Introduction
Mnemonic processes are controlled by selective modification (weakening or strengthening) of connections between neurons (1–5). To understand the precise molecular mechanisms by which this remarkably complex network encodes a given episode during learning is arguably one of the major challenges in modern neuroscience (6). Two kinds of memory storage mechanisms have been described: short-term memory (STM),3 which lasts only a few minutes or hours, and long-term memory (LTM), which persists for many weeks, months, years, and even a lifetime (7). Consolidation of LTM depends on de novo synthesis. Indeed, the first molecular distinction between STM and LTM emerged from studies with protein synthesis inhibitors >40 years ago: animals that were treated with drugs that block protein synthesis could not form LTM, yet their STM was preserved. More than a century ago, Dr. Santiago Ramón y Cajal, the great Spanish neuroanatomist, proposed that forming memories requires neurons to strengthen their connections with one another. Now, it is widely accepted that information is stored in the brain as changes in the strength of synaptic connections. Like LTM, long-lasting (but not short-lasting) changes in the strength of synaptic connections depend on new protein synthesis. Such changes can be observed when neuronal activity is recorded in brain slices with microelectrodes in vitro.
Synaptic plasticity refers to the ability of the synapse to strengthen or weaken in response to experience. The best studied forms of synaptic plasticity are long-term potentiation (LTP) and long-term depression (LTD), which refer to facilitation and depression of synaptic strength, respectively (8). LTP can be divided into two distinct temporal phases: early LTP (E-LTP), which depends on modification of pre-existing proteins, is usually induced by one tetanic train, and lasts 1–2 h, and late LTP (L-LTP), which requires new protein synthesis, is induced by repetitive tetanic trains, and lasts for several hours (9). There is emerging evidence that local protein synthesis at dendrites could play a key role in long-lasting forms of synaptic plasticity (10). Recent genetic and molecular studies have cast new light on the molecular mechanisms underlying protein synthesis-dependent synaptic plasticity and memory storage. We discuss here some of the molecular mechanisms by which translational control regulates changes in synaptic strength and memory storage.
Translational Control in Eukaryotes
Translational control is defined as a change in the rate of translation of the mRNA. Translation is a complex process that is divided into three steps: initiation, elongation, and termination. Initiation is the step at which the ribosome is recruited to the mRNA and is thought to be the major rate-limiting step in the translation process under most circumstances and a frequent target for regulation (11, 12). Translation of mRNA into protein begins after assembly of Met-tRNAi, mRNA, and the 40 S and 60 S ribosomal subunits into an 80 S ribosome in which the Met-tRNAi is positioned in the ribosomal P site occupied by the initiation codon. The initiation process consists of several key events: (i) formation of the 43 S ribosomal initiation complex, (ii) binding of the mRNA to the 43 S ribosomal complex to form the 48 S ribosomal initiation complex, (iii) movement of the 48 S ribosomal complex along the 5′-UTR) and base pairing of the initiation AUG codon with the anticodon of Met-tRNAi, and (iv) 80 S complex formation (12).
As an early step in translation initiation, the eukaryotic initiation factor eIF2 (which is composed of three subunits, α, β, and γ) binds Met-tRNAi and GTP to form a ternary complex (eIF2·Met-tRNAi·GTP) (Fig. 1). The 40 S ribosomal subunit, which is associated with other eIFs, binds to the ternary complex to form a 43 S ribosomal complex.
FIGURE 1.
Regulation of synaptic plasticity, learning, and memory via eIF2α phosphorylation. The ternary complex comprising the three eIF2 subunits (α, β, and γ), GTP, and Met-tRNAi associates with the 40 S ribosomal subunit. “Recycling” of the eIF2 ternary complex is mediated by eIF2B, which catalyzes the conversion of GDP to GTP on eIF2. This activity is regulated by eIF2α phosphorylation on Ser51; phosphorylated eIF2α inhibits eIF2B-mediated exchange of GTP for GDP, thus decreasing general translation initiation. A, under standard conditions, there is sufficient supply of the ternary complex. This leads to optimal global translation. Under these conditions, the rate of ATF4 mRNA translation is low, and subsequently, CREB-dependent transcription is increased. This leads to optimal transcription of synaptic plasticity-associated genes, and the threshold for L-LTP and LTM is low. B, under conditions in which eIF2α is phosphorylated, the amount of ternary complex is reduced. ATF4 mRNA is translated at a high rate, and thus, the threshold for L-LTP and LTM is high.
