer R. A., de Groot K., Layrolle P. 2003. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B Appl. Biomater. 67, 655–665. ( 10.1002/jbm.b.10057) [DOI] [PubMed] [Google Scholar]
- Berkhout T. A., et al. 1997. SR-12813 lowers plasma cholesterol in beagle dogs by decreasing cholesterol biosynthesis. Atherosclerosis 133, 203–212. ( 10.1016/S0021-9150(97)00131-7) [DOI] [PubMed] [Google Scholar]
- Betz O. B., et al. 2009. The repair of critical size bone defects using expedited, autologous BMP-2 gene activated fat implants. Tissue Eng. Part A 16, 1093–1101. ( 10.1089/ten.tea.2009.0656) [DOI] [PubMed] [Google Scholar]
- Borghouts J. M., Otto A. J. 1978. Silicone sheet and bead implants to correct the deformities of inadequately healed orbital fractures. Br. J. Plast. Surg. 31, 254–258. ( 10.1016/S0007-1226(78)90096-6) [DOI] [PubMed] [Google Scholar]
- Buck B. E., Malinin T. I., Brown M. D. 1989. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin. Orthop. Relat. Res. 240, 129–136. [PubMed] [Google Scholar]
- Campbell A. A., Fryxell G. E., Linehan J. C., Graff G. L. 1996. Surface-induced mineralization: a new method for producing calcium phosphate coatings. J. Biomed. Mater. Res. 32, 111–118. () [DOI] [PubMed] [Google Scholar]
- Carson J. S., Bostrom M. P. 2007. Synthetic bone scaffolds and fracture repair. Injury 38(Suppl. 1), S33–S37. ( 10.1016/j.injury.2007.02.008) [DOI] [PubMed] [Google Scholar]
- Cartmell S. 2008. Controlled release scaffolds for bone tissue engineering. J. Pharm. Sci. 98, 430–441. ( 10.1002/jps.21431) [DOI] [PubMed] [Google Scholar]
- Chambers T. J. 2000. Regulation of the differentiation and function of osteoclasts. J Pathol 192, 4–13. () [DOI] [PubMed] [Google Scholar]
- Chen P. R., Chen M. H., Lin F. H., Su W. Y. 2005. Release characteristics and bioactivity of gelatin-tricalcium phosphate membranes covalently immobilized with nerve growth factors. Biomaterials 26, 6579–6587. ( 10.1016/j.biomaterials.2005.03.037) [DOI] [PubMed] [Google Scholar]
- Chou Y. F., Chiou W. A., Xu Y., Dunn J. C., Wu B. M. 2004. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials 25, 5323–5331. ( 10.1016/j.biomaterials.2003.12.037) [DOI] [PubMed] [Google Scholar]
- Chung I. H., Yamaza T., Zhao H., Choung P. H., Shi S., Chai Y. 2009. Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells 27, 866–877. ( 10.1002/stem.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A., et al. 2006. Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372. ( 10.1182/blood-2005-07-2657) [DOI] [PubMed] [Google Scholar]
- Delaisse J. M., Eeckhout Y., Vaes G. 1984. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem. Biophys. Res. Commun. 125, 441–447. ( 10.1016/0006-291X(84)90560-6) [DOI] [PubMed] [Google Scholar]
- Demers C. N., Tabrizian M., Petit A., Hamdy R. C., Yahia L. 2002. Effect of experimental parameters on the in vitro release kinetics of transforming growth factor beta1 from coral particles. J. Biomed. Mater. Res. 59, 403–410. ( 10.1002/jbm.1256) [DOI] [PubMed] [Google Scholar]
- Du C., Schneider G. B., Zaharias R., Abbott C., Seabold D., Stanford C., Moradian-Oldak J. 2005. Apatite/amelogenin coating on titanium promotes osteogenic gene expression. J. Dent. Res. 84, 1070–1074. [DOI] [PubMed] [Google Scholar]
- Endo K. 1995. Chemical modification of metallic implant surfaces with biofunctional proteins (part 2). Corrosion resistance of a chemically modified NiTi alloy. Dent. Mater. J. 14, 199–210. [DOI] [PubMed] [Google Scholar]
- Esenwein S. A., Esenwein S., Herr G., Muhr G., Kusswetter W., Hartwig C. H. 2001. Osteogenetic activity of BMP-3-coated titanium specimens of different surface texture at the orthotopic implant bed of giant rabbits. Chirurg 72, 1360–1368. ( 10.1007/s001040170043) [DOI] [PubMed] [Google Scholar]
