Abstract
TGFβ induces fibrosis in healing corneal wounds, and in vitro corneal keratocytes up-regulate expression of several fibrosis-related genes in response to TGFβ. Hyaluronan (HA) accumulates in healing corneas, and HA synthesis is induced by TGFβ by up-regulation of HA synthase 2. This study tested the hypothesis that HA acts as an extracellular messenger, enhancing specific fibrotic responses of keratocytes to TGFβ. HA synthesis inhibitor 4-methylumbelliferone (4MU) blocked TGFβ induction of HA synthesis in a concentration-dependent manner. 4MU also inhibited TGFβ-induced up-regulation of α-smooth muscle actin, collagen type III, and extra domain A-fibronectin. Chemical analogs of 4MU also inhibited fibrogenic responses in proportion to their inhibition of HA synthesis. 4MU, however, showed no effect on TGFβ induction of luciferase by the 3TP-Lux reporter plasmid. Inhibition of HA using siRNA to HA synthase 2 reduced TGFβ up-regulation of smooth muscle actin, fibronectin, and cell division. Similarly, brief treatment of keratocytes with hyaluronidase reduced TGFβ responses. These results suggest that newly synthesized cell-associated HA acts as an extracellular enhancer of wound healing and fibrosis in keratocytes by augmenting a limited subset of the cellular responses to TGFβ.
Keywords: Carbohydrate Function, Extracellular Matrix Proteins, Fibronectin, Hyaluronate, p38, Signal Transduction, siRNA, Transforming Growth Factor β (TGFβ), Cornea, Keratocyte
Introduction
Keratocytes of the corneal stroma are responsible for the synthesis of the stromal extracellular matrix and thus play an essential role in maintenance of corneal transparency. Under normal in vivo conditions, keratocytes are characteristically quiescent, exhibiting a dendritic morphology with extensive intercellular contacts. In response to stromal injury, such as trauma, keratectomy, or photoablation by excimer laser, keratocytes near the injured site become activated to fibroblasts, altering their morphology, becoming migratory and mitotic, reducing expression of the characteristic keratan sulfate-containing proteoglycans, and eventually transforming into α-smooth muscle actin (αSMA)4-expressing myofibroblasts (1). Along with this transition, atypical components of the extracellular matrix are observed, including hyaluronan, biglycan, the extra domain A (EDA) splice form of fibronectin, collagen types I and III, and SPARC (secreted protein acidic and rich in cysteine) (1–5). These fibrotic matrix components contribute to the loss of transparency associated with corneal scars.
TGFβ has been identified as one of the most important factors involved in corneal fibrosis (6). A major function of TGFβ is to regulate the expression of genes, the products of which contribute to the formation and degradation of extracellular matrix. In monolayer cultures, primary bovine and rabbit keratocytes maintain a dendritic morphology and express corneal keratan sulfate-containing proteoglycans when cultured in serum-free medium. In the presence of TGFβ, keratocytes differentiate into myofibroblasts (2, 3, 7). These cells become mitotic, exhibit a spread morphology, develop focal adhesions, express α5β1 integrin, and develop prominent actin stress fibers containing αSMA. In addition, myofibroblasts exhibit reduced synthesis of keratan sulfate and abundant secretion of extracellular matrix-containing components identified in corneal scars. These include collagen types I and III, biglycan, EDA-FN, and HA (2, 3).
HA is a high molecular weight linear polymer of disaccharides containing glucuronic acid and N-acetylglucosamine present in most animal tissues. HA is not present in normal transparent cornea but appears in the stroma in response to a variety of types of corneal injury or pathology (8). Our previous studies found HA not to be secreted by quiescent keratocytes in vitro but rapidly induced in response to TGFβ, reaching a maximum rate of synthesis within 12 h after TGFβ exposure (9). Changes in secretion of dermatan sulfate, keratan sulfate, EDA-FN, and collagen occur in these cells only after several days of exposure to TGFβ (2, 3).
HA in mammalian cells is the product of hyaluronan synthase (HAS), of which there are three isoforms (HAS1, HAS2, and HAS3) (10). Each isoform is the product of a separate gene. Our previous study found keratocytes to secrete HA in response to TGFβ as a result of the up-regulation of HAS2 mRNA. HAS2 mRNA increased within 2 h of TGFβ exposure and reached a maximum by 5–6 h. On the other hand, alterations in mRNA for collagen III, EDA-FN, and biglycan required several days of stimulation by TGFβ (2, 3, 9).
Extracellular HA is known to exert a variety of biological effects via direct interaction with cell surface receptors CD44 and RHAMM. These effects include metastatic potential of tumor cells, cell motility, secretion of chemokines, mediation of the inflammatory responses, and cell division (11–13). The level and rapidity of the up-regulation of HAS2 mRNA in keratocytes suggest the possibility that rapid secretion of HA could play an active role in mediating the later responses in the sequence of myofibroblastic activation. Initial responses to corneal wounding involve migration of keratocytes toward the site of the injury followed by mitosis (14, 15). HA has a well documented role in stimulation of mitotic and migratory activity in a variety of cells (16, 17). Thus, the HA secreted by keratocytes in response to mitogens and cytokines could play a functional role in initiating responses of these cells to inflammation and/or wound healing in vivo. In this study we have tested the hypothesis that the phenotypic features exhibited by the corneal myofibroblasts in response to TGFβ, particularly the secretion of fibrotic matrix components, are dependent on HA secretion. We report that inhibition of HA secretion with competitive chemical inhibitors of HAS or blocking HAS up-regulation using RNA inhibition was effective in reducing a number of fibrotic responses to TGFβ in keratocytes.
