Abstract
Bone-anchored titanium implants have revolutionized oral healthcare. Surface properties of oral titanium implants play decisive roles for molecular interactions, cellular response and bone regeneration. Nevertheless, the role of specific surface properties, such as chemical and phase composition and nanoscale features, for the biological in vivo performance remains to be established. Partly, this is due to limited transfer of state-of-the-art preparation techniques to complex three-dimensional geometries, analytical tools and access to minute, intact interfacial layers. As judged by the available results of a few randomized clinical trials, there is no evidence that any particular type of oral implant has superior long-term success. Important insights into the recruitment of mesenchymal stem cells, cell–cell communication at the interface and high-resolution imaging of the interface between the surface oxide and the biological host are prerequisites for the understanding of the mechanisms of osseointegration. Strategies for development of the next generation of material surface modifications for compromised tissue are likely to include time and functionally programmed properties, pharmacological modulation and incorporation of cellular components.
Keywords: titanium, osseointegration, oral implant, surface characterization, gene expression, ultrastructure
1. Introduction
Titanium implants have revolutionized oral healthcare. The first generation of successfully used clinical titanium implants, which were machined with a smooth surface texture, now approach 50 years in clinical use. The second generation of clinically used implants underwent chemical and topographical modifications, usually resulting in a moderately increased surface topography. Many of these oral implant systems now approach 15 years of clinical use. The reader interested in the epidemiology and pathophysiology of osseointegration is referred to Esposito et al. (1998a,b).
Particularly intriguing is that many of the currently used oral titanium implants are less well characterized with respect to their surface properties. This means that it is not known whether the improved bone response is due to the surface roughness or the surface composition. Further, somewhat surprisingly, there is yet not enough hard evidence (randomized clinical trials) to tell whether the second generation of implants has a better clinical performance than the machined implants used earlier. Nevertheless, experimental evidence from in vitro and in vivo studies strongly suggests that some types of surface modifications promote a more rapid bone formation than do machined surfaces. This could depend on an altered surface chemistry and/or an increased texture on the micrometre scale (Albrektsson & Wennerberg 2004; Ellingsen et al. 2006).
Recent studies also show that surfaces with nanoscale features show additional biological effects in vitro and in vivo, e.g. by producing an integration, which ultrastructurally may be described as bonding on the atomic scale between the oxide and apatite nanocrystals (Palmquist et al. 2010). Such surfaces, intentionally modified with respect to microscale and nanoscale features, may represent the next generation of oral implant systems if possible to transfer to complex three-dimensional geometries. Hitherto, micro- and nano-fabricated surfaces have not reached the clinical evidence stage.
The coating of titanium with, for example, different types of calcium phosphates may improve bone integration even further. Early attempts to use thick calcium phosphate coatings have never reached commercial and clinical success in dentistry and are therefore not the focus of the present review. A review of ultrathin calcium phosphate coatings suggests that such alterations improve bone integration without the previously claimed drawbacks of thick coatings (Junker et al. 2009). Nevertheless, clinical studies are needed.
Ten years ago a number of scientific issues related to titanium were discussed in a textbook format (Brunette et al. 2001). Several of these questions were concentrated on the one hand on the modification of titanium surfaces and their characterization, and on the other hand, on the biological response to such surfaces. Ten years later, significant advancements in these areas have been made, both in terms of analytical and experimental methodology and in understanding of the biological mechanisms involved. Advances in interface methodology and improvements in the basic understanding of interface biology in vivo will be reviewed in this article.
The development of future generations of oral implants for compromised tissue conditions will most probably entail tailored modifications of the material surface, local release of pharmacological substances and the recruitment and delivery of cells to the host site. Such an approach will require a deepened understanding of the relationships between the host defence and regeneration as well as access to the interface for diagnostic and monitoring purposes.
The rest of the paper is divided into four sections. Section 2 deals with the surface characteristics of oral titanium implants. In §3, interface biology of oral implants is discussed. Section 4 provides clinical outcome of oral implants and §5 concludes with a summary and future outlook.
2. Surface characteristics of oral titanium implants
Surface characteristics play a special role for the biological performance of implants. Whereas mechanical properties such as Young's modulus and fatigue properties are mainly determined by the bulk of the material, chemical and biological interactions between the material and the host tissue are closely associated with the material surface properties. These interactions include early events such as binding of water molecules, ions and biomolecules, as well as mineralization at the implant surface. The result of these early interactions will be a conditioning layer with which the cells will eventually interact. The original surface can thus be regarded as one of the factors that will determine the tissue regeneration around the implant.
The surface properties of any material will be different from those of the bulk, for different reasons. One reason is a fundamental characteristic of surfaces. The creation of a surface inevitably involves breaking of the chemical bonds that keep the material together. A freshly created surface represents an energetically unfavourable situation, often referred to as high surface energy. When the surface is exposed to an ambient, the surface energy will rapidly be lowered by binding of, and reactions with, molecules from the ambient. For metals such as titanium, this involves reaction with air oxygen to form a thin surface oxide. Secondly, and more important for implants, the surface characteristics are strongly influenced by the method of surface preparation, handling and storage. During preparation of the implant, the material surface is subjected to various process chemicals that will leave residues at the surface. If the preparation involves elevated temperature, this will result in growth of surface oxide. Sterilization and storage in a sterile package are also likely to influence the surface, for example, via transfer of molecules from the packaging material to the implant surface. The close connection between surface preparation and resulting surface characteristics means that in order to produce reproducible implant surfaces, all aspects of the manufacturing process and ensuing logistics need to be carefully controlled.
Surface properties of interest for implants can broadly be divided into structural properties and chemical properties. For titanium oral implants most of these properties are associated with the surface oxide that always covers the metal. The chemical composition of surface oxides on titanium is usually titanium dioxide, TiO2. The surface oxide is chemically stable and corrosion-resistant, which makes titanium surfaces quite stable under normal physiological conditions. The surface oxide may also contain varying amounts of other intentional or non-intentional (impurity) substances. Furthermore, titanium implant surfaces are covered by various amounts of organic molecules, originating from adsorbed molecules from air, process residues or packaging materials. The latter will, to a great extent, influence the wetting properties of the surface and thereby also important interactions such as protein adsorption. Surface oxides on titanium can vary in thickness from a few nanometres up to several micrometres, depending on the method of preparation and the temperatures involved. They can have a microstructure that is amorphous (non-crystalline) or crystalline. At least three different crystalline TiO2 phases are possible, anatase, rutile and brookite, all of which have different chemical properties.
The effect of the phase composition of the surface oxide is a largely unexplored field as there are difficulties of analysing the often very thin oxide layer of the implants with regards to phase composition. There exist a variety of methods allowing the identification of phase composition of the surface oxide layer. However, many of these methods require flat surfaces and/or rather thick oxides, which are crystalline. Few techniques are available for the analysis of the native oxides of screw-shaped, clinically used titanium implants, having complex macro-geometries and increased micro-roughnesses.
With Raman spectroscopy (where visible light scattering is analysed) a thicker and crystalline oxide is required to overcome the noise level in order to identify the phase of the oxide (Sul et al. 2002). In the case of amorphous oxides, no information is retrieved and it is difficult to distinguish between amorphous or crystalline oxides thinner than a few tens of nanometres. The same applies to X-ray diffraction (where the scattering of X-rays is analysed): a larger flat area is needed to overcome the noise level. With grazing incident or thin film X-ray diffraction thinner oxides could be analysed. However, these measurements will be significantly disturbed by micro-roughness as the angle becomes smaller. The use of transmission electron microscopy (TEM) allows accurate measurements of the lattice parameters as well as analysis of the microstructure and grain sizes of the surface layer by high resolution TEM (HRTEM). In addition, electron diffraction can be used for phase identification even on nanoscale features. However, these techniques require tedious sample preparation. With electropolishing, a top view sample could be prepared by dissolving the bulk metal leaving an electron transparent titanium oxide window (Ask et al. 1990; Radegran et al. 1991). In order to get information regarding possible gradients throughout the oxide a cross-section sample is needed. This could be obtained either by grinding and polishing (Conforto et al. 2004) or by a focused ion beam (FIB; Jarmar et al. 2008a,b). A novel tool in modern scanning electron microscopy (SEM) is electron backscattering diffraction detection. This technique allows electron diffraction analysis of surface films, without extensive sample preparation (Trager-Cowan et al. 2002; Bhattacharyya & Eades 2009). However, the usefulness of this technique for acquiring structural information of real implant surfaces yet remains to be demonstrated.
