Abstract
Conducting polymers (CPs) have attracted much interest as suitable matrices of biomolecules and have been used to enhance the stability, speed and sensitivity of various biomedical devices. Moreover, CPs are inexpensive, easy to synthesize and versatile because their properties can be readily modulated by (i) surface functionalization techniques and (ii) the use of a wide range of molecules that can be entrapped or used as dopants. This paper discusses the various surface modifications of the CP that can be employed in order to impart physico-chemical and biological guidance cues that promote cell adhesion/proliferation at the polymer–tissue interface. This ability of the CP to induce various cellular mechanisms widens its applications in medical fields and bioengineering.
Keywords: conducting polymers, biomedical engineering, biomedical devices
1. Introduction
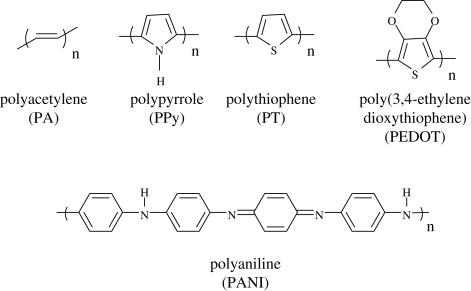
Conducting polymers (CPs) are a special class of polymeric materials with electronic and ionic conductivity. They can be used in the dry or wet state (Xu et al. 2005) owing to their electronic conductivity, their porous structure or because of their processibility in microstructuring processes (Schultze & Karabulut 2005). The structures of the widely used CPs are depicted in figure 1. A range of biomedical applications for CPs are currently being considered, including the development of artificial muscles (Otero & Sansinena 1998), controlled drug release (Abidian et al. 2006; Abidian & Martin 2009), neural recording (Abidian et al. 2009) and the stimulation of nerve regeneration (Schmidt et al. 1997). Moreover, electrically active tissues including the brain, heart and skeletal muscle provide opportunities to couple electronic devices and computers with human or animal tissues to create therapeutic body–machine interfaces (Warren et al. 1989). The conductive and semiconducting properties of the CPs make them an important class of materials for a wide range of applications. The important properties of various CPs and their potential applications are discussed in table 1. The origin of electrical conduction in CPs has been ascribed to the formation of nonlinear defects such as solitons, polarons or bipolarons formed during either doping or polymerization of a monomer (Saxena & Malhotra 2002; Tschmelak et al. 2005). Conductivity in CPs arises from the presence of conjugated double bonds along the backbone of an otherwise insulated structure. In conjugation, the bonds between the carbon atoms are alternatively single and double. Every bond in the backbone contains a localized ‘sigma’ (s) bond, which forms a strong chemical bond and every double bond also contains a less strongly localized ‘pi’ (p) bond (Wise et al. 1998; Heeger et al. 2000). Conductivity is imparted to these polymers through the use of a dopant ion, which must be introduced to the structure to carry charge in the form of extra electrons. The dopant neutralizes the unstable backbone when the polymer is in the oxidized form. On application of a potential across the film, a flux of ions either in or out of the film, dependent on dopant charge and motility, disrupts the stable backbone, resulting in the passage of charge through the polymer film. Conducting oligomers of pyrrole and thiophene connected by ester linkages have been considered for the creation of temporary scaffolds for cell attachment and proliferation for tissue engineering applications. In addition, these scaffolds are biodegradable (Rivers et al. 2002). The possibility of growing cells on CPs has proven the biocompatibility of these polymers (Schmidt et al. 1997; Garner et al. 1999a,b). Further, recently the biocompatibility of PPy and PEDOT films and PPy and PEDOT nanotubes was evaluated using a dorsal root ganglion model (Abidian et al. 2010). The implantation of CPs in vivo for several weeks has led to only minimal inflammation, again pointing to low toxicities and good tissue compatibility (Schmidt et al. 1997; Garner et al. 1999a,b; Rivers et al. 2002). Moreover, Abidian et al. (2009) successfully demonstrated that PEDOT nanotubes could record neuronal spikes about 30 per cent more than control sites with a high signal-to-noise ratio (SNR) for seven weeks post-implantation in vivo. Although there have been a number of reviews on CPs with regard to biomedical applications (Rivers et al. 2002; Zelikin et al. 2002; Gizdavic-Nikolaidis et al. 2004a,b; Schultze & Karabulut 2005; Abidian et al. 2006; Ahuja et al. 2007; Guimard et al. 2007a,b), this review focuses solely on the various tissue engineering and drug-delivery applications. Moreover, this review accentuates the various surface functionalization techniques that can be used in order to modify the physico-chemical, electrical and mechanical properties of the CPs so as to improve their potential biomedical applications.
Figure 1.
Chemical structures of the various conducting polymers.
Table 1.
Properties of the conducting polymer (CP).
| conducting polymer | synthesis technique | properties | applications |
|---|---|---|---|
| polypyrrole (PPy) | electrochemical and chemical synthesis | high conductivity (up to 160 S cm−1) when doped with iodine (Masada & Asano 2003); opaque, brittle, amorphous material | biosensors, antioxidants, drug delivery, bioactuators, neural prosthetics, cardiovascular application |
| polythiophenes (PT) | electrochemical and chemical synthesis | good electrical conductivity and optical property | biosensors, food industry |
| polyaniline (PANI) | electrochemical and chemical synthesis | belongs to the semiflexible rod polymer family; requires simple doping/dedoping chemistry; exists as bulk films or dispersions; high conductivity up to 100 S cm−1 | biosensors, antioxidants, drug delivery, bioactuators, food industry, cardiovascular application |
| poly(3,4-ethylenedioxythiophene) (PEDOT) | electrochemical and chemical synthesis | high temperature stability; ability to suppress the so-called ‘thermal runaway’ of the capacitor; transparent conductor; moderate band gap and low redox potential; conductivity up to 210 S cm−1 | biosensors, antioxidants, drug delivery, neural prosthetics |
2. Applications
2.1. Drug delivery
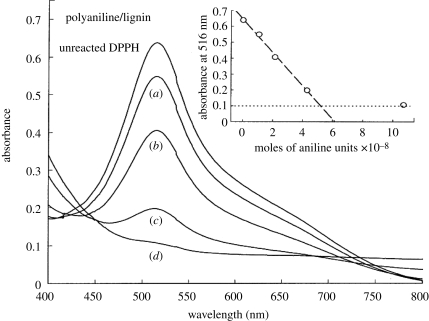
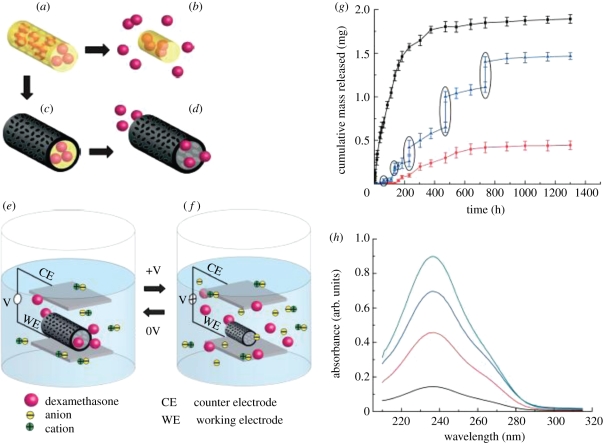
Current drug-delivery systems are effective at the controlled release of drugs. However, the application is still narrowed to targeting cell clusters rather than the individual cells (Venugopal et al. 2009). Developing novel drug-delivery approaches will open up contemporary avenues that were previously unsuited to traditional oral formulations. The use of CPs in the areas of bioanalytical sciences is of immense interest since their biocompatibility opens up the possibility of using them in in vivo biosensor applications (Adeloju & Wallace 1996) for continuous monitoring of drugs or metabolites in biological fluids (Harwood & Pouton 1996), or as a means of opening up the field to a variety of new analytes (Ahuja et al. 2007). The commercially available soluble CPs polyaniline (PANI) grafted to lignin, poly(anilinesulphonic acid) and polypyrrole (PPy) are effective scavengers of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical. This property may be particularly beneficial in tissues suffering from oxidative stress, where the ability to lower the excessive levels of reactive radical species is desirable. This free radical-reducing ability of CPs is matched by efficient scavenging of DPPH radicals, with two and four radicals scavenged per aniline or pyrrole monomer unit during the 30 min test period of the DPPH assay, as shown in figure 2, indicating the potential for CPs to be effective antioxidants when present in biological media (Gizdavic-Nikolaidis et al. 2004a,b). The various vitamins and polyphenol free radical-scavenging antioxidants present in beverages, fruits and vegetables are currently of great interest, as these antioxidants may offer protection against various diseases, such as cardiovascular diseases and cancer (Gizdavic-Nikolaidis et al. 2004a,b). Li et al. (2005a,b) employed a layered hydrogel (HG) CP composite for drug-delivery applications which was capable of supporting higher concentrations of biomolecules such as heparin. Controlled drug release can also be facilitated using a change in CP redox state to increase permeation of drugs such as dexamethasone (Stassen et al. 1995; Pernaut & Reynolds 2000). Electrical stimulation of CPs has been used to release a number of therapeutic proteins and drugs like nerve growth factor (NGF; Hodgson et al. 1996), dexamethasone (Abidian et al. 2006; Wadhwa et al. 2006) and heparin (Li et al. 2005a). An accelerated release of heparin from the HG immobilized onto PPy films was reported when PPy was electrically stimulated (Li et al. 2005a). Another study demonstrated the use of poly(3,4-ethylenedioxythiophene) nanotubes (PEDOT NT) polymerized on top of electrospun poly(lactic-co-glycolic acid) (PLGA) nanofibres for the potential release of the drug dexamethasone. Here, dexamethasone was incorporated within the PLGA nanofibres and then PEDOT was polymerized around the dexamethasone-loaded PLGA nanofibres. As the PLGA fibres degraded, dexamethasone molecules remained inside the PEDOT nanotubes. These PEDOT nanotubes favoured controlled drug release upon electrical stimulation. This is because of the change in volume of the PEDOT nanotube upon electrical stimulation owing to the expulsion of anions. Figure 3 demonstrates the incorporation and release mechanism of dexamethasone from PEDOT nanotubes due to electrical stimulation (Abidian et al. 2006). Although the CPs possess immense potential in drug-delivery applications, they have certain disadvantages attributed to the initial burst release of the drug and the hydrophobic nature of the polymer, which limits their application. Nevertheless, the drug-delivery systems are of immense scientific interest and give hope for the treatment of cancer and also minimum invasive techniques for several neural and cardiovascular applications.
Figure 2.
Visible spectra of DPPH radicals in methanol after 30 min exposure to nil, (a) 0.005 µl, (b) 0.01 µl, (c) 0.02 µl and (d) 0.05 µl of a 20% (w/v) solution of short-chain polyaniline grafted to lignin (Gizdavic-Nikolaidis et al. 2004a,b).
Figure 3.
Schematic of the controlled release of dexamethasone: (a) dexamethasone-loaded electrospun PLGA, (b) hydrolytic degradation of PLGA fibres leading to release of the drug and (c) electrochemical deposition of PEDOT around the dexamethasone-loaded electrospun PLGA fibre slows down the release of dexamethasone (d). (e) PEDOT nanotubes in a neutral electrical condition. (f) External electrical stimulation controls the release of dexamethasone from the PEDOT nanotubes owing to contraction or expansion of the PEDOT. By applying a positive voltage, electrons are injected into the chains and positive charges in the polymer chains are compensated. To maintain overall charge neutrality, counter ions are expelled towards the solution and the nanotubes contract. This shrinkage causes the drugs to come out of the ends of tubes. (g) Cumulative mass release of dexamethasone from: PLGA nanoscale fibres (black squares), PEDOT-coated PLGA nanoscale fibres (red circles) without electrical stimulation and PEDOT-coated PLGA nanoscale fibres with electrical stimulation of 1 V applied at the five specific times indicated by the circled data points (blue triangles). (h) UV absorption of dexamethasone-loaded PEDOT nanotubes after 16 h (black), 87 h (red), 160 h (blue) and 730 h (green). The UV spectra of dexamethasone have peaks at a wavelength of 237 nm. Data are shown ±s.d. (n = 15 for each case; Abidian et al. 2006).