- Filmon R., Grizon F., Basle M. F., Chappaard D. 2002. Effects of negatively charged groups (carboxymethyl) on the calcification of poly(2-hydroxyethyl methacrylate). Biomaterials 23, 3053–3059. ( 10.1016/S0142-9612(02)00069-8) [DOI] [PubMed] [Google Scholar]
- Gerson S. L. 1999. Mesenchymal stem cells: no longer second class marrow citizens. Nat. Med. 5, 262–264. ( 10.1038/6470) [DOI] [PubMed] [Google Scholar]
- Giannoudis P. V., Einhorn T. A., Marsh D. 2007. Fracture healing: the diamond concept. Injury 38(Suppl. 4), S3–S6. ( 10.1016/S0020-1383(08)70003-2) [DOI] [PubMed] [Google Scholar]
- Gretzer C., Emanuelsson L., Liljensten E., Thomsen P. 2006. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J. Biomater. Sci. Polym. Ed. 17, 669–687. ( 10.1163/156856206777346340) [DOI] [PubMed] [Google Scholar]
- Habraken W. J., Wolke J. G., Jansen J. A. 2007. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 59, 234–248. ( 10.1016/j.addr.2007.03.011) [DOI] [PubMed] [Google Scholar]
- Hafeman A. E., Li B., Yoshii T., Zienkiewicz K., Davidson J. M., Guelcher S. A. 2008. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharm. Res. 25, 2387–2399. ( 10.1007/s11095-008-9618-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar Z. S., Hamdy R. C., Tabrizian M. 2009a Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol. Lett. 31, 1817–1824. ( 10.1007/s10529-009-0099-x) [DOI] [PubMed] [Google Scholar]
- Haidar Z. S., Hamdy R. C., Tabrizian M. 2009b Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 31, 1825–1835. ( 10.1007/s10529-009-0100-8) [DOI] [PubMed] [Google Scholar]
- Haidar Z. S., Tabrizian M., Hamdy R. C. 2009c A hybrid rhOP-1 delivery system enhances new bone regeneration and consolidation in a rabbit model of distraction osteogenesis. Growth Factors 28, 44–55. ( 10.3109/08977190903367788) [DOI] [PubMed] [Google Scholar]
- Hausman M. R., Schaffler M. B., Majeska R. J. 2001. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 29, 560–564. ( 10.1016/S8756-3282(01)00608-1) [DOI] [PubMed] [Google Scholar]
- Healy K. E., Ducheyne P. 1992. Hydration and preferential molecular adsorption on titanium in vitro. Biomaterials 13, 553–561. ( 10.1016/0142-9612(92)90108-Z) [DOI] [PubMed] [Google Scholar]
- Heary R. F., Schlenk R. P., Sacchieri T. A., Barone D., Brotea C. 2002. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery 50, 510–516; discussion 516–517 ( 10.1097/00006123-200203000-00015) [DOI] [PubMed] [Google Scholar]
- Hollinger J. O., Kleinschmidt J. C. 1990. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1, 60–68. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Bottemiller B. L., Turner R. T., Rader J. I., Baylink D. J. 1981. Parathyroid hormone stimulates bone formation and resorption in organ culture: evidence for a coupling mechanism. Proc. Natl Acad. Sci. USA 78, 3204–3208. ( 10.1073/pnas.78.5.3204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon O., Song S. J., Kang S. W., Putnam A. J., Kim B. S. 2007. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(l-lactic-co-glycolic acid) scaffold. Biomaterials 28, 2763–2771. ( 10.1016/j.biomaterials.2007.02.023) [DOI] [PubMed] [Google Scholar]
- Jiang X. Q., Sun X. J., Lai H. C., Zhao J., Wang S. Y., Zhang Z. Y. 2009. Maxillary sinus floor elevation using a tissue-engineered bone complex with beta-TCP and BMP-2 gene-modified bMSCs in rabbits. Clin. Oral Implants Res. 20, 1333–1340. ( 10.1111/j.1600-0501.2009.01755.x) [DOI] [PubMed] [Google Scholar]
- Jung R. E., Glauser R., Scharer P., Hammerle C. H., Sailer H. F., Weber F. E. 2003. Effect of rhBMP-2 on guided bone regeneration in humans. Clin. Oral Implants Res. 14, 556–568. ( 10.1034/j.1600-0501.2003.00921.x) [DOI] [PubMed] [Google Scholar]
- Kanczler J. M., Oreffo R. O. 2008. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cell. Mater. 15, 100–114. [DOI] [PubMed] [Google Scholar]