Another key eIF involved in the recruitment of the ribosome to the mRNA is the eIF4F complex. It consists of three subunits: (i) eIF4E, the cap-binding protein; (ii) eIF4A, an ATP-dependent helicase that unwinds the secondary structure in the 5′-UTR; and (iii) eIF4G, a modular scaffolding protein that bridges the mRNA to the ribosome through interactions with eIF3 (which is bound to the 40 S ribosomal subunit) (2, 13). Once bound to the 5′-end of the mRNA, the 43 S ribosomal complex scans the 5′-UTR until the initiation AUG codon is encountered to form a 48 S initiation complex. This is followed by joining of the 60 S subunit after the release of eIFs, which is dependent on eIF5, a GTPase-activating protein that hydrolyzes the GTP bound to eIF2 (12). After initiation is completed, elongation factors are recruited to carry out the elongation of the polypeptide chain (14). Upon recognition of a stop codon, termination factors promote the release of the polypeptide chain from the mRNA and ribosome.
Two major mechanisms control translation initiation: (a) phosphorylation of the translation initiation factor eIF2α, which inhibits the formation of the ternary complex, and (b) eIF4F formation, as controlled by the mammalian target of rapamycin (mTOR), which promotes the recruitment of the 43 S ribosome to the 5′-end of the mRNA. Translation initiation can also be regulated at the mRNA 3′-end by controlling the poly(A) tail through the RNA-binding protein CPEB (cytoplasmic polyadenylation element-binding protein).
eIF2α Phosphorylation: A Master Switch of Learning and Memory
Phosphorylation of eIF2α on Ser51 blocks the GDP/GTP exchange reaction, which is catalyzed by eIF2B and is required to reconstitute a functional ternary complex for a new round of translation, thus causing a decrease in general translation initiation (Fig. 1B) (15, 16). In higher eukaryotes, the phosphorylation of eIF2α on Ser51 is a highly dynamic, regulated process that is controlled by four kinases and two phosphatase complexes. The four kinases are PKR (protein kinase activated by double-stranded RNA), HRI (hemin-regulated inhibitor kinase), PERK (PKR-like endoplasmic reticulum kinase), and GCN2 (general control non-derepressible-2 kinase) (Fig. 1B). The different kinases are activated by distinct forms of cellular stress (17, 18).
Two phosphatase complexes dephosphorylate eIF2α (reviewed in Ref. 19). One complex consists of the eIF2α-specific regulatory subunit CReP (constitutive repressor of eIF2α phosphorylation) and PPIc (protein phosphatase I catalytic subunit). The other complex consists of the related regulatory subunit GADD34 (growth arrest and DNA damage-inducible gene 34) and PPIc. The regulatory subunits are thought to provide the specificity for the phosphatase complex for eIF2α.
Although eIF2α phosphorylation suppresses general translation, it paradoxically stimulates the translation of a subset of mRNAs that contain short upstream open reading frames in their 5′-UTR (16, 19). The molecular mechanism underlying this unique translational control was elucidated in great detail for the transcriptional activator GCN4 mRNA in yeast (reviewed in Ref. 16 and 20). In mammalian cells, translation of the GCN4 metazoan counterpart, the transcriptional modulator ATF4 (activating transcription factor 4; also termed CREB2), is enhanced in response to eIF2α phosphorylation (21, 22). Interestingly, ATF4 and its homologs play an important role as repressors of CREB (cAMP response element-binding protein)-mediated gene expression in the brain, which is known to be required for long-lasting changes in synaptic plasticity and LTM (23–25).