2.2. Bioactuators
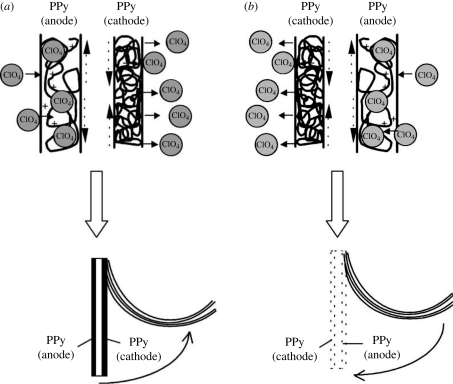
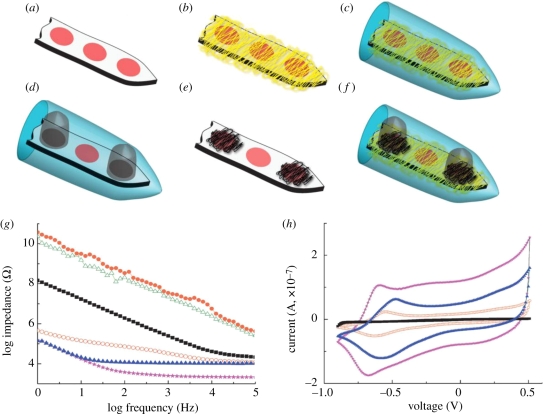
Bioactuators are devices that are used to create mechanical force, which in turn can be used as artificial muscles. The phenomenon of change in the volume of the CP scaffold upon electrical stimulation has been employed in the construction of bioactuators. In artificial muscle applications, two layers of CP are placed in a triple layer arrangement, where the middle layer comprises a non-conductive material (Otero & Sansihena 1997; Otero & Cortes 2003). When current is applied across the two CP films, one of the films is oxidized and the other is reduced. The oxidized film expands owing to the inflow of dopant ions, whereas the reduced film expels the dopant ions and in the process shrinks, as depicted in figure 4 (Otero & Cortes 2003). Ions can enter the polymer either in the oxidized state as shown in equation (2.1) or in the reduced state as in equation (2.2) (Smela 2003),
| 2.1 |
and
| 2.2 |
where P+ represents the doped oxidized state of the polymer and P0 represents the undoped reduced state of the polymer. P+ (A−) indicates that the anion A− (counter ion) is incorporated in the polymer as the dopant ion and P0 (AC) indicates that a cation is inserted during reduction. The combined effect of simultaneous expansion and contraction is translated into a mechanical force that bends the polymer, which mimics the effect of muscles in biological systems (Gandhi et al. 1995). CP actuators have many features that make them ideal candidates for artificial muscles, including that they (i) can be electrically controlled, (ii) have a large strain which is favourable for linear, volumetric or bending actuators, (iii) possess high strength, (iv) require low voltage for actuation (1 V or less), (v) can be positioned continuously between minimum and maximum values, (vi) work at room/body temperature, (vii) can be readily microfabricated and are light weight, and (viii) can operate in body fluids (Smela 2003). PPy, PANI and PPy–PANI composites and composites of these polymers with carbon nanotubes (CNT), i.e. PANI–CNT and PANI–CNT–PPy, have all been explored for their ability to function as actuators. Of these materials, PPy–PANI produced the highest work per cycle, which is desirable for strong mechanical properties. PPy actuators have also been fabricated, in which the forces created through doping and undoping the CPs are used to produce movements and create artificial muscles (Spinks et al. 2005a,b). For bioactuator applications, advantages of CPs include their strength, their ability to function at room or physiological temperatures and their ability to work with liquid electrolytes, such as body fluids (Spinks et al. 2005a,b, 2006).
Figure 4.
The triple layer device (polypyrrole(ClO−4)/non-conducting and adherent polymer/polypyrrole(ClO−4)) and its macroscopic movement produced as a consequence of volume change in the polypyrrole films. (a) A current flows and the left polypyrrole film acting as the anode is swelled by the entry of the hydrated counter ions (ClO−4). Simultaneously, the right film acting as the cathode contracts and shrinks because of the expulsion of the counter ions. These volume changes and the constant length of the non-conducting film promote the movement of the triple layer towards the polypyrrole film that is being contracted. (b) By changing the direction of the current, the movement takes place in the opposite direction. The muscle works in LiClO4 aqueous solution (Otero & Cortes 2003).
2.3. Tissue engineering applications
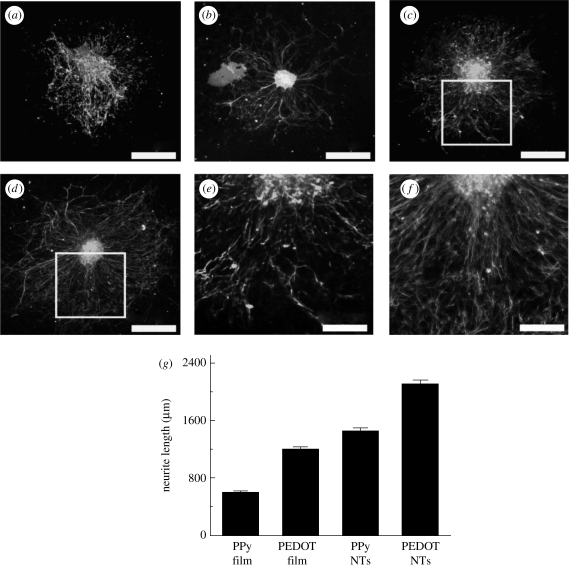
The general CP properties desired for tissue engineering applications include conductivity, reversible oxidation, redox stability, biocompatibility, hydrophobicity, three-dimensional geometry and surface topography. CPs are widely used in tissue-engineering applications because of their ability to subject cells to an electrical stimulation. Studies have addressed cell compatibility when a current or voltage is applied to PPy (Schmidt et al. 1997). Although little evidence of cytotoxicity was seen after long-term exposure to current (e.g. 96 h exposure to 1 mA), shorter periods of exposure to current have not been found to have negative effects on cells in culture (Williams & Doherty 1994). However, recently, PEDOT has received a lot of attention owing to its higher electrical conductivity and chemical stability. For example, Abidian et al. (2010) demonstrated the biocompatibility of PPy and PEDOT, in film and nanotube morphology, by culturing neuronal cells using an in vitro dorsal root ganglion model. Figure 5 shows the neurite outgrowth and neuronal cell proliferation on the PPy and PEDOT substrates. An advantage offered by CPs is that the electrochemical synthesis allows direct deposition of a polymer on the electrode surface while simultaneously trapping the protein molecules (Bartlett & Whitaker 1988). However, the hydrophobicity of CPs is a major limitation for successfully entrapping proteins and maintaining their bioactivity. Different approaches have been analysed to overcome this drawback, including the use of monomers that contain both redox centres and hydrophilic chains (Cosnier et al. 2003), blends with polyelectrolytes (Hodgson et al. 1996) and CP–HG composites (Brahim et al. 2002; Brahim & Guiseppi-Elie 2005).
Figure 5.
Dorsal root ganglion explants cultured on conducting polymer (CP) films and nanotubes. Ganglia on PPy film (a) and PPy nanotubes (c) degraded and had shorter and more branched neurites than PEDOT film (b) and PEDOT nanotubes (d), respectively. The extent of branching is best observed at higher magnification of PPy nanotubes (e) and PEDOT nanotubes (f). CP nanotubes produced longer neurites than their corresponding films, with PEDOT nanotubes producing the longest neurites overall (g). Column height represents the mean while error bars reflect the standard error of the mean for 10 neurites per condition (n = 10; Abidian et al. 2010). Scale bars (a–d), 400 µm; (e,f), 200 µm.
2.3.1. Neural applications
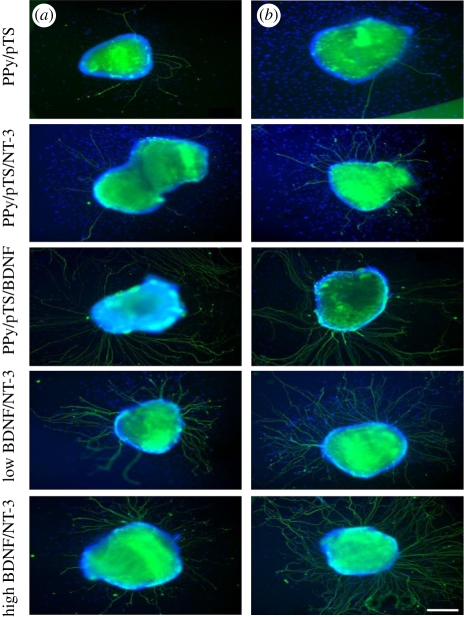
Neural probe applications require materials with high surface area, hydrophobicity and cell specificity to improve and maintain good SNR for detection of neuron signals. Typical neuroprosthetic implants are usually fabricated from platinum, gold or alloys of these metals and iridium oxide, but the minimal interaction of these materials with neural tissue limits their ability to provide optimal stimulation and recording from neural cells. While these neuroprostheses are used to electrically evoke responses in specific neural tissue through controlled stimulation paradigms, they can also perform recording functions that output information on the status of the neural–tissue interface. Neural interfaces ideally have intimate contact between the excitable tissue and the electrode to maintain signal quality and activation of neural cells. The impact of biological inclusions on polymer properties and their performance in neural prosthetics requires a greater understanding of the CP film characteristics for long-term performance. Neural recording electrodes have demonstrated clinical potential in assisting paralysed patients to control artificial limbs (Schwartz 2004). Neural electrode functionality can be increased by modifying the surface of the electrode sites with low impedance conductive polymer coatings with nanoscale roughness or porosity (Yang & Martin 2004a,b) and through the incorporation of cell adhesion peptides (Cui et al. 2001), proteins (Buchko et al. 2001; He & Bellamkonda 2005) or anti-inflammatory drugs (Abidian et al. 2006). Optimizing the electrode interface will however require a trade-off between the desired electrical, mechanical, chemical and biological properties. Schmidt et al. (1997) showed that PC12 cells cultured on PPy films and subjected to electrical stimulation showed a significant increase in neurite lengths when compared with the passive control. However, this neurite outgrowth was significant for only the first 24 h. Beyond this time frame, no significant difference was observed with passive controls. Electrical stimulation with neural electrodes is used clinically to improve conditions such as hearing loss (cochlear implants) (Edgerton et al. 1982) and Parkinson's disease (deep brain stimulators) (Kringelbach et al. 2007). It was found that, when PPy CP was coated onto cultured substrates on which cochlear explants are cultured, the neurite growth improved upon the incorporation of neurotrophins like NT-3 and brain-derived neurotrophic factor (BDNF), as shown in figure 6. Such CP-based materials provide a biocompatible substrate for storage and release of neurotrophins and also to help protect auditory neurons from degradation after sensorineural hearing loss and encourage neurite outgrowth towards the electrodes. This study suggests that this technique can be further used for cochlear implants (Thompson et al. 2010). In another study, the authors designed a neural probe, which could also be used as a neural scaffold, from PPy doped with polystyrenesulphonate (PSS) or sodium dodecylbenzenesulphonate (NaDBS; George et al. 2005).
Figure 6.
Representative images of cochlear neural explants grown on PPy/pTS polymers with and without neurotrophin. Neurites were visualized by immunocytochemistry with a neurofilament-200 primary antibody and a fluorescent secondary antibody (green). Cell nuclei are labelled with DAPI (blue). (a) In the absence of neurotrophin (PPy/pTS), very few neurites were observed from explants, while explants grown on PPy/pTS containing neurotrophin demonstrated increased numbers of sprouting neurites. A greater number of neurites per explant was observed on explants grown on the electrically stimulated PPy films (b). These images were taken after 4 days of explant culture. Scale bar, 200 µm (Thompson et al. 2010).
