Preface
Mechanotransduction research has focused historically on how externally applied forces can affect cell signalling and function. A growing body of evidence suggests that contractile forces generated internally by the actomyosin cytoskeleton are also important in regulating cell behaviour, and suggest a broader role for mechanotransduction in biology. While the molecular basis for these cellular forces in mechanotransduction are being pursued in cell culture, researchers are also beginning to appreciate their contribution to in vivo developmental processes. Here we examine the role for mechanical forces and contractility in regulating cell and tissue structure and function during development.
Introduction
Although early conceptions of embryogenesis focused on the structural rearrangements that give rise to complex morphological body plans, as well as the mechanical origins of such rearrangements 1, 2, much of our modern description of the process is presented in terms of spatiotemporally coordinated changes in gene expression patterning. Only recently have investigators begun to integrate these two approaches to provide early hints of a more global model that incorporates the contribution of mechanics to our modern molecular model of development.
The early developmental stages from egg to a detailed body plan differ between species, but in general are often characterized by common structural rearrangements (Box 1). At the cellular level, one can describe many of these stereotypic events as emerging from the coordinated and iterative regulation of many basic cellular processes including proliferation, differentiation, and spatial rearrangements (Box 1). In addition to the indispensable functions of different genetic programs and soluble morphogens in regulating proliferation, differentiation, and physical rearrangements, these cellular processes are also regulated by mechanical forces. Much work has uncovered how mechanical forces are transduced into biochemical signals (mechanotransduction), and how mechanotransduction in turn impacts numerous cell functions 3. In parallel, recent studies in vivo have also begun to characterize the forces that cells might experience during development.
Box 1. Key developmental steps of embryogenesis
Throughout development, and particularly during embryogenesis, there is a tight coupling between changes in gene expression, cell shape, and multicellular organization. Zygotic cell proliferation gives rise to a blastula, which then forms an inner cell mass in order to become a blastocyst. Gastrulation is the process by which the blastocyst is transformed into a gastrula, which displays different germ layers (in most organisms, three—the mesoderm, ectoderm, and endoderm). Gastrulation consists of several different steps. First, after progenitor cells sort, apical constriction and internalization movements position the nascent mesoderm and endoderm beneath the prospective ectoderm. Then epiboly events (including intercalation) expand and thin these nascent germ layers. Finally, convergence and extension mediolaterally narrows and anterioposteriorly lengthens the embryo, respectively, to form the gastrula. After gastrulation, the gastrula undergoes several movements that ultimately give rise to specialized tissues and organs of the embryo. The key cellular processes of embryogenesis— proliferation, differentiation, and spatial organization changes—are labeled in italics. While this review is intended to be general, the specific sketches here of various stages are modeled on X. laevis embryogenesis.
In this Review, we explore our nascent understanding of mechanical forces during embryogenesis and examine how these forces might regulate basic cellular processes (proliferation, differentiation, and organizational changes) specifically within the broader context of embryogenesis. For this reason, this review is not tailored to one specific species, but rather is written to be a general perspective. Drawing from both in vitro and in vivo studies from several model systems, we explore how actomyosin-mediated contractile forces regulate these cellular processes, and discuss how they might be mechanistically controlled during development. By focusing specifically on how forces in embryogenesis might drive changes in cell proliferation, differentiation, and organizational changes associated with development, we hope to synthesize recent data within a broader picture of the biology of mechanotransduction.
Biomechanics during embryogenesis
Two principal factors contribute to mechanical stresses that are experienced by cells and influence cell behaviour in early development—the mechanical stiffness of the local tissue environment and the contractile activity of the cells pulling on that environment. Stiffness and contractility both contribute to the cellular mechanical stresses essential for mechanotransduction. Cells routinely contract to pull on the scaffolds to which they are attached (the ECM or other cells), thereby generating tension within the cell, or an internal mechanical stress. The magnitude of such stress is affected both by strength of contractile activity in the cell and the substrate stiffness. In development, understanding the interplay between cellular contractile activity, stiffness of surrounding tissues, and the resultant deformations and mechanical stresses is critical for refining our model of embryogenesis.
Stiffness of embryos
In vivo there is evidence that stiffness is important during embryogenesis. For example, during Xenopus laevis gastrulation, convergence and extension movements can only occur if the mesoderm and notochord remain stiff enough to resist buckling 4, 5. In addition, during this same process, the involuting marginal zone actively stiffens so that this tissue does not collapse or deform during gastrulation 6. Whether these changes in tissue stiffness at various stages are strictly to provide mechanical strength for morphogenetic events or also present a mechanotransduction stimulus to orchestrate other cellular processes (such as proliferation or differentiation) remains unclear. Nonetheless, these data suggest that mechanisms exist to modulate tissue stiffness, and, furthermore, that these changes in stiffness are required for development to proceed appropriately.
Because of the technical challenges of accurately measuring mechanical parameters in vivo (Box 2), only a few studies have directly measured embryo stiffness. Stiffness is determined empirically by applying a defined force across a specific area (i.e., stress), and then measuring the resulting deformation (i.e., strain). The slope of the stress vs. strain plot is the stiffness, reported in units of Pascals (Pa). Stiffness values (of different tissues) during X. laevis gastrulation range from 3 to 14.2 Pa 6. Likewise, the estimated stiffness of Ambystoma mexicanum early-stage embryos is about 20 Pa 7. Stiffness of tissues in embryos is low in comparison to adult tissues 6–8, which range from 17 Pa (human fat) to 310 MPa (rat Achilles tendon) 9. Given the apparent importance of stiffness to cell function, it will be especially meaningful to develop approaches to track how stiffness values change in different tissues during the many movements and tissue rearrangements that occur in embryogenesis.
Box 2. Techniques used to study mechanics in embryos
Characterizing mechanics is difficult at the single cell level since cells are dynamic—they generate and respond to force. At the embryo level, however, this task becomes even more complicated. Embryos offer several additional challenges including increased tissue fragility, difficulty in defining regions of interest in a small embryo, and the continuous dynamic movements that cause gross tissue deformations 7, 24. However, several methods have been used to understand tissue mechanics during embryogenesis. Most of these methods rely on applying forces to explanted embryonic tissues and observing their behavior to define the mechanical parameters. For example, in the stress-relaxation test, tissue explants of starting length Lo are compressed to length L and the force required to maintain L is determined 6. Similar tests can be performed with parallel plates that compress the explanted tissue and measure its viscoelastic responses 101, 102. Conversely, cantilever tissue testers separate the embryonic epithelia from the embryo; cantilever wires are then used to elongate the tissue at a constant true strain rate. Force is then determined by measuring the bending of the wires 7. In addition, a fiber optic system has also been described that uses a flexible cantilevered optical fiber probe to apply force or deformation to an explanted tissue. The probe tip position and deflection measure tissue deformation and force, respectively 103. Another method commonly used to assess the mechanical contributions of different tissues is laser ablation. By locally cutting a tissue, one can determine indirectly whether that tissue was under tension (by observing tissue pulling away from the cutting site like a spring) 18, 19, 21, 22, 26.
While all of the above methods require external interventions, there are more recently described methods that do not require contact between a probe and the tissue. Particle tracking microrheology has been modified for use in C. elegans embryos. In this procedure, nanoparticles are microinjected into zygotes and particle movement is monitored to determine the local viscoelastic properties (including diffusion coefficient and shear viscocity) 104. In addition, a micromanipulation assay has recently been described in D. melanogaster embryos. Ferrofluid can be injected into specific locations in the embryo and then magnetic tweezers used to manipulate the magnetized cells to apply tissue deformations 60. Further refinement of this methods and other methods amenable to single cells will undoubtedly shed light on tissue mechanics during embryogenesis.