- Karageorgiou V., Kaplan D. 2005. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474–5491. ( 10.1016/j.biomaterials.2005.02.002) [DOI] [PubMed] [Google Scholar]
- Kawai M., Maruyama H., Bessho K., Yamamoto H., Miyazaki J., Yamamoto T. 2009. Simple strategy for bone regeneration with a BMP-2/7 gene expression cassette vector. Biochem. Biophys. Res. Commun. 390, 1012–1017. ( 10.1016/j.bbrc.2009.10.099) [DOI] [PubMed] [Google Scholar]
- Keselowsky B. G., Bridges A. W., Burns K. L., Tate C. C., Babensee J. E., LaPlaca M. C., Garcia A. J. 2007. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials 28, 3626–3631. ( 10.1016/j.biomaterials.2007.04.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S., Mayr-Wohlfart U., Ignatius A., Puhl W., Claes L., Gunther K. P. 2003. The impact of bone morphogenetic protein-2 (BMP-2), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF) on osseointegration, degradation and biomechanical properties of a synthetic bone substitute. Z. Orthop. Ihre. Grenzgeb. 141, 472–480. ( 10.1055/s-2003-41569) [DOI] [PubMed] [Google Scholar]
- Kilpadi K. L., Chang P. L., Bellis S. L. 2001. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J. Biomed. Mater. Res. 57, 258–267. () [DOI] [PubMed] [Google Scholar]
- Kim H. M., Miyaji F., Kokubo T., Nakamura T. 1996. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J. Biomed. Mater. Res. 32, 409–417. () [DOI] [PubMed] [Google Scholar]
- Kimelman N., Pelled G., Helm G. A., Huard J., Schwarz E. M., Gazit D. 2007. Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 13, 1135–1150. ( 10.1089/ten.2007.0096) [DOI] [PubMed] [Google Scholar]
- Klein C. P., Patka P., Wolke J. G., de Blieck-Hogervorst J. M., de Groot K. 1994. Long-term in vivo study of plasma-sprayed coatings on titanium alloys of tetracalcium phosphate, hydroxyapatite and alpha-tricalcium phosphate. Biomaterials 15, 146–150. ( 10.1016/0142-9612(94)90264-X) [DOI] [PubMed] [Google Scholar]
- Koc O. N., et al. 1999. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp. Hematol. 27, 1675–1681. ( 10.1016/S0301-472X(99)00101-0) [DOI] [PubMed] [Google Scholar]
- Kokubo T. 1991. Bioactive glass ceramics: properties and applications. Biomaterials 12, 155–163. ( 10.1016/0142-9612(91)90194-F) [DOI] [PubMed] [Google Scholar]
- Kokubo T., Ito S., Huang Z. T., Hayashi T., Sakka S., Kitsugi T., Yamamuro T. 1990. Ca,P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 24, 331–343. ( 10.1002/jbm.820240306) [DOI] [PubMed] [Google Scholar]
- Kretlow J. D., Mikos A. G. 2007. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 13, 927–938. ( 10.1089/ten.2006.0394) [DOI] [PubMed] [Google Scholar]
- Krout A., Wen H. B., Hippensteel E., Li P. 2005. A hybrid coating of biomimetic apatite and osteocalcin. J. Biomed. Mater. Res. A 73, 377–387. ( 10.1002/jbm.a.30310) [DOI] [PubMed] [Google Scholar]
- Kyriakides T. R., Leach K. J., Hoffman A. S., Ratner B. D., Bornstein P. 1999. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl Acad. Sci. USA 96, 4449–4454. ( 10.1073/pnas.96.8.4449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey D. C., Simmons P. J., Graves S. E., Hamilton J. A. 2008. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage/OARS, Osteoarthritis Res. Soc. 17, 735–742. ( 10.1016/j.joca.2008.11.011) [DOI] [PubMed] [Google Scholar]
- Lee S. H., Shin H. 2007. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 59, 339–359. ( 10.1016/j.addr.2007.03.016) [DOI] [PubMed] [Google Scholar]
- Lee S. H., Kim B. S., Kim S. H., Kang S. W., Kim Y. H. 2004. Thermally produced biodegradable scaffolds for cartilage tissue engineering. Macromol. Biosci. 4, 802–810. ( 10.1002/mabi.200400021) [DOI] [PubMed] [Google Scholar]