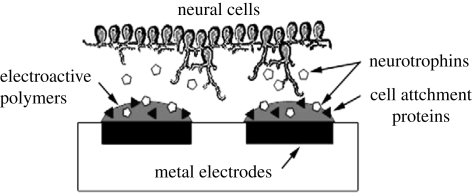
Conductive neural interfaces tailored for cell interaction by incorporation of bioactive factors produce superior neuroprosthetic devices with improved charge transfer capabilities. A study examined the effect of entrapping NGF within the CP PEDOT during electrodeposition to create a polymer capable of stimulating neurite outgrowth from proximal neural tissue (Green et al. 2009a,b). The incorporation of NGF can modify the biological interactions of the electrode without compromising the conductive properties or the morphology of the polymeric film, as shown in figure 7. Besides neurotropic growth factors, other biomolecules such as laminin peptides can be used to dope PEDOT, creating CPs with cell attachment properties (Green et al. 2009a,b). It was observed that the anodic peak potential (Epa) values upon incorporation of NGF to the CP PPy was found to shift to more positive values, increasing the applied charge, and thereby indicating more deposition of PPy and NGF (Gomez et al. 2007). Moreover, at higher current density, it was noticed that the amount of NGF incorporated was less than at lower current densities because of either slow diffusion or denaturing of the incorporated NGF when excessive current was applied (Gomez et al. 2007). Kim et al. (2006) have reported the incorporation of both collagen and NGF into PPy, and confirmed the activity of both biomolecules through the neurite outgrowth of PC12 cells. CP coatings aim to improve the electrode–tissue communication by providing a high surface area material more conducive to cell and tissue integration. Surface modification of neural electrodes with CP films is intended to improve probe sensitivity while suppressing detrimental immune responses and promoting neural tissue regeneration by angiogenesis, neurite extension and synapse formation. George et al. (2005) examined PPy biocompatibility and found that neurons and glial cells enveloped the PPy implant, as shown in figure 8. Studies have proved that PEDOT nanotubes can improve the signal quality of recording sites and the long-term performance of chronically implanted neural microelectrodes in vivo in rats for up to seven weeks (Abidian et al. 2007). The authors have further confirmed that the PEDOT nanotubes enhanced the quality of recording signals (Abidian et al. 2009). In this paper, the researchers measured in vivo electrochemical impedance spectroscopy (EIS), noise level, quality of unit activity and analysed local field potentials (LFPs), as shown in figure 9. They demonstrated that electrodes modified with PEDOT nanotubes registered high-quality unit activity (SNR > 4) on 30 per cent more sites than controls (uncoated), primarily as a result of a reduced noise floor. They also showed that sites modified with PEDOT nanotubes had significantly low frequency artefact in LFP recordings. The Nyquist plots of in vivo EIS measurements revealed that the PEDOT nanotubes may be used as a novel method for biosensing to indicate the transition between acute and chronic responses in brain tissue (Abidian et al. 2009). The same group also demonstrated that the EIS measurements for the uncoated electrode acted as a pure capacitor while the CP nanotube-coated electrode acted as a capacitor at frequencies less than 100 Hz and as a resistor at frequencies more than 100 Hz, as shown in figure 10a. Figure 10b illustrates PPy and PEDOT nanotubes on the surface of the electrode. It was observed that the value of resistance was the largest in PPy, and varied according to the following sequence: PPy > PPy NTs > PEDOT > PEDOT NTs (Abidian & Martin 2008).
Figure 7.
Schematic of a desirable bioactive CP electrode array with tailored cell response for both cell adherence and neural cell outgrowth (Green et al. 2009a,b).
Figure 8.
A fluorescently labelled section of neural tissue in an implant lumen: (a) neural tissue in the lumen of the Teflon implants and (b) neural tissue in the PPy lumen where the glia has reformed and neurons are present; scale bar, 100 µm; green, glia; red, neurons (George et al. 2005).
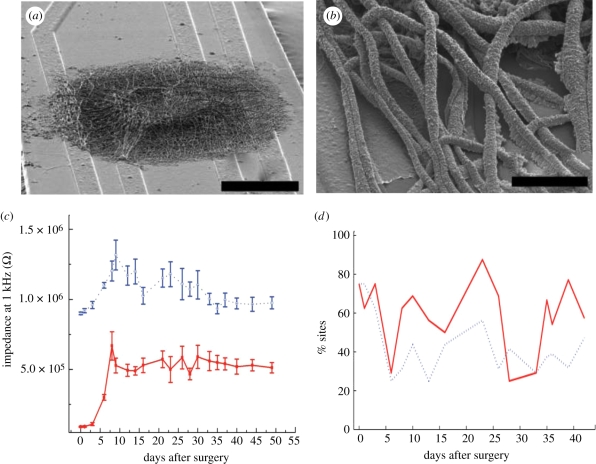
Figure 9.
(a,b) SEM images of PEDOT nanotubes. Scale bars, (a) 50 µm; (b) 500 nm. (c) Impedance magnitude at 1 kHz over time after surgery. (d) Average RMS noise, and percentage of sites recording quality units with SNR > 4 (Abidian et al. 2009). Solid line, PEDOT NTs; dotted line, control.
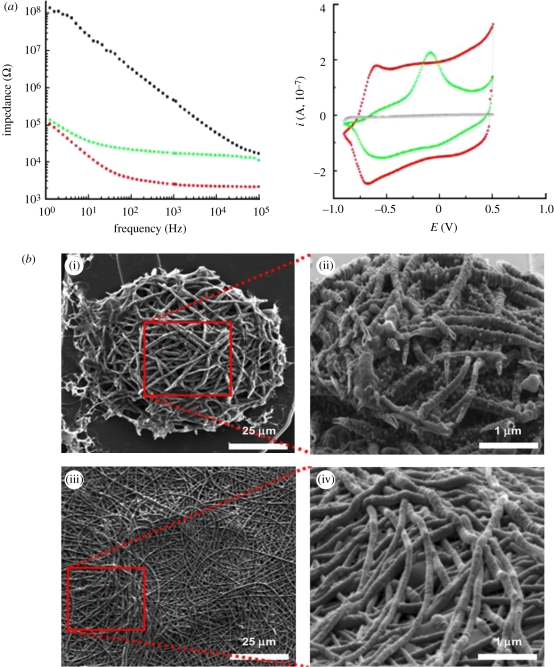
Figure 10.
Electrical properties of neural microelectrodes modified with CP nanotubes. (a) Impedance spectroscopy over a frequency range of 1–105 Hz: bare gold (black), PPy nanotubes (green) and PEDOT nanotubes (red). Cyclic voltammetry: bare gold (black), PPy nanotubes (green) and PEDOT nanotubes (red). Deposition charge density = 1.44 C cm−2, scan rate = 0.1 V s−1 (Abidian & Martin 2008). (b) Scanning electron micrographs of electropolymerized PPy and PEDOT nanotubes on neural microelectrode sites. (i) Top view of PPy nanotubes, (ii) three-dimensional view of PPy nanotubes, (iii) top view PEDOT nanotubes and (iv) three-dimensional view of PEDOT nanotubes. PPy nanotubes with deposition charge density 1.44 C cm−2 (Abidian & Martin 2008).
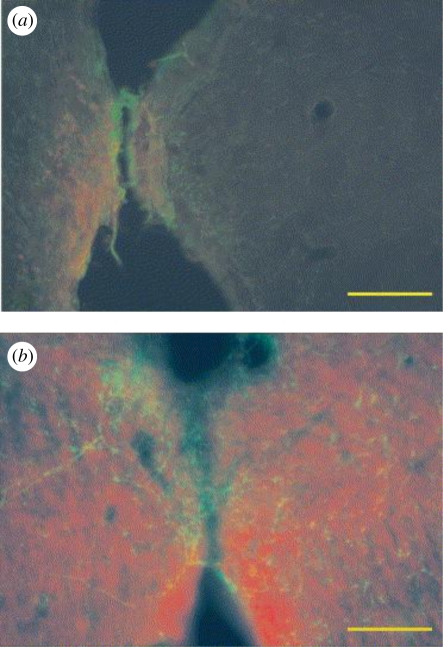
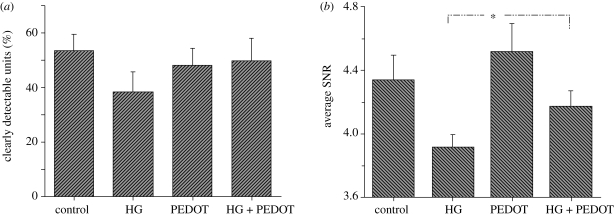
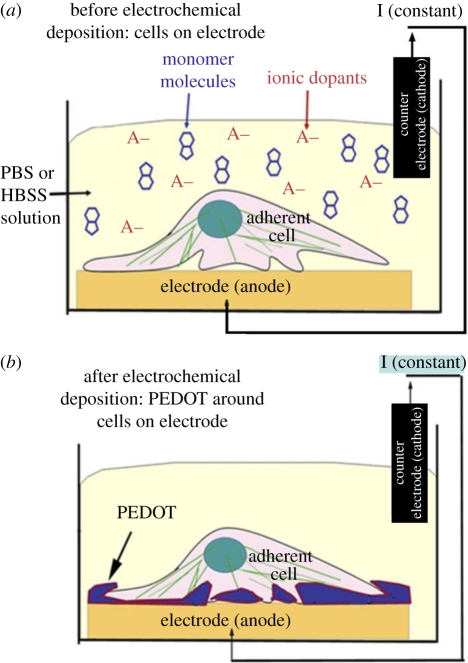
The role of PEDOT in improving the recording functionality of the HG-coated neural electrodes was analysed. A significant loss in recording functionality was observed with thicker HG coatings, as determined by the number of clearly detectable units (30% with 80 µm thick coatings) and average SNRs (3.91 with 80 µm thick coatings). However, deposition of the CP PEDOT on the electrode sites restored the lost functionality of the electrodes, as shown in figure 11. These CP/HG coatings have the potential to improve long-term performance of the neural electrodes not only by improving the electrode biocompatibility but also by facilitating more efficient signal transmission (Kim et al. 2009). In another study, PEDOT was electrochemically polymerized directly in the presence of neural cells seeded on electrodes, resulting in the formation of a CP matrix around and onto the adhered cells (Richardson-Burns et al. 2007). Further they investigated whether an intimate interface can be formed between living neurons and a CP towards establishment of the direct, functional contact that is required for communication between a bioelectronic device and its target tissue (Hutzler & Fromherz 2004; Merz & Fromherz 2005). It was found that intimate interactions exist between the CP and the neuronal membrane, revealed as the PEDOT surface was covered by delicate filopodia and neurites, characteristic of the existence of a unique cell–polymer–electrode interface, as indicated in figure 12 (Richardson-Burns et al. 2007). This system may be an ideal candidate material for the development of a new generation of biosensors and ‘smart’ bioelectrodes. Two studies have characterized the electrochemical deposition of PEDOT and PEDOT–MeOH (poly(hydroxymethylated-3,4-ethylenedioxythiophene)) on neural probes (Cui & Martin 2003; Xiao et al. 2004). Both forms of PEDOT were doped with the laminin-derived DCDPGYIGSR peptide and it was observed that rat glial cells preferentially grew on a PEDOT–DCDPGYIGSR-modified surface (Guimard et al. 2007a,b). Abidian and co-workers described a successful example of hybrid coating including nanostructured CP, drug-loaded nanofibres and HG coating for neural microelectrodes. The design and characterization of a multi-functional, hybrid nanostructured interface for neural microelectrodes that is soft, has low impedance, has high charge density and is capable of controlled drug release was noticed. The design includes: (i) biodegradable electrospun nanofibres for the controlled release of drugs and to provide a scaffold for the formation of CP nanotubes and (ii) HG layers for the sustained release of drugs, and to provide a scaffold for the formation of nanostructured cloud-like CPs. The fabrication process and optical and SEM images of coating on a neural microelectrode are illustrated in figure 13a,b. These HG coatings provide a mechanical buffer layer between the hard silicon-based probe and the soft brain tissue, a scaffold for growing the CP within the hydrogel matrix and a diffusion barrier for controlling drug release (Abidian & Martin 2009).
Figure 11.