Using mouse genetics and pharmacology, Costa-Mattioli et al. (17) demonstrated that eIF2α phosphorylation plays a crucial role in the conversion of STM to LTM. In the hippocampus of eIF2α+/S51A mice, which are heterozygous for the S51A mutation, the phosphorylation of eIF2α is reduced by ∼50%, and the formation of LTM is enhanced as determined by a variety of behavioral tasks (17). For instance, eIF2α+/S51A mice show enhanced spatial LTM when tested in the Morris water maze, where animals use visual cues to find the location of a hidden platform in a circular pool. The enhanced LTM correlates with facilitated LTP in eIF2α+/S51A mice and also in mice lacking GCN2, the major eIF2α kinase in the brain (17, 26). Thus, decreased eIF2α phosphorylation strengthens long-lasting synaptic transmission, which underlies LTM consolidation. Conversely, hippocampal infusion with a small molecule inhibitor (Sal003), which prevents eIF2α dephosphorylation, blocks both L-LTP and LTM formation (17). Jiang et al. (27) recently ruled out the possibility that the effects of eIF2α phosphorylation on LTM and LTP occur during development. Using a new pharmacogenetic mouse model in which eIF2α phosphorylation is selectively increased in CA1 hippocampal neurons (from adult animals) in a time-dependent and inducible manner, they demonstrated that both L-LTP and LTM were impaired (27).
How does eIF2α phosphorylation control L-LTP and LTM? Recent evidence supports the idea that eIF2α phosphorylation regulates L-LTP and LTM storage through translational control of specific mRNAs, such as ATF4 mRNA. For instance, although general translation is not altered in CA1 neurons, ATF4 protein is increased in the CA1 region from mice in which eIF2α is phosphorylated (27). Consistent with these data, Sal003, which increases eIF2α phosphorylation, failed to suppress L-LTP in slices from ATF4 knock-out mice compared with WT mice (17). Thus, eIF2α phosphorylation causes up-regulation of ATF4 mRNA translation and consequently blocks the expression of long-lasting synaptic plasticity genes and hence memory formation.
mTOR and eIF4F Complex in Synaptic Plasticity and Memory
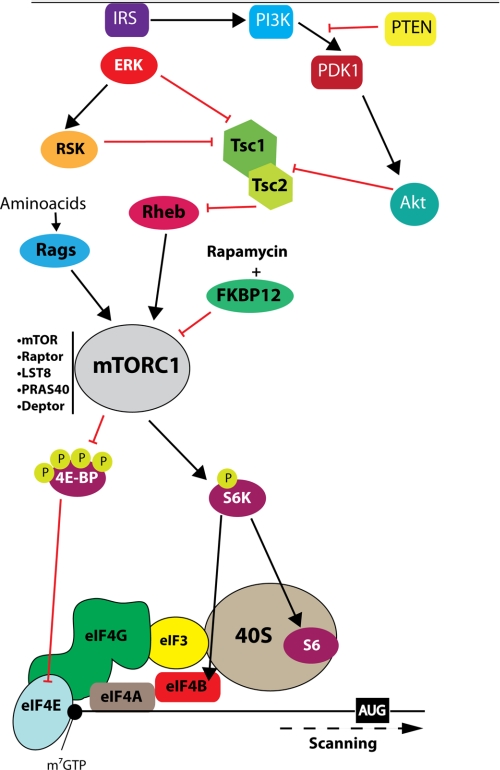
mTOR, a PI3K-like kinase that phosphorylates a variety of proteins, integrates a large number of extracellular stimuli and intracellular cues to effect anabolic outputs in all cells. In synapses, mTOR is thought to be important for the activation of translation in response to neuronal activity (reviewed in Ref. 2; see Ref. 4). Rapamycin, which is a specific inhibitor of mTORC1 (mTOR complex 1; see below), inhibits translation of a subset of mRNAs, possibly at synapses (28, 29). mTORC1 contains Raptor (regulatory-associated protein of mTOR), LST8 (also known as GβL (G-protein β-subunit-like protein)), and PRAS40 (proline-rich Akt substrate of 40 kDa). mTORC1 is sensitive to the drug rapamycin and regulates translation rates (30).