MATERIALS AND METHODS
Cell Culture
Primary keratocytes were obtained from fresh bovine corneas (Pel-Freez Biologicals) by collagenase digestion as described previously (18). The cells were diluted in a serum-free 1:1 mixture of DMEM and Ham's F-12 medium (DME/F12; Sigma-Aldrich) containing antibiotics (penicillin, 100 units/ml; streptomycin 100 μg/ml; gentamicin, 50 μg/ml; and amphotericin B, 2.5 μg/ml) and cultured on tissue culture-treated plastic at 4 × 104 cells/cm2 (not induced) or 2 × 104 cells/cm2 (induced) in a humidified atmosphere containing 5% CO2. The media were changed after 24 h of culture to DME/F12 1% (v/v) platelet-poor horse serum with or without 2 ng/ml recombinant human TGFβ1 (Sigma-Aldrich). Human corneal cells were expanded from collagenase digests of eye bank corneas in Jiang medium as described previously (19). Passage 2 cells were plated in Jiang medium at 2 × 104 cells/cm2 and transferred to serum-free DME/F12 medium after 18 h. After a further 24 h, the cells were treated with TGFβ in platelet-poor horse serum as described above, for 48 h in the presence or absence of 400 μm 4MU.
Quantitative Reverse Transcriptase PCR
The cells were collected by centrifugation after scraping into cold saline, and RNA was isolated using RNeasy Mini kit (Qiagen). RNA was transcribed, and the relative abundance of mRNA was compared with 18S ribosomal RNA using an ABI7700 thermocycler as described previously (3). Primers for human mRNA (not described previously) were as follows: biglycan: forward, TGGCTCCCAAGGGTGCAGGT, and reverse, TGCCTCTGGGTGGCTGTGGT; CSPG2: forward, GCTGCCCCGAGCCTTTCTGG, and reverse, GAAGGATGCGGAGGGTGCGG; Col5A1: forward, AACCCCTACATCCGCGCCCT, and reverse, CATGAAGCAAGCCGGCCCCA; Col8A1: forward, ACGTGCTCAAGGGAGCTCACAC, and reverse, TCCTGTGACACCTCTGGGGCTG; LamC2: forward, AGCAGAGGGGCCACCTCCAT, and reverse, ATGGCTCCCGAGCCCTGAGA; osteoglycin: forward, CCTTCCCACCCATACACATC, and reverse, TGGCACCACTGAGTCATCAT; Col1A1: forward, TCTGGCGCTCCCATGGCTCT, and reverse, GCCCTGCGGCACAAGGGATT; Col3A1, forward, TGGTGCCCCTGGTCCTTGCT, and reverse, TACGGGGCAAAACCGCCAGC; FN1, forward, CTGGCCTGGAACCGGGAACC, and reverse, AGGGTGGGTGACGAAAGGGG.
HA Assay
HA was detected in cell culture medium using FACE analysis as described previously (2). FACE bands were identified by comparison with fragments of purified standard chondroitin sulfate and hyaluronan (Sigma-Aldrich). Alternately, HA was quantified directly in culture media using a sandwich ELISA based on capture of biotinylated aggrecan (Hyaluronan DuoSet; R & D Systems) according to the manufacturer's instructions, using the fluorescent substrate Quanta Blue (Pierce).
Immunoblotting
The cells were lysed in 1× SDS sample buffer, and protein concentration was determined using Micro BCA assay (Pierce). Equal amounts of lysate protein were separated on 4–20% SDS-PAGE gels and then transferred to PVDF membranes. Equal transfer of proteins was examined by staining the membrane with Ponceau S and by immunoblotting for α-tubulin (clone B511; Sigma-Aldrich) or cyclophilin B (PA1-077; Affinity Bioreagents). EDA-FN and αSMA were detected by immunoblotting with monoclonal antibody against EDA-FN (Axyll) or monoclonal antibody against αSΜΑ (clone asm-1; Sigma-Aldrich). p38 MAPK was detected using Cell Signaling Technologies antibody L53F8 and phospho-p38 MAPK with 4631S.
Immunohistology
For immunofluorescent analysis, the cells after 4 days of TGFβ treatment were fixed in room temperature 3% paraformaldehyde in PBS and stained for EDA-fibronectin or αSMA as described previously (2). Photographs were acquired on a Bio-Rad laser scanning confocal microscope using a 60× oil objective.
Cell-based ELISA
To compare inhibition of HA, αSMA, and EDA-FN by 4MU homologs, primary keratocytes were cultured in 96-well plates (12,000 cells/well) and treated for 4 days with TGFβ as described above in the presence of six different concentrations of four different inhibitors: 4MU, esculetin, 4-methylesculetin, and coumarin. After 4 days, media were taken for direct determination of HA by ELISA, and the cells were rinsed in PBS, fixed in 3% paraformaldehyde in PBS for 20 min, and then permeabilized with cold 1% Triton X-100 in TBS. EDA-FN and αSMA were detected in six wells of each condition by ELISA with monoclonal primary antibodies and HRP-labeled secondary antibody, developed with QuantaBlu fluorogenic peroxidase substrate (Pierce), and quantified using a microplate fluorometer. Parallel plates were incubated with Calcein AM vital dye (Invitrogen) to determine live cell number used to normalize ELISA data. Dead cells were determined by staining cultures with propidium iodide followed by micrography and quantitative image analysis. No significant change in live or dead cells was detected in any of the incubation conditions. Inhibition curves were fit using Graph Pad software with nonlinear regression analysis to determine 50% end points and standard error.
Gene Expression
To determine TGFβ signaling, primary keratocytes were plated in 12-well dishes at 2 × 105 cells/well in serum-free DME/F12. After 24 h the cells were transfected with 300 ng/well TGFβ-inducible reporter plasmid (p3TP-Lux; Origene) and 50 ng/well of control reporter plasmid (pRL-SV40; Promega) using Lipofectamine LTX reagent (Invitrogen). After 24 h, the cells were incubated in serum-free DME/F12 with 2 ng/ml of TGFβ1 with or without 500 μm 4MU. The cells were harvested 24 h later and assayed for luciferase expression using a luciferase assay system (Promega) as described by the manufacturer. Total light emission was measured during the initial 10 s of the reaction using a luminometer (Gen 5; BioTek). Firefly luciferase activity in each sample (3TP-Lux) was corrected using Renilla luciferase activity (pRL-SV40) in the same cells.