The results of surface phase analysis for implants found in the literature are scarce since only a few TEM (Ask et al. 1990; Conforto et al. 2004; Jarmar et al. 2008a,b; Palmquist et al. 2010) and Raman (Sul et al. 2002; Lewandowska et al. 2007) analyses have been performed. The clinically and commercially available oral implants show large differences in the phase composition, ranging from thin amorphous oxides grown on titanium hydride via thin rutile oxide to thicker anatase/amorphous oxides (Conforto et al. 2004; Jarmar et al. 2008a,b).
In addition to chemical and phase composition, a particularly important structural property of oral implants is the surface topography, or surface roughness. Depending on the scale of roughness, this will be determined by the oxide or by the bulk material. For thick oxides the surface structure may be completely dominated by the oxide, whereas in other cases it is a combination of a micrometre scale rough metal surface covered by a thin oxide with nanometre scale roughness. Whereas it is well established that surface roughness on the micrometre scale plays an important role for cellular reactions, tissue healing and implant fixation (Albrektsson & Wennerberg 2004), the role of surface topography on the nanometre scale has not yet been explored in a systematic manner.
The variety of surface characteristics that are possible for titanium surfaces opens up opportunities for modifying titanium implant surfaces to enhance their biological performance. Currently used clinical titanium oral implants display a wide variety of microstructural and chemical properties. A complete review of all their properties is beyond the scope of this paper, but to illustrate this some examples are given in figures 1 and 2. Different mechanical, chemical and optical methods such as machining, blasting, acid etching, electrochemical oxidation and laser treatment are used to produce titanium implant surfaces with various surface topographies, oxide thicknesses, crystallinities and compositions. Although extensive documentation of the in vitro and in vivo performance of most of these titanium surfaces exists from different experimental models, it is difficult to draw any firm conclusions regarding possible differences in their performance in a clinical situation.
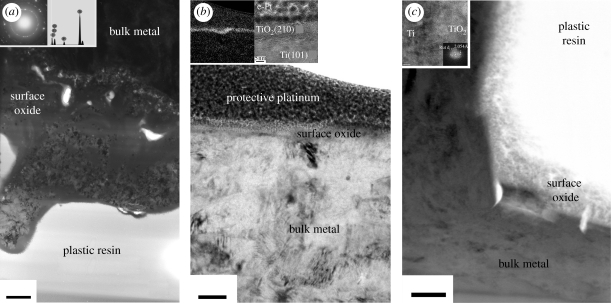
Figure 1.
SEM images at 5000× magnification. (a) Anodic oxidized surface (TiUnite, Nobel Biocare) showing volcanoes porous structure. (b) Machined surface (Fixture Original, Brånemark Integration) showing ridges and valleys along the turning direction (adapted from Jarmar et al. 2008a,b). (c) Laser-modified surface showing a combined surface roughness in both the micro- and nanoscale. (Scale bars, 5 µm.)
Figure 2.
TEM images at various magnifications. (a) Anodic oxidized surface (TiUnite, Nobel Biocare) showing a 4–8 µm thick oxide layer with a porous interface to the bulk metal. The oxide is both crystalline in anatase phase (electron diffraction inset) and amorphous (scale bar, 1 µm). Further, the oxide contains phosphorus according to EDS (inset). (b) Machined surface (Fixture Original, Brånemark Integration) showing a thin oxide (10 nm) distinguished by EFTEM for oxygen (inset). Further, the crystal phase composition was identified by HRTEM to be rutile (scale bar, 50 nm). (c) Laser-modified surface showing a 200–300 nm thick surface oxide (scale bar, 200 nm). The oxide consists of nanosized crystals according to HRTEM (inset); however, it is difficult to distinguish between the rutile and anatase phase for this surface. ((b,c) Adapted from Jarmar et al. (2008a,b) and Palmquist et al. (2010).)
Different types of coatings can also be used to further modify the surface. One interesting coating material for dental and other bone-anchored implants is calcium phosphate/hydroxyapatite (HA). HA coatings can be applied with different techniques. Early examples were made with plasma spraying, which is based on melting the coating material and spraying it onto the material to be coated, which produces rough and thick coatings. Early experience with these coatings indicated that they are prone to adhesion failure and cracking. An alternative technology is evaporation coating, such as physical vapour deposition (PVD). In PVD, the coating is evaporated by sputtering of a HA source, and the coating is deposited atom by atom. The technique can be used to make very thin (approx. nanometre) and up to micrometre thick coatings with good adhesion (Mohammadi et al. 2003, 2004). PVD is, however, a line-of-sight process and, therefore, less well suited for coating of implants with complex geometries. HA coatings can also be produced in wet chemical processes, such as sol–gel coating and biomimetic coating by immersion in simulated body fluid or phosphate buffered saline (Kokubo et al. 2001; Xia et al. 2010). By varying the solution composition, immersion time and temperature, these techniques offer multiple possibilities to vary the thickness, morphology and composition of the coatings. The biological significance of such modifications, however, remains to be investigated.
Another possibility to modify or coat the implant surface is the use of immobilized molecules, as covalently bonded and/or self-assembled monolayers. Examples of monolayer coatings include silane coatings and thiols with different end groups, which offer a wide variety of surface chemical properties. They can be used to modulate the hydrophilicity of the surface and to introduce specific chemical functional groups (e.g. methyl, hydroxyl or carboxyl groups) at the surface, in an attempt to modulate the biological response (Kalltorp et al. 2000). Belonging to this group of modifications are also other approaches aiming at targeting specific biological processes. Peptide sequences, such as RGD, have been used as monolayer modifications and shown to promote cell adhesion (Garcia et al. 2002). Another interesting approach is to incorporate and/or attach molecules and pharmacological substances for local release from the implant or graft material. An example is the use of bisphosphonates, which downregulate osteoclast activity and result in faster bone regeneration around implants (Wermelin et al. 2008; Bigi et al. 2009). Another example is the local application of simvastatin in conjunction with ceramic bone void fillers, increasing the recruitment of osteoprogenitors, promoting bone formation and inhibiting osteoclastic activity (Park 2009; Nyan et al. 2010). Although the clinical success of monolayer modified implants remains to be demonstrated, it can be anticipated that different types of monolayer coatings with, for example, proteins or other biologically active substances designed to target specific biological mechanisms and signalling pathways will be a strong line of development towards the next generation of implants.
3. Interface biology of oral implants
3.1. Cell and molecular biology
Despite the wide use of titanium and the substantially growing research on the development of new titanium surfaces and/or modification of existing surfaces, a detailed understanding of the mechanisms of osseointegration is still lacking. Bone regeneration around oral titanium implants has classically been regarded as similar to that observed after injury or fracture. This healing is based traditionally on the succeeding phases of inflammation, regeneration and remodelling with possible overlapping at certain occasions. However, major differences exist between the two types of healing (i.e. in absence or presence of titanium implant) both on the macro-level and on the cellular and molecular level. In the presence of an implant, the implant itself will act as an osteoconductive substrate decreasing the size of the defect to be bridged by new tissue. A crucial factor is the effect of the titanium surface on the different biological components that come in contact with the surface as soon as the implant is introduced into the surgically prepared defect. Based on results from mainly in vitro studies, the surface influences the initial sequences of protein adsorption, platelet adhesion and haemostasis, complement activation, inflammation and osteogenic cell response (Nygren et al. 1997; Boyan et al. 1998; Park & Davies 2000; Schneider et al. 2003; Masaki et al. 2005; Tan et al. 2006; Linderback et al. 2010). A major question is how early molecular and cellular events are influenced by material surface properties in vivo, and how these early events influence the organization of the surrounding tissue and its interlocking or bonding with the material surface and, in turn, the ability of this interface to adapt to long-term continuous load interactions.
