Abstract
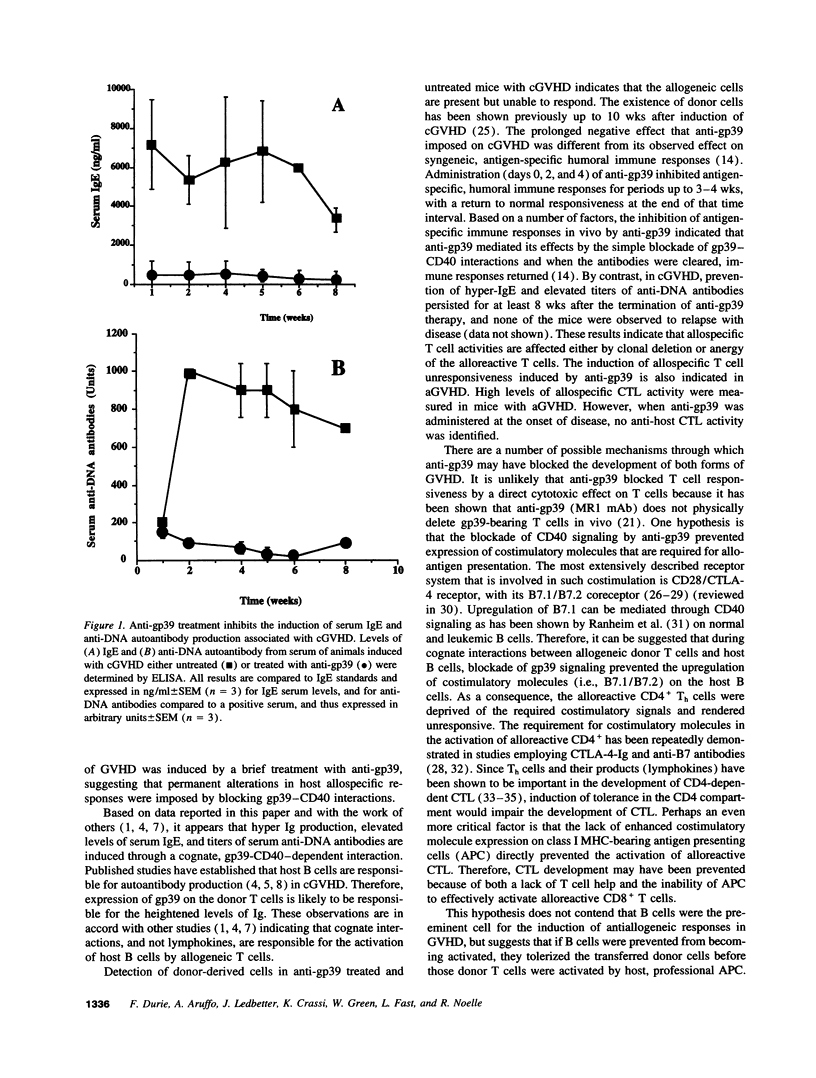
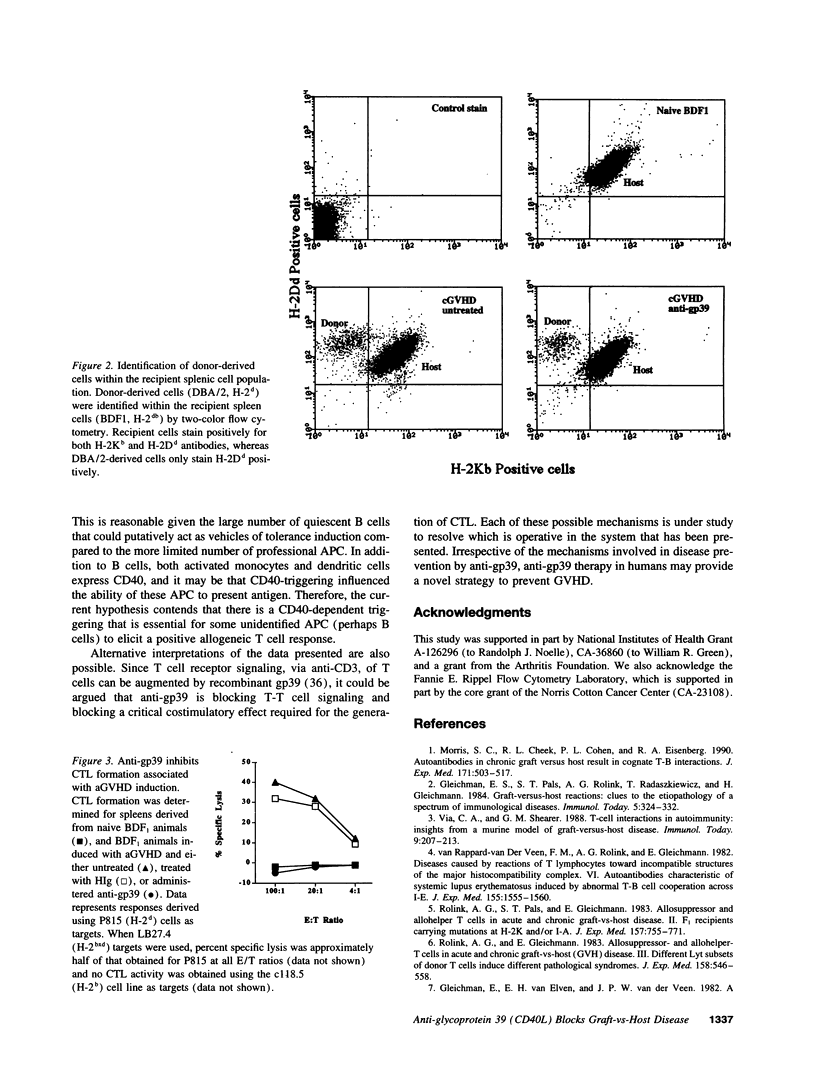
Chronic and acute graft-versus-host disease (cGVHD and aGVHD) result from donor cells responding to host disparate MHC alleles. In cGVHD (H-2d-->H-2bd), heightened polyclonal immunoglobulin production is due to the interaction of donor allospecific helper T cells (Th) and the host B cells. In vivo administration of antibody to the ligand for CD40, gp39, blocked cGVHD-induced serum anti-DNA autoantibodies, IgE production, spontaneous immunoglobulin production in vitro, and associated splenomegaly. Antibody production remained inhibited for extended periods of time after termination of anti-gp39 administration. Antiallogeneic CTL responses induced in a GVHD were also prevented by the in vivo administration of anti-gp39 as was the associated splenomegaly. These data suggest that CD40-gp39 interactions are critical in GVHD and that CD40-gp39 may be a valuable ligand-receptor pair for targeting immunotherapeutic agents to control GVHD.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Armitage R. J., Conley M. E., Rosenblatt H., Jenkins N. A., Copeland N. G., Bedell M. A., Edelhoff S., Disteche C. M., Simoneaux D. K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Tough T. W., Macduff B. M., Fanslow W. C., Spriggs M. K., Ramsdell F., Alderson M. R. CD40 ligand is a T cell growth factor. Eur J Immunol. 1993 Sep;23(9):2326–2331. doi: 10.1002/eji.1830230941. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L. S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993 Jan 29;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Bonnefoy J. Y., Gauchat J. F., Fischer A., de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- Durie F. H., Fava R. A., Foy T. M., Aruffo A., Ledbetter J. A., Noelle R. J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993 Sep 3;261(5126):1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- Fast L. D. Identification of a single non-H-2 gene regulating graft-versus-host disease response. J Immunol. 1990 Jun 1;144(11):4177–4182. [PubMed] [Google Scholar]
- Foy T. M., Shepherd D. M., Durie F. H., Aruffo A., Ledbetter J. A., Noelle R. J. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993 Nov 1;178(5):1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann E., Van Elven E. H., Van der Veen J. P. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur J Immunol. 1982 Feb;12(2):152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D., Grosmaire L. S., Kullas C. D., Chalupny N. J., Braesch-Andersen S., Noelle R. J., Stamenkovic I., Ledbetter J. A., Aruffo A. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992 Dec;11(12):4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. A., Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982 Mar 1;155(3):768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P., Traunecker A., Hubele S., Inui S., Lanzavecchia A., Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992 Oct;22(10):2573–2578. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- Lee R. S., Grusby M. J., Glimcher L. H., Winn H. J., Auchincloss H., Jr Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994 Mar 1;179(3):865–872. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Morris S. C., Cheek R. L., Cohen P. L., Eisenberg R. A. Allotype-specific immunoregulation of autoantibody production by host B cells in chronic graft-versus host disease. J Immunol. 1990 Feb 1;144(3):916–922. [PubMed] [Google Scholar]