The best studied function of mTORC1 is regulation of translation (2, 31). mTORC1 controls translation by regulating the formation of the eIF4F complex through 4E-BPs (eIF4E-binding proteins), which are small molecular weight repressor proteins that compete with eIF4G for binding to eIF4E and cause inhibition of cap-dependent translation (32). mTORC1 phosphorylates the 4E-BPs, which causes their dissociation from eIF4E, thus stimulating the assembly of the eIF4F complex and subsequently translation (Fig. 2). In contrast, the inhibition of mTORC1 leads to dephosphorylation of 4E-BPs and inhibition of eIF4F complex formation. An additional mechanism by which mTORC1 is thought to regulate translation is through phosphorylation of S6Ks (S6 protein kinase; at Thr389), which stimulates the activity of eIF4B, a translation factor that cooperates with eIF4F to facilitate ribosome recruitment to the mRNA (33).
FIGURE 2.
mTOR signaling pathway. mTOR is a critical downstream target of the PI3K signaling pathway. The insulin receptor substrate proteins (IRS) “transmit” signals from the insulin and insulin-like growth factor-1 receptors through PI3K and PDK1 (PI3K-dependent kinase 1), which phosphorylates Akt. mTOR forms two distinct protein complexes. mTORC1, which regulates translation, is sensitive to rapamycin and is defined by the scaffolding protein Raptor. mTORC1 phosphorylates 4E-BPs, S6K1, S6K2, and PRAS40. Akt can activate mTOR through phosphorylation and inhibition of TSC2 of the tuberous sclerosis complex. Phosphorylation of TSC2 leads to Rheb (Ras homolog enriched in brain) activation, which in turn activates mTOR. Cap-dependent translation through formation of the eIF4F complex can be regulated by mTORC1 inputs through 4E-BPs and S6Ks.
Most of the evidence for mTORC1 signaling in long-lasting synaptic plasticity and memory formation is based on the finding that rapamycin suppresses changes in synaptic strength in brain slices in vitro (34–37) and partially blocks LTM in vivo (38, 39). However, it is noteworthy that rapamycin does not block L-LTP in vivo in the dentate gyrus (40), and exceptionally high doses of rapamycin are required to block contextual fear memories in mice (41). In addition, mutant mice lacking downstream targets of mTORC1 exhibit altered synaptic plasticity and memory. Surprisingly, S6K1−/− mice display impaired protein synthesis-independent E-LTP but normal protein synthesis-dependent L-LTP (42, 43), demonstrating that S6K does not control the translation of mRNAs that underlie L-LTP. In contrast, in Aplysia neurons, S6K, but not 4E-BP, appears to be the major effector of mTORC1 for long-term facilitation (44). In mammals, S6K appears to be involved mainly in the regulation of long-lasting decreases in synaptic strength because metabotropic glutamate receptor-induced LTD is enhanced in slices from S6K2−/− mice (but not in those from S6K1−/− mice) (42). Lack of S6K1 leads to a variety of complex behavioral phenotypes, which include hypoactive behavior, impaired short-term fear memory, deficient conditioned taste aversion and spatial memory and decreased contextual fear memory 7 days (but not 1 day) after training, and reduced latent inhibition of conditioned taste aversion and normal spatial memory as determined by the Morris water maze (43).
Genetic deletion of 4E-BP2, the other major mTORC1 downstream target in the brain, also leads to alterations in synaptic plasticity and behavioral learning. 4E-BP2−/− mice show enhanced LTP when induced with a weak tetanic train, whereas L-LTP induced by four tetanic trains is impaired. Lack of 4E-BP2 not only alters LTP but also changes LTD because metabotropic glutamate receptor LTD is facilitated in these mice (45–47). A number of behavioral abnormalities in spatial memory, associative memory, fear conditioning, and even working memory were observed in mice deficient in 4E-BP2 (46, 47). Although these studies demonstrate that 4E-BP2 is important for synaptic plasticity and memory, it is not clear whether 4E-BPs are the major mTORC1 downstream effectors in the brain.
Interestingly, 4E-BP2 is not phosphorylated in the adult mammalian brain, likely due to deamidation, a spontaneous post-translational modification that occurs 4 weeks postnatally only in the brain (48). Thus, how eIF4F complex formation is regulated in the brain in response to neuronal activity remains to be determined.