- Lee M., Chen T. T., Iruela-Arispe M. L., Wu B. M., Dunn J. C. 2007. Modulation of protein delivery from modular polymer scaffolds. Biomaterials 28, 1862–1870. ( 10.1016/j.biomaterials.2006.12.006) [DOI] [PubMed] [Google Scholar]
- Lewandrowski K. U., Rebmann V., Passler M., Schollmeier G., Ekkernkamp A., Grosse-Wilde H., Tomford W. W. 2001. Immune response to perforated and partially demineralized bone allografts. J. Orthop. Sci. 6, 545–555. ( 10.1007/s007760100011) [DOI] [PubMed] [Google Scholar]
- Li P. 2003. Biomimetic nano-apatite coating capable of promoting bone ingrowth. J. Biomed. Mater. Res. A 66, 79–85. ( 10.1002/jbm.a.10519) [DOI] [PubMed] [Google Scholar]
- Li R. H., Wozney J. M. 2001. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 19, 255–265. ( 10.1016/S0167-7799(01)01665-1) [DOI] [PubMed] [Google Scholar]
- Li J., Beaussart A., Chen Y., Mak A. F. 2007. Transfer of apatite coating from porogens to scaffolds: uniform apatite coating within porous poly(dl-lactic-co-glycolic acid) scaffold in vitro. J. Biomed. Mater. Res. A 80, 226–233. ( 10.1002/jbm.a.31096) [DOI] [PubMed] [Google Scholar]
- Lichtman M. A. 1981. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp. Hematol. 9, 391–410. [PubMed] [Google Scholar]
- Lidgren L. 2003. The bone and joint decade 2000–2010. Bull. World Health Organ. 81, 629 (doi:S0042-96862003000900002) [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. R., Daluiski A., Einhorn T. A. 2002. The role of growth factors in the repair of bone. Biology and clinical applications. J. Bone Joint Surg. Am. 84-A, 1032–1044. [DOI] [PubMed] [Google Scholar]
- Liu Y., Layrolle P., de Bruijn J., van Blitterswijk C., de Groot K. 2001. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J. Biomed. Mater. Res. 57, 327–335. () [DOI] [PubMed] [Google Scholar]
- Liu Y., Hunziker E. B., Layrolle P., Van Blitterswijk C., Calvert P. D., de Groot K. 2003. Remineralization of demineralized albumin-calcium phosphate coatings. J. Biomed. Mater. Res. A 67, 1155–1162. ( 10.1002/jbm.a.20019) [DOI] [PubMed] [Google Scholar]
- Liu Y., de Groot K., Hunziker E. B. 2004a Osteoinductive implants: the mise-en-scene for drug-bearing biomimetic coatings. Ann. Biomed. Eng. 32, 398–406. ( 10.1023/B:ABME.0000017536.10767.0f) [DOI] [PubMed] [Google Scholar]
- Liu Y., Hunziker E. B., Layrolle P., De Bruijn J. D., De Groot K. 2004b Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 10, 101–108. ( 10.1089/107632704322791745) [DOI] [PubMed] [Google Scholar]
- Liu Y., de Groot K., Hunziker E. B. 2005. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 36, 745–757. ( 10.1016/j.bone.2005.02.005) [DOI] [PubMed] [Google Scholar]
- Liu Y., Li J. P., Hunziker E. B., de Groot K. 2006. Incorporation of growth factors into medical devices via biomimetic coatings. Phil. Trans. R. Soc. A 364, 233–248. ( 10.1098/rsta.2005.1685) [DOI] [PubMed] [Google Scholar]
- Liu Y., Enggist L., Kuffer A. F., Buser D., Hunziker E. B. 2007a The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials 28, 2677–2686. ( 10.1016/j.biomaterials.2007.02.003) [DOI] [PubMed] [Google Scholar]
- Liu Y., Huse R. O., de Groot K., Buser D., Hunziker E. B. 2007b Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 86, 84–89. [DOI] [PubMed] [Google Scholar]
- Luginbuehl V., Meinel L., Merkle H. P., Gander B. 2004. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 58, 197–208. ( 10.1016/j.ejpb.2004.03.004) [DOI] [PubMed] [Google Scholar]
- Luong L. N., Hong S. I., Patel R. J., Outslay M. E., Kohn D. H. 2006. Spatial control of protein within biomimetically nucleated mineral. Biomaterials 27, 1175–1186. ( 10.1016/j.biomaterials.2005.07.043) [DOI] [PubMed] [Google Scholar]
- Lutolf M. P., Weber F. E., Schmoekel H. G., Schense J. C., Kohler T., Muller R., Hubbell J. A. 2003. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 21, 513–518. ( 10.1038/nbt818) [DOI] [PubMed] [Google Scholar]