(a) The average percentage of clearly detectable units and (b) the average SNRs recorded from various modifications of the electrodes including unmodified electrode (control), 30 µm of HG-coated electrode (HG), PEDOT-deposited electrode (PEDOT), PEDOT deposition on the electrode sites under 30 µm HG coating (HG + PEDOT). Asterisks denote significance between HG and HG + PEDOT (Kim et al. 2009).
Figure 12.
(a) The electrochemical deposition cell and the neural cell monolayer cultured on the surface of the metal electrode prior to polymerization. (b) PEDOT polymerized around living cells (Richardson-Burns et al. 2007).
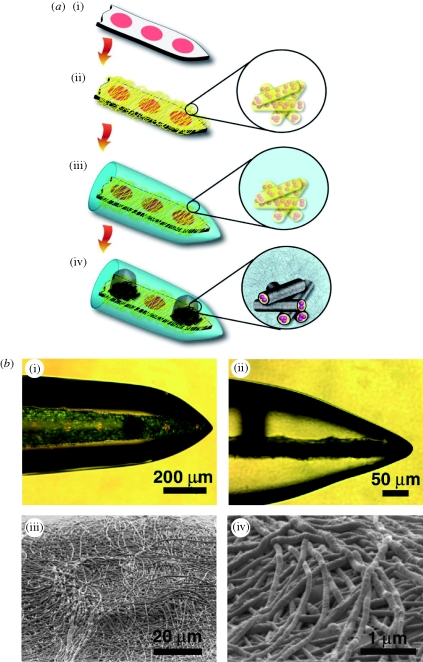
Figure 13.
The fabrication process for multi-functional polymer coatings on neural microelectrodes: (a) (i) uncoated microelectrode, (ii) electrospinning of DEX-loaded biodegradable nanofibres, (iii) alginate hydrogel coating, and (iv) electrochemical polymerization of PEDOT on the electrode sites around the DEX-loaded electrospun biodegradable nanofibres, and within the hydrogel scaffold (Abidian et al. 2009). (b) (i) Optical micrograph of deposited PEDOT (black) on the electrode site, (ii) side view of (i) showing vertical growth of PEDOT from an electrode site and through the alginate hydrogel scaffold (black), and (iii) SEM image of the electrode site after dissolving the alginate coating and electrospun nanofibres. This image shows that PEDOT had grown around the electrospun nanofibres to form PEDOT NTs. (iv) Higher magnification image of (iii) (Abidian et al. 2009).
PEDOT oxidation is performed in the presence of alcohol polymerization or post implantation may induce the formation of sulphonic acid esters. In particular, methyl and ethyl methanesulphonate esters are known genotoxins and carcinogens in rats and mice (IARC 2004), whereas the toxicity of p-toluene sulphonate esters has been generally established (Glowienke et al. 2005). Unfortunately, these materials are not selective enough for neurons and over time the neural signal decreases. Such decreases in conductivity continue to be a challenge in neuroprosthetic devices. Recording and stimulating neural prostheses can improve our understanding of the organization and operation of the nervous system and may lead to improved prosthetic devices for tackling some of mankind's most debilitating disorders, including deafness, paralysis, blindness, epilepsy and Parkinson's disease.
2.3.2. Cardiovascular applications
The complexity of the electrical conduction pathways in the heart has received tremendous attention in the field of cardiac tissue engineering. The cardiac cells ought to form the proper intercellular connections and matrix architecture so as to enable the electrical impulses to be transmitted in the appropriate direction at normal speed (Zimmermann et al. 2000). Further, the contraction of the cardiac muscle is driven by the waves of electrical impulses generated, which induces the mechanical stretch in the native heart. Any impairment in the electrical signal conducting pathway leads to cardiovascular diseases (Zimmermann et al. 2002). This is where a CP scaffold-based approach can be potentially useful. Li et al. (2006) demonstrated the potential for using PANI as an electroactive polymer in the culture of excitable cells for cardiac and/or neuronal tissue engineering applications. Various approaches of covalently attaching oligopeptides to PANI and electrospinning PANI–gelatin blend nanofibre scaffold were analysed as potential candidates for cardiac tissue engineering applications using H9c2 myoblast cells, as shown in figure 14 (Li et al. 2006). In addition, a PPy–heparin composite was found to be a good substrate for endothelial cell growth (Garner et al. 1999a,b), and a PPy–hyaluronic acid (HA) composite seemed to promote vascularization (Collier et al. 2000). Besides, PPy doped with erythrocytes can be used for the detection of blood rhesus factor via the rhesus factor antigens present on the cell surface (Campbell et al. 1999). It was found that the erythrocyte-containing PPy bound more antibody than the unmodified PPy, as evidenced by ELISA (Richardson-Burns et al. 2007). Tryptophan and oligopeptide (YIGSR)-modified PANI as well as PANI–collagen complexes showed that H9c2 cardiac cell viability was preserved and that biocompatibility was enhanced using these modified CP scaffolds (Meng-yan et al. 2007). The mechanical properties of the CP construct play a very important role in cardiac applications. Attempts have been made to improve the mechanical properties of CPs by creating composites or blends and doping with large molecules that possess the desired mechanical properties to mimic the native myocardium (Li et al. 2006).
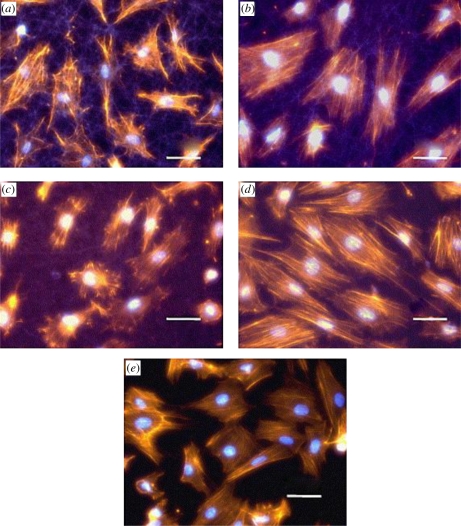
Figure 14.
Morphology of H9c2 myoblast cells at 20 h of post-seeding on: (a) gelatin fibre, (b) 15 : 85 PANI–gelatin blend fibre, (c) 30 : 70 PANI–gelatin blend fibre, (d) 45 : 55 PANI–gelatin blend fibres, and (e) glass matrices. Staining: nuclei, bisbenzimide; actin cytoskeleton, phalloidin; fibres, autofluorescence; original magnification 400× (Li et al. 2006). Scale bars, (a–e) 50 µm.
3. Surface functionalization of conducting polymers
3.1. Physical–chemical modifications
Surface modification of the CP for incorporating biomolecules has been achieved by both physical and chemical modifications. Such modifications can be used to generate both physical and chemical guidance cues, which can be employed for the desired biomedical application. Chemical modification has been extensively investigated using biomolecules as dopants (Cui et al. 2003), or by immobilizing bioactive moieties on the surface of the material (Zhong et al. 2001). Physical modification has been explored by increasing surface roughness using various methods, such as creating microporous films using polystyrene sphere templates, fabricating composites of nanoparticles and polylactide (Yang & Martin 2004a,b), growing CPs within HGs (Kim et al. 2003; Abidian & Martin 2009; Abidian et al. 2009) and blending with biomolecules to yield ‘fuzzy’ structures (Cui et al. 2003).
Kim et al. (2004) have shown that PPy with an underlying layer of HG applied to cortical electrodes had a lower impedance and improved cell attachment properties than PPy/PSS alone. This technique can be used to alter CP surface topography. Surface functionalization of tosylate-doped PPy films may be represented by means of (i) grafting acrylic acid (this molecule bearing a COOH functional moiety could also covalently bind matrix molecules and/or growth factors, which may in turn favourably influence cell adhesion and/or proliferation as indicated in figure 15 (Mattioli-Belmonte et al. 2002)), (ii) co-electrospinning or blending with natural proteins to form conducting nanofibres or films, and (iii) preparing CPs using natural biopolymers, such as collagen and gelatin as templates.
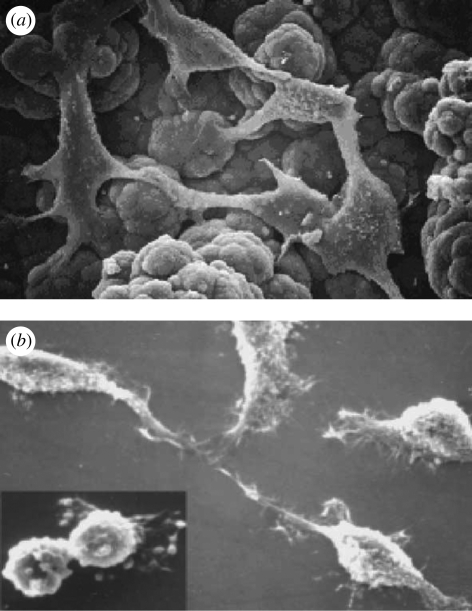
Figure 15.
SEM micrographs of NCTC keratinocytes (a) cultured onto self-standing oxidized PPy film (original magnification 2400 × ) and (b) grown onto thin oxidized PPy film showing different cell morphologies (original magnification 1000×). Inset: mitotic figure (original magnification 1500×; Mattioli-Belmonte et al. 2002).
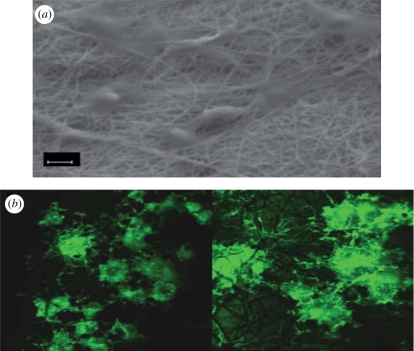
The electrode coatings employed are usually soft (Yang & Martin 2006) and can be tailored at the micrometre, nanometre and molecular scale to have fibrillar, nodular, fuzzy, tubular (Abidian et al. 2006) or porous surface morphologies (Kim et al. 2004; Yang & Martin 2004a,b; Yang et al. 2005). Thus, most tissue- and device-compatible surface modifications of the electrode would be inducing electrical activity, bioactivity, mechanical softness and topological features on a similar scale to that of cells in tissues. Cell-templated polymer coatings were analysed using neuron-templated PEDOT coatings after the removal of cells and cell material from the PEDOT matrix after polymerization around the cells. This cell-templated polymer surface was found to encourage the cells in the host tissue to re-populate the cell-shaped holes and send processes into the various topographical features on the template-like tunnels, troughs, crevasses and caves. This would provide for very intimate contact between cells and the CP (as depicted in figure 16), ensuring continuous electrical contact between the electrode and the tissue (Richardson-Burns et al. 2007). Moreover, the topographical patterns can provide contact guidance cues to primary neurons (Gomez et al. 2007). The surface roughness properties of the CP films can be tailored by modifying the CP synthesis temperature. Fine-tuning the surface roughness characteristics is significant because rougher topography correlates with increased surface area, which would increase signal conduction by increasing the interface with neurons. For example, PPy films synthesized at a lower temperature (41°C) were rougher than the same films made at 251°C (Guimard et al. 2007a,b). It was also demonstrated that the hippocampal neurons cultured on PPy microchannels polarized faster, meaning that more cells had defined axons when compared with controls on unmodified PPy, thereby proving the role of topography (Schultze & Karabulut 2005). Kmecko et al. (2006) demonstrated that the addition of carbon nanotubes as dopants to PPy and PEDOT CP favours the formation of nodules and increases the surface roughness.
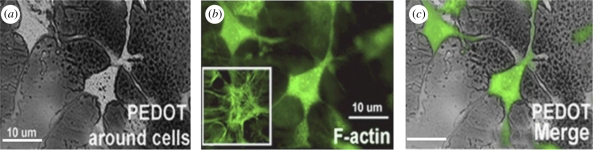
Figure 16.
(a) Optical image showing thin film of PEDOT around MCC 4 h after polymerization. (b) Fluorescent microscope image of MCC stained with phalloidin–Oregon green (green fluorescence) to detect F-actin. Inset shows the normal morphology of the F-actin cytoskeleton in control cells. (c) Merged image (Richardson-Burns et al. 2007).