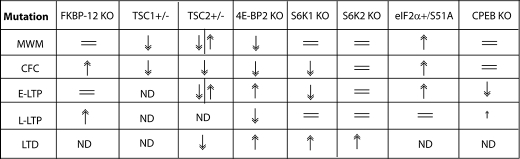
Genetic deletions of the tuberous sclerosis complex (TSC) proteins, which enhance mTORC1 activity, such as TSC2+/− and TSC1+/−, as well as FKBP12−/− (immunosuppressant drug FK506-binding protein 12), generate disparate phenotypes with regard to plasticity and memory (Table 1). In TSC2+/− mice, an E-LTP-inducing protocol generates a robust L-LTP, but long-term contextual fear memory is impaired. In contrast, in FKBP12−/− mice, which exhibit enhanced mTORC1 activity, similar to TSC2+/− mice, E-LTP is normal, and long-term contextual fear memory is enhanced (41, 49, 50). Thus, it remains unclear whether the effect of mTORC1 upstream regulators on plasticity and memory is through mTORC1 or another downstream target.
TABLE 1.
Long-lasting plasticity and memory phenotypes from mice with mutations in proteins that regulate translation
Normal ( ), enhanced (↑), or impaired (↓) long-lasting synaptic plasticity and/or memory is compared with that of WT control mice. ND, no data; MWM, Morris water maze; CFC, contextual fear conditioning; KO, knock-out.
), enhanced (↑), or impaired (↓) long-lasting synaptic plasticity and/or memory is compared with that of WT control mice. ND, no data; MWM, Morris water maze; CFC, contextual fear conditioning; KO, knock-out.
CPEB in Synaptic Plasticity and Memory
CPEB plays an important role in synaptic plasticity and memory formation. There are four related CPEBs in mammals (51). CPEBs are localized mainly postsynaptically, where they are thought to control local translation (52). Synaptic activity triggers polyadenylation and translation of the mRNA for the α-subunit of calcium/calmodulin-dependent protein kinase II, which contains a cytoplasmic polyadenylation element (CPE) in its 3′-UTR (53–55). Mice expressing the calcium/calmodulin-dependent protein kinase II α-subunit mRNA, which lacks the 3′-UTR, show impaired L-LTP and LTM (56). E-LTP, but not L-LTP, is impaired in hippocampal slices from mice lacking CPEB-1. Unexpectedly, CPEB-1−/− mice exhibit normal LTM (57). However, memory extinction, which is thought to be important for the formation of new memories, is impaired in the mutant mice (58).
CPEB-1 is thought to control synaptic plasticity through c-Jun, whose expression is reduced in the hippocampus of CPEB-1−/− mice (59), and transcriptionally regulates growth hormone. Indeed, growth hormone induces a deficient L-LTP in hippocampal slices from CPEB-1−/− mice compared with WT controls.
CPEB is also found in Aplysia sensory neurons, and its decrease by an antisense oligonucleotide led to impaired long-term synaptic strength (60). In vitro and in vivo studies demonstrated that, unlike its mammalian counterparts, Aplysia CPEB exhibits prion-like properties (60, 61). In addition, the neurotransmitter serotonin, which enhances synaptic strength, switches Aplysia CPEB from a monomeric to a multimeric state, and a neutralizing antibody against the multimeric state blocks long-term synaptic strength in Aplysia neurons (61). It would be interesting to investigate whether the multimeric state, but not the monomeric state in response to activity (serotonin), promotes the translation of specific mRNAs locally at synapses.
In summary, eIF2α and CPEB modulate synaptic plasticity and memory through translational control of the transcription factors ATF4 and c-Jun, respectively, suggesting that translational control of transcription may be a common mechanism by which translation controls memory processes.