Tissue stiffness may arise from several different factors, including the stiffness of the cells (which is usually regulated by the cytoskeleton 10), the strength of cell-ECM or cell-cell contacts, the biochemical identity of ECM proteins, and ECM organization and maturation. It is proposed that during convergence and extension movements in X. laevis, stiffness arises primarily from changes in the cytoskeleton and the ECM 6. Given the dramatic changes in cell-cell and cell-matrix adhesion that also occur during this complex rearrangement of cells with respect to one another, it is likely that changes in adhesion also contribute to tissue stiffness. However, because manipulations that target any one of these systems (the cytoskeleton, cell adhesions, or ECM) often feed back to affect all three, it has been difficult to develop an appropriate in vivo model for how these different factors independently contribute to a tissue’s stiffness.
In vitro, stiffness of the ECM has emerged as an important regulator of cell function. Decreasing substrate stiffness appears to dramatically alter cell structure in many cell types, reducing cell spreading against the substrate, the formation of focal adhesions, and stress fibers 11. Changes in stiffness also have potent effects on behaviour. In direct contrast to traditional culture on plastic, many cells types maintain a more differentiated phenotype when cultured on less stiff substrates more reflective of the stiffness of their in vivo tissue environment 9, 12–14. Mesenchymal stem cell (MSC) specification to different lineages is also strongly influenced by substrate stiffness 15. When MSCs are cultured on soft substrates, which resemble the stiffness of brain tissue, genetic profiling suggests that these cells undergo neuronal differentiation. However, on substrates of intermediate stiffness similar to striated muscle, MSCs differentiate into myoblasts. On stiff substrates, which mimick bone stiffness, MSCs undergo osteogenesis 15. These in vitro studies highlight the significant influence that stiffness can have on cell function and suggest that changes in stiffness may also regulate cell function during embryogenesis.
Cell-generated forces during embryogenesis
In addition to changes in stiffness, various internal and external forces also contribute to mechanical stresses during embryogenesis. For the purposes of this review, we define these forces at the cellular level. That is, “internal forces” refers to contractile forces generated internally by the actomyosin cytoskeleton, whereas “external forces” refer to forces generated outside of the cell responding to the force. Therefore, by these definitions, cell-generated forces in one part of a mechanically active tissue (where the force is internal) may cause passive deformation of a neighbouring tissue, such that that tissue responds to an external force. However, it is important to note that, at the tissue level, the definition of external forces refers to forces developed outside of the system (for example, intracardiac fluid forces that are required for embryonic cardiogenesis 16). However, while these types of external tissue forces are also critical during embryonic development 16, their analysis is outside the scope of this review.
An example of cellular internal forces (cell-generated contractile forces) regulating embryogenesis is demonstrated in the X. laevis dorsal involuting and non-involuting marginal zones. Cultured explants of these tissues still converge and extend, suggesting that the tissue itself, and not external forces generated in a different place in the embryo, actively regulate these movements in gastrulation 17. Although there are several methods used to observe and measure cell-generated forces at the in vitro single cell level (Box 3), these methods are difficult to translate to embryos (Box 2). Therefore, it has been difficult to directly measure forces generated in vivo.
Box 3. Techniques used to study mechanics in single cells
Two main approaches are commonly used to study cell mechanics. Cell-generated forces can be measured or external forces can be applied to cells and their responses recorded in order to obtain information about the cellular mechanical parameters. Both approaches have been used successfully in single cells and in embryos (see Box 2).
To observe cell-generated forces in single cells, Harris et al. first introduced wrinkling substrates more than twenty-five years ago 105. In this method, cells are cultured on thin films of silicone that wrinkle when cells pull on them 105. Over the years, this procedure has been modified to be more quantitative. By plating cells on thin micropatterned elastomer substrates, cells will distort the substrates (and thus the patterns) so their displacements can be mapped and cell-generated forces calculated 106. In addition, traction force microscopy also allows quantitative measures of force. In this technique, fluorescent beads are embedded in a flexible non-wrinkling material. As the cells pull the underlying material, the bead displacements are tracked, which can then be used to calculate cell-generated forces 107.
Laser tweezers and microneedles are capable of both measuring cell-generated forces and applying forces to cells. The laser tweezer technique uses a focused laser beam to physically hold an ECM-coated bead on cells. The amount of cell-generated force that is required to move the bead out of the laser trap can be calculated 108, 109. In addition, the strength of the laser trap can be increased to apply increasing force to the cell 108. Microneedles are arrays of elastic posts that act as microcantilevers. When cells plated on these post arrays apply forces, the posts bend. Force can then be calculated by measuring the bending (post deflection) 58. Forces can also be applied to cells using a magnetic modification of this system, whereby nanowires are interspersed in the posts so that a magnetic field will induce torque in the nanowires, causing post deflection to apply external force to the attached cell 110. To understand how forces dynamically regulate cell behavior, these methods have been used in conjunction with studies on focal adhesion formation and migration.
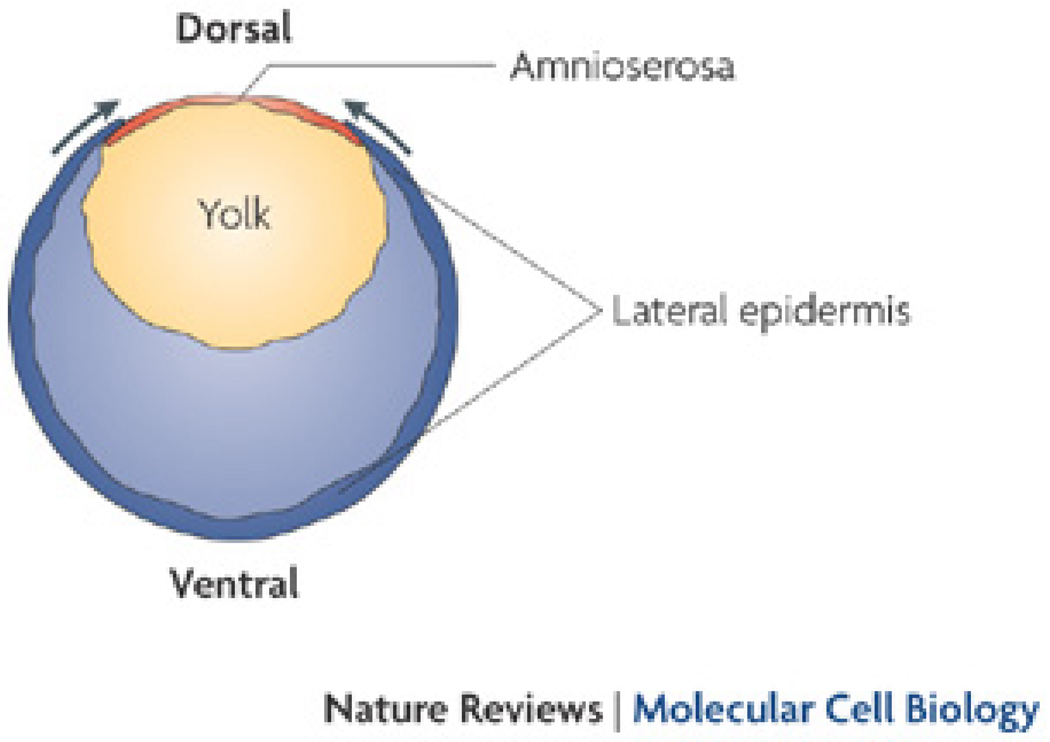
One method that has been used to give insight into the forces required for embryogenesis is laser ablation 18–24; Box 2). Studies using this approach in Caenorhabditis elegans and Drosophila melanogaster have reported changes in global movements that are too fast to be explained by signal transduction cascades, suggesting such movements are mechanical in nature 20, 23, 25. In conjunction with laser ablation studies, quantitative mechanical modelling has been used to form hypotheses about how these forces regulate movements in the embryo 21, 23. For example, these types of laser ablation studies have been used to examine how different tissues mechanically contribute to D. melanogaster dorsal closure 21, 22. Four forces have been proposed to contribute to dorsal closure: 1) the leading edge of the lateral epidermis is under tension and behaves like a “supracellular purse-string,”2) the amnioserosa in under tension, 3) the amnioserosa contracts, and 4) tension in the ventral lateral epidermis opposes the dorsally-located contraction 22, 23 (Fig. 1). These forces most likely arise from nonmuscle myosin II-dependent contraction 21, 22, because restoration of myosin motor activity in any one of the three areas that generate tension can restore dorsal closure in myosin-mutant embryos 26.