The structure of the intact bone/titanium interface is now well characterized down to the nanoscale (Palmquist et al. 2008, 2009, 2010). However, the early cellular and molecular activities determining the early tissue response and bone formation at the bone/implant interface are not yet fully understood. Several questions about the effects of the physico-chemical properties of the surface on the cellular pheno- and genotypes at the immediate vicinity of the surface need to be answered. Furthermore, the molecular mechanism of how and in which sequence the cells are recruited and become adherent to the surface has to be defined. Moreover, it is of great importance to understand the effect of the material surface on cell–cell communication during the early phase of osseointegration.
Using the highly sensitive quantitative polymerase chain reaction in conjunction with the well-documented rat tibia model of osseointegration it has been revealed that gene expression of the interfacial cells is immediately and differently influenced by titanium implants depending on their surface properties (Omar et al. 2010a,b). This model relied on analysing the gene expression of cells adherent to screw titanium implants during the first hours and days after implantation (table 1). The model was further combined with immunohistochemistry and SEM to confirm the presence of specific cell types at the interface. These results showed that the higher expression of monocyte chemoattractant protein-1 (MCP-1) was coupled with higher expression of pro-inflammatory cytokines TNF-α (3 h and 1 day after implantation) and IL-1β (1 day and 6 days after implantation) at machined implant surfaces. On the other hand, the expression of the chemokine receptor CXCR4, a receptor for stromal derived factor-1α (SDF-1α), was highly expressed at oxidized surfaces as early as 12 h after implantation. These results were corroborated by SEM and immunohistochemical observations showing the coexistence of monocytes/macrophages and mesenchymal stem cells (MSCs) at the interfacial region with predominance of MSCs at the oxidized surface. It has been shown that early peak expression of SDF-1α during the first day after tissue injury was associated with the highest MSCs homing to the injury site (Cheng et al. 2008). The role of SDF-1α/CXCR4 chemotactic axis in mediating the recruitment of progenitor cells is attracting considerable attention to reveal the paradigm of mesenchymal cell recruitment to different sites of healing (Ceradini et al. 2004; Ma et al. 2005; Wang et al. 2008; Kitaori et al. 2009).
Table 1.
Gene expression at oxidized and machined implant surfaces during the first week of implantation in rat tibia. The results are presented as a fold-difference between the oxidized (Ox) and machined (Ma) implants.
| expressing cells and biological roles | 12 h | 1 day | 3 days | 6 days | |
|---|---|---|---|---|---|
| Ox/Ma | Ox/Ma | Ox/Ma | Ox/Ma | ||
| RUNX2 | differentiation of MSCs towards osteoblastic lineage | not analysed | 1.83 | 6.086* | not analysed |
| ALP | osteoblasts, osteoprogenitors and others | not analysed | 1.628 | 5.118* | 1.847* |
| ↑phosphate concentration | |||||
| ↓inhibitors of crystals growth | |||||
| calcium binder | |||||
| CATK | osteoclasts and preosteoclasts | not analysed | 1.039 | 3.988* | 0.862 |
| catabolize collagen and elastin | |||||
| degradation and remodelling of extracellular matrix | |||||
| TNF-α | macrophages and inflammatory cells and cells of mesenchymal origin | 1 | 0.565* | 1.841 | 0.607 |
| modulates proliferation and apoptosis | |||||
| inflammation | |||||
| promote osteoclastic degradation | |||||
| MCP-1 | recruitment of monocytes/macrophages | 1.37 | 0.49 | not analysed | not analysed |
| CXCR4 | recruitment of various cell types including MSCs | 11.87* | 0.64 | not analysed | not analysed |
*p < 0.05.
Attachment to a surface is one of the critical first steps in the cell response to a biomaterial (Boyan et al. 2001). In vivo, the cellular attachment is meditated via a protein-rich layer and using several families of adhesion receptors including heterodimeric molecules, the integrins. T-shaped hollow titanium implants treated with sulphuric and hydrochloric acids showed higher expressions and peaks of β1 and β3 integrins in the surrounding bone after one week in rat femur compared with turned titanium and non-implant defect (Ogawa & Nishimura 2003). Cells adherent to oxidized surfaces showed upregulation of integrin β1 during the 24 h of implantation (Omar et al. 2010a), which underscores the importance of analysing cells adherent to the implants in addition to their presence in the surrounding tissue. In addition to osteogenic cells, monocytes and other cells also express integrin β1. On the other hand, integrin β2, expressed mainly by leukocytes (Stewart et al. 1995) and not by cells of osteoblastic lineage (Hughes et al. 1993), was higher at the oxidized implants after 12 h of implantation. This integrin has also been shown to be expressed by osteoclast progenitors (Hayashi et al. 2008). The results from a previous study (Omar et al. 2010b) showing a higher expression of osteoclastic marker (cathepsin K) at the oxidized surface suggested that the higher expression of β2 integrin may be due to the early presence of osteoclasts in the interface.
Inflammation at the in vivo bone/implant interface has not received similar attention as that given to the soft tissue/implant interface. Histological studies in bone revealed that macrophages and multinucleated cells from monocytic lineage are present in machined titanium implants (Sennerby et al. 1993a,b) and HA-coated implants (van Blitterswijk et al. 1985; Muller-Mai et al. 1990) during the early stage after implantation. These cells are known to express a wide range of pro-inflammatory and anti-inflammatory cytokines, growth and differentiation factors and chemotactic mediators. Major pro-inflammatory cytokine, TNF-α, was upregulated after 3 h at the machined implants compared with oxidized ones (Omar et al. 2010a). Higher expression was also observed at that surface after 1 day. Downregulatory effect was also observed on the expression of IL-6 at coin-shaped titanium implants blasted with TiO2 particles and subsequently treated with hydrofluoric acid (HF; Monjo et al. 2008). On the other hand this surface was associated with higher expression of anti-inflammatory cytokine IL-10, eight weeks after implantation in rabbit cortical bone. Since many cell types, including osteoblasts, can express these cytokines and growth factors it is still important to define which cell type is responsible for these changes in gene expression. For instance, insulin-like growth factor (IGF-1) is an important factor for osteogenic differentiation that can be expressed by cells from both monocytic and osteogenic lineages; the expression of IGF-1 was also upregulated at HF surface during the eight week evaluation period. In vitro studies have demonstrated that monocyte cell line expressed BMP-2 (Champagne et al. 2002), which contributed principally to the osteogenic differentiation; however, there are not yet in vivo data showing the expression of BMP-2 from monocytes during osseointegration. It is of great importance to apply antibody-labelling strategies such as immunohistochemistry and fluorescence assisted cell sorting in order to confirm such assumptions.
The regulation of gene expression at implant surfaces in vivo is a complex process. It is probably that material properties influence the gene expression of several factors by affecting key factors, such as RUNX2, which is a crucial transcription factor in the differentiation of mesenchymal cells towards the osteoblastic lineage. This factor has also been shown to contribute to the osteoclastic differentiation (Enomoto et al. 2003). The higher expression of osteoblast markers alkaline phosphatase (ALP) and osteocalcin (OC) and osteoclast marker cathepsin K (CATK) was parallel to a higher expression of RUNX2 at the oxidized surfaces compared with machined ones after 3 days of implantation (Omar et al. 2010b). Similar results were demonstrated for HF surface in comparison to similar surface without acid etching (Guo et al. 2007). In the latter study, RUNX2 expression was evident after 7 days in rat tibia and was associated with higher expression of ALP and bone sialoprotein (BSP). Regulatory effect on the expression of RUNX2 was also confirmed for the HF surface in rabbit cortical bone in parallel with higher expression of collagen 1 and OC after eight weeks of implantation (Monjo et al. 2008). Machined implants inserted in transgenic mice showed higher expression of bone BSP after local administration of virus encoding for osterix, which is a downstream factor of RUNX2 (Xu et al. 2009). Although all of these studies suggest fast and strong influence of the different material surface properties on the expression of critical switching factors, it is still not revealed in which way and which specific surface properties contribute to such effects.