Studies have found that fibroblasts attached and grew well on the PPy nanoparticle–PLA composites with an increased viability when stimulated at currents of 10–50 mA. This material retained biocompatibility in vivo even after maintaining electroconductivity for long periods of time (15% of conductivity retained after 1000 h; Wang, Z. et al. 2003, 2004; Wang, X. et al. 2004). Similarly, PANI–chitosan nanocomposites and PANI–gelatin cross-linked composites have been explored to increase biocompatibility and enhance surface characteristics. It was found that varying the ratio of PANI to gelatin allowed for the optimization of conductivity and fibre diameter, where cells were found to prefer fibres of smaller diameter (Cheng et al. 2005; Li et al. 2006).
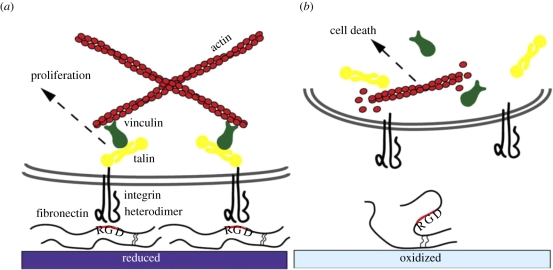
Svennersten et al. (2009) compared the cell adhesion behaviour between the reduced and the oxidized states of PEDOT–tosylate electrode surfaces. Figure 17 depicts the proposed molecular mechanism that mediates cell adhesion and proliferation on the reduced electrode while inhibiting adhesion and proliferation on the oxidized electrode. This is because the latter induces alteration of the fibronectin (Fn) conformation, which severely compromises cell adhesion and viability. This work proved that cell adhesion and proliferation can be controlled by electrochemical modulation of surface parameters. Electrochemically active polymers were also explored as the surface switch material to regulate surface wetting (Salto et al. 2008). Electrochemical switching in polymers is expected to affect the strength and the density of dipoles and the binding characteristics of doping ions. Thus, by modifying the electrochemical switch, it is possible to achieve electronic control over the surface tension (Isaksson et al. 2004; Sun et al. 2004; Causley et al. 2005; Robinson et al. 2006). It has also been reported that the wetting and adhesive properties of an electroactive polymer can be varied by changing its oxidation state.
Figure 17.
The proposed mechanism for cell interaction with (a) the reduced and (b) the oxidized PEDOT–tosylate surfaces (Svennersten et al. 2009).
Functionalization of CPs with different biomolecules has allowed biomedical engineers to modify CPs with biological sensing elements, and to turn on and off different signalling pathways required for several cellular processes to create CPs that enhance cell proliferation/differentiation. Dopants can also be used as intermediate ‘tethers’ to allow further modification of CPs. For example, doping with poly(glutamic acid) (PGlu) provides a carboxylic acid pendant group, which can be functionalized further by covalent linking using carbodiimide chemistry to any amino group, such as those found in polylysine and laminin (Song et al. 2006). Three-dimensional nanofibrous PPy/SIBS (poly(styrene—β-isobutylene—β-styrene) mats were fabricated using a vapour phase polymerization method. It was demonstrated that the PC12 cells cultured on these films had a higher cell density and more firm adhesion, and exhibited a spreading polygonal shape, suggesting good phenotypic spreading as shown in figure 18. Owing to these favourable properties, the three-dimensional PPy/SIBS scaffolds can be used as nerve growth guidance channels (Liu et al. 2008). Sanghvi et al. (2005) functionalized the surface of chlorine-doped PPy to anchor peptide molecules that promote nerve regeneration, blood vessel growth or other biological processes. Moreover, peptide-modified PANI surfaces have also shown excellent cell adhesion. PANI films with YIGSR and RYSGI peptides covalently attached to them showed higher PC12 cell proliferation than the control PANI films (Meng-yan et al. 2007). Several studies have focused on doping PPy with peptides derived from extracellular matrix proteins including laminin-derived peptides, such as p31 (CDPGYIGSR; Stauffer & Cui 2006), p20 (RNIAEIIKDI; Stauffer & Cui 2006) and YIGSR-containing sequences (DCDPGYIGSR; Cui et al. 2003) and silk-like polymer having FN fragments (SLPF; Cui et al. 2001).
Figure 18.
(a) SEM image of PC12 cells adhered to a PPy/SIBS nanofibrous mat and (b) fluorescence microscope images of phalloidin-stained PC12 cells grown on PPy/SIBS nanofibres (Liu et al. 2008).
3.2. Electrical property modification
Doping is the process of oxidizing (p-doping) or reducing (n-doping) a neutral polymer and providing a counter anion or cation (i.e. dopant), respectively. Upon doping, a CP system with a net charge of zero is produced owing to the close association of the counter ions with the charged CP backbone. This process introduces charge carriers in the form of charged polarons (i.e. radical ions) or bipolarons (i.e. dications or dianions) into the polymer. The attraction of electrons in one repeat unit to the nuclei in the neighbouring units yields charge mobility along the chains and between chains, often referred to as ‘electron hopping’. The ordered movement of these charge carriers along the conjugated CP backbone produces electrical conductivity. The smaller the band gap (i.e. distance between conducting band and valence band) energy for a CP, the more conductive it is considered to be (Guimard et al. 2007a,b).
The electrochemical properties of the CP can be varied by changing the dopant concentration. Electrical conductivities can be varied by as much as 15 orders of magnitude by changing dopant concentrations so that control is feasible over the entire range from insulator to semiconductor and then to metal (Malhotra & Singhal 2003). The commonly used dopants include aromatic sulphonate variants para-toluenesulphonate (pTS), PSS and sodium benzenesulphonate (BS) to dope polymers (Yamato et al. 1995; Cui et al. 2001; Wang, X. et al. 2004; Wang, Z. et al. 2004). Other suitable dopants for oxidation polymerization include buffer salts, I2, BF4, perchlorates and FeCl3. Biological dopants include laminin peptide sequences, HA, or SLPFs and polysaccharides (Collier et al. 2000; Finkenstadt 2005). The potential neural applications of the CP PEDOT were shown by fabricating electropolymerized PEDOT onto gold electrodes with a neural transmitter of glutamate (Glu) as the dopant (Peramo et al. 2008). The resultant PEDOT/Glu coating has lower impedance over a wide range of frequencies than bare gold, which is significant for obtaining high-quality electrical signals. Moreover, the coating exhibits good electrical activity in phosphate-buffered saline, implying that it can establish a bridge between the modified metal electrode and brain tissue (Peramo et al. 2008). Abidian and co-workers have compared the electrical properties of different combinations of CP within the HG, nanotubes in terms of EIS and the capacity of the charge density, as shown in figure 19. They have shown that the minimum impedance occurred in the case of combination of PEDOT nanotubes and PEDOT within the HG. The impedance decreased significantly at 1 kHz from 783 (bare gold) to 2.5 kΩ (PEDOT NTs + HG + PEDOT). It was also demonstrated that the capacity of the charge density increased significantly, by about two orders of magnitude, from 1.28 to 223.8 mC cm−2 in the case of PEDOT NTs + HG + PEDOT (Abidian & Martin 2009).
Figure 19.
(a) Uncoated electrodes, (b) DEX-loaded electrospun PLDL75G25A nanofibres on the electrodes (PLGA NFs), (c) alginate hydrogel coating of DEX-loaded electrospun PLDL75G25A nanofibres on the electrodes (HG + PLGA NFs), (d) cloudy PEDOT inside the alginate hydrogel on particular electrodes (HG + PEDOT), (e) PEDOT NTs on the electrodes (PEDOT NTs), (f) PEDOT NTs and cloudy PEDOT inside the hydrogel on the electrodes (PEDOT NTs + HG + PEDOT), (g) EIS of bare gold (black squares), PLGA NFs (green open triangles), PLGA NFs + HG (orange circles), HG + PEDOT (red open circles), PEDOT NTs (blue triangles) and PEDOT NTs + HG + PEDOT (pink stars) with an applied deposition charge density of 2.88 C cm−2 and (h) CV of bare gold (black squares), HG + PEDOT (red hollow circles), PEDOT NTs (blue triangles) and PEDOT NTs + HG + PEDOT (purple stars); the potential was swept from −0.9 to 0.5 V at a scan rate of 100 mV s−1 (Abidian et al. 2009).
The choice of the dopant material varies depending on the application desired. For example, tetraethyl tosylate can be used as a dopant with PPy to obtain thick, self-standing films with good mechanical properties (Wynne & Street 1985). However, a major drawback of employing dopants is the possible diffusion of the dopant into the culture medium with effects on cytotoxicity and deterioration of the electrical characteristics of the CP film itself. For instance, dodecyl sulphate-doped PPy films undergo structural changes after one week of soaking in deionized water (Cui et al. 2003). The uptake of positive ions from the medium in the case of large, entrapped dopants like PSS poses a threat to the cellular mechanisms. For example, in PPy-doped PSS, an uptake of positive ions such as Na+ from the medium is speculated to affect several cellular processes, including protein adsorption and the cell cycle (Wong et al. 1994). PSS is widely used as a dopant in combination with PPy because of its relatively inert nature, its stability once deposited and biocompatibility upon implantation (Wang, X. et al. 2004; Wang, Z. et al. 2004). Other studies have also explored the modification of PPy via different dopants, including doping with dermatan sulphate for increasing keratinocyte viability (Ateh et al. 2006), doping with heparin for increasing endothelial cell proliferation (Garner et al. 1999a,b) and doping with laminin-derived peptides to control neuron and astrocyte adhesion (Stauffer & Cui 2006).
Studies have been carried out to compare the various physical properties and conductivities of the CP PPy films in the presence of different dopants. It was demonstrated that PPy films doped with PSS, a polyanionic molecule, have more hydrophilic surfaces than those films doped with monoanionic dopants such as Cl and ToS. PSS-doped PPy films serve as a more suitable candidate for long-term implants such as neural probes. This may be owing to the presence of free charges in the longer strands of PSS in the PSS-doped films, whereas no such free charges exist in the Cl- and ToS-doped films. It was also shown that PSS-doped PPy films had a smooth surface with the roughness remaining almost constant for all thicknesses. ToS-doped PPy films also maintained essentially constant roughness, but samples thicker than a few hundred nanometres showed an increase in roughness as they become thicker. Chloride-doped PPy films were much rougher than PSS- and ToS-doped PPy, and its roughness rapidly increased with thickness. Moreover, ToS-doped PPy films showed conductivities 10 times higher than either PSS-doped or Cl-doped films. Hence, ToS-doped PPy films can be used in applications requiring high conductivity. According to the surface morphology of the doped CP film, the morphology of the cells cultured on these films was also found to differ. Schwann cells grown on PSS-doped PPy films seem to prefer a thin, spindle-like morphology, whereas films doped with the other two dopants seem to prefer a more spread morphology. Thus, depending on the desired application, the dopant molecule can be chosen accordingly (Guimard et al. 2007a,b).
The range of possible dopants is vast as long as the selected dopant is charged. Alternatively, covalent methods can be used to more permanently functionalize CPs. The monomer can be synthesized with desired functional groups and then polymerized, or post-polymerization covalent modification is also possible. It is important to note that the steric effects of any incorporated functional group may disrupt the planarity of the conjugated system, which could in turn decrease conductivity.