Figure 1. Forces that regulate Drosophila melanogaster dorsal closure.
Shown is a diagram of a cross-section of a D. melanogaster embryo at the early stages of dorsal closure. The surface of the embryo (including the lateral epidermis and amnioserosa) is thought to be under tension throughout this stage, in part as a result of contractile activity of the cells within these tissues 22. The arrows represent movement of the tissue that results from these forces. Modified with permission from 22.
Recent work using laser microdissection in D. melanogaster embryos has revealed another unexpected source of force generation, apoptosis. Although apoptosis was known to occur during dorsal closure to remove supernumary cells, Toyama et al. found that, in addition to this role, apoptosis also contributes between half and a third of the forces needed during dorsal closure 27. Interestingly, in vitro studies in epithelial monolayers have revealed that neighbouring cells increase contractility to actively extrude the apoptotic cell 28 from the monolayer. Therefore, it has been suggested that these apoptotic events in D. melanogaster embryos might act as “triggers” of local contraction that can spread through the amnioserosa to propogate force generation required for dorsal closure to proceed 27, 29.
Proliferation and mechanical stress
Proliferation is an absolute requirement for development because it provides the necessary cellular mass for developing tissues. Proliferation must be tightly regulated during embryogenesis so that cells do not grow uncontrollably, which would, amongst other consequences, disrupt the shape of the embryo and its developing tissues.
Mechanical feedback regulates proliferation
Wang and Riechmann have recently described a mechanism to explain how localized proliferation is controlled by mechanical stresses during D. melanogaster egg chamber morphogenesis 30. As the epithelial cyst grows, epithelial cells proliferate to increase the surface area of the epithelium 30. Localized myosin activity at the apical face of the epithelia leads to increased tension in the growing epithelial cyst, which results in localized proliferation to regulate tissue growth during oogenesis 30. Furthermore, the cyst deforms when myosin activity is reduced or absent but blocking cyst growth suppresses these deformations, demonstrating a link between cyst tissue growth and cell proliferation 30. These data suggest that tensional stresses increase proliferation, whereas compression slows growth 30, 31. Recent mathematical models corroborate that this type of mechanical feedback could stabilize growth to maintain D. melanogaster tissue shape and form 32, 33.
This mechanical feedback model was actually first proposed over twenty-five years ago by Ingber and colleagues, who suggested that the tensional and compressional forces transmitted through a tissue may continually feedback to regulate tissue shape and form 34. Our lab has reported experimental evidence to support these models, showing that regions of high tensional stress in epithelial monolayers correlate with increased proliferation in vitro 35. Inhibition of myosin-generated tension or disruption of cell-cell contacts relaxes these regions of stress, leading to inhibition of proliferation. These studies demonstrate that tissue form and forces can in fact feedback to regulate growth 35. In addition, these data suggest it is not contractility per se that directly regulates proliferation, but rather the resultant mechanical stresses associated with contractility that can transmit through a tissue. In this regard, it is important to note that mechanical feedback-regulated proliferation is likely to apply to embryogenesis only after the earliest stages of blastocyst formation. Although cell division is necessary to form a blastocyst from the zygote, the shortened cell cycle that controls proliferation at this stage is regulated independent of cell-cell interactions 36, 37.
Cytoskeletal tension regulates cell proliferation
Indeed, several lines of evidence implicate cytoskeletal tension as a strong regulator of proliferation. For example, smooth muscle cells on low stiffness substrates or with contractility inhibited decrease proliferation 38, 39. The small GTPase RhoA regulates contractility 40 and is also required for proliferation 41. The RhoA effector, Rho Kinase (ROCK), induces contractility through phosphorylation of myosin light chain and myosin light chain phosphatase to increase myosin ATPase activity 42–44. In vitro, inhibition of ROCK in many diverse cell types inhibits proliferation 39, 45, 46, whereas activation of ROCK is necessary and sufficient to induce G1-S-phase cell-cycle progression 47. The RhoA/ROCK pathway appears to regulate proliferation, at least in part, though its effects on contractility and force generation, since inhibition of myosin also blocks proliferation in vitro 35, 48. This contractile regulation of proliferation is also observed in models of blood vessel mechanotransduction. In vivo, blood vessels are subjected to various strains created by pulse pressure. In vitro models mimicking these forces demonstrate that stretch is also a potent activator of RhoA signalling and proliferation in endothelial and smooth muscle cells 49 50. However, RhoA or ROCK inhibition blocks stretch-dependent proliferation 49, again demonstrating the requirement for forces and contractility in proliferation.