Recent studies on early osseointegration (hours–days) have demonstrated that the upregulation of genes responsible for bone formation ALP and OC was coupled with upregulation of genes expressed by osteoclasts indicating that the bone remodelling phase is triggered much earlier than what has previously been assumed (Omar et al. 2010b). Similar results were observed with other surfaces where the over expression of collagen 1 and OC was coupled with higher expression of TRAP in cells adherent to coin titanium implants retrieved from rabbit tibia after eight weeks of implantation (Monjo et al. 2008). An intimate cross-talk is established between osteogenic cells and osteoclasts, e.g. the surface receptor RANK on osteoclasts recognizes and binds to osteoblast membrane-associated factor (RANKL) during the osteoclastic differentiation from the monocytic lineage (Katagiri & Takahashi 2002). Therefore, it is possible that differentiation of one type of these cells would be coupled with differentiation of the other type at the implant surface. Taken together, results suggest that active bone resorption and bone formation are processes taking place over a wide time range and starting already during the first days after implantation. Indeed, studies evaluating the gene expression of interfacial cells in combination with other structural and biomechanical data, also at later time points, are needed to further explore the role of early interfacial events for long-term functional performance of the implant.
3.2. Ultrastructure
Understanding the bonding mechanisms between bone and implant surface requires the application of high-resolution imaging and elemental detection in intact implant–tissue specimens. The method of choice is TEM where a variety of analyses are available and readily performed. For imaging, different contrast phenomena could be used to highlight different aspects, bright field, dark field and high angular annular dark field (HAADF). For elemental analysis most TEM instruments are equipped with energy dispersive X-ray spectroscopy (EDS) and some with electron energy loss spectroscopy (EELS) where elemental information at nano-level resolution may be provided. Further, with EELS, energy filtered TEM (EFTEM) could be performed as well as analysis of the electronic bonding state of the elements, adding information regarding the molecular structure. For structural analysis electron diffraction and HRTEM reveal the crystalline structure by analysis of the lattice parameter.
The associated problems and scarcity of publications within the field of biomaterial lies with the cumbersome sample preparation. The requirement of the TEM sample is that it has to be electron transparent, which means a thickness less than 100 nm. The conventional sample preparation technique within the biological sciences is to cut thin samples of embedded tissue blocks with an ultramicrotome and diamond knife; however, this technique could not cut the implant material (Albrektsson et al. 1981). Therefore, different methods to circumvent this problem have been suggested. By using titanium-coated plastic implants the intact interface could be cut with a diamond knife as the titanium coating is thin enough (Albrektsson et al. 1982). However, this method is strictly limited to experimental research and could not be used on clinically retrieved implants. Further, the lack of bulk properties and the surface characteristics of the sputtered titanium coating hampered comparisons with clinically used implants. To be able to analyse clinically relevant implants a method with gentle removal of the implant after embedding and prior to sectioning was introduced (Thomsen & Ericson 1985). Questions regarding where the separation actually occurs were raised and surface analysis of the removed implant showed a low amount of residuals and concluded that the separation occurred at the bone–implant interface (Lausmaa & Linder 1988). These observations have recently been confirmed for smooth, machined implants (Palmquist et al. 2009). However, the fracture technique is restricted to smooth implants where fractures occur at the interface to the implant. To be able to cut intact interfaces of clinically relevant implants another approach was developed where the bulk metal of the implant was removed by electrochemical dissolution, leaving the oxide layer intact prior to cutting ultrathin samples (Sennerby et al. 1991). With this technique both the oxide layer and bone were present in the samples but due to demineralization of the interfacial bone induced by the electrochemical process, the technique is restricted to soft-tissue interfaces (Bjursten et al. 1990). Yet another method to remove the bulk metal leaving the oxide layer has been performed by sawing and grinding (Hemmerle & Voegel 1996), but this method is restricted to thicker coatings such as plasma-sprayed titanium. With FIB microscopy the problem with cutting the implant is overcome and true intact section containing bulk metal, oxide layer and eventual coatings and bone tissue could be prepared (Engqvist et al. 2006) and could also be combined with preparation of ground sections for histological analysis (Palmquist et al. 2010).
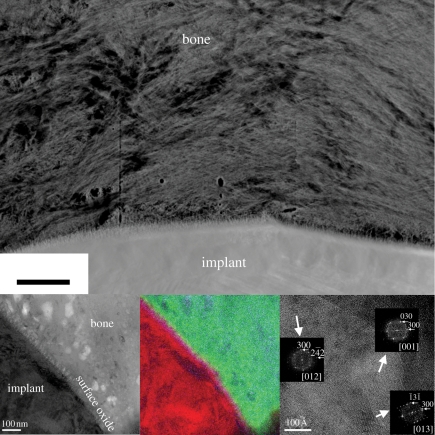
The interfaces between bone tissue and titanium implant surface described in the literature show some common patterns as well as some differences depending on the sample preparation method and research group. Most of the published data are built on the separation technique for sample preparation and the suggested interfaces consist of either mineralized bone in direct contact with the implant surface or with interposed layers in the nanoscale. These interposed layers could either be electron dense (Steflik et al. 1999) or electron lucent (Albrektsson et al. 1985) and sometimes referred to as a cement-like line (Davies 2007). With the use of FIB for sample preparation where an intact interface could be analysed as both implant and tissue are present in the sample, it has recently been shown that the interface is mineralized both for machined (Palmquist et al. 2008) and laser modified implants (Palmquist et al. 2010). Further, it has also been demonstrated that the bonding mechanisms are different between different implant surfaces where micro- and nanostructured laser-modified implant surfaces attain a strong bonding at the nano-level (figure 3). However, systematic interface analysis has to be performed with altered surface characteristics, such as surface topography and surface phase composition, in order to further understand how the bone tissue bonds to the implant surface.
Figure 3.
HAADF STEM image of the interface between laser-modified implant and rabbit bone after eight weeks of healing. EFTEM images as zero-loss image, corresponding superposed element map for calcium, oxygen and titanium and HRTEM of the interfacial bone as insets showing a fully mineralized interface and bone bonding (scale bar, 300 nm). (Adapted from Palmquist et al. 2010.)
4. Clinical outcome of oral implants
Osseointegrated dental implants are available in different materials, body shapes, diameters, lengths, platforms, surface properties and coatings. In particular, the area of implant surface modification and coating has been subjected to aggressive marketing aimed at establishing the superiority of a given surface over the others. It is therefore important to know whether there are surface modifications, implant shapes or particular materials that can improve clinical results. This summary of a Cochrane systematic review (Higgins & Green 2009) looks at whether there are differences in clinical performance among different implant types. For a detailed description of the review the reader is referred to the original publication (Esposito et al. 2007).
Six hypotheses were tested.
— There is no difference between implants with different surface preparations, but having similar shape and material.
— There is no difference between implants with different shapes, but having similar surface preparation and material.
— There is no difference between implants made of different materials, but having similar surface preparation and shape.
— There is no difference between various implant types differing in surface preparation and/or shape and/or material.
— There is no difference in the occurrence of early failures between turned and roughened dental implants.
— There is no difference in the occurrence of peri-implantitis between turned and roughened dental implants after 3 and 5 years in function.