Future Questions
Since the first studies linking learning and memory to translation (62–64), our understanding of the relationship between translational control and cognitive functions has significantly advanced. The links between memory and translational control are summarized in Table 1. In the next decades, there is a plethora of research questions to be addressed. Some of the questions are as follows. What is the role of microRNAs in memory processes? Given that 4E-BP2 cannot be phosphorylated in the adult brain, how is eIF4F complex-mediated translational control accomplished? Perhaps, other unidentified eIF4E-binding proteins are involved. Is mTORC1 involved in plasticity and memory? Genetic deletions that enhance mTOR activity, such as TSC1+/−, FKBP12 knock-out, or 4E-BP2 knock-out mice, generate disparate phenotypes with regard to plasticity and memory (Table 1). Thus, there is a need to obtain direct genetic evidence supporting the role for mTORC1 in these processes.
Acknowledgments
We thank Arkady Khoutorsky and Rachel Jeffrey for critical reading of the manuscript.
This is the eighth article in the Thematic Minireview Series on Protein Synthesis. This minireview will be reprinted in the 2010 Minireview Compendium, which will be available in January, 2011.
- STM
- short-term memory
- LTM
- long-term memory
- LTP
- long-term potentiation
- LTD
- long-term depression
- E-LTP
- early LTP
- L-LTP
- late LTP
- mTOR
- mammalian target of rapamycin
- TSC
- tuberous sclerosis complex.
REFERENCES
- 1.Collingridge G. L., Isaac J. T., Wang Y. T. (2004) Nat. Rev. Neurosci. 5, 952–962 [DOI] [PubMed] [Google Scholar]
- 2.Costa-Mattioli M., Sossin W. S., Klann E., Sonenberg N. (2009) Neuron 61, 10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudai Y. (2004) Annu. Rev. Psychol. 55, 51–86 [DOI] [PubMed] [Google Scholar]
- 4.Richter J. D., Klann E. (2009) Genes Dev. 23, 1–11 [DOI] [PubMed] [Google Scholar]
- 5.Zukin R. S., Richter J. D., Bagni C. (2009) Front. Neural Circuits 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva A. J., Zhou Y., Rogerson T., Shobe J., Balaji J. (2009) Science 326, 391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGaugh J. L. (2000) Science 287, 248–251 [DOI] [PubMed] [Google Scholar]
- 8.Bear M. F., Malenka R. C. (1994) Curr. Opin. Neurobiol. 4, 389–399 [DOI] [PubMed] [Google Scholar]
- 9.Kandel E. R. (2001) Science 294, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 10.Sutton M. A., Schuman E. M. (2006) Cell 127, 49–58 [DOI] [PubMed] [Google Scholar]
- 11.Mathews M. B., Sonenberg N., Hershey J. W. B. (2007) in Translational Control in Biology and Medicine (Mathews M. B., Sonenberg N., Hershey J. W. B., eds) pp. 1–40, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 12.Pestova T. V., Lorsch J. R., Hellen C. U. T. (2007) in Translational Control in Biology and Medicine (Mathews M. B., Sonenberg N., Hershey J. W. B., eds) pp. 87–128, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 13.Gingras A. C., Raught B., Sonenberg N. (1999) Annu. Rev. Biochem. 68, 913–963 [DOI] [PubMed] [Google Scholar]
- 14.Herbert T. P., Proud C. G. (2007) in Translational Control in Biology and Medicine (Mathews M. B., Sonenberg N., Hershey J. W. B., eds) pp. 601–624, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 15.Dever T. E. (2002) Cell 108, 545–556 [DOI] [PubMed] [Google Scholar]