Knockdown of HAS2 expression with siRNA was carried out by electroporation as described previously (9) or by lipofection using end-modified 21-bp siRNA (StealthTM; Invitrogen). For the latter, 16 pmol of total siRNA was complexed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol and then incubated with 4 × 105 uncultured primary keratocytes from collagenase digests in a volume of 0.4 ml of Advanced DMEM (Invitrogen) without antibiotics. The mixture was incubated 2–4 h in 2 ml of polypropylene tubes at 37 °C with mixing and then plated at 4 × 104 cells/cm2 in serum-free medium on plastic dishes precoated with FNC coating mix (Athena ES). After 24 h the cells were treated with 1% platelet-poor horse serum and 2 ng/ml TGFβ1 in DME-F12 medium for 1–3 days.
Hyaluronidase
Keratocytes in serum-free DME/F12 were stimulated with 10 ng/ml PDGF for 24 h. The cells were rinsed and treated for 15 min with 500 μg/ml testicular hyaluronidase (3,000 USP/NF units/mg of protein; Worthington) at 37 °C. Afterward the cells were rinsed four times in warm DME/F12 and returned to culture in the presence of 10 ng/ml PDGF and 2 ng/ml TGFβ for the times indicated. Equivalent inhibition was observed using hyaluronidase from Streptomyces hyalurolyticus and hyaluronidase SD from Streptococcus dysgalactgiae, enzymes with little chondroitinase activity compared with testicular hyaluronidase (data not shown).
Statistical Analysis
Data were obtained from three to six replicate cultures (as indicated in the figure legends) from a single batch of primary cells. Curve fitting and statistical analyses were carried out using the Prism software (Graph Pad Inc., La Jolla, CA). Comparisons involving two samples used an unpaired two-tailed t test with a 95% confidence interval. Multiple samples were compared using a one-sided analysis of variance with a Tukey post analysis to compare individual pairs of data. The results of each analysis were confirmed using a minimum of three different cell preparations.
RESULTS
4MU Inhibits TGFβ-induced HA Biosynthesis
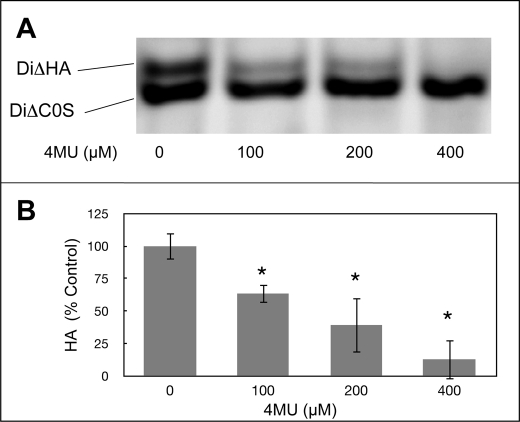
Our previous study (9) showed HA synthesis to be strongly induced in keratocytes by TGFβ. In the current study we observed 4MU, a competitive inhibitor of HA biosynthesis, to block the HA induction. FACE gel analysis after 4 days of TGFβ treatment showed a concentration-dependent decrease of secreted HA in response to 4MU, but no decrease in chondroitin-dermatan sulfate (Fig. 1A). Quantitative analysis of this effect, shown in Fig. 1B, documented a linear decrease with suppression of >90% HA secretion by 4MU concentrations of 400 μm. Vital dye staining showed 4MU to have no effect on the viability of the cells at any of the concentrations used (not shown).
FIGURE 1.
4MU inhibits secretion of hyaluronan by keratocytes. Primary bovine keratocytes were cultured 4 days in medium containing 1% platelet-poor horse serum and 2 ng/ml TGβ1 in the presence of different concentrations of 4MU. A, HA and chondroitin sulfate from the culture medium from equal numbers of cells were detected by FACE as described under “Materials and Methods.” The HA disaccharide (DiΔHA) was decreased by 4MU but not chondroitin sulfate (DiΔC0S). B, quantification of HA bands from gels similar to that shown in A. The bars show S.D. of triplicate samples. The asterisks indicate significant reduction (p < 0.05) compared with a sample with no 4MU using analysis of variance. No HA was detected in the absence of TGFβ (not shown).
Fibrotic Response to TGFβ
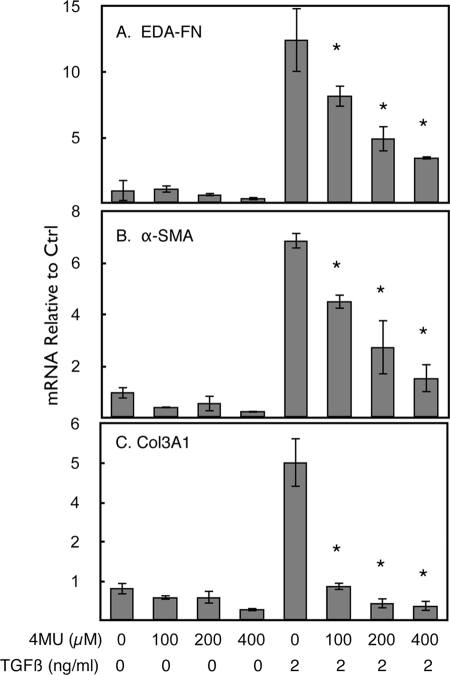
TGFβ is widely recognized as an inducer of fibrotic extracellular matrix both in vivo and in vitro. Our previous studies identified a number of matrix components present in corneal wounds to be induced by TGFβ in cultured keratocytes (3). As shown in Fig. 2, mRNAs for EDA-FN (Fig. 2A), αSMA (Fig. 2B), and collagen type III (Fig. 2C) were induced 7–50-fold after 4 days of TGFβ treatment. In each case, the presence of 4MU reduced or blocked this induction in a concentration-dependent manner. 4MU had little effect on the expression levels of these genes in the absence of TGFβ.