All randomized controlled clinical trials (RCTs) with a follow-up of at least 1 year in function of osseointegrated dental implants replacing missing teeth comparing different implant types were reached on the Cochrane Oral Health Group's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE. Handsearching included several dental journals. No language restriction was applied and attempts were made to retrieve unpublished data. The last electronic search was conducted on 13 June 2007. Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted in duplicate and independently by two review authors. Results were expressed as random-effects models using mean differences for continuous outcomes and risk ratios (RR) for dichotomous outcomes with 95% CI. Outcome measures were (i) implant failures including implant fracture and other mechanical complications not allowing use of the implants, (ii) radiographic marginal bone level changes on intraoral radiographs taken with a paralleling technique, and (iii) occurrence of peri-implantitis defined as implants affected by progressive marginal bone loss with signs of infection.
Forty different RCTs were identified. Sixteen of these RCTs, reporting results from a total of 771 patients, were suitable for inclusion in the review. Eighteen different implant types were compared with a follow-up ranging from 1 to 5 years. All implants were made in commercially pure titanium and had different shapes and surface preparations.
- — Trials comparing implants with different surface preparations, but having similar shape and material (three trials).
- Brånemark Mark III implants: turned versus TiUnite oxide surface (Fröberg et al. 2006).
- Brånemark Mark IV implants: turned versus TiUnite oxide surface (Schincaglia et al. 2007).
- 3i implants: full versus dual Osseotite surface (Stavropoulos et al. 2007).
- — Trials comparing implants with different shapes, but having similar surface preparation and material (three trials).
- Brånemark Mark II type versus Brånemark conical transmucosal implants (Gatti & Chiapasco 2002).
- Brånemark standard versus Brånemark Mark IV prototype implants (Friberg et al. 2003).
- ITI cylindrical versus ITI tapered (Lang et al. 2007).
— Trials comparing implants with different materials, but having similar surface preparation and shape (no trials).
- — Trials comparing implants with different surface preparation and/or shape and/or material (10 trials).
- Astra versus Brånemark implants (Åstrand et al. 1999).
- Astra versus ITI implants (Kemppainen et al. 1997).
- Brånemark versus IMZ implants (Batenburg et al. 1998).
- Brånemark TiUnite versus Southern implants (Payne et al. 2004).
- IMZ versus ITI implants (Batenburg et al. 1998; Heijdenrijk et al. 2002).
- ITI versus Southern implants (Payne et al. 2003).
— Early failures between turned and roughened surfaces (seven trials; Batenburg et al. 1998; Åstrand et al. 1999, 2002; Moberg et al. 2001; Wennström et al. 2004; Fröberg et al. 2006; Schincaglia et al. 2007).
— Peri-implantitis between turned and roughened surfaces at 3 years (three trials; Åstrand et al. 1999, 2002; Moberg et al. 2001).
On a ‘per patient’ rather than ‘per implant’ basis no significant differences were observed between various implant types for implant failures. There were statistically significant differences for peri-implant bone level changes on intraoral radiographs in three comparisons in two trials. In one trial there was more bone loss only at 1 year for IMZ implants compared with Brånemark (mean difference 0.60 mm; 95% CI 0.01 to 1.10) and ITI implants (mean difference 0.50 mm; 95% CI 0.01 to 0.99; Batenburg et al. 1998). In the other trial Southern implants displayed more bone loss at 5 years than Steri-Oss implants (mean difference −0.35 mm; 95% CI −0.70 to −0.01; Tawse-Smith et al. 2002). However this difference disappeared in the meta-analysis. More implants with rough surfaces were affected by peri-implantitis (RR 0.80; 95% CI 0.67–0.96) meaning that turned implant surfaces had a 20 per cent reduction in risk of being affected by peri-implantitis over a 3 year period (figure 4).
Figure 4.
Forest plot illustrating the number of patients affected by peri-implantitis when comparing implants with a turned (machined) or rough surface after 3 years in function (Esposito et al. 2007).
Based on the available results of RCTs, there is limited evidence showing that implants with relatively smooth (turned) surfaces are less prone to lose bone owing to chronic infection (peri-implantitis) than implants with rougher surfaces. On the other hand, there is no evidence showing that any particular type of dental implant has superior long-term success. These findings are based on a few RCTs, often at high risk of bias, with few participants and relatively short follow-up periods. More RCTs should be conducted, with follow-up of at least 5 years including a sufficient number of patients to detect a true difference. Such trials should be reported according to the CONSORT recommendations (www.consort-statement.org/).
5. Summary and future outlook
Currently used clinical dental implants show a wide variety of surface characteristics, both in terms of structural and chemical properties. Whereas the materials for the first generation of dental implant were selected mainly on the basis of their mechanical properties and corrosion resistance under physiological conditions, the current ones can be regarded as the second generation. Their surfaces have been modified—mainly with respect to surface topography, but in some cases also chemical composition—in order to achieve a better biological response. From experimental studies and clinical experience, there is extensive empirical knowledge about the role of such surface modifications for biological responses. However, this knowledge is very fragmented, and we are still far from a situation where we understand the different biological processes taking place at the interface, and how these are influenced and can be controlled by specific surface properties. The development of the next, third generation of dental implants will require further knowledge about the interface biology on a molecular and cellular level. Implant surfaces modified with or releasing biologically active substances, new microscopic and analytical tools, and the new, emerging understanding about stem and progenitor cells will play an important role in acquiring the knowledge necessary for specifying the optimal properties of the next generation of oral implants for a given clinical indication.
Acknowledgements
The financial support from the Swedish Research Council (grants K2006-73X-09 495-16-3 and K2009-52X-09 495-22-3), BIOMATCELL VINN Excellence Center of Biomaterials and Cell Therapy, Region Västra Götaland ALF/LUA (ALFGBG-11 128), and IBCT (part of GöteborgBIO) grant is gratefully acknowledged.
Footnotes
One contribution to a Theme Supplement ‘Scaling the heights—challenges in medical materials: an issue in honour of William Bonfield, Part II. Bone and tissue engineering’.