- Luttikhuizen D. T., Harmsen M. C., Van Luyn M. J. 2006. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 12, 1955–1970. ( 10.1089/ten.2006.12.1955) [DOI] [PubMed] [Google Scholar]
- Milovancev M., Muir P., Manley P. A., Seeherman H. J., Schaefer S. 2007. Clinical application of recombinant human bone morphogenetic protein-2 in 4 dogs. Vet. Surg. 36, 132–140. ( 10.1111/j.1532-950X.2007.00245.x) [DOI] [PubMed] [Google Scholar]
- Mistry A. S., Cheng S. H., Yeh T., Christenson E., Jansen J. A., Mikos A. G. 2009. Fabrication and in vitro degradation of porous fumarate-based polymer/alumoxane nanocomposite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 89, 68–79. ( 10.1002/jbm.a.32010) [DOI] [PubMed] [Google Scholar]
- Mistry A. S., Pham Q. P., Schouten C., Yeh T., Christenson E. M., Mikos A. G., Jansen J. A. 2010. In vivo bone biocompatibility and degradation of porous fumarate-based polymer/alumoxane nanocomposites for bone tissue engineering. J. Biomed. Mater. Res. A 92, 451–462. ( 10.1002/jbm.a.32371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. C., Ehrlich M. G., McAllister S. C., Machan J. T., Hart C. E., Voigt C., Lesieur-Brooks A. M., Weber E. W. 2009. Recombinant human platelet-derived growth factor-BB augmentation of new-bone formation in a rat model of distraction osteogenesis. J. Bone Joint Surg. Am. 91, 1973–1984. ( 10.2106/JBJS.H.00540) [DOI] [PubMed] [Google Scholar]
- Moreau M. F., Gallois Y., Basle M. F., Chappard D. 2000. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials 21, 369–376. ( 10.1016/S0142-9612(99)00193-3) [DOI] [PubMed] [Google Scholar]
- Mourino V., Boccaccini A. R. 2009. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 7, 209–227. ( 10.1098/rsif.2009.0379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell R. D., Mooney M. P., Siegel M. I., Losken A. 1993. Osseous guided tissue regeneration using a collagen barrier membrane. J. Oral Maxillofac. Surg. 51, 1004–1012. (doi:S0278239193001211) [DOI] [PubMed] [Google Scholar]
- Murakami N., Saito N., Takahashi J., Ota H., Horiuchi H., Nawata M., Okada T., Nozaki K., Takaoka K. 2003. Repair of a proximal femoral bone defect in dogs using a porous surfaced prosthesis in combination with recombinant BMP-2 and a synthetic polymer carrier. Biomaterials 24, 2153–2159. ( 10.1016/S0142-9612(03)00041-3) [DOI] [PubMed] [Google Scholar]
- Murphy W. L., Kohn D. H., Mooney D. J. 2000a Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J. Biomed. Mater. Res. 50, 50–58. () [DOI] [PubMed] [Google Scholar]
- Murphy W. L., Peters M. C., Kohn D. H., Mooney D. J. 2000b Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 21, 2521–2527. ( 10.1016/S0142-9612(00)00120-4) [DOI] [PubMed] [Google Scholar]
- Muscolo D. L., Ayerza M. A., Aponte-Tinao L. A., Ranalletta M. 2006. Use of distal femoral osteoarticular allografts in limb salvage surgery. Surgical technique. J. Bone Joint Surg. Am. 88(Suppl. 1, Pt 2), 305–321. [DOI] [PubMed] [Google Scholar]
- Nagano M., Kitsugi T., Nakamura T., Kokubo T., Tanahashi M. 1996. Bone bonding ability of an apatite-coated polymer produced using a biomimetic method: a mechanical and histological study in vivo. J. Biomed. Mater. Res. 31, 487–494. () [DOI] [PubMed] [Google Scholar]
- Nair M. B., Varma H. K., John A. 2009. Triphasic ceramic coated hydroxyapatite as a niche for goat stem cell-derived osteoblasts for bone regeneration and repair. J. Mater. Sci. Mater. Med. 20(Suppl. 1), S251–S258. ( 10.1007/s10856-008-3598-8) [DOI] [PubMed] [Google Scholar]
- Nie H., Soh B. W., Fu Y. C., Wang C. H. 2008. Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery. Biotechnol. Bioeng. 99, 223–234. ( 10.1002/bit.21517) [DOI] [PubMed] [Google Scholar]