Linking proliferation and cell shape changes
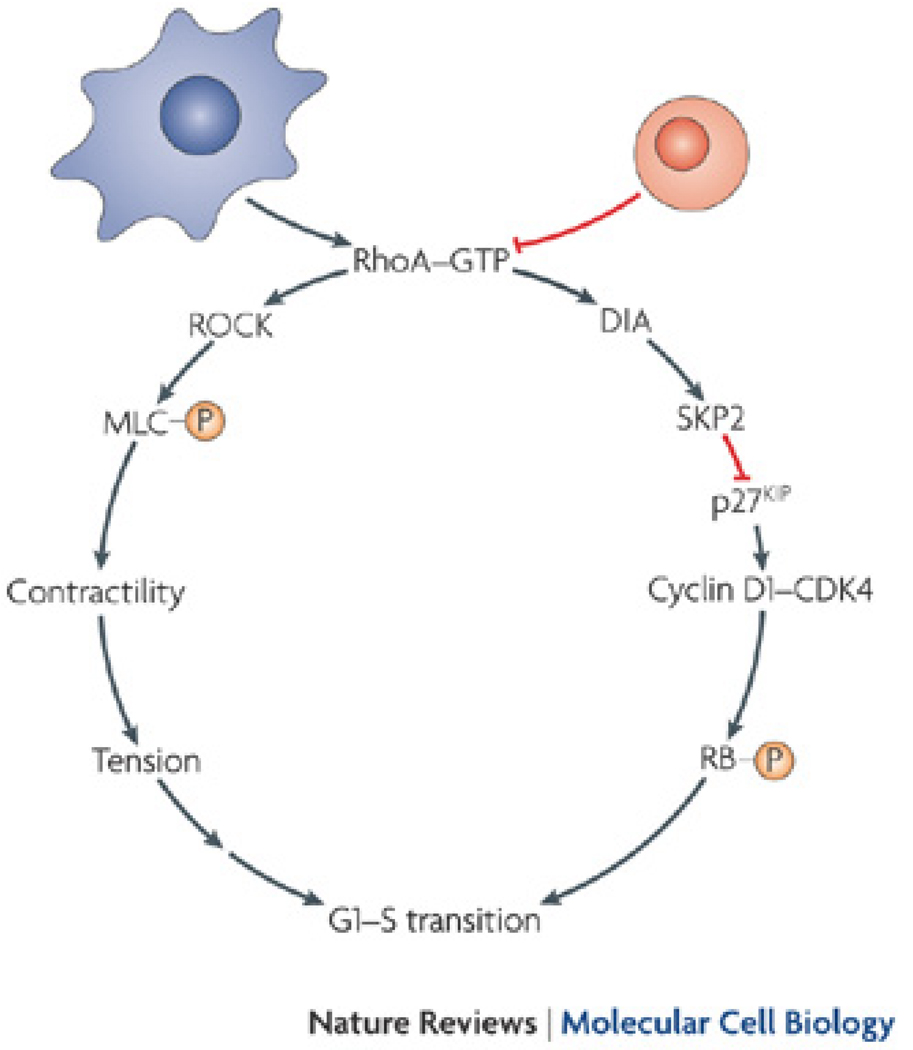
Changes in cell shape and morphology are required at most steps of embryogenesis 31. Although these changes are usually described as being a result of myosin-driven cell movements or upregulation of specific genes 51, it is important to recognize the instructive role of cell shape. Folkman and Moscona were the first to show that cell proliferation could be regulated by changes in cell shape 52. Using poly(2-hydroxyethyl methacrylate) to modulate cell shape, they found that DNA synthesis increases with cell spreading and flattening against the substrate, suggesting that cell shape plays a key—and then under appreciated—role in growth regulation 52. Early studies using changes in ECM density to control cell shape also reported shape-regulated proliferation 53. Sophisticated microcontact printing techniques were later used to adjust the extent of cell spreading without changing ECM density in order to confirm that cell shape per se imparts proliferative cues 54. Mechanistically, it appears that cell shape regulates proliferation in late G1 phase by regulating the small GTPase RhoA and its effector mDia; restricting cell spreading blocks RhoA and mDia-dependent Skp2 expression that would normally ubiquitinate and degrade p27kip 55, 56. p27kip is an inhibitor of the cyclin D1/cdk4 complex; when it is degraded, cyclin D1/cdk4 is free to phosphorylate Rb and allow cell cycle progression in spread cells 55, 56 (Fig. 2). Regulation of proliferation by cell shape also appears to be mediated through the effects of cell shape on ROCK-mediated contractility. We have demonstrated that restricting cell spreading suppresses RhoA activity and cellular force generation, and that constitutively activated RhoA rescues proliferation in unspread cells 57, 58. This suggests a model in which cell shape regulates RhoA-GTP levels to control mDia and ROCK activity, which both contribute to cell proliferation (Fig. 2).
Figure 2. Cell shape regulates proliferation through the small GTPase RhoA.
Restricting cell spreading decreases proliferation through the regulation of RhoA activity. RhoA promotes G1/S phase transition and cell proliferation through two pathways. First, the RhoA effector, ROCK, increases myosin light chain phosphorylation to generate cellular contractility. This generates tension in the cell, which is required for proliferation 57, 58. Second, the RhoA effector, mDia, activates Skp2 to inhibit p27kip. Since p27kip can no longer degrade the cyclin D1/cdk4 complex, the complex is free to phosphorylate Rb, leading to G1/S phase transition 55, 56. Because restricting cell shape decreases RhoA activity in some cell types, these two pathways are not activated. Without contractility and tension generation and Skp2 activity, G1/S phase transition is blocked and proliferation is reduced.
The regulation of proliferation by cell shape and forces is particularly intriguing because, as discussed above, there are many events during embryogenesis that involve dramatic changes in cell shape, structure, and mechanics. In the adult, it is thought that muscle, skin, and other soft tissues within a limb react to tensional forces generated by the growing long bones, and not just to soluble cues, by increasing proliferation. This model is borne out in orthopedic settings in which lengthening a limb bone results in coordinated growth of all of the surrounding soft tissue 59. Thus, like in adult tissues, we hypothesize that the local stresses and shape changes generated during later embryogenesis could provide local proliferative controls that can maintain tissue mass homeostasis.
Mechanotransduction and differentiation
Differentiation is necessary during many stages of development so that ultimately cells can perform their specific functions. Both mechanical forces and cell-generated contractility regulate differentiation in vitro and in vivo.
Twist is mechanically regulated in vivo
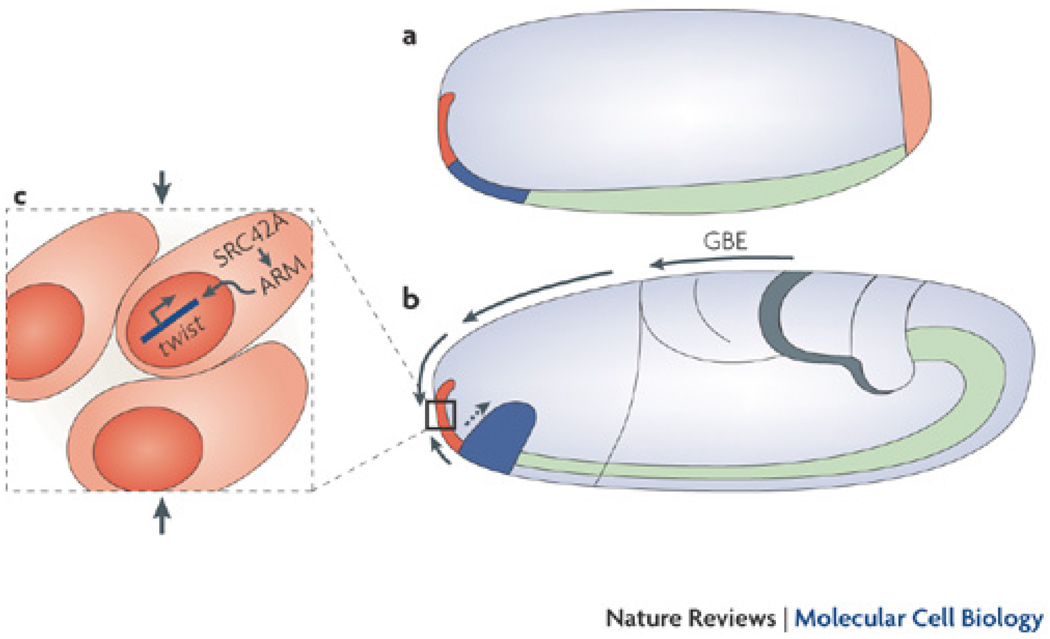
Intriguing evidence from D. melanogaster suggests that mechanotransduction may regulate differentiation in vivo. During gastrulation, germband extension (GBE) causes an endogenous compression of stomodeal cells, which correlates with an increase in Twist expression 60, 61. Twist is one of the genes that controls formation of the digestive tract 62 and regulates apical constriction during mesoderm/midgut invagination 63. Uniaxial stretching of D. melanogaster embryos causes an upregulation of Twist expression, suggesting that Twist is sensitive to mechanical perturbations during GBE (Fig. 3) 61.