- 16.Hinnebusch A. G., Dever T. E., Asano K. (2007) in Translational Control in Biology and Medicine (Mathews M. B., Sonenberg N., Hershey J. W. B., eds) pp. 225–268, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 17.Costa-Mattioli M., Gobert D., Stern E., Gamache K., Colina R., Cuello C., Sossin W., Kaufman R., Pelletier J., Rosenblum K., Krnjević K., Lacaille J. C., Nader K., Sonenberg N. (2007) Cell 129, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dever T. E., Dar A. C., Sicheri F. (2007) in Translational Control in Biology and Medicine (Mathews M. B., Sonenberg N., Hershey J. W. B., eds) pp. 319–345, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 19.Ron D., Walter P. (2007) Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 20.Hinnebusch A. G. (2005) Annu. Rev. Microbiol. 59, 407–450 [DOI] [PubMed] [Google Scholar]
- 21.Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. (2000) Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 22.Vattem K. M., Wek R. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abel T., Martin K. C., Bartsch D., Kandel E. R. (1998) Science 279, 338–341 [DOI] [PubMed] [Google Scholar]
- 24.Bartsch D., Ghirardi M., Skehel P. A., Karl K. A., Herder S. P., Chen M., Bailey C. H., Kandel E. R. (1995) Cell 83, 979–992 [DOI] [PubMed] [Google Scholar]
- 25.Chen A., Muzzio I. A., Malleret G., Bartsch D., Verbitsky M., Pavlidis P., Yonan A. L., Vronskaya S., Grody M. B., Cepeda I., Gilliam T. C., Kandel E. R. (2003) Neuron 39, 655–669 [DOI] [PubMed] [Google Scholar]
- 26.Costa-Mattioli M., Gobert D., Harding H., Herdy B., Azzi M., Bruno M., Bidinosti M., Ben Mamou C., Marcinkiewicz E., Yoshida M., Imataka H., Cuello A. C., Seidah N., Sossin W., Lacaille J. C., Ron D., Nader K., Sonenberg N. (2005) Nature 436, 1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Z., Belforte J. E., Lu Y., Yabe Y., Pickel J., Smith C. B., Je H. S., Lu B., Nakazawa K. (2010) J. Neurosci. 30, 2582–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casadio A., Martin K. C., Giustetto M., Zhu H., Chen M., Bartsch D., Bailey C. H., Kandel E. R. (1999) Cell 99, 221–237 [DOI] [PubMed] [Google Scholar]
- 29.Martin K. C., Casadio A., Zhu H., Yaping E., Rose J. C., Chen M., Bailey C. H., Kandel E. R. (1997) Cell 91, 927–938 [DOI] [PubMed] [Google Scholar]
- 30.Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 31.Jacinto E., Hall M. N. (2003) Nat. Rev. Mol. Cell Biol. 4, 117–126 [DOI] [PubMed] [Google Scholar]
- 32.Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr., Sonenberg N. (1994) Nature 371, 762–767 [DOI] [PubMed] [Google Scholar]
- 33.Shahbazian D., Roux P. P., Mieulet V., Cohen M. S., Raught B., Taunton J., Hershey J. W., Blenis J., Pende M., Sonenberg N. (2006) EMBO J. 25, 2781–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C. H., Yeh S. H., Lin C. H., Lu K. T., Leu T. H., Chang W. C., Gean P. W. (2001) Neuron 31, 841–851 [DOI] [PubMed] [Google Scholar]
- 35.Man H. Y., Wang Q., Lu W. Y., Ju W., Ahmadian G., Liu L., D'Souza S., Wong T. P., Taghibiglou C., Lu J., Becker L. E., Pei L., Liu F., Wymann M. P., MacDonald J. F., Wang Y. T. (2003) Neuron 38, 611–624 [DOI] [PubMed] [Google Scholar]
- 36.Opazo P., Watabe A. M., Grant S. G., O'Dell T. J. (2003) J. Neurosci. 23, 3679–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang S. J., Reis G., Kang H., Gingras A. C., Sonenberg N., Schuman E. M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dash P. K., Orsi S. A., Moore A. N. (2006) J. Neurosci. 26, 8048–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tischmeyer W., Schicknick H., Kraus M., Seidenbecher C. I., Staak S., Scheich H., Gundelfinger E. D. (2003) Eur. J. Neurosci. 18, 942–950 [DOI] [PubMed] [Google Scholar]