- Ohgushi H., Caplan A. I. 1999. Stem cell technology and bioceramics: from cell to gene engineering. J. Biomed. Mater. Res. 48, 913–927. () [DOI] [PubMed] [Google Scholar]
- Oliveira A. L., Malafaya P. B., Costa S. A., Sousa R. A., Reis R. L. 2007. Micro-computed tomography (micro-CT) as a potential tool to assess the effect of dynamic coating routes on the formation of biomimetic apatite layers on 3D-plotted biodegradable polymeric scaffolds. J. Mater. Sci. Mater. Med. 18, 211–223. ( 10.1007/s10856-006-0683-8) [DOI] [PubMed] [Google Scholar]
- Ono I., Gunji H., Kaneko F., Saito T., Kuboki Y. 1995a Efficacy of hydroxyapatite ceramic as a carrier for recombinant human bone morphogenetic protein. J. Craniofac. Surg. 6, 238–244. [DOI] [PubMed] [Google Scholar]
- Ono I., Gunji H., Suda K., Kaneko F., Murata M., Saito T., Kuboki Y. 1995b Bone induction of hydroxyapatite combined with bone morphogenetic protein and covered with periosteum. Plast. Reconstr. Surg. 95, 1265–1272. ( 10.1016/S0278-2391(96)90322-5) [DOI] [PubMed] [Google Scholar]
- Oreffo R. O. 2004. Growth factors for skeletal reconstruction and fracture repair. Curr. Opin. Invest. Drugs 5, 419–423. [PubMed] [Google Scholar]
- Park M. S., Kim S. S., Cho S. W., Choi C. Y., Kim B. S. 2006. Enhancement of the osteogenic efficacy of osteoblast transplantation by the sustained delivery of basic fibroblast growth factor. J. Biomed. Mater. Res. B Appl. Biomater. 79, 353–359. ( 10.1002/jbm.b.30549) [DOI] [PubMed] [Google Scholar]
- Potter J. K., Ellis E. 2004. Biomaterials for reconstruction of the internal orbit. J. Oral Maxillofac. Surg. 62, 1280–1297. ( 10.1016/j.joms.2004.04.018) [DOI] [PubMed] [Google Scholar]
- Prabu P., Dharmaraj N., Aryal S., Lee B. M., Ramesh V., Kim H. Y. 2006. Preparation and drug release activity of scaffolds containing collagen and poly(caprolactone). J. Biomed. Mater. Res. A 79, 153–158. ( 10.1002/jbm.a.30715) [DOI] [PubMed] [Google Scholar]
- Prockop D. J. 1997. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74. ( 10.1126/science.276.5309.71) [DOI] [PubMed] [Google Scholar]
- Qu H., Wei M. 2008. The effect of temperature and initial pH on biomimetic apatite coating. J. Biomed. Mater. Res. B Appl. Biomater. 87, 204–212. ( 10.1002/jbm.b.31096) [DOI] [PubMed] [Google Scholar]
- Rasmusson I. 2006. Immune modulation by mesenchymal stem cells. Exp. Cell Res. 312, 2169–2179. ( 10.1016/j.yexcr.2006.03.019) [DOI] [PubMed] [Google Scholar]
- Ratner B. D., Bryant S. J. 2004. Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 6, 41–75. ( 10.1146/annurev.bioeng.6.040803.140027) [DOI] [PubMed] [Google Scholar]
- Reese J. S., Koc O. N., Gerson S. L. 1999. Human mesenchymal stem cells provide stromal support for efficient CD34+ transduction. J. Hematother. Stem Cell Res. 8, 515–523. ( 10.1089/152581699319966) [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Meyerson H., Anderson J. M. 2008. Quantitative in vivo cytokine analysis at synthetic biomaterial implant sites. J. Biomed. Mater. Res. A 89A, 152–159. ( 10.1002/jbm.a.31939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N., Takaoka K. 2003. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 24, 2287–2293. ( 10.1016/S0142-9612(03)00040-1) [DOI] [PubMed] [Google Scholar]
- Schliephake H., Aref A., Scharnweber D., Bierbaum S., Roessler S., Sewing A. 2005a Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin. Oral Implants Res. 16, 563–569. ( 10.1111/j.1600-0501.2005.01143.x) [DOI] [PubMed] [Google Scholar]
- Schliephake H., Scharnweber D., Dard M., Sewing A., Aref A., Roessler S. 2005b Functionalization of dental implant surfaces using adhesion molecules. J. Biomed. Mater. Res. B Appl. Biomater. 73, 88–96. ( 10.1002/jbm.b.30183) [DOI] [PubMed] [Google Scholar]
- Schmitz J. P., Hollinger J. O. 1986. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 205, 299–308. [PubMed] [Google Scholar]