References
- Albrektsson T., Wennerberg A. 2004. Oral implant surfaces: part 1—review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 17, 536–543. [PubMed] [Google Scholar]
- Albrektsson T., Brånemark P. I., Hansson H. A., Lindstrom J. 1981. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 52, 155–170. [DOI] [PubMed] [Google Scholar]
- Albrektsson T., Brånemark P. I., Hansson H. A., Ivarsson B., Jönsson U. 1982. Ultrastructural analysis of the interface zone of titanium and gold implants. In Advances in biomaterials, vol. 4 (eds Albrektsson T., Lee A. J. C., Brånemark P. I.), pp. 167–177. Chichester, UK: Wiley. [Google Scholar]
- Albrektsson T., Hansson H. A., Ivarsson B. 1985. Interface analysis of titanium and zirconium bone implants. Biomaterials 6, 97–101. ( 10.1016/0142-9612(85)90070-5) [DOI] [PubMed] [Google Scholar]
- Ask M., Rolander U., Lausmaa J., Kasemo B. 1990. Microstructure and morphology of surface oxide films on Ti–6Al–4V. J. Mater. Res. 5, 1662–1667. ( 10.1557/JMR.1990.1662) [DOI] [Google Scholar]
- Åstrand P., Engquist B., Dahlgren S., Engquist E., Feldmann H., Gröndahl K. 1999. Astra Tech and Brånemark System implants: a prospective 5-year comparative study. Results after one year. Clin. Implant Dent. Relat. Res. 1, 17–26. ( 10.1111/j.1708-8208.1999.tb00087.x) [DOI] [PubMed] [Google Scholar]
- Åstrand P., Engquist B., Anzén B., Bergendal T., Hallman M., Karlsson U., Kvint S., Lysell L., Rundcranz T. 2002. Nonsubmerged and submerged implants in the treatment of the partially edentulous maxilla. Clin. Implant Dent. Relat. Res. 4, 115–127. ( 10.1111/j.1708-8208.2002.tb00161.x) [DOI] [PubMed] [Google Scholar]
- Batenburg R. H. K., Meijer H. J. A., Raghoebar G. M., Van Oort R. P., Boering G. 1998. Mandibular overdentures supported by two Brånemark, IMZ or ITI implants. A prospective comparative preliminary study: one-year results. Clin. Oral Implants Res. 9, 374–383. ( 10.1034/j.1600-0501.1996.090603.x) [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A., Eades J. A. 2009. Use of an energy filter to improve the spatial resolution of electron backscatter diffraction. Scanning 31, 114–121. ( 10.1002/sca.20150) [DOI] [PubMed] [Google Scholar]
- Bigi A., Boanini E., Capuccini C., Fini M., Mihailescu I. N., Ristoscu C., Sima F., Torricelli P. 2009. Biofunctional alendronate-hydroxyapatite thin films deposited by matrix assisted pulsed laser evaporation. Biomaterials 30, 6168–6177. ( 10.1016/j.biomaterials.2009.07.066) [DOI] [PubMed] [Google Scholar]
- Bjursten L. M., Emanuelsson L., Ericson L. E., Thomsen P., Lausmaa J., Mattsson L., Rolander U., Kasemo B. 1990. Method for ultrastructural studies of the intact tissue–metal interface. Biomaterials 11, 596–601. ( 10.1016/0142-9612(90)90085-5) [DOI] [PubMed] [Google Scholar]
- Boyan B. D., Batzer R., Kieswetter K., Liu Y., Cochran D. L., Szmuckler-Moncler S., Dean D. D., Schwartz Z. 1998. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1α,25-(OH)2D3. J. Biomed. Mater. Res. 39, 77–85. () [DOI] [PubMed] [Google Scholar]
- Boyan B., Dean D., Lohmann C., Cochran D., Sylvia V., Schwartz Z. 2001. The titanium-bone cell interface in vitro: the role of the surface in promoting osteointegration. In Titanium in medicine (eds Brunette D. M., Tengvall P., Textor M., Thomsen P.), pp. 561–585. New York, NY: Springer. [Google Scholar]
- Brunette D. M., Tengvall P., Textor M., Thomsen P. (eds) 2001. Titanium in medicine: material science, surface science, engineering, biological responses, and medical applications. Berlin, Germany: Springer. [Google Scholar]
- Ceradini D. J., et al. 2004. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 10, 858–864. ( 10.1038/nm1075) [DOI] [PubMed] [Google Scholar]
- Champagne C. M., Takebe J., Offenbacher S., Cooper L. F. 2002. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 30, 26–31. ( 10.1016/S8756-3282(01)00638-X) [DOI] [PubMed] [Google Scholar]
- Cheng Z., Liu X., Ou L., Zhou X., Liu Y., Jia X., Zhang J., Li Y., Kong D. 2008. Mobilization of mesenchymal stem cells by granulocyte colony-stimulating factor in rats with acute myocardial infarction. Cardiovasc. Drugs Ther. 22, 363–371. ( 10.1007/s10557-008-6110-2) [DOI] [PubMed] [Google Scholar]
- Conforto E., Caillard D., Aronsson B. O., Descouts P. 2004. Crystallographic properties and mechanical behaviour of titanium hydride layers grown on titanium implants. Phil. Mag. 84, 631–645. ( 10.1080/14786430310001627386) [DOI] [Google Scholar]
- Davies J. E. 2007. Bone bonding at natural and biomaterial surfaces. Biomaterials 28, 5058–5067. ( 10.1016/j.biomaterials.2007.07.049) [DOI] [PubMed] [Google Scholar]
- Ellingsen J. E., Thomsen P., Lyngstadaas S. P. 2006. Advances in dental implant materials and tissue regeneration. Periodontol. 2000 41, 136–156. ( 10.1111/j.1600-0757.2006.00175.x) [DOI] [PubMed] [Google Scholar]
- Engqvist H., Botton G. A., Couillard M., Mohammadi S., Malmstrom J., Emanuelsson L., Hermansson L., Phaneuf M. W., Thomsen P. 2006. A novel tool for high-resolution transmission electron microscopy of intact interfaces between bone and metallic implants. J. Biomed. Mater. Res. A 78A, 20–24. ( 10.1002/jbm.a.30696) [DOI] [PubMed] [Google Scholar]
- Enomoto H., et al. 2003. Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2-/- mice by RANKL transgene. J. Biol. Chem. 278, 23 971–23 977. ( 10.1074/jbc.M302457200) [DOI] [PubMed] [Google Scholar]
- Esposito M., Hirsch J. M., Lekholm U., Thomsen P. 1998a Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral Sci. 106, 527–551. ( 10.1046/j.0909-8836..t01-2-.x) [DOI] [PubMed] [Google Scholar]
- Esposito M., Hirsch J. M., Lekholm U., Thomsen P. 1998b Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur. J. Oral Sci. 106, 721–764. ( 10.1046/j.0909-8836..t01-6-.x) [DOI] [PubMed] [Google Scholar]
- Esposito M., Murray-Curtis L., Grusovin M. G., Coulthard P., Worthington H. V. 2007. Interventions for replacing missing teeth: different types of dental implants. Cochrane database of systematic reviews, vol. 4 Chichester, UK: Wiley. [DOI] [PubMed] [Google Scholar]
- Friberg B., Jisander S., Widmark G., Lundgren A., Ivanoff C. J., Sennerby L., Thorén C. 2003. One-year prospective three-center study comparing the outcome of a ‘soft bone implant’ (prototype Mk IV) and the standard Brånemark implant. Clin. Implant Dent. Relat. Res. 5, 71–77. ( 10.1111/j.1708-8208.2003.tb00186.x) [DOI] [PubMed] [Google Scholar]
- Fröberg K. K., Lindh C., Ericsson I. 2006. Immediate loading of Brånemark System implants: a comparison between TiUnite and turned implants placed in the anterior mandible. Clin. Implant Dent. Relat. Res. 8, 187–197. ( 10.1111/j.1708-8208.2006.00017.x) [DOI] [PubMed] [Google Scholar]
- Garcia A. J., Schwarzbauer J. E., Boettiger D. 2002. Distinct activation states of α5β1 integrin show differential binding to RGD and synergy domains of fibronectin. Biochemistry 41, 9063–9069. ( 10.1021/bi025752f) [DOI] [PubMed] [Google Scholar]
- Gatti C., Chiapasco M. 2002. Immediate loading of Brånemark implants: a 24-month follow-up of a comparative prospective pilot study between mandibular overdentures supported by conical transmucosal and standard MKII implants. Clin. Implant Dent. Relat. Res. 4, 190–199. ( 10.1111/j.1708-8208.2002.tb00171.x) [DOI] [PubMed] [Google Scholar]
- Guo J., Padilla R. J., Ambrose W., De Kok I. J., Cooper L. F. 2007. The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo. Biomaterials 28, 5418–5425. ( 10.1016/j.biomaterials.2007.08.032) [DOI] [PubMed] [Google Scholar]
- Hayashi H., Nakahama K., Sato T., Tuchiya T., Asakawa Y., Maemura T., Tanaka M., Morita M., Morita I. 2008. The role of Mac-1 (CD11b/CD18) in osteoclast differentiation induced by receptor activator of nuclear factor-κB ligand. FEBS Lett. 582, 3243–3248. ( 10.1016/j.febslet.2008.08.023) [DOI] [PubMed] [Google Scholar]
- Heijdenrijk K., Raghoebar G. M., Meijer H. J., Van Der Reijden W. A., Van Winkelhoff A. J., Stegenga B. 2002. Two-stage IMZ implants and ITI implants inserted in a single-stage procedure. Clin. Oral Implants Res. 13, 371–380. ( 10.1034/j.1600-0501.2002.130405.x) [DOI] [PubMed] [Google Scholar]
- Hemmerle J., Voegel J. C. 1996. Ultrastructural aspects of the intact titanium implant-bone interface from undecalcified ultrathin sections. Biomaterials 17, 1913–1920. ( 10.1016/0142-9612(95)00244-8) [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Green S. 2009. Cochrane handbook for systematic reviews of interventions 5.0.2 [updated September 2009]. See www.cochrane-handbook.org.