Figure 3. Mechanical regulation of Twist gene expression.
The left panel shows a D. melanogaster embryo at the beginning of gastrulation, while the right panel shows the embryo during germband extension. During gastrulation, the tissue-lengthening movements that occur during germband extension push the tissue posteriorly, causing tissue and buckling (see black arrow). At the same time, the endoderm (shown in blue) invaginates (see blue arrow). This causes compression of the adjacernt stomodeal cells (shown in red). Inset: It is hypothesized that this compression (black arrows) leads to the Src42A-dependent nuclear translocation of the Armadillo transcription factor, which increases Twist expression 60, 61. This model places Twist expression in the appropriate location to regulate midgut differentiation. Modified with permission from 61.
To test the role of mechanical forces on Twist expression, laser ablation of the D. melanogaster dorsal epithelium was used to prevent deformation of the future digestive track anterior pole cells of the embryo, which normally occurs during gastrulation. Laser ablation inhibited Twist expression in these cells and subsequent tissue invagination 61. Interestingly, recent work demonstrated that normal levels of stomodeal Twist expression in laser ablated embryos can be rescued by mimicking GBE-triggered endogenous deformation, by using either a micromanipulated needle or magnetic tweezers to compress the adjacent ferrofluid-injected tissue 60. Furthermore, Suppato et al. has used femtosecond laser pulse-induced ablation and third-harmonic generation microscopy (to visualize both velocity fields and cell movements during D. melanogaster GBE) to verify that active tissue movements in the ventral side of the embryo correlate with Twist mechanosensitive gene expression 25. Mechanistically, this compression led to force-dependent nuclear translocation of Armadillo (the fly homologue of β-catenin) to increase Twist expression in a Src42A-dependent manner 60, 61 (Fig. 3). Together these data suggest that compressive strain, or the decreased dimensions, caused by GBE-induced tissue deformations propagates through the dorsal tissue to control Twist expression during D. melanogaster early gastrulation 60, 61.
Twist regulation of D. melanogaster mesoderm invagination implies that Twist activity may feed back to regulate contractility during apical constriction. How might mechanically activated Twist expression, in turn, regulate cellular contraction? Twist is proposed to be a master regulator of cell shape changes during mesoderm invagination. Twist activates transcription of folded gastrulation (fog), an apically secreted protein that regulates cell shape changes during gastrulation 64. These cells receive this signal at their apical face, where this causes activation of a RhoA exchange factor RhoGEF2 51 through two cooperative mechanisms. RhoGEF2 is released from microtubules and is localized at the apical side of the cells 65; at the same time Twist-dependent upregulation of the transmembrane protein T48 is targeted to the apical membrane, where it binds to RhoGEF2 (through its PDZ-domain) 66. This apical localization of RhoGEF2 results enhanced Rho activity and activation of Rho’s effector, ROK. Asymmetrical ROK activity leads to polarized actin and myosin accumulation; thus the polarized actomyosin contracts at the apical side, leading to constriction 51. Because actin is tethered to adherens junctions, this contraction is hypothesized to cause the apical localization of these cell-cell contacts in D. melanogaster 51. It is important that contraction is properly regulated during apical constriction; this could be accomplished if the movements created by constriction activate Twist, leading to a positive feedback loop 67.
Contractility regulates differentiation
Further support that contractile forces are necessary in development is given by MSC lineage commitment and differentiation studies. A murine genetic knockout of p190-B RhoGAP, a negative regulator of RhoA activity, has defects in adipogenesis 68. p190-B RhoGAP-knockout fibroblasts show defective adipogenesis and enhanced myogenesis, suggesting that enhanced RhoA activity inhibits differentiation into the adipogeneic lineage 68. Our lab extended these studies to test the role of RhoA-mediated contractility in in vitro lineage commitment and differentiation in human MSCs. We found that RhoA and ROCK-generated contraction are required for MSC commitment into the osteoblast cell fate, and that this pathway inhibits MSC adipogenesis 48. Furthermore, it was previously observed that adipogenesis is inhibited in spread cells 69. We have also found that cell shape acts as a master regulator of this lineage switch 48. Our studies implicated RhoA and ROCK-generated contractility as the mediator of shape-regulated lineage commitment, whereby well spread cells increased contractility and osteogenesis and unspread cells suppressed contractility to promote adipogenesis 48. In addition, the MSC lineage differentiation effect of substrate stiffness (discussed above) is also dependent on contractility, as inhibition of non-muscle myosin II blocked differentiation into any of the lineages studied: neuronal, myogenic, and osteogenic 15. Together, these in vitro and in vivo studies support a central role for contractile forces in differentiation during development.
It is also clear that contractility regulates in vitro differentiation in adult tissues. More than twenty years ago, the Pitelka and Bissell groups reported that mammary epithelial cells form differentiated structures only when cultured on floating collagen gels, and not more rigid substrates, such as 2D glass, petri dishes, or even on collagen gels that remain attached to the dish 70, 71. This finding was significant because mammary cell culture on rigid 2D substrates is very different from the in vivo environment cells normally encounter 13. In addition, for proper mammary epithelial differentiation to occur, contraction of the floating collagen gel is required 72. This was a seminal observation, as it was later shown that many cell types contract their surrounding matrix during in vitro differentiation and morphogenesis 73–76. Furthermore, inhibition of myosin-mediated contractility blocks matrix contraction and differentiation, confirming that contractile force is required for differentiation in many different cell types and contexts in vitro 13, 15, 76, 77.
Thus, although the GBE-induced Twist regulation in D. melanogaster illustrates the intricate interactions between mechanical forces, gene expression, and differentiation in a developmental context, in vitro studies indicate that many other factors must be present to orchestrate the many complex movements demanded in early embryogenesis. The presence of such mechanotransduction mechanisms in adult stem cells and differentiated cells points to a clear gap in our understanding of their relevance to developmental biology.
Spatial organization of cells
The spatial organization of cells is regulated by morphogenetic movements and is critical for tissue structure and function. There are several examples of how mechanotransduction and contractile forces may regulate embryonic cellular movements that result in proper cell and tissue organization.
Cell-generated force in border cell migration
Mechanical forces are thought to be important in border cell migration, the process by which clusters of D. melanogaster follicle cells migrate down the center of the developing egg chamber towards the nurse cell/oocyte border during oogenesis 78 (Fig 4A). F-actin cytoskeletal dynamics regulate a critical checkpoint for this migration, as mutants of actin regulatory proteins disrupt border cell migration 79. F-actin dynamics regulate the activity of the transcription factor SRF and its cofactor MAL-D 80. SRF-mediated transcription regulates the expression of many proteins, including actin-regulatory proteins 81. Indeed, border cells expressing a MAL-D mutant show altered cytoskeletal rearrangement and F-actin dynamics leading to cell fragmentation, which prevents productive border cell migration 79, 82.