- 40.Panja D., Dagyte G., Bidinosti M., Wibrand K., Kristiansen A. M., Sonenberg N., Bramham C. R. (2009) J. Biol. Chem. 284, 31498–31511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehninger D., Han S., Shilyansky C., Zhou Y., Li W., Kwiatkowski D. J., Ramesh V., Silva A. J. (2008) Nat. Med. 14, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antion M. D., Hou L., Wong H., Hoeffer C. A., Klann E. (2008) Mol. Cell. Biol. 28, 2996–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antion M. D., Merhav M., Hoeffer C. A., Reis G., Kozma S. C., Thomas G., Schuman E. M., Rosenblum K., Klann E. (2008) Learn. Mem. 15, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weatherill D. B., Dyer J., Sossin W. S. (2010) J. Biol. Chem. 285, 12255–12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banko J. L., Hou L., Poulin F., Sonenberg N., Klann E. (2006) J. Neurosci. 26, 2167–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banko J. L., Merhav M., Stern E., Sonenberg N., Rosenblum K., Klann E. (2007) Neurobiol. Learn. Mem. 87, 248–256 [DOI] [PubMed] [Google Scholar]
- 47.Banko J. L., Poulin F., Hou L., DeMaria C. T., Sonenberg N., Klann E. (2005) J. Neurosci. 25, 9581–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bidinosti M., Ran I., Sanchez-Carbente M. R., Martineau Y., Gingras A. C., Gkogkas C., Raught B., Bramham C. R., Sossin W. S., Costa-Mattioli M., DesGroseillers L., Lacaille J. C., Sonenberg N. (2010) Mol. Cell 37, 797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goorden S. M., van Woerden G. M., van der Weerd L., Cheadle J. P., Elgersma Y. (2007) Ann. Neurol. 62, 648–655 [DOI] [PubMed] [Google Scholar]
- 50.Hoeffer C. A., Tang W., Wong H., Santillan A., Patterson R. J., Martinez L. A., Tejada-Simon M. V., Paylor R., Hamilton S. L., Klann E. (2008) Neuron 60, 832–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter J. D. (2007) Trends Biochem. Sci. 32, 279–285 [DOI] [PubMed] [Google Scholar]
- 52.Huang Y. S., Richter J. D. (2004) Curr. Opin. Cell Biol. 16, 308–313 [DOI] [PubMed] [Google Scholar]
- 53.Huang Y. S., Jung M. Y., Sarkissian M., Richter J. D. (2002) EMBO J. 21, 2139–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells D. G., Dong X., Quinlan E. M., Huang Y. S., Bear M. F., Richter J. D., Fallon J. R. (2001) J. Neurosci. 21, 9541–9548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L., Wells D., Tay J., Mendis D., Abbott M. A., Barnitt A., Quinlan E., Heynen A., Fallon J. R., Richter J. D. (1998) Neuron 21, 1129–1139 [DOI] [PubMed] [Google Scholar]
- 56.Miller S., Yasuda M., Coats J. K., Jones Y., Martone M. E., Mayford M. (2002) Neuron 36, 507–519 [DOI] [PubMed] [Google Scholar]
- 57.Alarcon J. M., Hodgman R., Theis M., Huang Y. S., Kandel E. R., Richter J. D. (2004) Learn. Mem. 11, 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berger-Sweeney J., Zearfoss N. R., Richter J. D. (2006) Learn. Mem. 13, 4–7 [DOI] [PubMed] [Google Scholar]
- 59.Zearfoss N. R., Alarcon J. M., Trifilieff P., Kandel E., Richter J. D. (2008) J. Neurosci. 28, 8502–8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Si K., Giustetto M., Etkin A., Hsu R., Janisiewicz A. M., Miniaci M. C., Kim J. H., Zhu H., Kandel E. R. (2003) Cell 115, 893–904 [DOI] [PubMed] [Google Scholar]
- 61.Si K., Choi Y. B., White-Grindley E., Majumdar A., Kandel E. R. (2010) Cell 140, 421–435 [DOI] [PubMed] [Google Scholar]
- 62.Agranoff B. W., Klinger P. D. (1964) Science 146, 952–953 [DOI] [PubMed] [Google Scholar]
- 63.Davis H. P., Squire L. R. (1984) Psychol. Bull. 96, 518–559 [PubMed] [Google Scholar]
- 64.Flexner J. B., Flexner L. B., Stellar E. (1963) Science 141, 57–59 [DOI] [PubMed] [Google Scholar]