FIGURE 2.
4MU blocks up-regulation of mRNA for fibrotic genes induced by TGFβ. Primary bovine keratocytes were untreated (first four samples) or treated with 2 ng/ml TGFβ in 1% platelet-poor horse serum for 4 days, and mRNA levels were determined by quantitative reverse transcriptase PCR as described under “Materials and Methods.” 4MU was present during treatment at the concentrations noted. A, EDA fibronectin. B, αSMA. C, collagen III α1 chain. The error bars represent S.D. of triplicate analyses. The asterisks show a significant (p < 0.05) decrease in mean values of 4MU+TGFβ-treated samples compared with TGFβ-treated control (Ctrl) as determined by analysis of variance.
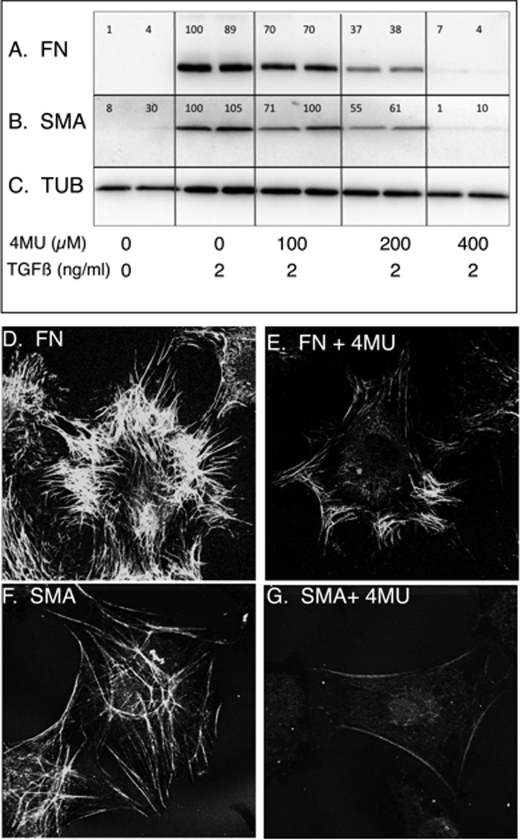
In Fig. 3 (A–C), induction of EDA-FN and αSMA proteins also underwent a significant reduction in response to 4MU, similar to the mRNA pools for these proteins. Immunostaining of cells treated with TGFβ showed accumulation of EDA-FN fibrils in the pericellular matrix (Fig. 3D) to be largely eliminated if the TGFβ treatment was carried out in the presence of 4MU (Fig. 3E). Similarly, αSMA accumulation in stress fibers of TGFβ-treated cells (Fig. 3F) was completely blocked in the presence of 400 μm 4MU (Fig. 3G). Cells incubated in medium without TGFβ did not exhibit EDA-FN or αSMA staining (not shown).
FIGURE 3.
Expression and localization of EDA-fibronectin and αSMA protein in response to 4MU. Proteins induced by 4 days of TGFβ treatment were immunodetected in equal amounts of cell lysate protein after separation by SDS-PAGE and electrotransfer to membranes as described under “Materials and Methods.” Results from duplicate cultures are shown. A, EDA-FN. B, αSMA (SMA) in the same cell lysates. C, α-tubulin (TUB) loading control was detected on the same membrane as FN. The numbers above the bands represent a normalized ratio between the FN or SMA and the tubulin bands. In D and E, immunostaining of EDA-fibronectin was photographed after 4 days of TGFβ treatment in the absence (D) or presence (E) of 400 μm 4MU. F, αSMA immunostained as cytoskeletal microfibrils in TGFβ-treated keratocytes. G, little αSMA was detected in cells treated with TGFβ in the presence of 400 μm 4MU.
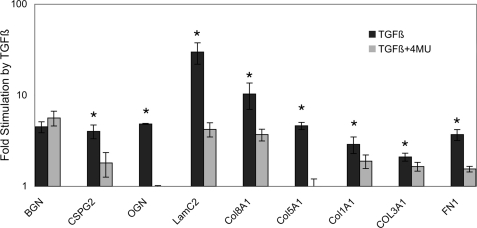
The most clinically relevant response of keratocytes to TGFβ is accumulation of extracellular matrix responsible for disruption of corneal transparency. Analysis of gene array data from human corneal fibroblasts (not shown) identified a number of extracellular matrix genes strongly up-regulated by TGFβ in vitro. Fig. 4 shows analysis of the effect of 4MU on the TGFβ response of nine corneal extracellular matrix components in human corneal fibroblasts. For eight of the nine genes, 4MU significantly reduced expression. Biglycan was the only exception. 4MU, therefore, appears to exert a broad inhibition of fibrotic response in both bovine and human corneal cells.
FIGURE 4.
4MU inhibits TGFβ up-regulation of extracellular matrix proteins in human corneal fibroblasts. Up-regulation of mRNA abundance by 4 days of TGFβ treatment was determined by quantitative reverse transcriptase PCR in low passage human corneal fibroblasts as described under “Materials and Methods.” Expression by untreated cells was set to 1, and the black bars show the relative increase in response to TGFβ treatment (note log scale). The gray bars show the same response in the presence of 400 μm 4MU. The error bars show S.D. of triplicate assays. The differences between the gray and black bars are significant (p < 0.05, by t test) except for biglycan. BGN, biglycan; CSPG2, versican; OGN, osteoglycin; LamC2, laminin gamma 2; Col8A1, collagen type VIII; Col1A1, collagen type I; COL3A1, collagen type III; FN1, fibronectin 1.