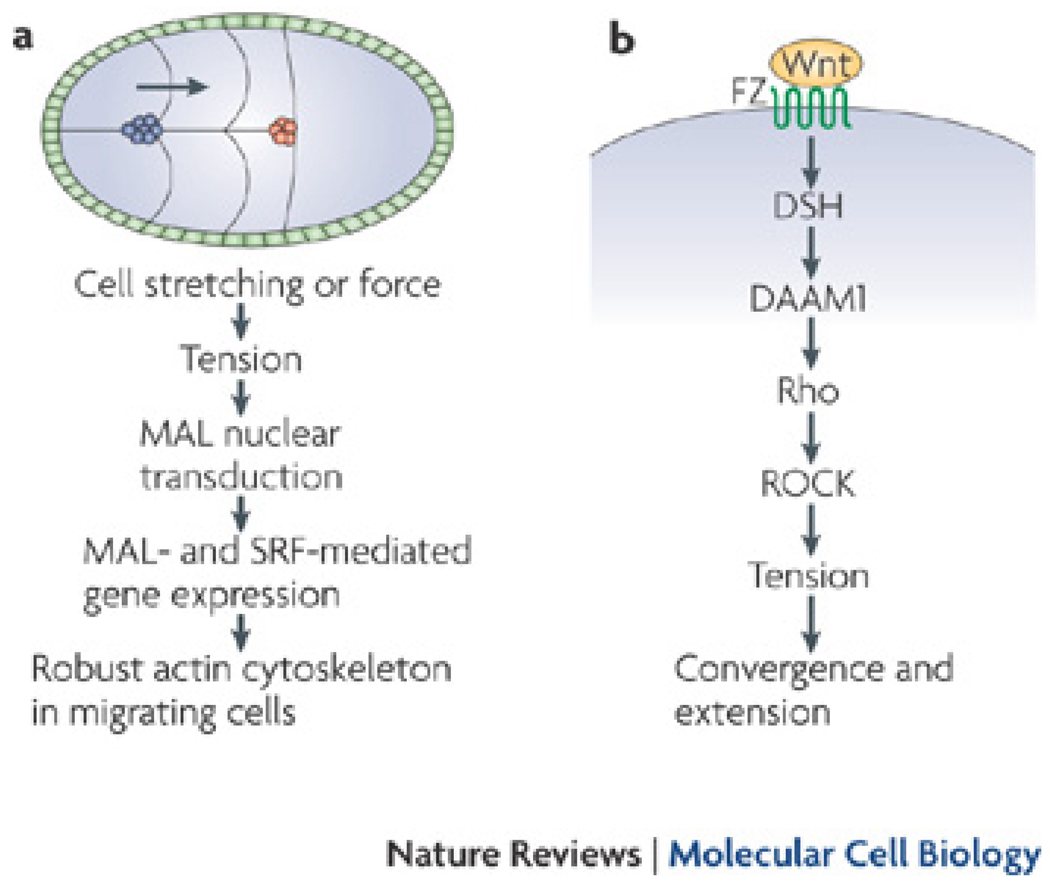
Figure 4. Forces regulate the spatial organization of cells.
A., During D. melanogaster oogenesis, border cell migration, follicle cells migrate down the midline of the egg chamber (blue cells are migrating cells, red cells denotes their final position). As cells are stretched or subjected to external force during migration, the tension generated causes the nuclear translocation of the SRF cofactor, MAL. Nuclear MAL and SRF can then regulate the expression of many genes, including genes required for cytoskeletal integrity. This model is proposed to allow cells to assemble and maintain a robust actin cytoskeleton during migration 82.B., The non-canonical Wnt pathway, also called the PCP (planar cell polarity) pathway, regulates many morphogenetic movements leading to cell and tissue spatial rearrangements during convergence and extension 89–91. When Wnt binds to the Frizzled (Fz) receptor, this activated Dishevelled (Dsh), which then activates Daam1, leading to RhoA activation and ROCK-generated contractility and cellular tension.
Interestingly, mechanical tension is proposed to regulate SRF-dependent gene expression in D. melanogaster. Analysis of MAL-D nuclear localization (which is an indicator of activity) demonstrated that MAL-D nuclear localization is most apparent in cells that appear stretched 82. In order to determine if stretching could directly regulate MAL-D nuclear translocation, slbo mutants were analysed for MAL-D localization. These mutants are unable to migrate; however, because during border cell migration, cells move as clusters (and not as individual cells), they can be “pulled” by other wild-type cells, likely through adhesion complexes 83. Mutant cells only showed MAL-D nuclear translocation when pulled by other wild-type cells 82. Therefore, the authors proposed that tension-induced MAL-D nuclear accumulation allows MAL-D and SRF to maintain gene expression required for a robust cytoskeleton, which is necessary for efficient migration and cellular differentiation 82 (Fig 4A). Interestingly, stretch-induced regulation of SRF also increases differentiation markers in vitro in vascular smooth muscle cells 84, suggesting that local stretch, generated by other cells, may be a common mechanism that has evolved to direct differentiation and morphogenesis in a number of tissues.
Wnt regulates contractility in embryogenesis
Wnts are secreted proteins that are essential regulators of development. They bind the Frizzled (Fz) family and members of the low-density-lipoprotein receptor-related protein (Lrp) family to activate several intracellular signalling cascades that regulate diverse cell behaviours 85. Wnt signalling is required for the establishment and maintenance of cell polarity during several processes during Caenorhabditis elegans gastrulation 86–88. Besides regulating polarity, Wnt signalling also modulates actin cytoskeletal organization and contractile forces 87. At the beginning of gastrulation, different progenitor cell types must separate. Individual cell types then undergo apical constriction that allows them to internalize, which results in the separation of the nascent germ layers. Wnt signalling leads to phosphorylation of myosin light chain in the apical cortex, generating localized contraction 87.
Wnt signalling also regulates convergence and extension movements in X. laevis and zebrafish 89–91 (Fig. 4B). During X. laevis elongation, Wnt/Fz signalling activates the cytoplasmic scaffolding protein Dishevelled and the formin Daam1 90, which leads to activation of the small GTPase Rho 90,92. Activation of Rho regulates cytoskeletal changes that contribute to planar cell polarity signalling 90. This planar cell polarity pathway (PCP) is also known as the non-canonical Wnt pathway and involves Wnt signalling to the GTPase Rho (Fig. 4B). In contrast to the canonical Wnt pathway, the PCP pathway is transcription-independent and contains different molecular players 93. Whether Wnt-Rho signalling leads to changes in contraction remains unclear. However, the PCP pathway signals to Rho and ROCK 93; ROCK is a potent regulator of contractility, therefore it is possible that the PCP pathway might also regulates cellular contraction.
Wnt regulation of Rho and actomyosin contraction has major implications for mechanotransduction during embryogenesis. There are some suggestions from in vitro studies that Wnt signalling may play a role in mechanotransduction. First, in osteoblasts, fluid shear activates Wnt pathways downstream of the early mechanosensitive genes integrin β1 (Igtb1) and cyclooxegenase-2 (Cox-2), which mediate signalling downstream of shear-regulated osteoblast proliferation 94. Second, the Wnt co-receptor Lrp5 is required for strain-induced mechanotransduction in osteoblasts 95. Strain also decreases the expression of the Wnt-Lrp5 inhibitor, Sost/sclerostin, suggesting that there are mechanisms to regulate Wnt activity downstream of mechanical cues. It is tempting to speculate that the various mechanical movements in embryogenesis could locally activate or modulate Wnt signalling, in a similar fashion to mechanical induction of Twist expression 60, 61. Mechanical regulation of Wnt signalling could be a very efficient way to create mechanical “checkpoints” that control Wnt signal activation and duration during embryogenesis, ensuring that certain structural criteria are met before the next stages of development are triggered.