3.3. Mechanical property modification
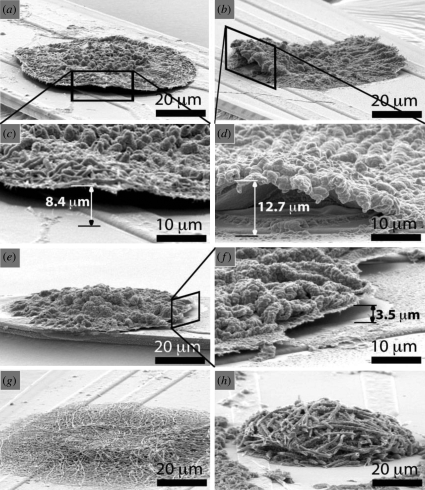
The tensile properties for CPs such as polyacetylene (Druy et al. 1980) and PPy (Diaz & Hall 1983) have been reported to be disappointing in comparison with a linear macromolecule. However, the moduli and tensile strengths have been reported to increase as a result of annealing (Machado et al. 1989). Besides, the mechanical properties have also been shown to depend on the method of synthesis of the CP (Diaz & Hall 1983). The addition of dopant molecules affects the mechanical properties of the CP. It has been reported that the addition of dopants like SbF5 and AsF5 caused a considerable reduction in the modulus and strength of the material. This may be because even though the addition of dopants causes an increase in the average cross-sectional area, no significant contribution was achieved towards the load-bearing capacity of the polymer, thereby lowering its modulus (Machado et al. 1989). Also, doping resulted in embrittlement and scission, because of the strong oxidizing potential of the dopants employed. Sometimes doping reduces the tensile modulus and attainable strain to about 1 per cent in cases where the effect of doping is greater, because of the lateral separation of the polymer chain required to accommodate the dopant molecule (Osada et al. 1992; Osada & Gong 1993). Modification of the mechanical properties can also be achieved through the variation of the alkyl chain length (Otero & Sansihena 1997). Also, solubility can be achieved through the addition of appropriate side chain groups. For example, the solubility of poly(3-alkyl thiophene) can be increased by incorporating side chain lengths greater than four carbon atoms (Wu et al. 1996). Mechanical properties of the CP can be varied by their surface morphology. For instance, it has been demonstrated that mechanical properties on the surface of electrodes varies in the case of nanotube morphology compared with films. Abidian et al. (2010) demonstrated that PPy and PEDOT nanotubes can adhere better to the surface of electrodes than their film counterparts. Figure 20 demonstrates the delamination of PPy and PEDOT films during electrical stimulation. Recent studies have shown that procedures such as melt processibility/stretching under increased temperature has allowed for the introduction of chain alignment and extension, thereby improving the mechanical property of the CP films (Caruso et al. 2009). Such mechanical property modifications are useful for biological applications like bioactuators.
Figure 20.
SEM images of conducting polymers after CV measurement on neural electrode: (a–d) PEDOT film; (e, f) PPy film on a neural electrode showing delamination on the edge of the polymer film. (c) Higher magnification image of (a). (d) Higher magnification image of (b). (f) Higher magnification image of (e). (g) PEDOT nanotubes. (h) PPy naotubes. PPy nanotubes and PEDOT nanotubes remained firmly attached to the neural electrode after CV measurement. The delamination height was measured as shown in (c, d and f). The delamination height was 3.7 ± 1.3 mm for PPy film and 13.4 ± 2.5 mm for PEDOT film (±s.d., n = 8). More delamination of PEDOT film was observed than for PPy film on the edges of electrode sites (p < 0.0001). PEDOT and PPy films and nanotubes were electropolymerized on the electrode site with a deposition charge density of 1.44 C cm−2. CV measurement was carried out by applying a scanning voltage from −0.9 V to 0.5 V with a scan rate of 100 mV s−1 for five cycles (Abidian et al. 2010).
4. Future perspectives
Future perspectives in neural development need to focus on creating CPs with multiple biological functionalities, and this will ultimately be achieved by combining both soluble signals such as growth factors like NGF and structural ligands like adhesion sequences derived from the extracellular matrix. The use of CPs as a means to electrically stimulate cells for tissue regeneration has also been widely explored. However, the largest limitation of using CPs in tissue regeneration is their inherent inability to naturally degrade. Erodible CPs (Zelikin et al. 2002) and CPs blended with degradable materials like polylactide, polyglycolide and their copolymers or ester linkages (Shi et al. 2004) have been created in an attempt to overcome this issue. Schmidt and co-workers proposed an alternative to create a CP composed of conductive and degradable backbone units. This polymer design is foreseen to allow for better control over polymer properties, such as degradation rate and conductivity (Guimard et al. 2007a,b).
Metal electrode materials used in active implantable devices are often associated with poor long-term stimulation and recording performance. Modification of these materials with CP coatings has been suggested as an approach for improving the tissue–electrode interface and increasing the effective lifetime of the implants. PPy film has been used as a coating polymer for Ti alloy substrates to improve osteointegration performances (De Giglio et al. 2001); such improvements, like its electropolymerization on the surface of metal alloys (De Giglio et al. 2001), also with the covalent attachment of an RGD (Arg-Gly-Asp)-containing peptide (De Giglio et al. 2000) or acrylic acid grafting (Cen et al. 2003), will enable the development of bioactive materials for potential use in orthopaedic applications (e.g. Ti orthopaedic prostheses). Metallic surfaces can be coated with CPs to impart the metallic surfaces with topography and functionality. This can be employed for generating more biocompatible and biofunctional interfaces on metallic medical implants such as stents (Weiss et al. 2004).
However, challenges facing CPs include poor electroactive stability and mechanical properties as well as control of the mobility, concentration and presentation of bioactive molecules. Strategies have been proposed to create electroactivity in the absence of external electric fields (i.e. tissue scaffolds and wireless extracellular and intracellular biosensors) to pursue medical bioelectric needs with ease.
5. Conclusion
CPs have immense applications in the fields of drug delivery, neuroprosthetic devices, cardiovascular applications, bioactuators, biosensors and the food industry. The surface modifications of CPs proposed should (i) facilitate the transport of charge carriers between the implanted device and resident tissue, (ii) mediate the large difference in mechanical modulus, (iii) lower the impedance to enhance the sensitivity of the recording site (the electrode), and (iv) have biocompatibility and stability in the physiological environment. Functionalization of CPs with different biomolecules/dopants has allowed biomedical engineers to modify CPs with biological sensing elements, and to turn on and off different signalling pathways required for several cellular processes, in order to create CPs that enhance cell proliferation/differentiation. CPs thus provide an excellent opportunity for fabricating highly selective, biocompatible, specific, stable, economic and handy biomedical devices.
Footnotes
One contribution to a Theme Supplement ‘Scaling the heights—challenges in medical materials: an issue in honour of William Bonfield, Part II. Bone and tissue engineering’.
References
- Abidian M. R., Martin D. C. 2008. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials 29, 1273–1283. ( 10.1016/j.biomaterials.2007.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidian M. R., Martin D. C. 2009. Multifunctional nanobiomaterials for neural interfaces. Adv. Funct. Mater. 19, 573–585. ( 10.1002/adfm.200801473) [DOI] [Google Scholar]
- Abidian M. R., Kim D. H., Martin D. C. 2006. Conducting polymer nanotubes for controlled drug release. Adv. Mater. 18, 405–409. ( 10.1002/adma.200501726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidian M. R., Salas L. G., Yazdan-Shahmorad A., Marzullo T. C., Martin D. C., Kipke D. R. 2007. In vivo evaluation of chronically implanted neural microelectrode arrays modified with poly(3,4-ethylenedioxythiophene) nanotubes. In Proc. 3rd Int. IEEE/EMBS Conf. on Neural Engineering, Kohala Coast, HI, 2–5 May 2007, pp. 61–64. ( 10.1109/CNE.2007.369612) [DOI] [Google Scholar]
- Abidian M. R., Ludwig K. A., Marzullo T. C., Martin D. C., Kipke D. R. 2009. Interfacing conducting polymer nanotubes with the central nervous system: chronic neural recording using poly(3,4-ethylenedioxythiophene) nanotubes. Adv. Mater. 21, 3764–3770. ( 10.1002/adma.200900887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidian M. R., Corey J. M., Kipke D. R., Martin D. C. 2010. Conducting-polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment, and neurite outgrowth of neural electrodes. Small 6, 421–429. ( 10.1002/smll.200901868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeloju S. B., Wallace G. G. 1996. Conducting polymers and the bioanalytical sciences: new tools for biomolecular communication. A review. Analyst 121, 699–703. [DOI] [PubMed] [Google Scholar]
- Ahuja T., Mir I. A., Kumar D. 2007. Biomolecular immobilization on conducting polymers for biosensing applications. Biomaterials 28, 791–805. [DOI] [PubMed] [Google Scholar]
- Ateh D. D., Vadgama P., Navsaria H. A. 2006. Culture of human keratinocytes on polypyrrole-based conducting polymers. Tissue Eng. 12, 645–655. [DOI] [PubMed] [Google Scholar]
- Bartlett P. N., Whitaker R. G. 1988. Modified electrode surface in amperometric biosensors. Med. Biol. Eng. Comput. 28, 10–17. ( 10.1007/BF02442675) [DOI] [PubMed] [Google Scholar]
- Brahim S., Guiseppi-Elie A. 2005. Electroconductive hydrogels: electrical and electrochemical properties of polypyrrole–poly (HEMA) composites. Electroanalysis 17, 556–570. ( 10.1002/elan.200403109) [DOI] [Google Scholar]
- Brahim S., Narinesingh D., Guiseppi-Elie A. 2002. Polypyrrole–hydrogel composites for the construction of clinically important biosensors. Biosens. Bioelectron. 17, 53–59. ( 10.1016/S0956-5663(01)00262-7) [DOI] [PubMed] [Google Scholar]
- Buchko C. J., Kozloff K. M., Martin D. C. 2001. Surface characterization of porous, biocompatible protein polymer thin films. Biomaterials 22, 1289–1300. ( 10.1016/S0142-9612(00)00281-7) [DOI] [PubMed] [Google Scholar]
- Campbell T. E., Hodgson A. J., Wallace G. G. 1999. Incorporation of erythrocytes into polypyrrole to form the basis of a biosensor to screen for Rhesus (D) blood groups and rhesus (D) antibodies. Electroanalysis 11, 215–222. ( 10.1002/(SICI)1521-4109(199904)11:4) [DOI] [Google Scholar]
- Caruso M. M., Davis D. A., Shen Q., Odom S. A., Sottos N. R., White S. W., Moore S. J. 2009. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798. ( 10.1021/cr9001353) [DOI] [PubMed] [Google Scholar]
- Causley J., Stitzel S., Brady S., Diamond D., Wallace G. 2005. Electrochemically-induced fluid movement using polypyrrole. Synth. Met. 151, 60–64. ( 10.1016/j.synthmet.2005.04.001) [DOI] [Google Scholar]