- Hughes D. E., Salter D. M., Dedhar S., Simpson R. 1993. Integrin expression in human bone. J. Bone Miner. Res. 8, 527–533. ( 10.1002/jbmr.5650080503) [DOI] [PubMed] [Google Scholar]
- Jarmar T., Palmquist A., Brånemark R., Hermansson L., Engqvist H., Thomsen P. 2008a Characterization of the surface properties of commercially available dental implants using scanning electron microscopy, focused ion beam, and high-resolution transmission electron microscopy. Clin. Implant Dent. Relat. Res. 10, 11–22. ( 10.1111/j.1708-8208.2007.00056.x) [DOI] [PubMed] [Google Scholar]
- Jarmar T., Palmquist A., Brånemark R., Hermansson L., Engqvist H., Thomsen P. 2008b Technique for preparation and characterization in cross-section of oral titanium implant surfaces using focused ion beam and transmission electron microscopy. J. Biomed. Mater. Res. A 87A, 1003–1009. ( 10.1002/jbm.a.31856) [DOI] [PubMed] [Google Scholar]
- Junker R., Dimakis A., Thoneick M., Jansen J. A. 2009. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin. Oral Implants Res. 20, 185–206. ( 10.1111/j.1600-0501.2009.01777.x) [DOI] [PubMed] [Google Scholar]
- Kalltorp M., Carlen A., Thomsen P., Olsson J., Tengvall P. 2000. Analysis of rat plasma proteins desorbed from gold and methyl- and hydroxyl-terminated alkane thiols on gold surfaces. J. Mater. Sci. Mater. Med. 11, 191–199. ( 10.1023/A:1008935826310) [DOI] [PubMed] [Google Scholar]
- Katagiri T., Takahashi N. 2002. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 8, 147–159. ( 10.1034/j.1601-0825.2002.01829.x) [DOI] [PubMed] [Google Scholar]
- Kemppainen P., Eskola S., Ylipaavalniemi P. 1997. A comparative prospective clinical study of two single-tooth implants: a preliminary report of 102 implants. J. Prosth. Dent. 77, 382–387. ( 10.1016/S0022-3913(97)70163-X) [DOI] [PubMed] [Google Scholar]
- Kitaori T., et al. 2009. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arth. Rheum. 60, 813–823. ( 10.1002/art.24330) [DOI] [PubMed] [Google Scholar]
- Kokubo T., Kim H. M., Kawashita M., Nakamura T. 2001. Process of calcification on artificial materials. Z. Kardiol. 90(Suppl. 3), 86–91. [DOI] [PubMed] [Google Scholar]
- Lang N. P., et al. 2007. Immediate implant placement with transmucosal healing in areas of aesthetic priority. A multicentre randomized-controlled clinical trial. I. Surgical outcomes. Clin. Oral Implants Res. 18, 188–196. ( 10.1111/j.1600-0501.2006.01371.x) [DOI] [PubMed] [Google Scholar]
- Lausmaa J., Linder L. 1988. Surface spectroscopic characterization of titanium implants after separation from plastic-embedded tissue. Biomaterials 9, 277–280. ( 10.1016/0142-9612(88)90098-1) [DOI] [PubMed] [Google Scholar]
- Lewandowska M., Roguska A., Pisarek M., Polak B., Janik-Czachor M., Kurzydlowski K. J. 2007. Morphology and chemical characterization of Ti surfaces modified for biomedical applications. Biomol. Eng. 24, 438–442. ( 10.1016/j.bioeng.2007.07.002) [DOI] [PubMed] [Google Scholar]
- Linderback P., Harmankaya N., Askendal A., Areva S., Lausmaa J., Tengvall P. 2010. The effect of heat- or ultra violet ozone-treatment of titanium on complement deposition from human blood plasma. Biomaterials 31, 4795–4801. ( 10.1016/j.biomaterials.2010.02.060) [DOI] [PubMed] [Google Scholar]
- Ma J., Ge J., Zhang S., Sun A., Shen J., Chen L., Wang K., Zou Y. 2005. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res. Cardiol. 100, 217–223. ( 10.1007/s00395-005-0521-z) [DOI] [PubMed] [Google Scholar]
- Masaki C., Schneider G. B., Zaharias R., Seabold D., Stanford C. 2005. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implants Res. 16, 650–656. ( 10.1111/j.1600-0501.2005.01170.x) [DOI] [PubMed] [Google Scholar]
- Moberg L. E., Köndell P. Å., Sagulin G. B., Bolin A., Heimdahl A., Gynther G. W. 2001. Brånemark System and ITI dental implant system for treatment of mandibular edentulism. A comparative randomized study: 3-year follow-up. Clin. Oral Implants Res. 12, 450–461. ( 10.1034/j.1600-0501.2001.120504.x) [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Esposito M., Hall J., Emanuelsson L., Krozer A., Thomsen P. 2003. Short-term bone response to titanium implants coated with thin radiofrequent magnetron-sputtered hydroxyapatite in rabbits. Clin. Implant Dent. Relat. Res. 5, 241–253. ( 10.1111/j.1708-8208.2003.tb00207.x) [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Esposito M., Hall J., Emanuelsson L., Krozer A., Thomsen P. 2004. Long-term bone response to titanium implants coated with thin radiofrequent magnetron-sputtered hydroxyapatite in rabbits. Int. J. Oral Maxillofac. Implants 19, 498–509. [PubMed] [Google Scholar]
- Monjo M., Lamolle S. F., Lyngstadaas S. P., Ronold H. J., Ellingsen J. E. 2008. In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 29, 3771–3780. ( 10.1016/j.biomaterials.2008.06.001) [DOI] [PubMed] [Google Scholar]
- Muller-Mai C. M., Voigt C., Gross U. 1990. Incorporation and degradation of hydroxyapatite implants of different surface roughness and surface structure in bone. Scanning Microsc. 4, 613–622; discussion 622–624. [PubMed] [Google Scholar]
- Nyan M., Miyahara T., Noritake K., Hao J., Rodriguez R., Kuroda S., Kasugai S. 2010. Molecular and tissue responses in the healing of rat calvarial defects after local application of simvastatin combined with alpha tricalcium phosphate. J. Biomed. Mater. Res. B 93B, 65–73. ( 10.1002/jbm.b.31559) [DOI] [PubMed] [Google Scholar]
- Nygren H., Tengvall P., Lundstrom I. 1997. The initial reactions of TiO2 with blood. J. Biomed. Mater. Res. A 34, 487–492. () [DOI] [PubMed] [Google Scholar]
- Ogawa T., Nishimura I. 2003. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implants 18, 200–210. [PubMed] [Google Scholar]
- Omar O., Lennerås M., Svensson S., Suska F., Emanuelsson L., Hall J., Nannmark U., Thomsen P. 2010a Integrin and chemokine receptor gene expression in implant-adherent cells during early osseointegration. J. Mater. Sci. Mater. Med. 21, 969–980. ( 10.1007/s10856-009-3915-x) [DOI] [PubMed] [Google Scholar]
- Omar O., Svensson S., Zoric N., Lennerås M., Suska F., Wigren S., Hall J., Nannmark U., Thomsen P. 2010b In vivo gene expression in response to anodically oxidized versus machined titanium implants. J. Biomed. Mater. Res. A 92A, 1552–1566. ( 10.1002/jbm.a.32475) [DOI] [PubMed] [Google Scholar]
- Palmquist A., Jarmar T., Emanuelsson L., Brånemark R., Engqvist H., Thomsen P. 2008. Forearm bone-anchored amputation prosthesis: a case study on the osseointegration. Acta Orthop. 79, 78–85. ( 10.1080/17453670710014806) [DOI] [PubMed] [Google Scholar]