- Morris S. C., Cheek R. L., Cohen P. L., Eisenberg R. A. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J Exp Med. 1990 Feb 1;171(2):503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Ledbetter J. A., Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992 Nov;13(11):431–433. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reap E. A., Sobel E. S., Cohen P. L., Eisenberg R. A. Conventional B cells, not B-1 cells, are responsible for producing autoantibodies in lpr mice. J Exp Med. 1993 Jan 1;177(1):69–78. doi: 10.1084/jem.177.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reap E. A., Sobel E. S., Jennette J. C., Cohen P. L., Eisenberg R. A. Conventional B cells, not B1 cells, are the source of autoantibodies in chronic graft-versus-host disease. J Immunol. 1993 Dec 15;151(12):7316–7323. [PubMed] [Google Scholar]
- Rolink A. G., Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J Exp Med. 1983 Aug 1;158(2):546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A. G., Pals S. T., Gleichmann E. Allosuppressor and allohelper T cells in acute and chronic graft-vs.-host disease. II. F1 recipients carrying mutations at H-2K and/or I-A. J Exp Med. 1983 Feb 1;157(2):755–771. doi: 10.1084/jem.157.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M., Waldschmidt T., Aruffo A., Ledbetter J. A., Noelle R. J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993 Sep 1;151(5):2497–2510. [PubMed] [Google Scholar]
- Rozendaal L., Pals S. T., Gleichmann E., Melief C. J. Persistence of allospecific helper T cells is required for maintaining autoantibody formation in lupus-like graft-versus-host disease. Clin Exp Immunol. 1990 Dec;82(3):527–532. doi: 10.1111/j.1365-2249.1990.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P., Anasetti C., Hansen J. A., Melrose J., Brunvand M., Bradshaw J., Ledbetter J. A., Linsley P. S. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993 Jan 1;177(1):165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Noelle R. J., Roy M., Shepherd D. M., Aruffo A., Ledbetter J. A., Boersma W. J., Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993 Nov 1;178(5):1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via C. S., Shearer G. M. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol Today. 1988 Jul-Aug;9(7-8):207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- van Rappard-van der Veen F. M., Rolink A. G., Gleichmann E. Diseases caused by reactions of T lymphocytes towards incompatible structures of the major histocompatibility complex. VI. Autoantibodies characteristic of systemic lupus erythematosus induced by abnormal T-B cell cooperation across I-E. J Exp Med. 1982 May 1;155(5):1555–1560. doi: 10.1084/jem.155.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Haas W. Distinct Ir genes for helper and killer cells in the cytotoxic response to H-Y antigen. J Exp Med. 1979 Nov 1;150(5):1134–1142. doi: 10.1084/jem.150.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]