- Silber J. S., Anderson D. G., Daffner S. D., Brislin B. T., Leland J. M., Hilibrand A. S., Vaccaro A. R., Albert T. J. 2003. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 28, 134–139. ( 10.1097/00007632-200301150-00008) [DOI] [PubMed] [Google Scholar]
- Smith J. D., Abramson M. 1974. Membranous vs endochondrial bone autografts. Arch. Otolaryngol. 99, 203–205. [DOI] [PubMed] [Google Scholar]
- Sokolsky-Papkov M., Agashi K., Olaye A., Shakesheff K., Domb A. J. 2007. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 59, 187–206. ( 10.1016/j.addr.2007.4.001) [DOI] [PubMed] [Google Scholar]
- Solheim E. 1998a Growth factors in bone. Int. Orthop. 22, 410–416. ( 10.1007/s002640050290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim E. 1998b Osteoinduction by demineralised bone. Int. Orthop. 22, 335–342. ( 10.1007/s002640050273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Malathong V., Bertozzi C. R. 2005. Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone. J. Am. Chem. Soc. 127, 3366–3372. ( 10.1021/ja043776z) [DOI] [PubMed] [Google Scholar]
- Stancu I. C., Filmon R., Cincu C., Marculescu B., Zaharia C., Tourmen Y., Basle M. F., Chappard D. 2004. Synthesis of methacryloyloxyethyl phosphate copolymers and in vitro calcification capacity. Biomaterials 25, 205–213. ( 10.1016/S0142-9612(03)00485-X) [DOI] [PubMed] [Google Scholar]
- Sterling H., Saginario C., Vignery A. 1998. CD44 occupancy prevents macrophage multinucleation. J. Cell Biol. 143, 837–847. ( 10.1083/jcb.143.3.837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetzer K., Cooper G., Gassner R., Kapucu R., Mundell R., Mooney M. P. 2002. Effects of fixation type and guided tissue regeneration on maxillary osteotomy healing in rabbits. J. Oral Maxillofac. Surg. 60, 427–436; discussion 436–427. ( 10.1053/joms.2002.31232) [DOI] [PubMed] [Google Scholar]
- Sun J. S., Lin F. H., Wang Y. J., Huang Y. C., Chueh S. C., Hsu F. Y. 2003. Collagen-hydroxyapatite/tricalcium phosphate microspheres as a delivery system for recombinant human transforming growth factor-β 1. Artif. Organs 27, 605–612. ( 10.1046/j.1525-1594.2003.07169.x) [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Yamamoto M., Tabata Y. 2005. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 26, 4856–4865. ( 10.1016/j.biomaterials.2005.01.012) [DOI] [PubMed] [Google Scholar]
- Takahashi K., van den Beucken J. J., Wolke J. G., Hayakawa T., Nishiyama N., Jansen J. A. 2008. Characterization and in vitro evaluation of biphasic calcium pyrophosphate–tricalciumphosphate radio frequency magnetron sputter coatings. J. Biomed. Mater. Res. A 84, 682–690. ( 10.1002/jbm.a.31341) [DOI] [PubMed] [Google Scholar]
- Tanahashi M., Matsuda T. 1997. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 34, 305–315. () [DOI] [PubMed] [Google Scholar]
- Tanahashi M., Yao T., Kokubo T., Minoda M., Miyamoto T., Nakamura T., Yamamuro T. 1995. Apatite coated on organic polymers by biomimetic process: improvement in its adhesion to substrate by glow-discharge treatment. J. Biomed. Mater. Res. 29, 349–357. ( 10.1002/jbm.820290310) [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Friedenstein A. 1983. Hemopoietic stromal microenvironment. Am. J. Hematol. 15, 195–203. ( 10.1002/ajh.2830150211) [DOI] [PubMed] [Google Scholar]
- Toth J. M., Boden S. D., Burkus J. K., Badura J. M., Peckham S. M., McKay W. F. 2009. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine (Phila Pa 1976) 34, 539–550. ( 10.1097/BRS.0b013e3181952695) [DOI] [PubMed] [Google Scholar]
- Tsai A. T., Rice J., Scatena M., Liaw L., Ratner B. D., Giachelli C. M. 2005. The role of osteopontin in foreign body giant cell formation. Biomaterials 26, 5835–5843. ( 10.1016/j.biomaterials.2005.03.003) [DOI] [PubMed] [Google Scholar]