- Cen L., Neoh K. G., Kang E. T. 2003. Surface functionalization of polypyrrole film with glucose oxidase and viologen. Biosens. Bioelectron. 18, 363–374. ( 10.1016/S0956-5663(02)00149-5) [DOI] [PubMed] [Google Scholar]
- Cheng D., Xia H., Chan H. S. 2005. Synthesis and characterization of surface-functionalized conducting polyaniline–chitosan nanocomposite. J. Nanosci. Nanotechnol. 5, 466–473. [DOI] [PubMed] [Google Scholar]
- Collier J. H., Camp J. P., Hudson T. W., Schmidt C. E. 2000. Synthesis and characterization of polypyrrole–hyaluronic acid composite biomaterials for tissue engineering applications. J. Biomed. Mater. Res. 50, 574–584. ( 10.1002/(SICI)1097-4636(20000615)50:4) [DOI] [PubMed] [Google Scholar]
- Cosnier S., Dawod M., Gorgy K., Da Silva S. 2003. Synthesis and electrochemical characterization of a new electropolymerizable hydrophilic viologen designed for enzyme wiring. Microchim. Acta 143, 139–145. ( 10.1007/s00604-003-0064-7) [DOI] [Google Scholar]
- Cui X. Y., Martin D. C. 2003. Electrochemical deposition and characterization of poly (3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens. Actuat. B: Chem. 89, 92–102. ( 10.1016/S0925-4005(02)00448-3) [DOI] [Google Scholar]
- Cui X., Lee V. A., Raphael Y., Wiler J. A., Hetke J. F., Anderson D. J. 2001. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 56, 261–272. [DOI] [PubMed] [Google Scholar]
- Cui X., Wiler J., Dzaman M., Altschuler R. A., Martin D. C. 2003. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials 24, 777–787. ( 10.1016/S0142-9612(02)00415-5) [DOI] [PubMed] [Google Scholar]
- De Giglio E., Sabbatini L., Colucci S., Zambonin G. 2000. Synthesis, analytical characterization, and osteoblast adhesion properties on RGD-grafted polypyrrole coatings on titanium substrates. J. Biomater. Sci. Polym. 11, 1073–1083. ( 10.1163/156856200743580) [DOI] [PubMed] [Google Scholar]
- De Giglio E., Guascito M. R., Sabbatini L., Zambonin G. 2001. Electropolymerization of pyrrole on titanium substrates for the future development of new biocompatible surfaces. Biomaterials 22, 2609–2616. ( 10.1016/S0142-9612(00)00449-X) [DOI] [PubMed] [Google Scholar]
- Diaz A. F., Hall B. 1983. Mechanical properties of electrochemically prepared polypyrrole films. IBM J. Res. Dev. 27, 342–347. [Google Scholar]
- Druy M. A., Tsang C. H., Brown N., Heeger A. J., MacDiarmid A. G. 1980. The evolution of polyacetylene morphology upon doping. J. Polym. Sci. Polym. Phys. Edn. 18, 429–441. [Google Scholar]
- Edgerton B. J., House W. F., Hitselberger W. 1982. Hearing by cochlear nucleus stimulation in humans. Ann. Otol. Rhinol. Laryngol. 91, 117–124. [PubMed] [Google Scholar]
- Finkenstadt V. L. 2005. Natural polysacharrides as electroactive polymers. Appl. Microbiol. Biotechnol. 67, 735–745. ( 10.1007/s00253-005-1931-4) [DOI] [PubMed] [Google Scholar]
- Gandhi M. R., Murray P., Spinks G. M., Wallace G. G. 1995. Mechanism of electromechanical actuation in polypyrrole. Synth. Met. 73, 247–256. ( 10.1016/0379-6779(95)80022-0) [DOI] [Google Scholar]
- Garner B., Georgevich A., Hodgson A. J., Liu L., Wallace G. G. 1999a Polypyrrole–heparin composites as stimulus-responsive substrates for endothelial cell growth. J. Biomed. Mater. Res. 44, 121–129. ( 10.1002/(SICI)1097-4636(199902)44:2) [DOI] [PubMed] [Google Scholar]
- Garner B., Hodgson A. J., Wallace G. G., Underwood P. A. 1999b Human endothelial cell attachment to and growth on polypyrrole–heparin is vitronectin dependent. J. Mater. Sci. Mater. Med. 10, 19–27. [DOI] [PubMed] [Google Scholar]
- George P. M., et al. 2005. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 26, 3511–3519. [DOI] [PubMed] [Google Scholar]
- Gizdavic-Nikolaidis M., Travas-Sejdic J., GrahamBowmaker A., Ralph Cooney P., Thompson C., Kilmartin P. A. 2004a The antioxidant activity of conducting polymers in biomedical applications. Curr. Appl. Phys. 4, 347–350. ( 10.1016/j.cap.2003.11.045) [DOI] [Google Scholar]
- Gizdavic-Nikolaidis M., Travas-Sejdic J., Graham Bowmaker A., Ralph Cooney P., Kilmartin P. A. 2004b Conducting polymers as free radical scavengers. Synth. Met. 140, 225–232. ( 10.1016/S0379-6779(03)00372-2) [DOI] [Google Scholar]
- Glowienke S., Frieauff W., Allmendinger T., Martus H. J., Suter W., Mueller L. 2005. Structure-activity considerations and in vitro approaches to assess the genotoxicity of methane-, benzene- and toluenesulfonic acid esters. Mutat. Res. 581, 23–34. ( 10.1016/j.mrgentox.2004.10.004) [DOI] [PubMed] [Google Scholar]
- Gomez N., Lee J. Y., Nickels J. D., Schmidt C. E. 2007. Micropatterned polypyrrole: a combination of electrical and topographical characteristics for the stimulation of cells. Adv. Funct. Mater. 17, 1645–1653. ( 10.1002/adfm.200600669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. A., Lovell N. H., Poole-Warren L. A. 2009a Cell attachment functionality of bioactive conducting polymers for neural interfaces. Biomaterials 30, 3637–3644. ( 10.1016/j.biomaterials.2009.03.043) [DOI] [PubMed] [Google Scholar]
- Green R. A., Lovell N. H., Poole-Warren L. A. 2009b Impact of co-incorporating laminin peptide dopants and neurotrophic growth factors on conducting polymer properties. Acta Biomater. 6, 63–71. ( 10.1016/j.actbio.2009.06.030) [DOI] [PubMed] [Google Scholar]
- Guimard N. K., Sessler J. L., Schmidt C. E. 2007a Design of a novel electrically conducting biocompatible polymer with degradable linkages for biomedical applications. Mater. Res. Soc. Symp. Proc. 950, 99–104. [Google Scholar]
- Guimard N. K., Gomezb N., Schmidt C. E. 2007b Conducting polymers in biomedical engineering. Prog. Polym. Sci. 32, 876–921. ( 10.1016/j.progpolymsci.2007.05.012) [DOI] [Google Scholar]
- Harwood G. W. J., Pouton C. W. 1996. Amperometric enzyme biosensors for the analysis of drug and metabolites. Adv. Drug. Deliv. Rev. 18, 163–191. ( 10.1016/0169-409X(95)00093-M) [DOI] [Google Scholar]
- He W., Bellamkonda R. V. 2005. Nanoscale neuro-integrative coatings for neural implants. Biomaterials 26, 2983–2990. ( 10.1016/j.biomaterials.2004.08.021) [DOI] [PubMed] [Google Scholar]
- Heeger A. J., MacDiarmid A. G., Shirakawa H. 2000. The Nobel Prize in chemistry, 2000: conductive polymers. Stockholm, Sweden: Royal Swedish Academy of Sciences. [Google Scholar]
- Hodgson A. J., John M. J., Campbell T., Georgevich A., Woodhouse S., Aoki T. 1996. Integration of biocomponents with synthetic structures—use of conducting polymer polyelectrolyte composites. Proc. SPIE. Int. Soc. Opt. Eng. 2716, 164–176. ( 10.1117/12.232137) [DOI] [Google Scholar]
- Hutzler M., Fromherz P. 2004. Silicon chip with capacitors and transistors for interfacing organotypic brain slice of rat hippocampus. Eur. J. Neurosci. 19, 2231–2238. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). 2004. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. In Monographs on the evaluation of carcinogenic risks to humans, vol. 71, p. 109 Lyon, France: International Agency for Research on Cancer. [PMC free article] [PubMed] [Google Scholar]
- Isaksson J., Tengstedt C., Fahlman M., Robinson N., Berggren M. 2004. A solid-state organic electronic wettability switch. Adv. Mater. 16, 316–320. ( 10.1002/adma.200306131) [DOI] [Google Scholar]
- Kim D. R., Abidian M., Martin D. C. 2003. Synthesis and characterization of conducting polymers grown in hydrogels for neural applications. In Proc. Symp. Architecture and Application of Biomaterials and Biomolecular Materials, Boston, MA, 1–4 December 2003, vol. 1 (eds Plant A. L., Uhrich K., Saltzmann W. M., Luo D., Chilkoti A.), pp. 55–60. Wareendale, PA: Materials Research Society. [Google Scholar]
- Kim D. H., Abidan M., Martin D. C. 2004. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J. Biomed. Mater. Res. A 71, 577–585. [DOI] [PubMed] [Google Scholar]
- Kim D. H., Richardson-Burns S. M., Hendricks J. L., Sequera C., Martin D. C. 2006. Effect of immobilized nerve growth factor on conductive polymers: electrical properties and cellular response. Adv. Funct. Mater. 17, 1–8. ( 10.1002/adfm.200500594) [DOI] [Google Scholar]
- Kim D. H., Wiler J. A., Anderson D. J., Kipke D. R., Martin D. C. 2009. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 6, 57–62. ( 10.1016/j.actbio.2009.07.034) [DOI] [PubMed] [Google Scholar]
- Kmecko T., Hughes G., Cauller L., Jeong-Bong L., Romero-Ortega M. 2006. Nanocomposites for neural interfaces. Res. Soc. Symp. Proc. Mater. Res. Soc. 926E, 0926-CC04-06. [Google Scholar]
- Kringelbach M. L., Jenkinson N., Owen S. L. F., Aziz T. Z. 2007. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635. ( 10.1038/nrn2196) [DOI] [PubMed] [Google Scholar]
- Li Y., Neoh K. G., Cen L., Kang E. T. 2005a Controlled release of heparin from polypyrrole–poly(vinyl alcohol) assembly by electrical stimulation. J. Biomed. Mater. Res. A 73A, 171–181. ( 10.1002/jbm.a.30286) [DOI] [PubMed] [Google Scholar]
- Li Y., Neoh K. G., Cen L., Kang E. T. 2005b Porous and electrically conductive polypyrrole–poly(vinyl alcohol) composite and its applications as a biomaterial. Langmuir 21, 10702–10709. ( 10.1021/la0514314) [DOI] [PubMed] [Google Scholar]
- Li M., Guo Y., Wei Y., MacDiarmid A. G., Lelkes P. I. 2006. Electrospinning polyaniline-containing gelatin nanofibers for tissue engineering applications. Biomaterials 27, 2705–2715. ( 10.1016/j.biomaterials.2005.11.037) [DOI] [PubMed] [Google Scholar]
- Liu X., Chen J., Gilmore K. J., Wallace G. G. 2008. 3D Bio-nanofibrous PPy/SIBS mats as platforms for cell culturing. Chem. Commun. 2008, 3729–3731. ( 10.1039/b804283g) [DOI] [PubMed] [Google Scholar]
- Machado J. M., Masse M. A., Karasz F. E. 1989. Anisotropic mechanical properties of uniaxially oriented electrically conducting poly(p-phenylene vinylene). Polymer 30, 1992–1996. [Google Scholar]
- Malhotra B. D., Singhal R. 2003. Conducting polymer based biomolecular electronic devices. Pramana 61, 331–343. [Google Scholar]
- Masada H., Asano D. K. 2003. Preparation and properties of polypyrrole. Synth. Met. 135, 43–44. [Google Scholar]
- Mattioli-Belmonte M., Virgili L., De Santis C. M., Giantomassi F., Gabbanelli F., Solomos G., Guerra R., Biagini G. 2002. Biocharacterization of Tosylate doped polypyrrole films for biomedical applications. Mater. Sci. Eng. C 25, 43–49. [Google Scholar]
- Meng-yan L., et al. 2007. Electroactive and nanostructured polymers as scaffold materials for neuonal and cardiac tissue engineering. Chin. J. Polym. Sci. 25, 331–339. ( 10.1142/S0256767907002199) [DOI] [Google Scholar]
- Merz M., Fromherz P. 2005. Silicon chip interfaced with a geometrically defined net of snail neurons. Adv. Funct. Mater. 15, 739–744. ( 10.1002/adfm.200400316) [DOI] [Google Scholar]
- Osada Y., Gong J. 1993. Stimuli-responsive polymer gels and their application to chemomechanical systems. Progr. Polym. Sci. 18, 187–226. [Google Scholar]