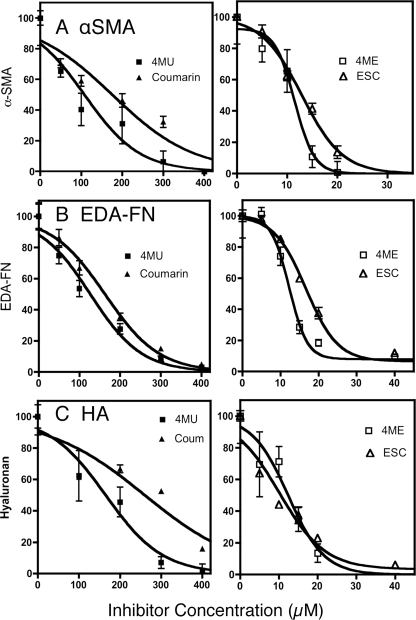
4MU is a derivative of the bicyclic ketone coumarin (7-hydroxy, 4-methyl coumarin). Recently several other coumarin derivatives including 6,7-dihydroxycoumarin (esculetin) and 6,7-dihydroxy 4-methylcoumarin (4-methylesculetin) were shown to be highly effective at blocking HA biosynthesis, whereas coumarin itself showed less inhibition of HA than 4MU (20). In Fig. 5 we examined the ability of these four related chemicals to inhibit αSMA, EDA-FN, and HA expression by TGFβ-stimulated keratocytes. Calculation of the concentrations required for IC50 for each response, summarized in Table 1, showed that esculetin and 4-methylesculetin were each more potent at inhibition of HA than 4MU. Similarly these compounds were significantly more effective at blocking expression of cell-associated EDA-FN and αSMA than 4MU. Coumarin, however, showed reduced inhibition of HA synthesis compared with 4MU and similarly required higher concentrations for inhibition of αSMA and FN. These results show that the potency of these compounds for blocking TGFβ-induced EDA-FN and αSMA accumulation correlates with their ability to block HA synthesis.
FIGURE 5.
Inhibition of TGFβ fibrotic response by coumarin analogs. Expression of cell-associated FN and αSMA in cultures of keratocytes treated with TGFβ for 4 days as in Fig. 3 was detected by cell-based ELISA, and HA was detected by ELISA from culture media. The values were normalized for cell number as determined by Calcein AM staining in parallel plates. Inhibition curves show relative responses compared with noninhibited samples. The error bars are S.D. of six individual sample wells. Coum, coumarin; 4ME, 4-methylesculetin; ESC, esculetin. The lines represent nonlinear curve fitting of data using statistical software as described under “Materials and Methods.”
TABLE 1.
EC50 inhibition concentrations (μm) for 4MU analogs
For each assay, the pairwise differences are significant (p < 0.01) except for 4-methylesculetin and esculetin, which did not differ from each other.
| HA | EDA-FN | αSMA | |
|---|---|---|---|
| 4-Methylesculetin | 12.4 ± 1.9 | 12.3 ± 0.4 | 11.5 ± 0.5 |
| Esculetin | 10.0 ± 2.9 | 16.7 ± 0.5 | 12.9 ± 0.6 |
| 4MU | 164 ± 13 | 127 ± 5.1 | 10 ± 9 |
| Coumarin | 261 ± 20 | 162 ± 8.5 | 175 ± 11 |
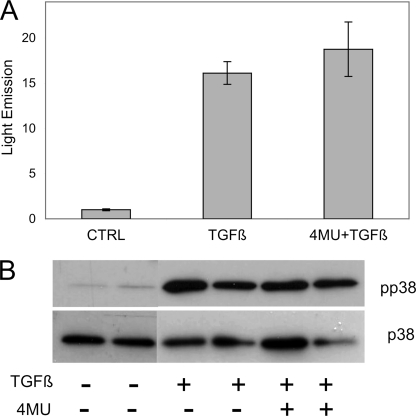
TGFβ Signaling
TGFβ is known to activate gene expression via the SMAD pathway, and up-regulation of αSMA by TGFβ has been linked to phosphorylation of p38 MAPK (21–23). To examine the effect of 4MU on early TGFβ signaling, primary keratocytes were transfected with a reporter plasmid 3TP-Lux in which luciferase transcription is driven by three TGFβ-responsive promoters (24, 25). As shown in Fig. 6A, TGFβ treatment for 24 h up-regulated luciferase activity ∼15-fold compared with mock-transfected cells not exposed to TGFβ. Treatment with 4MU had no effect on luciferase induction, indicating that 4MU did not directly activate this transcription by TGFβ signaling. Similarly, the presence of 4MU had no effect on phosphorylation of p38 MAPK in response to 1 h of treatment with TGFβ (Fig. 6B).
FIGURE 6.
4MU exerts no effect on initial TGFβ-induced signaling. A, light emission by 3TP-LUX, a luciferase reporter plasmid driven by TGFβ-responsive promoter, after 24 h TGFβ treatment stimulated light emission by ∼15-fold in primary keratocytes (p < 0.001). This reporter activity was not diminished by treatment with 500 μm 4MU. 3TP-Lux activity was normalized by co-transfection with control plasmid as described under “Materials and Methods.” The error bars show S.D. of quadruplicate analyses. The difference between TGFβ and TGFβ+4MU was not significant. B, duplicate cultures of primary keratocytes were treated for 1 h with TGFβ (as in Fig. 2) in the presence or absence of 400 μm 4MU. Equal amounts of protein from cell lysates were immunoblotted for phosphorylated p38 MAPK (pp38) using the nonphosphorylated p38 as a loading control. Coum, coumarin; 4ME, 4-methylesculetin; ESC, esculetin; CTRL, control.