Diverse roles for contractility
Contractile force is required for numerous processes during the movements that regulate the spatial organization of cells in embryogenesis. Additionally, myosin has recently been implicated in several other processes, demonstrating that cell-generated force has surprisingly diverse essential roles in embryogenesis. For example, myosin has been implicated in cell sorting at beginning of gastrulation. One model for how progenitor cell types separate is known as the differential adhesion hypothesis, which states that cell sorting is a consequence of different adhesive properties inherent to the cells 96. Krieg has recently revisited this phenomenon in zebrafish and found that differences in actomyosin-dependent cell-cortex tension, and not only differences in adhesion, are necessary and sufficient for progenitor sorting 97. Such studies demonstrate that, in addition to differential adhesion, local regulation of cytoskeletal tension provides another means to alter the interfacial energies that drive cell sorting.
Another unexpected role for myosin was demonstrated during D. melanogaster GBE, as cells change position to physically extend and lengthen the tissues during gastrulation. Neither cell shape changes nor regulated cell proliferation appear to regulate GBE 98. Rather, cell rearrangement is regulated by controlled global adherens junction remodelling arising from myosin II-mediated junction disassembly and assembly 99. The presence of myosin II at adherens junctions and its requirement for cell movements in GBE suggests that the local contractile forces between cells (and not external forces) drive D. melanogaster intercalation 99. While other mechanisms may also be in place to regulate cell rearrangements in other tissues and organisms 31, 100, together these data suggest that local cell-generated contractile forces are key regulators of cell function during embryogenesis.
Conclusions and future perspectives
While mechanotransduction classically refers to the response of cells to applied forces, we have come to appreciate the importance of forces that cells exert through regulated actomyosin contractility. These contractile forces allow cells to sense and respond to a variety of different mechanical and structural contexts, and appear to be required for many steps in embryogenesis. Because characterizing forces in vivo is a complicated and daunting task, it is important for the field to be able to draw from in vitro mechanotransduction studies to help explain complex developmental behaviours in vivo. Furthermore, understanding how cells sense and respond to mechanical cues is important not only for our understanding of embryogenesis, but also for diseases such as cancer, where the mechanical properties of the microenvironment are hypothesized to regulate tumorigenesis 13 (also see the Lammerding article in this issue).
In order to continue this type of comparative analysis, three major areas warrant further study in vitro. First, much of our understanding of cellular forces is based on measurements of those forces when cells are in isolation. Given that embryonic cells are almost always in contact with other cells, it is important that forces are also examined in multicellular contexts, and that the forces between cells are characterized, as well as the forces exerted on matrices. Second, the role of key developmental patterning genes (such as the Wnts, BMPs, and transcriptional regulators) in mechanotransduction should be further defined given their essential role in development. Finally, because we do not understand the constitutive behavior of embryonic tissues, the field is in dire need of sophisticated in vitro 3D experimental and conceptual models that will allow the detailed analysis of single cells embedded within tissues. These models should recapitulate specific aspects of embryogenesis, including physiological ranges of stiffness, in order to study how both forces and genetics cooperate to regulate cell rearrangements and tissue movements.
In parallel, to better understand how biomechanics controls development, we must have a more complete appreciation of the forces that are generated during embryogenesis. These forces, whether cell-generated or external to the tissue, should be capable of both mechanically deforming the embryo and causing changes in signal transduction and downstream cellular processes required for development. Once these forces are characterized, forces (of physiological magnitude and duration) can then be reapplied to the embryo to determine the physical and biochemical responses 60. In cases for which contractile forces are distributed throughout the embryo 21, 22, this analysis will be critical to understand how cells locally and globally respond to force. A detailed characterization of these forces, combined with quantitative modelling, will be necessary for the field to determine how mechanotransduction cooperates with other known pathways to regulate development. Therefore, to have a more complete understanding of development, our future challenge is to develop advanced in vitro and in vivo models in order to link the biochemical and biomechanical events of embryogenesis.
Acknowledgements
We apologize to authors whose papers could not be cited due to space limitations. We thank Ravi Desai, Jennifer Leight, and Dr. Lina Kwong for helpful comments. We acknowledge support from the National Institutes of Health (NIBIB, NHLBI, and NIGMS) and the Army Research Office Multidisciplinary University Research Initiative. M.A.W. acknowledges financial support from the Ruth L. Kirschstein National Research Service Award.
Glossary
- morphogen
diffusible signaling molecule usually presented in a concentration gradient; in development, morphogens regulate tissue patterning
- stiffness
the degree to which the surrounding adhesive scaffold resists deformation, also defined as the elastic modulus (E) of a material
- contractility
ability of a cell to shorten or shrink in response to a stimulus; generated by the motor myosin II using ATP hydrolysis to “walk”along actin filaments
- convergence
embryonic movements that narrow the tissue mediolaterally
- mesoderm
one of the three germ layers produced by gastrulation that gives rise to bone muscle, and connective tissue
- notochord
flexible rod of mesodermal cells that defines the axis of the embryo to provide support
- involuting marginal zone
the vegetal portion of the marginal zone (a region between the animal and vegetal hemispheres) of the X. laevis embryo that turns inside the embryo during involution
- mesenchymal stem cell (MSC)
multipotent stem cell that retains the ability to differentiate into multiple cell types
- dorsal closure
the process at the end of D. melanogaster embryogenesis whereby the two sides of epidermal tissue grow to close and cover the dorsal opening; during this time the underlying amnioserosa is also stretched to separate the yolk sac from the vitelline envelope
- proliferation
the process by which one mother cell undergoes mitosis to produce two identical daughter cells
- egg chamber (D. melanogaster)
consists of a germline cyst covered by a somatic epithelium; during morphogenesis the cyst grows in a proliferation-independent manner while the epithelium grows by proliferation
- blastocyst
A structure in early embryogenesis that contains the inner cell mass, which gives rise to the embryo
- poly(2-hydroxyethyl methacrylate) (polyHEMA)
a hydrophilic polymer that prevents cell attachment and spreading
- microcontact printing
a method in which an elastomeric stamp with relief features is used to transfer “inked” molecules (usually self-assembled monolayers or ECM proteins) onto the surface of a substrate through conformal contact
- differentiation
the process by which a cell becomes more specialized in order to perform particular functions; often defined by changes in gene expression,cell structure, and phenotype
- germband extension (GBE)
the process by which the D. melanogaster embryo lengthens and narrows during gastrulation
- apical constriction
apically localized actomyosin-driven bending of the tissue inwards to promote invagination
- forming
a protein that nucleates actin filaments to promote elongation
- intercalation
the process by which cells rearrange and exchange neighbors to result in one plane of cells; this thins and expands the tissue during epiboly and convergence/extension
References
- 1.Thompson DAW. On Growth and Form. Cambridge, U.K: Cambridge Univ. Press; 1917. [Google Scholar]
- 2.His W. Unsere Korperform und das Physiologische Problem Ihrer Entstehung. Germany: F.C.W. Vogel, Leipzig; 1874. [Google Scholar]
- 3.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 4.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Keller R, Jansa S. Xenopus Gastrulation without a blastocoel roof. Dev Dyn. 1992;195:162–176. doi: 10.1002/aja.1001950303. [DOI] [PubMed] [Google Scholar]
- 6.Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 7.Wiebe C, Brodland GW. Tensile properties of embryonic epithelia measured using a novel instrument. J Biomech. 2005;38:2087–2094. doi: 10.1016/j.jbiomech.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 8.von Dassow M, Davidson LA. Variation and robustness of the mechanics of gastrulation: the role of tissue mechanical properties during morphogenesis. Birth Defects Res C Embryo Today. 2007;81:253–269. doi: 10.1002/bdrc.20108. [DOI] [PubMed] [Google Scholar]
- 9.Levental I, Georges P, Janmey P. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 10.Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- 11.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engler AJ, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Hove JR, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 17.Keller R, Danilchik M. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development. 1988;103:193–209. doi: 10.1242/dev.103.1.193. [DOI] [PubMed] [Google Scholar]
- 18.Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol. 1986;117:156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- 19.Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124:2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 20.Hardin J. The role of secondary mesenchyme cells during sea urchin gastrulation studied by laser ablation. Development. 1988;103:317–324. doi: 10.1242/dev.103.2.317. [DOI] [PubMed] [Google Scholar]