- Osada Y., Okuzaki H., Hurl H. 1992. A polymer gel with electrically driven motility. Nature 355, 242–244. ( 10.1038/355242a0) [DOI] [Google Scholar]
- Otero T. F., Cortes M. T. 2003. A sensing muscle. Sens. Actuat. B 96, 152–156. ( 10.1016/S0925-4005(03)00518-5) [DOI] [Google Scholar]
- Otero T. F., Sansihena J. M. 1997. Bilayer dimensions and movement in artificial muscles. Bioelectrochem. Bioenerg. 42, 117–122. ( 10.1016/S0302-4598(96)05112-4) [DOI] [Google Scholar]
- Otero T. F., Sansinena J. M. 1998. Soft and wet conducting polymers for artificial muscles. Adv. Mater. 10, 491–494. [DOI] [PubMed] [Google Scholar]
- Peramo A., Urbanchek M. G., Spanninga S. A., Povlich L. K., Cederna P., Martin D. C. 2008. In situ polymerization of a conductive polymer in acellular muscle tissue constructs. Tissue Eng. Part A 14, 423–432. [DOI] [PubMed] [Google Scholar]
- Pernaut J. M., Reynolds J. R. 2000. Use of conducting electroactive polymers for drug delivery and sensing of bioactive molecules. A redox chemistry approach. J. Phys. Chem. B 104, 4080–4090. ( 10.1021/jp994274o) [DOI] [Google Scholar]
- Richardson-Burns S. M., Hendricks J. L., Brian F., Povlich L. K., Dong-Hwan K., Martin D. C. 2007. Polymerization of the conducting polymer poly (3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials 28, 1539–1552. ( 10.1016/j.biomaterials.2006.11.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers T. J., Hudson T. W., Schmidt C. E. 2002. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv. Funct. Mater. 12, 33–37. ( 10.1002/1616-3028(20020101)12:1) [DOI] [Google Scholar]
- Robinson L., Isaksson J., Robinson N. D., Berggren M. 2006. Electrochemical control of surface wettability of poly (3-alkylthiophenes). Surf. Sci. 600, 148–152. ( 10.1016/j.susc.2006.03.039) [DOI] [Google Scholar]
- Salto C., Saindon E., Bolin M., Kanciurzewska A., Fahlman M., Edwin Jager W. H., Tengvall P., Arenas E., Berggren M. 2008. Control of neural stem cell adhesion and density by an electronic polymer surface switch. Langmuir 24, 14 133–14 138. ( 10.1021/la8028337) [DOI] [PubMed] [Google Scholar]
- Sanghvi A. B., Miller K. P., Belcher A. M., Schmidt C. E. 2005. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat. Mater. 4, 496–502. ( 10.1038/nmat1397) [DOI] [PubMed] [Google Scholar]
- Saxena V., Malhotra B. D. 2002. Handbook of polymers in electronics, p. 3 Shrewsbury, UK: Rapra Technology. [Google Scholar]
- Schmidt C. E., Shastri V. R., Vacanti J. P., Langer R. 1997. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl Acad. Sci. USA 94, 8948–8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze J. W., Karabulut H. 2005. Application potential of conducting polymers. Electrochim. Acta 50, 1739–1745. ( 10.1016/j.electacta.2004.10.023) [DOI] [Google Scholar]
- Schwartz A. B. 2004. Cortical neural prosthetics. Annu. Rev. Neurosci. 27, 487–507. ( 10.1146/annurev.neuro.27.070203.144233) [DOI] [PubMed] [Google Scholar]
- Shi G., Rouabhia M., Wang Z., Dao L. H., Zhang Z. 2004. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 25, 2477–2488. ( 10.1016/j.biomaterials.2003.09.032) [DOI] [PubMed] [Google Scholar]
- Smela E. 2003. Conjugated polymer actuators for biomedical applications. Adv. Mater. 15, 481–494. ( 10.1002/adma.200390113) [DOI] [Google Scholar]
- Song H. K., Toste B., Ahmann K., Hoffman-Kim D., Palmore G. T. R. 2006. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: a new platform for characterizing neurite extension in complex environments. Biomaterials 27, 473–484. [DOI] [PubMed] [Google Scholar]
- Spinks G. M., Campbell T. E., Wallace G. G. 2005a Force generation from polypyrrole actuators. Smart Mater. Struct. 14, 406–412. ( 10.1088/0964-1726/14/2/015) [DOI] [Google Scholar]
- Spinks G. M., Xi B., Troung V. T., Wallace G. G. 2005b Actuation behavior of layered composites of polyaniline, carbon nanotubes and polypyrrole. Synth. Met. 151, 85–91. ( 10.1016/j.synthmet.2005.03.006) [DOI] [Google Scholar]
- Spinks G. M., Mottaghitalab V., Bahrami-Samani M., Whitten P. G., Wallace G. G. 2006. Carbon nanotube reinforced polyaniline fibres for high strength artificial muscles. Adv. Mater. 18, 637–640. ( 10.1002/adma.200502366) [DOI] [Google Scholar]
- Stassen I., Sloboda T., Hambitzer G. 1995. Membrane with controllable permeability for drugs. Synth. Met. 71, 2243–2244. ( 10.1016/0379-6779(94)03241-W) [DOI] [Google Scholar]
- Stauffer W. R., Cui X. T. 2006. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials 27, 2405–2413. ( 10.1016/j.biomaterials.2005.10.024) [DOI] [PubMed] [Google Scholar]
- Sun T., Wang G., Feng L., Liu B., Ma Y., Jiang L., Zhu Angew D. 2004. Reversible switching between superhydrophilicity and superhydrophobicity. Chem. Int. Ed. 43, 357–360. ( 10.1002/anie.200352565) [DOI] [PubMed] [Google Scholar]
- Svennersten K., Bolin M. H., Edwin Jager W. H., Berggren M., Richter-Dahlfors A. 2009. Electrochemical modulation of epithelia formation using conducting polymers. Biomaterials 30, 6257–6264. ( 10.1016/j.biomaterials.2009.07.059) [DOI] [PubMed] [Google Scholar]
- Thompson B. C., Richardson R. T., Moulton S. E., Evans A. J., Stephen O., Clark G. M., Wallace G. C. 2010. Conducting polymers, dual neurotrophins and pulsed electrical stimulation—dramatic effects on neurite outgrowth. J. Control. Rel. 141, 161–167. ( 10.1016/j.jconrel.2009.09.016) [DOI] [PubMed] [Google Scholar]
- Tschmelak J., et al. 2005. Automated Water Analyser Computer Supported System (AWACSS). Part II. Intelligent, remote-controlled, cost-effective, on-line, water-monitoring measurement system. Biosens. Bioelectron. 20, 1509–1519. ( 10.1016/j.bios.2004.07.033) [DOI] [PubMed] [Google Scholar]
- Venugopal J., Molamma P., Choon A. T., Deepika G., Giri Dev V. R., Ramakrishna S. 2009. Continuous nanostructures for the controlled release of drugs. Curr. Pharm. Des. 15, 1799–1808. [DOI] [PubMed] [Google Scholar]
- Wadhwa R., Lagenaur C. F., Cui X. T. 2006. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control. Rel. 110, 531–541. ( 10.1016/j.jconrel.2005.10.027) [DOI] [PubMed] [Google Scholar]
- Wang X., Gu X., Yuan C., Chen S., Zhang P., Zhang T., Yao J., Chen F., Chen G. 2004. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. 68A, 411–422. ( 10.1002/jbm.a.20065) [DOI] [PubMed] [Google Scholar]
- Wang Z., Roberge C., Wan Y., Dao L. H., Guidoin R., Zhang Z. 2003. A biodegradable electrical bioconductor made of polypyrrole nanoparticle/poly (d,l-lactide) composite: a preliminary in vitro biostability study. J. Biomed. Mater. Res. 66A, 738–746. [DOI] [PubMed] [Google Scholar]
- Wang Z., Roberge C., Dao L. H., Wan Y., Shi G., Rouabhia M., Guidoin R., Zhang Z. 2004. In vivo evaluation of a novel electrically conductive polypyrrole/poly(d,l-lactide) composite and polypyrrole coated poly(d,l-lactide-co-glycolide) membranes. J. Biomed. Mater. Res. 70A, 28–38. ( 10.1002/jbm.a.30047) [DOI] [PubMed] [Google Scholar]
- Warren L. F., Walker J. A., Anderson D. P., Rhodes C. G., Buckley L. J. 1989. A study of conducting polymer morphology. J. Electrochem. Soc. 136, 2286–2295. [Google Scholar]
- Weiss Z., Mandler D., Shustak G., Domb A. J. 2004. Pyrrole derivatives for electrochemical coating of metallic medical devices. J. Polym. Sci. Part A: Polym. Chem. 42, 658–667. ( 10.1002/pola.11097) [DOI] [Google Scholar]
- Williams R. L., Doherty P. J. 1994. A preliminary assessment of poly (pyrrole) in nerve guide studies. J. Mater. Sci. Mater. Med. 5, 429–433. ( 10.1007/BF00058978) [DOI] [Google Scholar]
- Wise D. L., Wnek G. E., Trantolo D. J., Cooper T. M., Gresser J. D., Marcel D. 1998. Electrical and optical polymer systems: fundamentals, methods and application, p. 1031 New York, NY: Marcel Dekker. [Google Scholar]
- Wong J. Y., Langer R., Ingber D. E. 1994. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc. Natl Acad. Sci. USA 91, 3201–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Tian-An C., Rieke R. D. 1996. A study of small band gap polymers: head-to-tail regioregular poly[3-(alkylthio)thiophenes] prepared by regioselective synthesis using active zinc. Macromolecules 29, 7671–7677. ( 10.1021/ma960946m) [DOI] [Google Scholar]
- Wynne K. J., Street G. B. 1985. Poly (pyrrol-2-ylium tosylate), electrochemical synthesis and physical and mechanical properties. Macromolecules 18, 2361–2368. [Google Scholar]
- Xiao Y. H., Cui X. Y., Hancock J. M., Bouguettaya M., Reynolds J. R., Martin D. C. 2004. Electrochemical polymerization of poly(hydroxymethylated-3,4 ethylenedioxythiophene) (PEDOT–MeOH) on multichannel neural probes. Sens. Actuat. B 99, 437–443. ( 10.1016/j.snb.2003.12.067) [DOI] [Google Scholar]
- Xu L. B., Chen W., Mulchandani A., Yan Y. 2005. Reversible conversion of conducting polymer films from superhydrophobic to superhydrophilic. Angew. Chem. Int. Ed. 44, 6009–6012. ( 10.1002/anie.200500868) [DOI] [PubMed] [Google Scholar]
- Yamato H., Ohwa M., Wernet W. 1995. Stability of polypyrrole and poly (3,4-ethylenedioxythiophene) for biosensor application. J. Electroanal. Chem. 397, 163–170. ( 10.1016/0022-0728(95)04156-8) [DOI] [Google Scholar]
- Yang J. Y., Martin D. C. 2004a Microporous conducting polymers on neural microelectrode arrays. I. Electrochemical deposition. Sens. Actuat. B Chem. 101, 133–142. ( 10.1016/j.snb.2004.02.056) [DOI] [Google Scholar]
- Yang J. Y., Martin D. C. 2004b Microporous conducting polymers on neural microelectrode arrays. II. Physical characterization. Sens. Actuat. A 113, 204–211. ( 10.1016/j.sna.2004.02.029) [DOI] [Google Scholar]
- Yang J. Y., Martin D. C. 2006. Impedance spectroscopy and nanoindentation of conducting poly(3,4-ethylenedioxythiophene) coatings on microfabricated neural prosthetic devices. J. Mater. Res. 21, 1124–1132. ( 10.1557/JMR.2006.0145) [DOI] [Google Scholar]
- Yang J. Y., Kim D. H., Hendricks J. L., Leach M., Northey R., Martin D. C. 2005. Ordered surfactant-templated poly (3,4-ethylenedioxythiophene) (PEDOT) conducting polymer on microfabricated neural probes. Acta Biomater. 1, 125–136. ( 10.1016/j.actbio.2004.09.006) [DOI] [PubMed] [Google Scholar]
- Zelikin A. N., Lynn D. M., Farhadi J., Martin I., Shastri V., Langer R. 2002. Erodible conducting polymers for potential biomedical applications. Angew. Chem. Int. Ed. 41, 141–144. ( 10.1002/1521-3773(20020104)41:1) [DOI] [PubMed] [Google Scholar]
- Zhong Y. H., Yu X. J., Gilbert R., Bellamkonda R. V. 2001. Stabilizing electrode-host interfaces: a tissue engineering approach. Rehabil. J. Res. Dev. 38, 627–632. [PubMed] [Google Scholar]
- Zimmermann W. H., Fink C., Kralish D., Remmers U., Weil J., Eschenhagen T. 2000. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 68, 106–114. [PubMed] [Google Scholar]
- Zimmermann W. H., et al. 2002. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation 106, I151–I157. ( 10.1161/01.cir.0000032876.55215.10) [DOI] [PubMed] [Google Scholar]