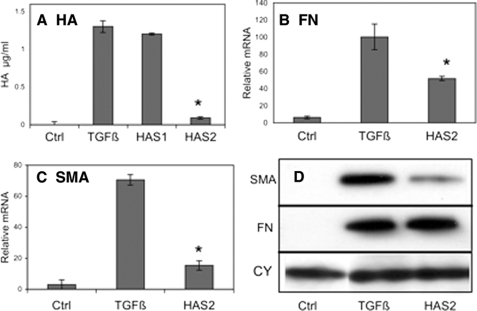
HAS2 Knockdown
We recently demonstrated that up-regulation of HAS2 mRNA in response to TGFβ could be largely eliminated by transfection of siRNA containing unique sequences of HAS2 (9). To test the involvement of HA in the TGFβ response, primary bovine keratocytes were transfected with siRNA to HAS2 or HAS1 mRNA. As we previously reported, the marked up-regulation of HA in response to TGFβ was inhibited >90% by HAS2 knockdown, but HAS1 knockdown had little effect (Fig. 7A). The HAS2 knockdown also significantly reduced stimulation of mRNA for EDA-FN (Fig. 7B) and for αSMA (Fig. 7C). The protein levels of αSMA also decreased in HAS2 siRNA transfected cells, and no alteration in cell-associated EDA-FN protein was observed.
FIGURE 7.
TGFβ stimulation of protein and mRNA for fibrotic markers is reduced by knockdown of HAS2 mRNA. Primary keratocytes were transfected with siRNA for HAS2, HAS1 or vehicle only and cultured 3 days in the absence (control, Ctrl) or presence of TGFβ as described for Fig. 2. A, as previously reported, knockdown of HAS2 mRNA, but not HAS1 RNA, markedly reduced HA expression (p < 0.01). B, EDA-fibronectin mRNA (FN) up-regulation by TGFβ was significantly reduced (p < 0.05) by HAS2 siRNA compared with nontransfected controls. RNA is expressed as fold increase from that in freshly isolated cells. C, stimulation of αSMA mRNA was similarly reduced by HAS2 siRNA transfection (p < 0.01). D, immunoblotting of αSMA and EDA-fibronectin as in Fig. 3 showed a decrease in αSMA protein in response to HAS2 knockdown but no apparent change in cell-associated EDA-FN. CY is a blot for cyclophilin (loading control) on the same membrane as FN. The error bars represent S.D. of triplicate analyses. Coum, coumarin; 4ME, 4-methylesculetin; ESC, esculetin. The asterisks show significant (p < 0.05, by t test) inhibition of TGFβ effect.
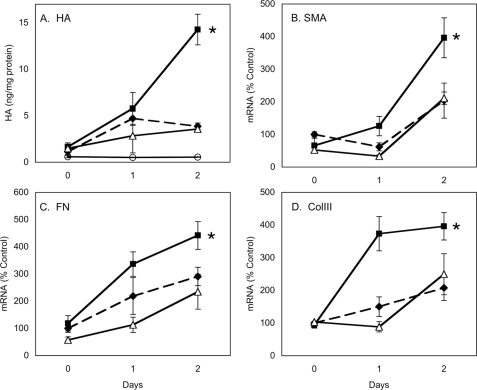
The results of these experiments suggest autocrine involvement of HA in activation of EDA-FN and αSMA gene expression; however, direct addition of HA to culture medium in either macromolecular or fragmented forms during TGFβ treatment had no effect on rescuing inhibition by 4MU or HAS2 knockdown (data not shown). Thus, soluble HA did not seem to be involved in stimulating the HA response. We hypothesized that the HA involved in the TGFβ response may be cell-associated. We therefore examined the effect of removal of cell-associated HA using a brief treatment with hyaluronidase. Because primary keratocytes do not synthesize HA in quiescent culture, the cells were pretreated for 24 h with PDGF, a cytokine previously found to up-regulate keratocyte HAS2 and HA secretion but not EDA-FN or αSMA expression (9). The cells were then treated for 15 min with hyaluronidase to remove cell-associated HA and exposed to PDGF plus TGFβ for an additional 48 h. As shown in Fig. 8, the PDGF pretreatment resulted in a significant stimulation of HA secretion as well as mRNA expression of EDA-FN and αSMA in comparison with cells not pretreated with PDGF (compare filled squares and filled diamonds). When the cells were subjected to brief hyaluronidase treatment before TGFβ treatment, the response pattern of HA, EDA-FN, and αSMA returned to the kinetics of cells not exposed to PDGF (compare filled diamonds with open triangles). These results suggest that the stimulating effect of PDGF was the result of cell-associated HA, induced by PDGF; such stimulation was removed by hyaluronidase treatment. Additionally we observed that HAS2 mRNA and cell-associated HA in hyaluronidase-treated cells responded with kinetics similar to that of untreated cells (data not shown), strengthening the hypothesis that the stimulation of response in PDGF-pretreated cells results from cell-associated HA. Similar results (not shown) were obtained with hyaluronidase preparations from Streptococcus and Streptomyces, as well as those shown with testicular hyaluronidase, supporting the idea that the response was the result of HA removal, not of contaminating glycosidases or proteases in the enzymes.
FIGURE 8.