- Vallet-Regi M., Romero A. M., Ragel C. V., LeGeros R. Z. 1999. XRD, SEM-EDS, and FTIR studies of in vitro growth of an apatite-like layer on sol–gel glasses. J. Biomed. Mater. Res. 44, 416–421. () [DOI] [PubMed] [Google Scholar]
- Vuyk H. D., Adamson P. A. 1998. Biomaterials in rhinoplasty. Clin. Otolaryngol. Allied Sci. 23, 209–217. ( 10.1097/PRS.0b013e3181c82f12) [DOI] [PubMed] [Google Scholar]
- Wang Y. J., Lin F. H., Sun J. S., Huang Y. C., Chueh S. C., Hsu F. Y. 2003. Collagen-hydroxyapatite microspheres as carriers for bone morphogenic protein-4. Artif. Organs 27, 162–168. ( 10.1046/j.1525-1594.2003.06953.x) [DOI] [PubMed] [Google Scholar]
- Weinstein S. L. 2000. 2000–2010: the bone and joint decade. J. Bone Joint Surg. Am. 82, 1–3. [PubMed] [Google Scholar]
- Wen H. B., de Wijn J. R., van Blitterswijk C. A., de Groot K. 1999. Incorporation of bovine serum albumin in calcium phosphate coating on titanium. J. Biomed. Mater. Res. 46, 245–252. () [DOI] [PubMed] [Google Scholar]
- Wernike E., Hofstetter W., Liu Y., Wu G., Sebald H. J., Wismeijer D., Hunziker E. B., Siebenrock K. A., Klenke F. M. 2009. Long-term cell-mediated protein release from calcium phosphate ceramics. J. Biomed. Mater. Res. A 92, 463–474. ( 10.1002/jbm.a.32411) [DOI] [PubMed] [Google Scholar]
- Whang K., Tsai D. C., Nam E. K., Aitken M., Sprague S. M., Patel P. K., Healy K. E. 1998. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J. Biomed. Mater. Res. 42, 491–499. () [DOI] [PubMed] [Google Scholar]
- Wilson C. J., Clegg R. E., Leavesley D. I., Pearcy M. J. 2005. Mediation of biomaterial–cell interactions by adsorbed proteins: a review. Tissue Eng. 11, 1–18. ( 10.1089/ten.2005.11.1) [DOI] [PubMed] [Google Scholar]
- Wu G., Liu Y., Iizuka T., Hunziker E. B. 2008. BMP-2-functionalized polymers for bone engineering: the influence of polymer geometry on biomimetic coating with a layer of calcium phosphate and on osteogenic potential. Amsterdam, The Netherlands: World Biomaterials Congress. [Google Scholar]
- Wu G., Hunziker E. B., Liu Y., Wismeijer D. 2009. Functionalization of Bio-Oss promotes bone formation and quells inflammation. J. Dent. Res. 88, 290. [Google Scholar]
- Wu G., Liu Y., Iizuka T., Hunziker E. B. In press Biomimetic coating of organic polymers with a protein-functionalized layer of calcium phosphate: the surface properties of the carrier influence neither the coating characteristics nor the incorporation mechanism or release kinetics of the protein. Tissue Eng. Part C Methods. ( 10.1089/ten.TEC.2009.588) [DOI] [PubMed] [Google Scholar]
- Yan W. Q., Nakamura T., Kawanabe K., Nishigochi S., Oka M., Kokubo T. 1997. Apatite layer-coated titanium for use as bone bonding implants. Biomaterials 18, 1185–1190. ( 10.1016/S0142-9612(97)00057-4) [DOI] [PubMed] [Google Scholar]
- Yang S., Leong K. F., Du Z., Chua C. K. 2001. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 7, 679–689. ( 10.1089/107632701753337645) [DOI] [PubMed] [Google Scholar]
- Yin Y. J., Luo X. Y., Cui J. F., Wang C. Y., Guo X. M., Yao K. D. 2004. A study on biomineralization behavior of N-methylene phosphochitosan scaffolds. Macromol. Biosci. 4, 971–977. ( 10.1002/mabi.200400063) [DOI] [PubMed] [Google Scholar]
- Yu X., Qu H., Knecht D. A., Wei M. 2009. Incorporation of bovine serum albumin into biomimetic coatings on titanium with high loading efficacy and its release behavior. J. Mater. Sci. Mater. Med. 20, 287–294. ( 10.1007/s10856-008-3571-6) [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wu G., Zhao J., Wang L., Sun P., Gu Z. 2010. RhBMP2/7 Heterodimer: an osteoblastogenesis-inducer of not higher-potency but lower-effective-concentration compared to RhBMP2 and RhBMP7 homodimers. Tissue Eng. Part A 16, 879–887. ( 10.1089/ten.TEA.2009.0312) [DOI] [PubMed] [Google Scholar]