- 21. Hutson MS, et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. Used sophisticated laser ablation techniques and mathematical modeling to demonstrate that multiple areas of theD. melanogasterembryo (including the amnioserosa and adjacent epithelium) contribute to the force generation needed for dorsal closure
- 22.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peralta XG, et al. Upregulation of forces and morphogenic asymmetries in dorsal closure during Drosophila development. Biophys J. 2007;92:2583–2596. doi: 10.1529/biophysj.106.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson L, Keller R. Measuring mechanical properties of embryos and embryonic tissues. Methods Cell Biol. 2007;83:425–439. doi: 10.1016/S0091-679X(07)83018-4. [DOI] [PubMed] [Google Scholar]
- 25.Supatto W, et al. In vivo modulation of morphogenetic movements in Drosophila embryos with femtosecond laser pulses. Proc Natl Acad Sci U S A. 2005;102:1047–1052. doi: 10.1073/pnas.0405316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 27. Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052.Provides the first evidence that apoptosis contributes between half to a third of the forces needed for D. melanogaster dorsal closure
- 28.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 29.Davidson LA. Developmental biology. Apoptosis turbocharges epithelial morphogenesis. Science. 2008;321:1641–1642. doi: 10.1126/science.1164583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–1355. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 31.Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–192. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- 32.Shraiman BI. Mechanical feedback as a possible regulator of tissue growth. Proc Natl Acad Sci U S A. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci U S A. 2007;104:3835–3840. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci U S A. 1981;78:3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nelson CM, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102.First experimental demonstration that the forces transmitted through epithelial monolayers can regulate localized proliferation
- 36.Hara K, Tydeman P, Kirschner M. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc Natl Acad Sci U S A. 1980;77:462–466. doi: 10.1073/pnas.77.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimelman D, Kirschner M, Scherson T. The events of the midblastula transition in Xenopus are regulated by changes in the cell cycle. Cell. 1987;48:399–407. doi: 10.1016/0092-8674(87)90191-7. [DOI] [PubMed] [Google Scholar]
- 38.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto H, et al. A p160ROCK-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth. J Hepatol. 2000;32:762–770. doi: 10.1016/s0168-8278(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 40.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 42.Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 43.Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 44.Kureishi Y, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 45.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z, Rivkees SA. Rho-associated kinases play an essential role in cardiac morphogenesis and cardiomyocyte proliferation. Dev Dyn. 2003;226:24–32. doi: 10.1002/dvdy.10212. [DOI] [PubMed] [Google Scholar]
- 47.Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol. 2006;26:4612–4627. doi: 10.1128/MCB.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 49.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101:e44–e52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 50.Numaguchi K, Eguchi S, Yamakawa T, Motley ED, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- 51.Dawes-Hoang RE, et al. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 52. Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0.Shows, for the first time, that the extent of cell spreading can regulate cell proliferation
- 53.Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 55.Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 57.Pirone DM, et al. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol. 2006;174:277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan JL, et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989:249–281. [PubMed] [Google Scholar]
- 60.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 61. Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1.Shows that mechanical deformation can generate Twist expression in D. melanogaster embryos
- 62.Reuter R, Leptin M. Interacting functions of snail, twist and huckebein during the early development of germ layers in Drosophila. Development. 1994;120:1137–1150. doi: 10.1242/dev.120.5.1137. [DOI] [PubMed] [Google Scholar]
- 63.Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- 64.Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 65.Rogers SL, Wiedemann U, Hacker U, Turck C, Vale RD. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr Biol. 2004;14:1827–1833. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 66.Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- 67.Brouzes E, Supatto W, Farge E. Is mechano-sensitive expression of twist involved In mesoderm formation? Biol Cell. 2004;96:471–477. doi: 10.1016/j.biolcel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 69.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 70.Emerman JT, Bartley JC, Bissell MJ. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981;134:241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- 71.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 72.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274.References 70–73 demonstrate that the presentation of the extracellular matrix regulates cell differentiation in vitro and show a key role for matrix contraction in differentiation
- 74.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore KA, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 76.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grinnell F, Ho CH, Lin YC, Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274:918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- 78.Montell DJ. The social lives of migrating cells in Drosophila. Curr Opin Genet Dev. 2006;16:374–383. doi: 10.1016/j.gde.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 80.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 81.Sun Q, et al. Defining the mammalian CArGome. Genome Res. 2006;16:197–207. doi: 10.1101/gr.4108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Somogyi K, Rorth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell. 2004;7:85–93. doi: 10.1016/j.devcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 83.Rorth P, Szabo K, Texido G. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol Cell. 2000;6:23–30. doi: 10.1016/s1097-2765(05)00008-0. [DOI] [PubMed] [Google Scholar]
- 84.Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol. 2005;83:869–875. doi: 10.1139/y05-061. [DOI] [PubMed] [Google Scholar]
- 85.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 86.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 87. Lee JY, et al. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr Biol. 2006;16:1986–1997. doi: 10.1016/j.cub.2006.08.090.Provides evidence that Wnt signaling directly regulates contractility to control apical constriction during C. elegans gastrulation
- 88.Wallingford JB, et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 89.Heisenberg CP, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 90.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 91.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 92.Liu W, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 94.Lau KH, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- 95.Sawakami K, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 96.Steinberg MS, Garrod DR. Observations on the sorting-out of embryonic cells in monolayer culture. J Cell Sci. 1975;18:385–403. doi: 10.1242/jcs.18.3.385. [DOI] [PubMed] [Google Scholar]
- 97. Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705.Demonstrates a key role for actomyosin-dependent cell-cortex tension in the regulation of cell sorting in zebrafish embryos
- 98.Irvine KD, Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994;120:827–841. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- 99. Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590.Suggests a model by which local forces at cell-cell boundaries cause junctional remodeling during intercalation of D. melanogaster embryos
- 100.Hardin J, Walston T. Models of morphogenesis: the mechanisms and mechanics of cell rearrangement. Curr Opin Genet Dev. 2004;14:399–406. doi: 10.1016/j.gde.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 101.Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys J. 1998;74:2227–2234. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–1620. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 103.Moore SW. A fiber optic system for measuring dynamic mechanical properties of embryonic tissues. IEEE Trans Biomed Eng. 1994;41:45–50. doi: 10.1109/10.277270. [DOI] [PubMed] [Google Scholar]
- 104.Daniels BR, Masi BC, Wirtz D. Probing single-cell micromechanics in vivo: the microrheology of C elegans developing embryos. Biophys J. 2006;90:4712–4719. doi: 10.1529/biophysj.105.080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 106.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 107.Munevar S, Wang Y, Dembo M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J. 2001;80:1744–1757. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5.Uses lazer tweezers to apply increased force to cells in order to show that cells strengthen their integrin-cytoskeletal linkages in response to increased matrix rigidity
- 109.Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- 110.Sniadecki NJ, et al. Magnetic microposts as an approach to apply forces to living cells. Proc Natl Acad Sci U S A. 2007;104:14553–14558. doi: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]