PDGF stimulation is eliminated by hyaluronidase. Primary keratocytes were preincubated for 24 h in 10 ng/ml PDGF (solid lines) or in serum-free medium without PDGF (dashed lines). After preincubation (day 0), the cells were washed and treated for 15 min with hyaluronidase as described under “Materials and Methods” (open triangles). The cultures were thoroughly rinsed and incubated for 2 days in the presence of 10 ng/ml PDGF and 2 ng/ml TGFβ. HA was detected in culture media (A), and the relative abundance of mRNA is shown for SMA (B), EDA-FN (C), or COL3A1 (D). The values in A show HA concentration in the culture media normalized to cell protein (ng of HA/mg of protein). The cultures without PDGF and TGFβ served as controls (open circles). B–D show mRNA abundance relative to untreated control cells normalized to 100. The error bars show S.D. of n = 6 for A and n = 3 in B–D. In each case the filled squares (PDGF pretreatment) are significantly greater than the other two values at day 2 (asterisks, p < 0.05, by analysis of variance).
DISCUSSION
In this study we observed that 4MU is able to block HA synthesis induced in keratocytes by TGFβ in a dose-dependent manner. This observation is consistent with previous studies documenting inhibition of HA synthesis by 4MU in a wide variety of cultured cells. The novel aspect of the experimental system in the current study is that keratocytes make little if any HA. As we showed previously, HA is induced in these cells by a number of cytokines and growth factors by up-regulation of HAS2 mRNA. This up-regulation is rapid, is detected by 2 h after induction with growth factors, and reaches a peak at 6 h. The HAS2 up-regulation is additionally stimulated by the simultaneous presence of TGFβ. Because keratocyte HA synthesis can be rapidly induced and because that induction can be blocked with 4MU, these cells provide an extremely sensitive experimental system for investigation of functional roles for HA. The current study shows that blocking HA induction with 4MU blocks induction of several molecules involved in fibrotic responses to TGFβ.
We found that 4MU inhibited TGFβ-induced EDA-FN, collagen III, and αSMA, as well as cytoskeletal and extracellular reorganization. Each of these responses is typical of cells involved in wound healing. Expression of αSMA defines the myofibroblast phenotype. Inhibition of myofibroblast formation and of cell division by 4MU has been reported in cultured lung fibroblasts (26, 27). Our current study adds significant new information beyond the effect on αSMA response. We identified at least eight extracellular matrix genes up-regulated by TGFβ to be inhibited by 4MU. This novel observation is important to understanding the mechanism of TGFβ-induced fibrosis and points to potential clinical relevance for 4MU or its analogs as anti-fibrotic agents.
Several lines of evidence support the idea that the effects of 4MU are related to its ability to block synthesis of HA. Examination of early TGFβ signaling events (Fig. 6) shows that 4MU is not a general inhibitor of TGFβ-induced responses in corneal cells. The response of the 3TP-Lux reporter plasmid to TGFβ was not altered (Fig. 6A), and the rapid phosphorylation of p38 MAPK, implicated in TGFβ induced fibrosis, was not blocked (Fig. 6B). These results are consistent with a recent study showing phosphorylation of SMAD3 to be unaltered by 4MU treatment of lung fibroblasts (26). Additionally, we found that the effects of 4MU on keratocytes were reversible on withdrawal of the drug (data not shown). These observations support the idea that the 4MU effect is limited in scope, and its effects are targeted via a specialized mechanism. The most well established molecular function of 4MU is a competitive inhibition of HA biosynthesis by direct interaction with HAS enzymes. We hypothesize that the effect of 4MU on fibrosis is a downstream result of this inhibition. This hypothesis is supported by the observations that analogs of 4MU have the ability to block induction of EDA-FN and αSMA in proportion with their ability to block HA synthesis (Table 1).
A direct role for HA in the TGFβ response is independently supported by the observation that knockdown of HAS2 with siRNA, previously characterized as specific, also mediated an inhibition in the TGFβ induction of mRNA for αSMA and EDA-FN. Finally, a third line of evidence suggesting involvement of HA in the induction of TGFβ-responsive genes came from the observation that hyaluronidase treatment of the cells before TGFβ exposure eliminated stimulation imparted by preincubation with PDGF. We previously found PDGF to up-regulate HAS2 and HA synthesis in these cells (9); thus reversal of the PDGF stimulation by hyaluronidase is consistent with the model that cell-associated HA has a direct role in mediating TGFβ up-regulation of a number of genes involved in fibrosis.
The data presented here are all consistent with the involvement of HA in the fibrotic response; however, they do not provide a molecular mechanism. 4MU was more effective at suppression of fibrotic responses than knockdown of HAS2, suggesting that 4MU may inhibit other components involved in the signaling cascade. Similarly, we were unable to rescue 4MU inhibition by the addition of soluble HA to the cultures, either as intact molecules or as fragments (data not shown). This and the effect of hyaluronidase treatment both suggest that the signaling may be initiated by cell-associated HA, possibly HA actually in the process of secretion by HAS2. Recent studies have shown 4MU not only blocks HA secretion but blocks HAS enzyme expression. Thus, this double effect on biosynthesis and secretion may account for the increased effectiveness of this compound compared with HAS2 knockdown (28). From our current data we hypothesize that a cell surface association of HA receptor (possibly CD44) and HAS2 is involved in stimulating the TGFβ-enhanced signaling event but that this signal may not directly involve the TGFβ receptor or its signaling via the SMAD proteins.
Keratocytes are normally quiescent cells expressing a unique extracellular matrix required for corneal transparency. In response to TGFβ, both in vivo and in vitro, these cells up-regulate mRNA and protein of a number of cell-associated and matrix proteins found in corneal scars. In this study we focused on three such proteins: αSMA, EDA-fibronectin, and collagen type III. None of these proteins are present in normal cornea, but they appear in healing corneal wounds and corneal scars. HA also appears in corneal healing in response to TGFβ but much earlier than other fibrotic and myofibroblast markers. The current study supports the idea that, in vivo, early secretion of HA by keratocytes enhances myofibroblast and scar tissue formation. Corneal scarring is permanent and accounts for blindness of millions of individuals worldwide. The role of HA in this process and the ability to block its synthesis with inhibitors such as 4MU therefore present a potential for therapeutic applications.