- Palmquist A., Lindberg F., Emanuelsson L., Brånemark R., Engqvist H., Thomsen P. 2009. Morphological studies on machined implants of commercially pure titanium and titanium alloy (Ti6Al4V) in the rabbit. J. Biomed. Mater. Res. B 91B, 309–319. ( 10.1002/jbm.b.31404) [DOI] [PubMed] [Google Scholar]
- Palmquist A., Lindberg F., Emanuelsson L., Brånemark R., Engqvist H., Thomsen P. 2010. Biomechanical, histological, and ultrastructural analyses of laser micro- and nano-structured titanium alloy implants: a study in rabbit. J. Biomed. Mater. Res. A 92A, 1476–1486. ( 10.1002/jbm.a.32439) [DOI] [PubMed] [Google Scholar]
- Park J. B. 2009. The use of simvastatin in bone regeneration. Med. Oral Patol. Oral Cir. Bucal 14, e485–e488. [PubMed] [Google Scholar]
- Park J. Y., Davies J. E. 2000. Red blood cell and platelet interactions with titanium implant surfaces. Clin. Oral Implants Res. 11, 530–539. ( 10.1034/j.1600-0501.2000.011006530.x) [DOI] [PubMed] [Google Scholar]
- Payne A. G., Tawse-Smith A., Thompson W. M., Kumara R. 2003. Early functional loading of unsplinted roughened surface implants with mandibular overdentures 2 weeks after surgery. Clin. Implant Dent. Relat. Res. 5, 143–153. ( 10.1111/j.1708-8208.2003.tb00196.x) [DOI] [PubMed] [Google Scholar]
- Payne A. G. T., Tawse-Smith A., Thompson W. M., Duncan W. J., Kumara R. 2004. One-stage surgery and early loading of three implants for maxillary overdentures: a one-year report. Clin. Implant Dent. Relat. Res. 6, 61–74. ( 10.111/j.1708-8208.2004.tb00028.x) [DOI] [PubMed] [Google Scholar]
- Radegran G., Lausmaa J., Mattsson L., Rolander U., Kasemo B. 1991. Preparation of ultra-thin oxide windows on titanium for TEM analysis. J. Electron Microsc. Tech. 19, 99–106. ( 10.1002/jemt.1060190110) [DOI] [PubMed] [Google Scholar]
- Schincaglia G. P., Marzola R., Scapoli C., Scotti R. 2007. Immediate loading of dental implants supporting fixed partial dentures in the posterior mandible: a randomized controlled split-mouth study—machined versus titanium oxide implant surface. Int. J. Oral Maxillofac. Implants 22, 35–46. [PubMed] [Google Scholar]
- Schneider G. B., Perinpanayagam H., Clegg M., Zaharias R., Seabold D., Keller J., Stanford C. 2003. Implant surface roughness affects osteoblast gene expression. J. Dent. Res. 82, 372–376. [DOI] [PubMed] [Google Scholar]
- Sennerby L., Ericson L. E., Thomsen P., Lekholm U., Astrand P. 1991. Structure of the bone-titanium interface in retrieved clinical oral implants. Clin. Oral Implants Res. 2, 103–111. ( 10.1034/j.1600-0501.1991.020302.x) [DOI] [PubMed] [Google Scholar]
- Sennerby L., Thomsen P., Ericson L. E. 1993a Early tissue response to titanium implants inserted in rabbit cortical bone. Part I. Light microscopic observations. J. Mater. Sci. Mater. Med. 4, 240–250. ( 10.1007/BF00122275) [DOI] [Google Scholar]
- Sennerby L., Thomsen P., Ericson L. E. 1993b Early tissue response to titanium implants inserted in rabbit cortical bone. Part II. Ultrastructural observations. J. Mater. Sci. Mater. Med. 4, 494–502. ( 10.1007/BF00120129) [DOI] [Google Scholar]
- Stavropoulos A., Karring T., Kostopoulos L. 2007. Fully vs. partially rough implants in maxillary sinus floor augmentation: a randomized-controlled clinical trial. Clin. Oral Implants Res. 18, 95–102. ( 10.1111/j.1600-0501.2006.01289.x) [DOI] [PubMed] [Google Scholar]
- Steflik D. E., Corpe R. S., Young T. R., Sisk A. L., Parr G. R. 1999. The biologic tissue responses to uncoated and coated implanted biomaterials. Adv. Dent. Res. 13, 27–33. ( 10.1177/08959374990130011101) [DOI] [PubMed] [Google Scholar]
- Stewart M., Thiel M., Hogg N. 1995. Leukocyte integrins. Curr. Opin. Cell Biol. 7, 690–696. ( 10.1016/0955-0674(95)80111-1) [DOI] [PubMed] [Google Scholar]
- Sul Y. T., Johansson C. B., Petronis S., Krozer A., Jeong Y., Wennerberg A., Albrektsson T. 2002. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: the oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials 23, 491–501. ( 10.1016/S0142-9612(01)00131-4) [DOI] [PubMed] [Google Scholar]
- Tan K. S., Qian L., Rosado R., Flood P. M., Cooper L. F. 2006. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials 27, 5170–5177. ( 10.1016/j.biomaterials.2006.05.002) [DOI] [PubMed] [Google Scholar]
- Tawse-Smith A., Payne A. G. T., Kumara R., Thomson W. M. 2001. One-stage operative procedure using two different implant systems: a prospective study on implant overdentures in the edentulous mandible. Clin. Implant Dent. Relat. Res. 3, 185–193. ( 10.1111/j.1708-8208.2001.tb00140.x) [DOI] [PubMed] [Google Scholar]
- Tawse-Smith A., Payne A. G. T., Kumara R., Thomson W. M. 2002. Early loading of unsplinted implants supporting mandibular overdentures using a one-stage operative procedure with two different implant systems: a 2-year report. Clin. Implant Dent. Relat. Res. 4, 33–42. ( 10.1111/j.1708-8208.2002.tb00149.x) [DOI] [PubMed] [Google Scholar]
- Thomsen P., Ericson L. E. 1985. Light and transmission electron microscopy used to study the tissue morphology close to implants. Biomaterials 6, 421–424. ( 10.1016/0142-9612(85)90104-8) [DOI] [PubMed] [Google Scholar]
- Trager-Cowan C., et al. 2002. Characterization of nitride thin films by electron backscatter diffraction. J. Microsc. 205, 226–230. ( 10.1046/j.1365-2818.2002.00996.x) [DOI] [PubMed] [Google Scholar]
- van Blitterswijk C. A., Grote J. J., Kuypers W., Blok-van Hoek C. J., Daems W. T. 1985. Bioreactions at the tissue/hydroxyapatite interface. Biomaterials 6, 243–251. ( 10.1016/0142-9612(85)90020-1) [DOI] [PubMed] [Google Scholar]
- Wang Y., Deng Y., Zhou G. Q. 2008. SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 1195, 104–112. ( 10.1016/j.brainres.2007.11.068) [DOI] [PubMed] [Google Scholar]
- Wennström J. L., Ekestubbe A., Gröndahl K., Karlsson S., Lindhe J. 2004. Oral rehabilitation with implant-supported fixed partial dentures in periodontitis-susceptible subjects. A 5-year prospective study. J. Clin. Periodontol. 31, 713–724. ( 10.1111/j.1600-051X.2004.00568.x) [DOI] [PubMed] [Google Scholar]
- Wermelin K., Aspenberg P., Linderback P., Tengvall P. 2008. Bisphosphonate coating on titanium screws increases mechanical fixation in rat tibia after two weeks. J. Biomed. Mater. Res. A 86A, 220–227. ( 10.1002/jbm.a.31583) [DOI] [PubMed] [Google Scholar]
- Xia W., Lindahl C., Lausmaa J., Borchardt P., Ballo A., Thomsen P., Engqvist H. 2010. Biomineralized strontium-substituted apatite/titanium dioxide coating on titanium surfaces. Acta Biomater. 6, 1591–1600. ( 10.1016/j.actbio.2009.10.030) [DOI] [PubMed] [Google Scholar]
- Xu B., Zhang J., Brewer E., Tu Q., Yu L., Tang J., Krebsbach P., Wieland M., Chen J. 2009. Osterix enhances BMSC-associated osseointegration of implants. J . Dent. Res. 88, 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]