Acknowledgment
We appreciate the help of Kira Lathrop.
This work was supported, in whole or in part, by National Institutes of Health Grant EY09368 (to J. L. F.) and Core Grant P30-EY08098. This work was also supported by Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh.
- αSMA
- α-smooth muscle actin
- HAS
- hyaluronan synthase
- HA
- hyaluronan
- 4MU
- 4-methylumbelliferone
- EDA
- extra domain A
- FN
- fibronectin
- FACE
- fluorophore-assisted carbohydrate electrophoresis.
REFERENCES
- 1.Fini M. E. (1999) Prog. Retin. Eye Res. 18, 529–551 [DOI] [PubMed] [Google Scholar]
- 2.Funderburgh J. L., Funderburgh M. L., Mann M. M., Corpuz L., Roth M. R. (2001) J. Biol. Chem. 276, 44173–44178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funderburgh J. L., Mann M. M., Funderburgh M. L. (2003) J. Biol. Chem. 278, 45629–45637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berryhill B. L., Kane B., Stramer B. M., Fini M. E., Hassell J. R. (2003) Exp. Eye Res. 77, 85–92 [DOI] [PubMed] [Google Scholar]
- 5.Cintron C., Hong B. S., Covington H. I., Macarak E. J. (1988) Invest. Ophthalmol. Vis. Sci. 29, 767–775 [PubMed] [Google Scholar]
- 6.Jester J. V., Barry-Lane P. A., Petroll W. M., Olsen D. R., Cavanagh H. D. (1997) Cornea 16, 177–187 [PubMed] [Google Scholar]
- 7.Jester J. V., Barry-Lane P. A., Cavanagh H. D., Petroll W. M. (1996) Cornea 15, 505–516 [PubMed] [Google Scholar]
- 8.Fitzsimmons T. D., Molander N., Stenevi U., Fagerholm P., Schenholm M., von Malmborg A. (1994) Invest. Ophthalmol. Vis. Sci. 35, 2774–2782 [PubMed] [Google Scholar]
- 9.Guo N., Kanter D., Funderburgh M. L., Mann M. M., Du Y., Funderburgh J. L. (2007) J. Biol. Chem. 282, 12475–12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itano N., Kimata K. (2002) IUBMB Life 54, 195–199 [DOI] [PubMed] [Google Scholar]
- 11.Adamia S., Maxwell C. A., Pilarski L. M. (2005) Curr. Drug. Targets Cardiovasc. Haematol. Disord. 5, 3–14 [DOI] [PubMed] [Google Scholar]
- 12.Turino G. M., Cantor J. O. (2003) Am. J. Respir Crit. Care. Med. 167, 1169–1175 [DOI] [PubMed] [Google Scholar]
- 13.Toole B. P. (2002) Glycobiology 12, 37R–42R [DOI] [PubMed] [Google Scholar]
- 14.Mohan R. R., Hutcheon A. E., Choi R., Hong J., Lee J., Ambrósio R., Jr., Zieske J. D., Wilson S. E. (2003) Exp. Eye Res. 76, 71–87 [DOI] [PubMed] [Google Scholar]
- 15.Zieske J. D., Guimarães S. R., Hutcheon A. E. (2001) Exp. Eye Res. 72, 33–39 [DOI] [PubMed] [Google Scholar]
- 16.Turley E. A., Noble P. W., Bourguignon L. Y. (2002) J. Biol. Chem. 277, 4589–4592 [DOI] [PubMed] [Google Scholar]
- 17.Itano N., Atsumi F., Sawai T., Yamada Y., Miyaishi O., Senga T., Hamaguchi M., Kimata K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funderburgh M. L., Mann M. M., Funderburgh J. L. (2008) Mol. Vis. 14, 308–317 [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y., Sundarraj N., Funderburgh M. L., Harvey S. A., Birk D. E., Funderburgh J. L. (2007) Invest. Ophthalmol. Vis. Sci. 48, 5038–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morohashi H., Kon A., Nakai M., Yamaguchi M., Kakizaki I., Yoshihara S., Sasaki M., Takagaki K. (2006) Biochem. Biophys. Res. Commun. 345, 1454–1459 [DOI] [PubMed] [Google Scholar]
- 21.Meyer-Ter-Vehn T., Gebhardt S., Sebald W., Buttmann M., Grehn F., Schlunck G., Knaus P. (2006) Invest. Ophthalmol. Vis. Sci. 47, 1500–1509 [DOI] [PubMed] [Google Scholar]
- 22.Meyer-Ter-Vehn T., Katzenberger B., Han H., Grehn F., Schlunck G. (2008) Invest. Ophthalmol. Vis. Sci. 49, 3955–3960 [DOI] [PubMed] [Google Scholar]
- 23.Sebe A., Leivonen S. K., Fintha A., Masszi A., Rosivall L., Kähäri V. M., Mucsi I. (2008) Nephrol. Dial. Transplant. 23, 1537–1545 [DOI] [PubMed] [Google Scholar]
- 24.Kim S., Lee Y., Seo J. E., Cho K. H., Chung J. H. (2008) Cell. Signal. 20, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 25.Li F., Cao Y., Townsend C. M., Jr., Ko T. C. (2005) World J. Surg. 29, 306–311 [DOI] [PubMed] [Google Scholar]
- 26.Webber J., Meran S., Steadman R., Phillips A. O. (2009) J. Biol. Chem. 284, 9083–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webber J., Jenkins R. H., Meran S., Phillips A., Steadman R. (2009) Am. J. Pathol. 175, 148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kultti A., Pasonen-Seppänen S., Jauhiainen M., Rilla K. J., Kärnä R., Pyöriä E., Tammi R. H., Tammi M. I. (2009) Exp. Cell. Res. 315, 1914–1923 [DOI] [PubMed] [Google Scholar]